Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Introduction to Chapter 29, Pediatric Thyroid Cancer.
Much like many childhood diseases, there are major differences in the diagnosis and management of pediatric patients with thyroid cancers compared with adults. This chapter highlights some of those differences and reviews the current approach to the etiology, diagnosis, and treatment of pediatric thyroid cancer. Thyroid cancer accounts for approximately 1% to 3% of all childhood malignancies and 7% of all pediatric head and neck tumors. Although thyroid malignancy in children is less common than thyroid cancer in adult patients, a similar annual increase in the number of thyroid cancer diagnoses has been reported in children, with an increase between 2% to 3.8% per year per the Surveillance, Epidemiology, and End Results (SEER) database. The largest increase is found in female patients between 15 to 19 years of age where thyroid cancer accounts for 8% of all cancers. Over 85% of childhood thyroid carcinomas are papillary thyroid cancer (PTC) with the remainder divided between follicular thyroid cancer (FTC) and medullary thyroid cancer (MTC), with the majority of MTC associated with multiple endocrine neoplasia type 2 (MEN 2).
For the majority of children and adolescents diagnosed with thyroid cancer or thyroid nodules, there is no identifiable risk factor. A cohort of pediatric patients is at higher risk of developing thyroid cancer—those with a history of exposure to ionizing radiation and those with a genetic predisposition. Genetic predisposition may present as a family history of isolated differentiated thyroid cancer (DTC; familial nonmedullary thyroid cancer) or with de novo or inherited mutations in oncogenes that increase the risk of developing thyroid malignancy.
The association between thyroid cancer and prior radiation exposure is well established and has been documented for decades. Initial observations about this relationship were made in patients receiving radiation for the treatment of benign diseases, such as tonsillar hypertrophy, thymic hyperplasia, acne, and tinea capitis. The development and use of nuclear weapons resulted in further understanding of the relationship between radiation exposure and the development of thyroid cancer after observations that residents exposed to nuclear fallout after atomic bomb testing and survivors of nuclear attacks in Hiroshima and Nagasaki, Japan, had an increased incidence of thyroid cancer. By 1954, nuclear power plants for commercial production of energy were in operation. By 2017, there were more than 450 nuclear power plants in operation across the world, and, fortunately, only three significant accidents (meltdowns) have occurred as of 2018: Three Mile Island, United States (1979), Chernobyl, Ukraine (1986), and Fukushima Daiichi, Japan (2011). Each accident has provided valuable lessons, but Chernobyl stands out as providing the most informative data on the risks of inhaled and ingested 131 I in the development of thyroid cancer in pediatric patients as the incidence of thyroid cancer increased by a factor of more than 60 in the populations of both Belarus and Ukraine. The initial cohort of pediatric patients who developed PTC after Chernobyl presented within 5 years of the accident and presented with clinically advanced disease, with regional lymph node (LN) metastases and pulmonary metastases in 60% and 24% of patients, respectively, and 50% of tumors exhibiting extrathyroidal extension.
Medical exposure to ionizing radiation is also associated with an increased risk of thyroid cancer and may occur during diagnostic, radiologic imaging, as well as part of treatment regimens for nonthyroid malignancy. Across the various imaging modalities, the increased use of computed tomography (CT) scans is believed to account for up to 47% of the collective effective dose of radiation exposure from diagnostic imaging across the world. Although radiation exposure resulting from a single CT scan is low, and this exposure is further reduced by using pediatric-specific protocols, cumulative exposure from serial CT imaging may contribute to an increased risk of thyroid nodules and thyroid cancer, especially in pediatric patients due to increased proliferative cellular activity observed during childhood.
In childhood cancer survivors who received therapeutic radiation therapy (RT), thyroid nodules develop at a rate of about 2% annually, reaching a peak incidence 15 to 25 years after treatment. Approximately 20% of long-term pediatric cancer survivors will have a > 1 cm nodule detected by ultrasound (US) that is not clinically apparent on physical examination alone. This risk is greatest after RT at a young age (< 10 years) and with doses up to 20 to 29 Gy. Overall, the standard incidence ratio for radiation-induced DTC ranges from 5- to 70-fold, with younger age and dose of exposure correlating with the highest risk.
