Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The word “plastic” is derived from the Greek word for “moldable.” Therefore, plastic surgery refers to surgery that focuses on form and function. Form is often related to appearance, which is why aesthetics usually plays an important role in plastic surgery. Although all surgeons repair function to some degree, plastic surgery emphasizes functions that improve quality of life, such as eating, speaking, self-confidence, and social interactions. Patients with craniofacial anomalies, for example, may be able to eat and breathe, but because one of the main functions of the face is to look like a face, such individuals often suffer from significant isolation and social anxiety. Similarly, although speech is not essential for survival, a patient with a cleft-related speech dysfunction may have profound difficulty communicating in peers, which again can lead to isolation.
Because many congenital deformities impact both function and appearance, problems encountered in pediatric plastic surgery can be distressing for patients and their families. The newborn’s anomalies are immediately apparent, causing emotions that range from guilt to fear. The face is so central to human identity and recognition that craniofacial abnormalities can affect bonding, integration, and socialization from infancy onward. As children learn to recognize self and non-self, apparent differences can lead to teasing and suffering that can affect normal psychological development. Defects that are cosmetic in adults can be devastating in children.
Many pediatric anomalies are associated with more extensive disorders or syndromes, making identification crucial for diagnosis and prognosis. Additionally, children are especially prone to trauma and other acquired abnormalities that require the surgeon to apply principles of reconstruction both for form and function, but also for growth. This chapter familiarizes the pediatrician or neonatologist with the most common pathologies of form and function that pediatric plastic surgeons treat. It is important to educate parents that although improvements are the rule rather than the exception, the severity of the defect, variability in wound healing, and the growth of the patient make the final result difficult to predict.
There is no more human quality than the familiar external form of the head and face, which is the domain of the plastic surgeon specializing in craniofacial surgery. Likewise, human speech is complex and shared by no other creature, and thus, palate and speech surgery are also within the same field. On the other hand, all animals share the common internal structures and functions involved with hearing and ear drainage, nasal drainage, airway, sight, and mastication. These are treated by non–plastic surgery subspecialists. The external shapes of the ear, nose, or lips are clearly the realm of the plastic surgeon.
This section divides the craniofacial arena into upper third, middle third, and lower third. The upper third deals with abnormalities of the skull and forehead, including deformational plagiocephaly, and both nonsyndromic and syndromic craniosynostosis. The middle third covers the maxilla, orbits, and the external ear and nose. The lower third includes the mandible and tongue. Cleft lip and nasal deformities and cleft palates are discussed separately.
Congenital anomalies are best understood from an embryologic perspective. Craniofacial development occurs 4 to 7 weeks after conception. At 4 weeks, the fetus has a clear cranial/caudal axis and differentiated endoderm, mesoderm, and ectoderm. On either side of the neural tube, the paraxial mesoderm divides into segmented tissue blocks called somitomeres —cephalically, and somites from the occiput caudally, which ultimately form the bones of the neurocranium, or protective vault of the brain. Simultaneously, mesenchymal differentiation of neural crest cells participates in the formation of the viscerocranium or the facial skeleton.
The neurocranium consists of plates that ultimately become the adult frontal, occipital, sphenoid, ethmoid, paired temporal, and paired parietal bones ( Fig. 23.1 ). They are separated by sutures and fontanelles that serve two main purposes: (1) to allow molding of the head as it passes through the birth canal during parturition, and (2) to allow for rapid increase of brain volume, which doubles in the first 6 months of life and again by 2 years old.
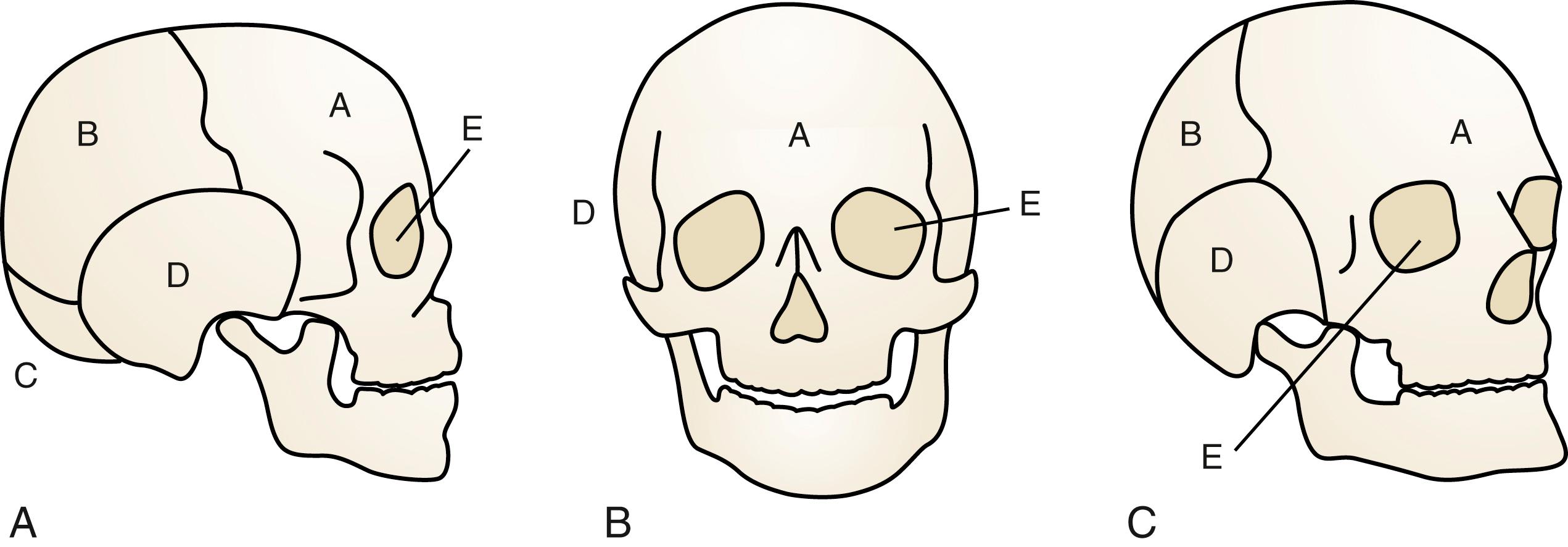