The second most common identifiable risk factor for the development of thyroid cancer is genetic predisposition, which may be divided into two broad categories: nonsyndromic and syndromic (thyroid cancer associated with other tumors). Familial nonmedullary thyroid cancer (FNMTC) is an inherited predisposition to DTC without an increased risk for the development of additional tumors (see Chapter 30 , Familial Nonmedullary Thyroid Cancer). In families with two or more first-degree relatives with DTC, there is an 8- to 10-fold increased risk of developing either PTC or FTC. Similar to sporadic DTC, 85% of FNMTC is composed of patients with PTC. The transmission pattern is most consistent with an autosomal dominant mode of inheritance; however, to date, a single germline locus has not been identified. Compared with sporadic DTC, FNMTC appears to present at a younger age and exhibits clinical anticipation between generations, whereas subsequent generation family members present with earlier and more invasive disease. In families with three or more affected individuals, US screening of other at-risk family members has been shown to detect disease at an earlier stage, allowing for less aggressive treatment.
Syndromic forms of thyroid cancer in which there is a familial genetic tumor predisposition to the development of thyroid malignancy and other tumors include PTEN hamartoma tumor syndrome (PHTS), familial adenomatous polyposis (FAP), DICER1 syndrome, Carney complex, and MEN 2 ( Table 29.1 ).
| Tumor Syndrome | Thyroid Pathology | Risk of Developing Thyroid Cancer | Other Cancers | Gene |
|---|---|---|---|---|
| PTEN Hamartoma Syndrome | Papillary thyroid cancer or follicular thyroid cancer | 35% (> 75% develop multinodular goiter) | Women—breast cancer and endometrial cancer (Cowden syndrome) Men and women—intestinal polyps, colorectal cancer, renal cell carcinoma, cutaneous melanoma |
PTEN |
| Familial adenomatous polyposis (FAP, also known as Gardner’s syndrome) | Papillary thyroid cancer (cribriform-morular variant) | 12% | Intestinal polyps, colorectal carcinoma, small bowel cancer, hepatoblastoma, osteomas, dental and cutaneous abnormalities (cysts and dermoid tumors), hypertrophy of the retinal pigment epithelium (CHRPE) | APC |
| DICER1 syndrome | Papillary thyroid cancer or follicular thyroid cancer | 16% (> 50% develop multinodular goiter) | Pleuropulmonary blastoma (birth to age 5 years), Sertoli-Leydig cell tumors, cystic nephroma, Wilms tumor, eye and nose tumors, botryoid embryonal rhabdomyosarcoma, pituitary blastoma | DICER1 |
| Carney complex | Papillary thyroid cancer or follicular thyroid cancer | < 5% (> 50% develop follicular adenomas) | Lentigines, nerve sheath tumors (schwannomas), myxomas, primary, pigmented nodular adrenocortical disease, growth hormone-secreting pituitary adenomas, large-cell calcifying Sertoli-cell tumors (males) | PRKAR1A |
| Multiple endocrine neoplasia, type 2 | Medullary thyroid cancer | > 98% | Parathyroid adenoma, pheochromocytoma | RET proto-oncogene |
Additional risk factors for the development of thyroid nodules and thyroid cancer include iodine intake and a personal history of autoimmune thyroiditis. The roles of iodine deficiency and iodine excess in thyroid cancer tumorigenesis have both been examined. Current data suggest that iodine deficiency is a weak initiator but a strong promoter of DTC, in particular for FTC, likely secondary to chronic thyroid-stimulating hormone (TSH) stimulation. Autoimmune thyroiditis, both hypothyroidism (Hashimoto’s thyroiditis) and hyperthyroidism (Graves’ disease), have also been associated with increased risk.