The longitudinal suture between the paired parietal bones is the sagittal suture. Anteriorly, the sagittal suture becomes the anterior fontanelle where it intersects the paired coronal sutures that separate the frontal bones from the parietal bones. Posteriorly, the sagittal suture becomes the posterior fontanelle where it meets the oblique L -shaped lambdoid sutures. Finally, the metopic suture runs longitudinally between the two paired frontal bones ( Fig. 23.2 ). Closure of the posterior fontanelle occurs within the first 6 months of life, whereas the anterior fontanelle closes between 12 and 18 months old. The metopic suture closes at about 7 months old, and it completely fuses such that the adult frontal bone has no evidence of a former metopic suture. The sagittal and coronal sutures are next to fuse in adulthood, in a posterior-to-anterolateral direction. Prenatal or postnatal premature fusion is called craniosynostosis, which causes abnormal skull shape by restricting growth in the direction of the fusion, and can lead to abnormal intracranial pressure as the brain grows against a fixed restriction.
The facial skeleton, or viscerocranium, is supported on a scaffold of 14 bones: the vomer; the mandible; and the paired nasal, maxilla, lacrimal, zygoma, palatal, and inferior nasal concha. By the end of the fourth gestational week, the neural crest–derived mesenchyme differentiates to form three facial prominences: the maxillary, mandibular, and frontonasal. Over the course of the next 2 weeks, migration and fusion result in the sculpture of the facial features supported by the underlying bony face. The frontal/nasal prominence gives rise to the forehead, bridge of the nose, and medial and lateral nasal prominences that further define the lower nose. The maxillary prominence evolves into the cheeks, palate, and lateral aspect of the upper lip; and the mandibular prominence develops into the lower lip. Abnormal migration, fusion, or disruption of established events during this cascade results in anomalies.
Cleft lip, nose, and palate are the most frequent presentations of orofacial clefts, which are the most common congenital anomalies in the United States (Basseri et al., 2011; Centers for Disease Control and Prevention, 2006; Parker et al., 2010) ( Table 23.1 ). Approximately 1 in 700 individuals in the United States is affected, or about 15,000 live births per year. Although the cleft may be a component of an identifiable syndrome, it more commonly occurs as a solitary nonsyndromic defect. The clefting of the lip always affects the shape of the nose as well. Facial clefting is frequently categorized into cleft lip with or without cleft palate and the isolated cleft palate. Epidemiologically, a distinction is notable between the two with respect to incidence, race, and sex. Approximately 1 in 700 live births is affected with a cleft lip with or without cleft palate, occurring twice as often in males. Asians constitute the largest population of affected infants, followed by the white population, and then African Americans. Cleft palate has a lower incidence of approximately 1 in 1000 live births, with slightly more females affected, but no ethnic predilection.
| Cleft Lip/Nose ± Cleft Palate | Isolated Cleft Palate | |
|---|---|---|
| Incidence | 1 in 700 | 1 in 1500 |
| Sex | M > F (2:1) | F > M (3:2) |
| Race | Asian > Caucasian > African (4:2:1) | No difference |
| Syndromic association | 15% | 50% |
Embryologically, at the end of the fifth week the maxillary prominences grow medially, and the medial nasal prominences are displaced toward the midline, where they fuse and ultimately form the premaxilla, which contains the philtrum of the upper lip, the portion of the upper jaw carrying the incisors, and the triangular primary (anterior) palate. Simultaneously, maxillary prominences develop outgrowths called the palatine shelves, which fuse in the midline, forming the secondary (posterior) palate. The primary and secondary palates join at the incisive foramen to separate the nasal and oral cavities. Failure of fusion of the palatine shelves results in secondary palatal clefting, whereas partial or complete lack of fusion of the maxillary prominence with the medial nasal prominence on one or both sides results in lip clefting with or without clefting of the primary and secondary palates ( Fig. 23.3 ). This developmental cascade is complete by the 12th week of gestation. During this vulnerable period, anatomic interference (malposition of the tongue due to mandibular hypoplasia, as in Pierre Robin sequence), miscues in cell differentiation and migration, or teratogens (phenytoin, retinoids, steroids, lithium, and maternal smoking) may lead to clefting in the developing fetus.
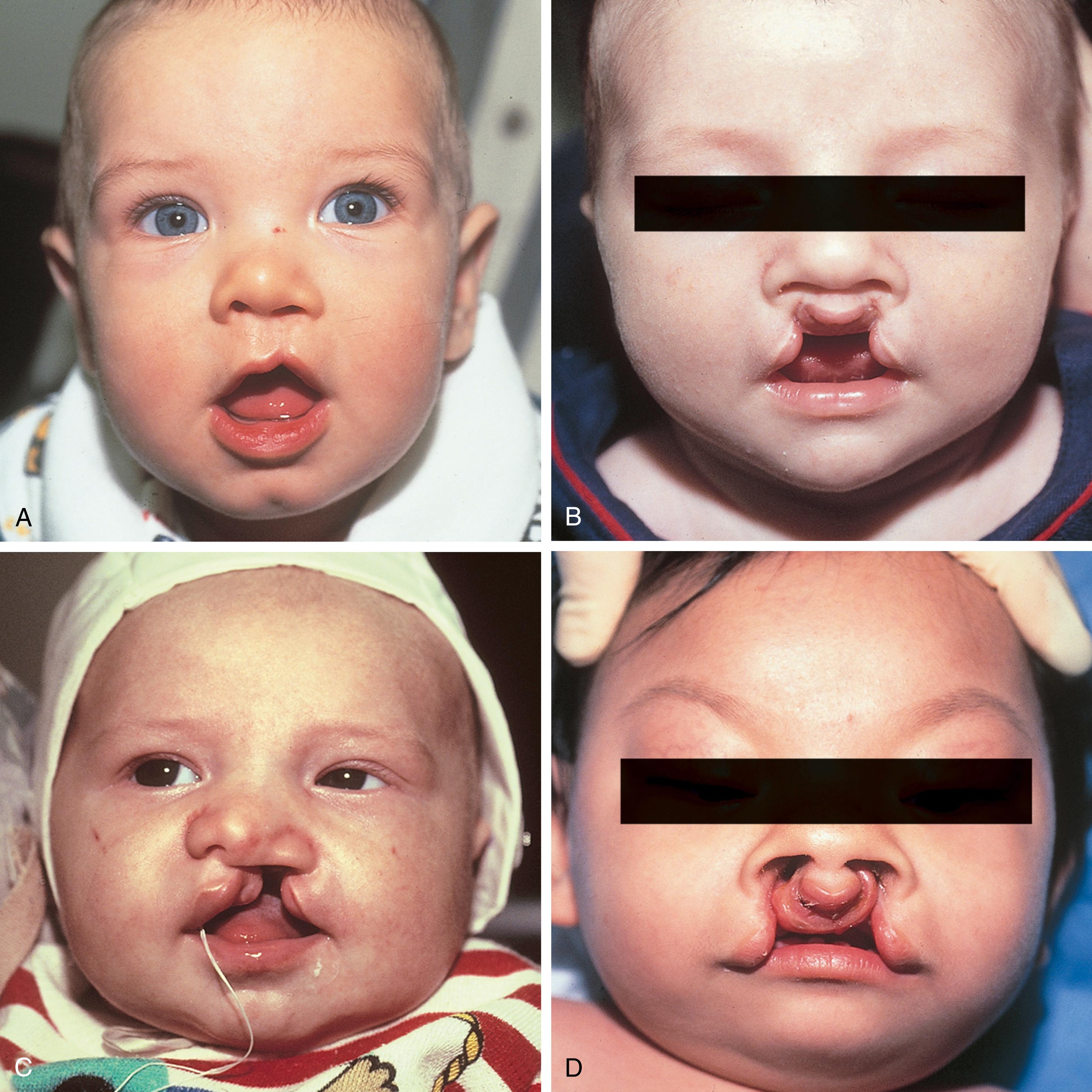
Initial difficulties develop from the inability of the infant to create an airtight seal to suckle effectively, which is related to the size of the clefts. Nasal regurgitation is frequent. Upright positioning and adaptive feeding nipples can assist with preventing regurgitation and achieving effective closure around the nipple to obtain a seal. Breastfeeding is possible for some infants with clefting; in fact, because the breast is more compliant, the infant may fare better in creating a seal around the breast. As with any newborn, infant weight and growth should be monitored closely; however, it is not unusual for poor weight gain to be noted in the first 2 to 3 months of life in this patient population. Thereafter, infants typically progress well and are able to make up weight.
Cleft palates lead to disruption of the muscular levator veli palatini and tensor veli palatine slings, which can disrupt eustachian tube ventilation and lead to frequent bouts of otitis media. Persistent effusions may result in conductive hearing loss with subsequent delays in speech and language development. Careful monitoring of middle ear effusions is crucial and the placement of tympanostomy tubes by the pediatric otolaryngologist is often required to allow adequate drainage. Indeed, hearing loss may be identified in up to one-third of children with palatal clefting, underscoring the importance of continued audiology surveillance through adulthood.