PTC and its variants are the most common histologic subtype of childhood thyroid cancer (> 85%; see Chapter 41 , Surgical Pathology of the Thyroid Gland). PTC subtypes include classic PTC (CPTC), follicular variant PTC (FVPTC), and, additionally, more invasive forms of PTC, including diffuse sclerosing variant PTC (DSVPTC), widely invasive (diffuse) follicular variant PTC, solid-trabecular PTC (SPTC), and tall-cell variant PTC (TCVPTC). In radiation-induced tumors, a solid-trabecular growth pattern is more common than a dominant papillary growth pattern. The remainder of thyroid cancers diagnosed in children and adolescents are FTC, MTC, and, very rarely, poorly DTC.
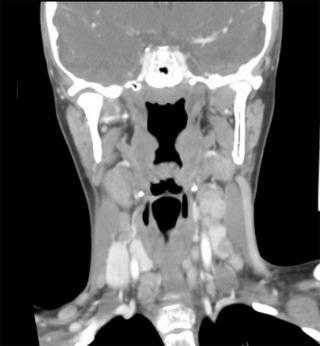
Within each variant, the histologic appearance of thyroid cancer in children is similar to adults. Childhood PTC has a high predilection to invade lymphatics, giving rise to multifocal disease through intraglandular spread in 30% to 80% of patients, a high incidence of regional LN metastases in up to 70% to 90% of patients ( Figures 29.1 and 29.2 ), and an increased rate of distant metastasis, primarily to the lungs, in 8% to 20% of patients at the time of diagnosis. Additionally, PTC can directly invade local structures, such as the esophagus, trachea, recurrent laryngeal nerve (RLN), and blood vessels, creating significant local morbidity.
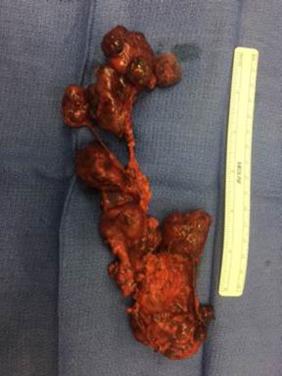
DTC usually presents as a firm, partially encapsulated or nonencapsulated, infiltrative mass. Encapsulated CPTC appears to have a similar risk for regional LN metastasis compared with partially or unencapsulated CPTC; however, under strict criteria for diagnosis, encapsulated FVPTC (EFVPTC) has a lower risk for intrathyroidal or LN metastasis. The most indolent form of EFVPTC is referred to as a noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP)— a recent change in nomenclature to designate the benign behavior of this neoplasm subtype. Although most data suggest that NIFTPs are indolent, there are reports that associated LN metastasis may arise, emphasizing the need to ensure the diagnosis is used following strict criteria and that surveillance may still be appropriate to ensure noninvasive behavior.
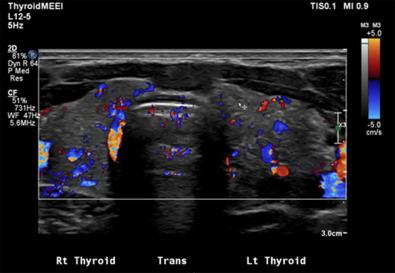
Invasive or aggressive forms of PTC, including DSVPTC, widely invasive (diffuse) follicular variant PTC, SPTC, and TCVPTC, are associated with an increased risk of regional and distant metastasis. DSVPTC typically does not present as a distinct thyroid nodule; rather, it displays diffuse infiltrative invasion of part or all of the thyroid with innumerable microcalcifications (the “snow-storm” appearance on US; Figure 29.3 ) (see section dedicated to DSVPTC at the end of this chapter). Other forms of invasive PTC may display similar behavior, including aggressive local invasion with extrathyroidal and extranodal extension and an increased risk of pulmonary metastasis. On occasion, a mixed histology will be present. In these cases, one should direct treatment and surveillance based on the most invasive variant.
FTC, like PTC, is a well-differentiated cancer that produces thyroglobulin (Tg) and has the capacity to transport iodine across the cell membrane via the sodium-iodine symporter (NIS). FTC is less common than PTC but shows an increased incidence in iodine deficient regions of the world as well as in certain thyroid tumor predisposition syndromes, in particular PHTS. In contrast to PTC, FTC metastasizes hematogeneously. The risk of local and distant metastasis is determined by the extent of capsular invasion as well as the extent of metastasis into blood vessels in the tumor capsule and pericapsular space. Within pediatrics, minimally invasive FTC defined as microscopic invasion into the capsule with < 4 capsular or pericapsular blood vessels showing evidence of invasion is more common than widely invasive FTC. Angioinvasive FTC is associated with an increased risk of distant metastasis with higher rates of recurrence and poorer outcomes in tumors with metastasis into > 4 blood vessels. Due to the rarity of FTC in pediatrics, it is unclear if the size criteria established in adults, FTC > 4 cm, increases the risk of invasive behavior. Widely invasive FTC and Hürthle cell carcinoma are uncommon in pediatrics but are associated with an increased risk of regional and distant metastasis (lungs and bone).
Medullary thyroid cancer (MTC), unlike thyroid cancers of follicular cell origin, originate from the parafollicular C cells. These cells are derived from neural crest derivatives and do not express the TSH-receptor, Tg, or NIS. Thus MTC does not respond to TSH suppressive therapy, and 131 I therapy is ineffective in the treatment of MTC. In addition, calcitonin, along with carcinoembryonic antigen (CEA) rather than Tg, is secreted and used as a marker of tumor progression. With uncommon exception, MTC in pediatrics is associated with MEN 2 with only case reports of sporadic MTC. MTC tumorigenesis follows the same pattern of development in pediatrics as in adults, starting with C-cell hypertrophy (CCH) with progression to MTC. The CT level is predictive of MTC development, progression, and metastasis (see Chapter 26 , Sporadic Medullary Thyroid Carcinoma, and Chapter 27 , Syndromic Medullary Thyroid Cancer: MEN 2A and MEN 2B). Surgical management of MTC in children is similar to its management in adult patients, with early total thyroidectomy recommended for patients with American Thyroid Association (ATA) “high” and “highest” risk RET proto-oncogene mutations.
Alterations in the oncogenes (mutations and fusions) that drive tumorigenesis in DTC and MTC are similar between pediatric and adult patients. However, differences in clinical behavior of these tumors, most importantly, maintenance of cellular differentiation, suggest that there are likely differences in gene expression and downstream signaling pathways between pediatric and adult DTC. The consequences of these differences are reflected by increased disease-specific mortality in adult patients with regional and distant metastasis compared with pediatric patients with similar disease burden.
In DTC, thyroid tumorigenesis and progression are associated with somatic point mutations of BRAF, DICER1 , and the RAS genes, as well as fusions involving the rearranged during transfection ( RET ), neurotrophic tyrosine receptor ( NTRK ), and anaplastic lymphoma ( ALK ) kinases, with resultant constitutive activation of the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) signaling pathways (see Chapter 18 , Molecular Pathogenesis of Thyroid Neoplasia). With uncommon exceptions, these oncogene mutations are mutually exclusive events, and there is a fairly predictable relationship between oncogenic genotype and histopathologic phenotype, with RET-PTC (RET/PTC) rearrangements and B-rapidly accelerated fibrosarcoma (BRAF) point mutations common in PTC, paired-box gene 8 (PAX8) -peroxisome proliferator-activated receptor gamma (PPARγ) common in FTC, and RAS , DICER1 , and PTEN mutations found across the spectrum of thyroid tumors, from benign follicular adenomas to FVPTC, FTC, and poorly differentiated thyroid carcinoma. Additional point mutations and fusions have been reported and more recent data in adults suggest that second or third mutations, in particular telomerase reverse transcriptase (TERT) + BRAF or RAS and EIF1AX + RAS, are associated with tumor progression and dedifferentiation. In pediatrics, RET/PTC and NTRK -fusion genes are associated with an increased risk of invasive disease, although there are no data to suggest that these alterations are associated with increased disease-specific mortality. In children, BRAF mutations may not increase the risk of invasive or refractory disease. A summary of the most common oncogenes and their association with DTC and clinical behavior is provided in Table 29.2 .