Plastic surgery reconstruction of facial clefts is a multistep endeavor, depending on the type and degree of deformity. The goals are aesthetic improvement, maintenance of normal maxillofacial growth, and restoration of palatal function in an effort to assist normal phonation. Reparative strategies continue to evolve, and controversy exists as to optimal timing and optimal procedure. Typically, the cleft lip/nose deformity repair is scheduled at 3 to 6 months old. For wide unilateral and bilateral clefts, most centers will preoperatively narrow the cleft to obtain better results. Some centers perform a lip adhesion, or simpler approximation of the defect, when the infant is younger than 3 months old; others preoperatively narrow the cleft defect by presurgical orthopedics, or customized intraoral mouth/nosepieces adjusted by pediatric orthodontists.
In some centers, palate repair may be combined with the initial lip repair, but in most, the palate repair is performed several months later, from 9 to 12 months old. Optimal hearing and speech acquisition evolve when palatal integrity is restored before the second birthday. As such, palate closure is typically performed before 2 years old despite concerns that early surgical interventions involving the midface may have a negative impact on maxillofacial growth and result in midface retrusion. Studies continue to try to define intervals when palatal procedures can be performed with a minimum of untoward effects.
Unique errors of speech articulation are common to patients with cleft palate and are more likely to develop in children who undergo delayed palatal repair. Hypernasal speech, also called velopharyngeal insufficiency, may occur in patients in whom the soft palate is foreshortened and allows air to escape into the posterior nasal vestibule. For these patients, a pharyngeal flap procedure may be performed whereby a peninsula of the posterior pharynx is attached to the soft palate. This recruited tissue serves to lengthen the palate and substantially alleviates hypernasal speech.
As the child develops an awareness of self, the lip scar resulting from earlier repair often becomes an issue, and revisions can be performed to improve the upper lip’s appearance. Between ages 7 and 10, during the time of mixed dentition, an alveolar bone graft is usually indicated to permit normal eruption of the canine on the affected side(s), as well as to restore the maxillary arch, provide nasal support, and close the alveolar cleft.
The end of adolescence marks the completion of the child’s facial skeletal growth. At this time, residual nasal deformities, which usually involve a broad and inferiorly displaced nasal ala, may be targeted by a formal rhinoplasty. Malalignment of the upper and lower jaws may additionally exist because of deficient maxillary growth. Once mandibular growth is complete, surgical advancement of the midface can be performed to restore normal occlusion.
As this brief discussion outlines, the family of a newborn with a facial cleft can anticipate numerous procedures spanning the entire childhood of their infant before reaching the end of the restorative journey. The impact of these interventions on the child is certainly profound.
The general term plagiocephaly, derived from the Greek word plagio meaning “oblique,” describes an asymmetric cranium. Etiologies can be intrinsic, such as genetic factors causing premature suture synostosis, or extrinsic, such as mechanical factors in utero or postnatally.
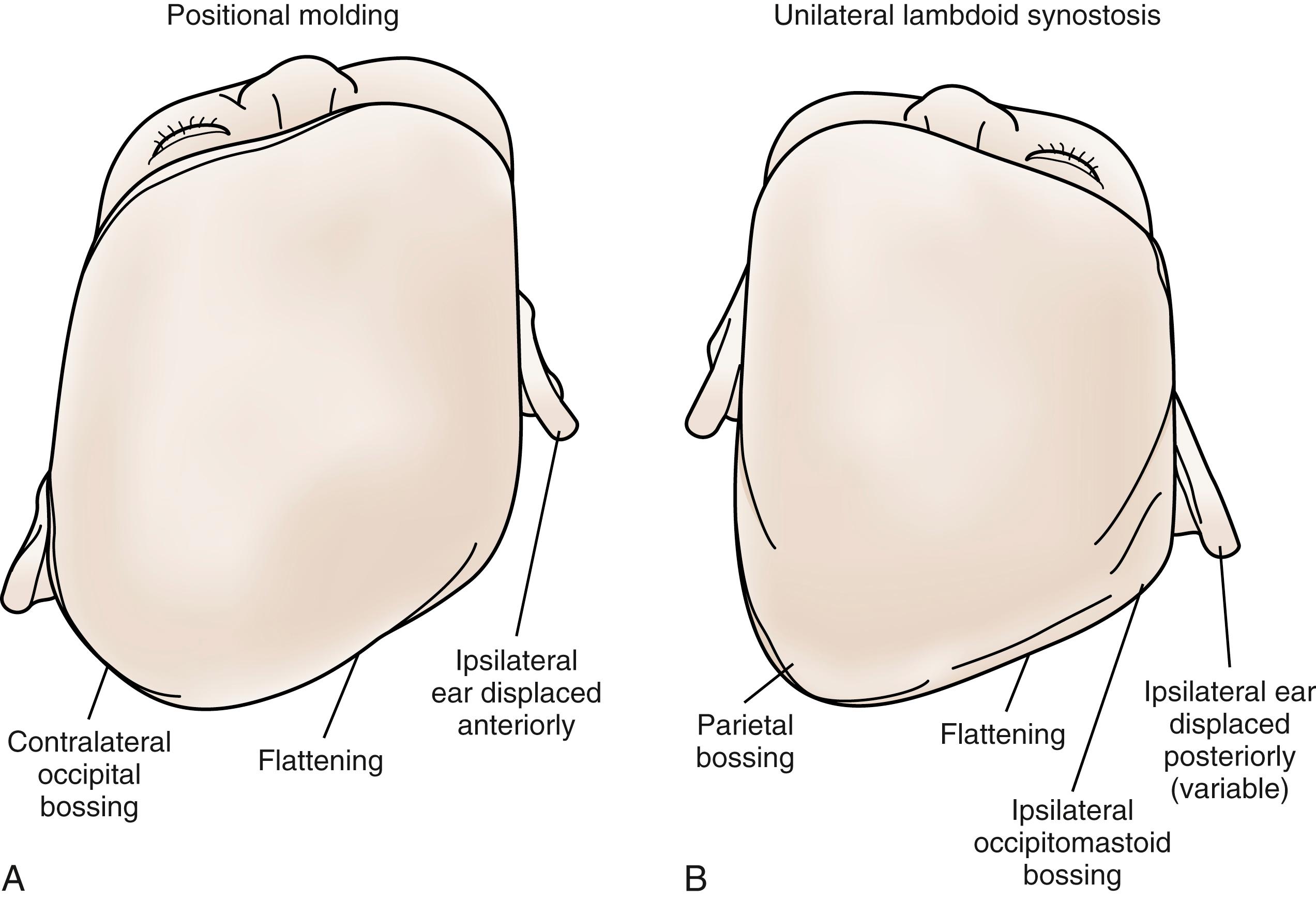
Deformational plagiocephaly refers to perinatal occipital flattening due to mechanical forces, with resulting changes in the malleable infant craniofacial skeleton. About 10% of cases are congenital, from pressure causes such as multiple gestations or reduced maternal pelvic volume. Postnatally, occipital flattening is acquired from persistent supine sleep positions. The high incidence (5% to 48% of healthy newborns) is because newborns cannot lift and center their heads until 3 months of life, when neuromotor control has matured. In addition, supine positioning has become even more popular since the 1990s, when the American Academy of Pediatrics (AAP) recommended supine sleeping to reduce the incidence of sudden infant death syndrome. In fact, compliance rates with the “Safe to Sleep” campaign correlate with the incidence of deformational plagiocephaly. Other associated factors are male gender, multiparity, and torticollis, of which the latter is associated with up to 20% of infants with deformational plagiocephaly.
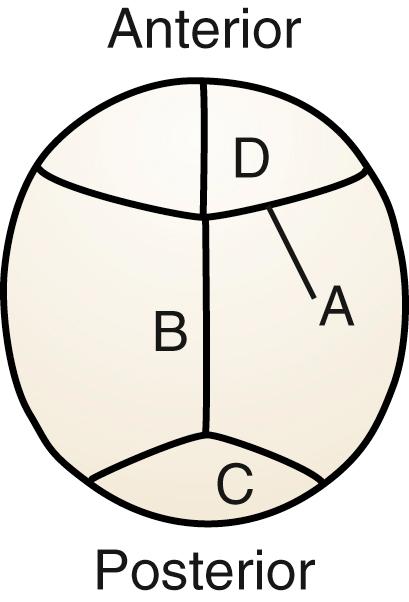
Right-sided deformational plagiocephaly is more common, possibly due to right-handed mothers holding infants in a right-side-down position to nurse, causing pressure and flattening of the right occiput. Regardless of the side, once a preferential supine position develops, it becomes habitual and difficult to correct. History usually confirms a normal head at birth and acquired asymmetry that worsens with time. As the occiput becomes flatter, up to 80% of infants will have anterior displacement of the ipsilateral forehead, with concomitant increase in the height of the ipsilateral palpebral fissure, anterior displacement of the ipsilateral ear, and anterior displacement of the ipsilateral cheek, which can be seen from the anterior view ( Fig. 23.4 ). These changes are shaped like a parallelogram on vertex view ( Fig. 23.5 ). Posteriorly, the mastoid skull bases should be symmetric; otherwise, there would be suspicion for a true unilateral lambdoid synostosis, described later.