| Oncogene | Increased Risk of Differentiated Thyroid Cancer | Increased Risk of Invasive Disease |
|---|---|---|
| BRAF RET - PTC fusion NTRK -fusion BRAF -fusion |
Yes Yes Yes Yes |
Adults (yes) Pediatrics (no) Yes Yes (limited data) Yes (very limited data) |
| DICER1 RAS PAX8 - PPARγ |
Yes, but also found in benign disease (NIFTP and FA) Yes (FTC) |
No May be associated with dedifferentiated disease (uncommon in pediatrics) No |
| TSHR , THADA , GNAS | No | Not applicable |
Overall, MTC in adults may either develop secondary to sporadic, somatic mutations (75%) or be associated with germline RET mutations (25%). Sporadic MTC is uncommon in pediatrics and is most commonly associated with somatic mutations in the RET proto-oncogene and RAS, as well as several additional genes, including fusion genes involving anaplastic lymphoma kinase ( ALK ). Familial MTC is one of several tumors syndromes that define MEN 2. MEN 2 is caused by activating mutations in the RET proto-oncogene that are transmitted via the germline in an autosomal dominant pattern of inheritance. The expression and penetrance of the tumors associated with MEN 2, including MTC, parathyroid adenomas, and pheochromocytoma, correlate with the specific RET proto-oncogene codon mutation (i.e., there is a correlation between genotype and phenotype; see Chapter 27 , Syndromic Medullary Thyroid Carcinoma: MEN 2A and MEN 2BMTC). Mutations in codon M918T are associated with the most aggressive form of MTC and designated as “highest” risk under the ATA guidelines with a risk of MTC developing before 1 year of age. MEN 2B is also associated with early signs and symptoms, including alacrima (the inability or decreased ability to make tears), constipation (associated with ganglioneuromatosis), and hypotonia, (feeding difficulties with failure to thrive, club feet, hip dislocation). The more classically defining symptoms, including oral and lip mucosal neuromas and elongated, marfanoid facies, are not clinically evident until school age, around 5 years of age. Because in MEN 2B germline mutations in codon 918 are more often de novo , recognition of the early clinical signs and symptoms is critically important to diagnose the syndrome before MTC metastasis, which often occurs before 4 years of age. Fifty percent of patients will develop a pheochromocytoma; however, unlike patients with MEN 2A, there does not appear to be an increased risk for developing hyperparathyroidism.
There are multiple codon mutations associated with MEN 2A, with the highest risk of developing MTC associated with mutations in C634 and A883F. The remaining mutations carry an increased risk of developing MTC, although for individual patients, the course from CCH to MTC may be quite indolent with MTC not developing until the third, fourth, or later decades of life. There is a 10% to 50% risk of developing hyperparathyroidism and/or pheochromocytoma, defined by the individual codon mutation. Two additional features associated with MEN 2A are cutaneous lichen amyloidosis, a pruritic, plaque-like rash typically on the upper back associated with mutations in C634 and V804M, and Hirschsprung’s disease, associated with mutations in C609, C611, C618, and C620.
The clinical presentation of pediatric DTC can be variable. Small thyroid masses may be incidentally noted on imaging studies obtained for another indication. Larger lesions can present as a neck lump notable on physical examination. Owing to the attachment of the thyroid gland to the airway, associated tumors classically elevate along with the laryngo-tracheal complex as the patient swallows. In the context of thyroid cancer, masses located more laterally in the neck raise concern for disease metastatic to the lateral neck nodes. Involvement of cervical LNs in the central neck may be less easily distinguishable from the primary lesion or thyroid gland by physical examination alone. Imaging studies, including cervical US and, in select cases, cross-sectional imaging, can further demonstrate the relationship between the thyroid gland, associated intrathyroidal masses, and worrisome cervical lymphadenopathy. Clinical suspicion for thyroid cancer is higher in patients with a history of neck radiation, autoimmune thyroiditis, select cancer predisposition syndromes, or a family history of thyroid cancer. Along with obtaining a comprehensive patient and family history, the initial clinical evaluation should include a complete head and neck examination, including confirmation of the status of vocal fold mobility through fiberoptic laryngoscopy preoperatively.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here