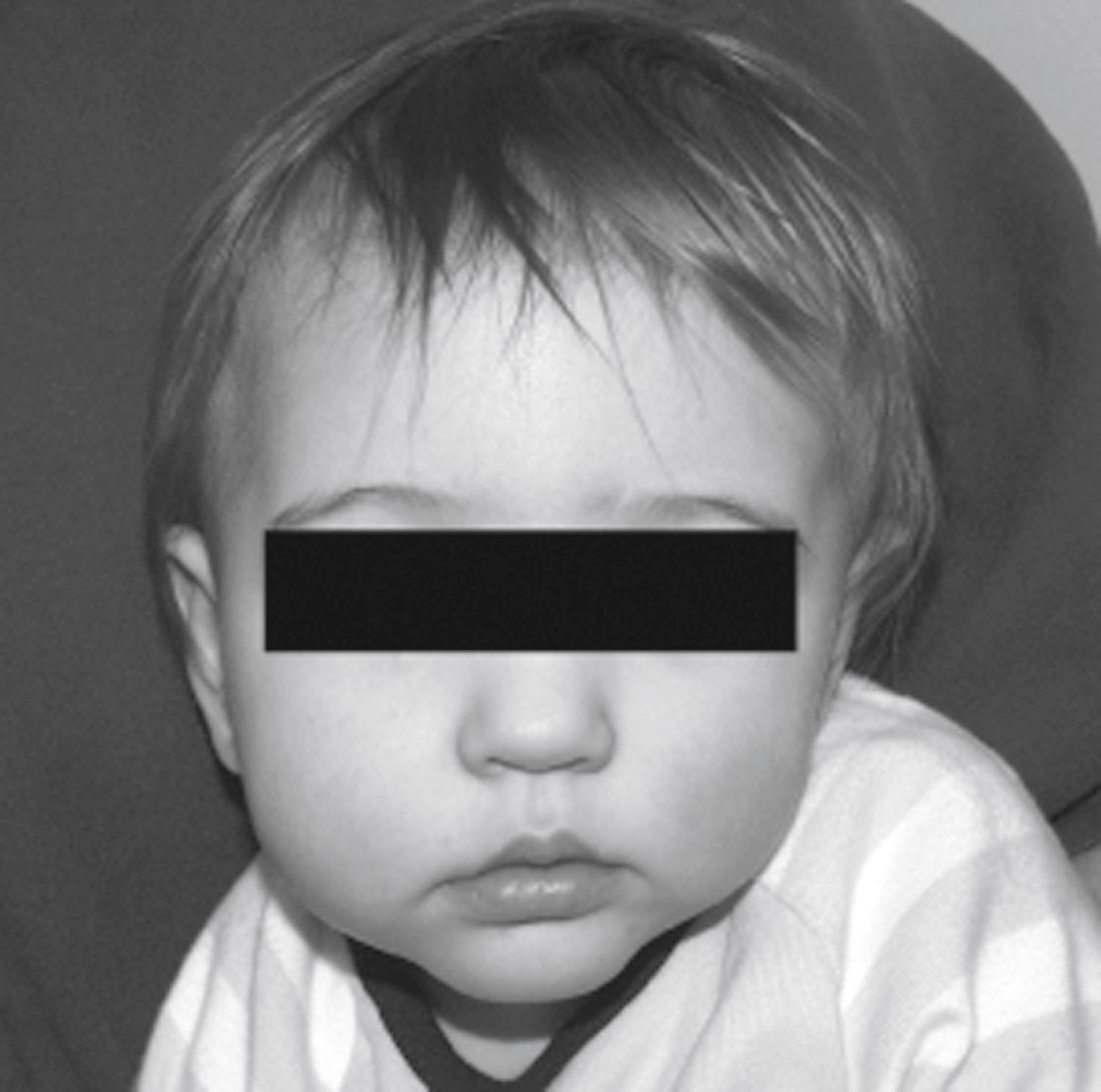
Torticollis is often associated with deformational plagiocephaly, with patients typically having contralateral sternocleidomastoid muscle (SCM) “stiffness” rather than true muscle atrophy or fibrosis. For a given SCM, muscle contraction twists the head contralaterally but tilts the head ipsilaterally. In typical deformational plagiocephaly, the contralateral SCM is foreshortened and resists full extension, yielding a contralateral head tilt and ipsilateral head twist that makes the mandibular midline appear to be deviated ipsilaterally to the side of the occipital flattening. Physical examination can reveal tenderness of this contralateral SCM or resistance and agitation on contralateral head twist.
Treatment is by careful monitoring and prevention. Whenever not sleeping, infants should be placed prone (“tummy time”) to decrease preferential supine positioning and to increase shoulder girdle strength. Parents should alternate infant supine positions during feeding and sleeping. During feeding, pressure should be avoided on the side of the flattened occiput. Changing the position of stimuli in the crib may also influence the infant to turn to a different side. Although a rolled-up towel or foam pinned to the clothes on one side will prevent the infant from sleeping on that side, care must be exercised to avoid materials in cribs that pose risks to the sleeping infant. If there is torticollis, neck exercises should be performed at each diaper change so that the chin can touch each shoulder for at least 10 seconds. Physical therapy may need to be employed. These infants are monitored monthly until 6 months old.
For infants older than 6 months or with more severe craniofacial deformities that are not improved with positioning, external cranioplasty with an orthotic device (helmet) is a more intense treatment method. Before 10 months old, an orthotic helmet worn 23 or more hours per day allows the malleable infant skull to grow into the shape of the symmetric helmet. Infants are typically monitored every 2 to 3 months for contour and neurologic development. After 10 months old, helmet therapy has minimal effect.
Craniosynostosis is defined as premature closing of the sutures between the cranial bones during development, resulting in deformities of the skull. Primary craniosynostosis originates from pathology of the involved suture, whereas secondary craniosynostosis results from dysgenesis of the underlying brain that then misdirects cranial expansion. The latter usually does not require plastic surgical skull reconstruction because the defect is in the brain itself.
Normal sutural bone growth occurs in a direction perpendicular to the suture’s axis. Therefore, when a suture is fused and normal growth is interrupted, growth is restricted in a direction perpendicular to the suture’s axis while compensatory growth occurs in the direction parallel to the suture. The growth abnormality caused by premature fusion of a particular suture leads to a characteristic head shape pattern. For example, sagittal craniosynostosis results in abnormal growth parallel to the fused sagittal suture leading to anteroposterior elongation and temporal narrowing, resulting in a scaphocephaly, or “boat-shaped head.” Simple craniosynostosis refers to single suture fusion, whereas complex craniosynostosis is multiple suture fusion ( Table 23.2 ).
| Name | Suture(s) Involved | Shape |
|---|---|---|
| Acrocephaly | Bilateral coronal | Skull height greater anteriorly, slanting downward posteriorly |
| Brachycephaly | Bilateral coronal | Wide, taller skull shortened in anteroposterior dimension |
| Oxycephaly | Bilateral coronal | Taller skull, shortened width and anteroposterior dimension |
| Turricephaly | Bilateral coronal | Tall skull |
| Plagiocephaly | Unilateral coronal or unilateral lambdoidal | Asymmetrical skull |
| Scaphocephaly | Sagittal | Anteroposterior elongation with bitemporal narrowing |
| Trigonocephaly | Metopic | Narrow, triangular, ridged forehead |
| Kleeblattschädel | Bilateral coronal, lambdoidal, and metopic | Cloverleaf deformity |
The incidence of craniosynostosis is approximately 1 in 2500 live births across ethnic populations but varies between genders depending on the sutures involved. In simple craniosynostosis, genetics and fetal environment may both play roles, because twins and infants delivered from breech position have a higher incidence. The genes involved in many of the syndromic craniosynostoses are known and are described later.
On physical examination, normal patent sutures (which may overlap slightly) move when palpated by the examiner’s thumbs. Prematurely fused sutures can have a palpable ridge. To confirm, a computed tomography (CT) scan of the face and skull with fine cuts and three-dimensional reconstructions will reveal the skeletal pathoanatomy.
Increased intracranial pressure is the main concern of craniosynostosis, although its clinical significance and risk are likely low in simple craniosynostosis and high in complex and syndromic craniosynostoses. Intracranial hypertension (ICH) could potentially lead to developmental delay and even blindness. Therefore, along with routine neurologic and developmental examinations, at least yearly pediatric ophthalmology funduscopic examinations are mandatory, and they are required more frequently if symptoms of ICH occur. In cases of syndromic craniosynostosis and midface hypoplasia, patients should also be routinely evaluated for midface retrusion causing airway obstruction, obstructive sleep apnea and exposure keratopathy.
Treatment usually occurs before 12 months old, while the skull is relatively malleable and the dura can stimulate osteogenesis. Surgery consists of a bicoronal scalp incision to expose the calvarium; the plastic surgeon then draws the outlines of the bony pieces to make, and the neurosurgeon then elevates the pieces off the brain. When the deformity extends to the supraorbital rim, the elevation of the fronto-orbital bar for reconstruction adds considerable length to the operation because both the brain and globes must be protected. The plastic surgeon then reassembles the skull pieces with absorbable plates, screws, and sutures to reshape the head. Many centers also offer limited-incision, endoscopically assisted suturectomy, in which the fused suture is simply cut open and then the patient’s head placed postoperatively into a molding orthotic helmet to allow growth into a normal shape; but this must be done before 6 months old. Regardless of treatment, patients need to be monitored postoperatively at least yearly, for neurologic, ophthalmologic, and developmental changes, and for recurrence of craniofacial deformities.
Up to 70% of simple, isolated craniosynostoses occur sporadically. Autosomal dominant and recessive familial patterns have been identified in 8% of cases. If one parent and child are affected, subsequent pregnancies are quoted to have a 50% incidence risk. Simple sagittal synostosis is the most commonly encountered simple craniosynostosis, representing 57% of cases. Coronal synostosis can be unicoronal or bicoronal and is less common. Metopic synostosis is still less frequent, although it is rising in incidence; and true lambdoid synostosis is extremely rare.
Sagittal synostosis, the premature fusion of the sagittal suture, leads to increased anteroposterior length and biparietal narrowing, which is known as scaphocephaly ( Fig. 23.6 ). Isolated nonsyndromic sagittal synostosis is the most common form of craniosynostosis. It is almost always sporadic, with only 2% of patients having a genetic etiology. A 4:1 male predominance is recognized, with no race predilection. There is low risk for ICH and abnormal brain development, because the remaining cranial sutures allow compensatory expansion of the neurocranium.
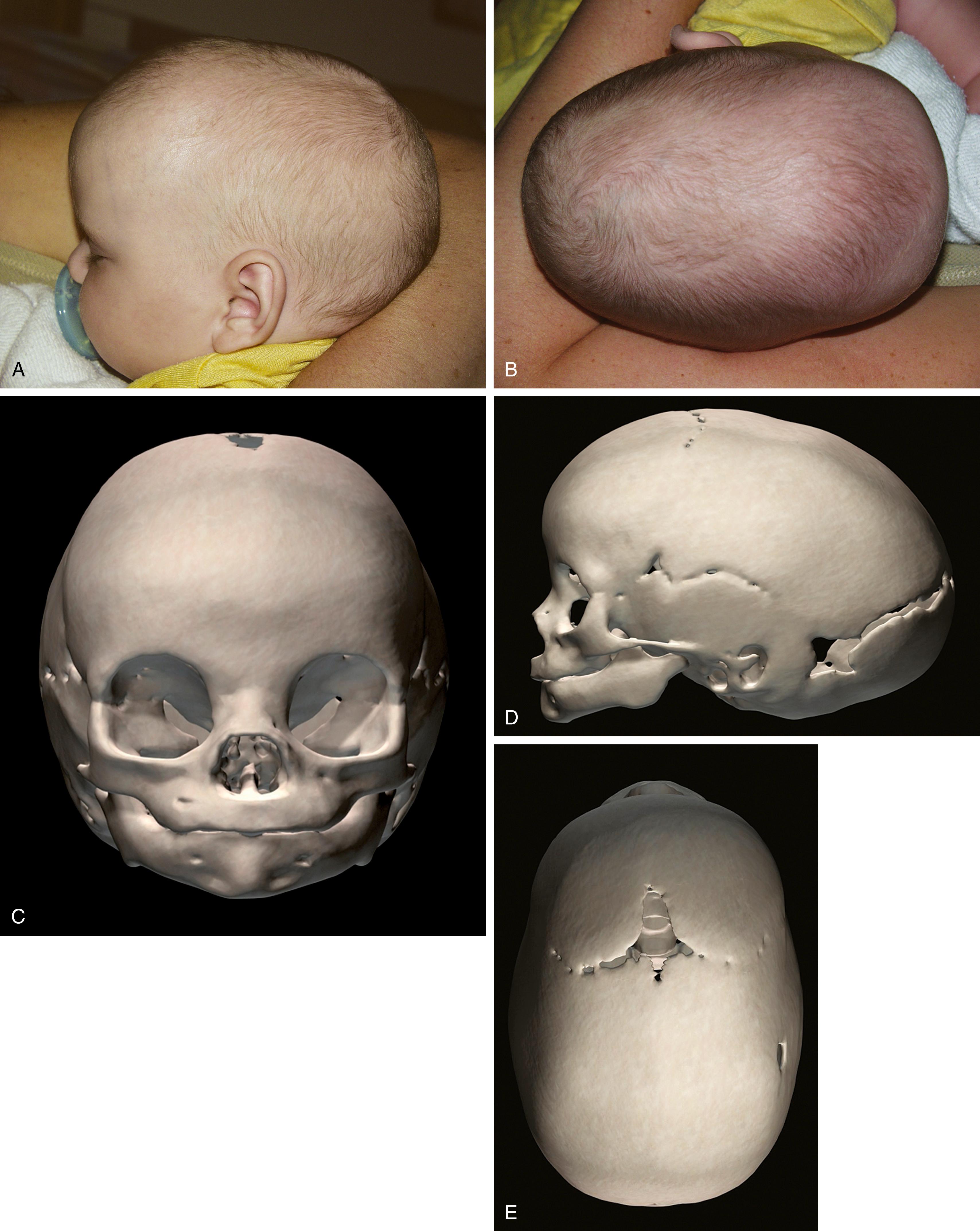
Sagittal suture synostosis can range from predominantly anterior fusion to predominantly posterior fusion, to complete fusion, causing slightly different skull shapes. Isolated anterior sagittal suture fusion will manifest frontal bossing, whereas posterior sagittal suture fusion will exhibit occipital bossing. Bossing of both the frontal and occipital domains with associated biparietal narrowing results from complete fusion of the suture.
A ridged sagittal suture may be palpable as discussed previously, but intracranial and ophthalmic risks are low. The goals of repair are therefore appearance-related, to reduce the anterior and posterior prominences while widening the biparietal dimension. Anterior and posterior vault remodeling can be staged or simultaneous. Endoscopic strip suturectomy is also frequently successful.
The metopic suture is the first cranial suture to fuse, typically at about 7 months old. It is the only suture that disappears and is indiscernible in the adult skull. Significantly premature fusion leads to a keel-shaped trigonocephalic head ( Fig. 23.7 ). Metopic synostosis has an incidence of between 1:2500 and 1:15,000 births, accounting for 10% to 20% of isolated craniosynostoses. Males are affected more often than females at a ratio of about 3:1. Physical examination shows the keel-shaped forehead with hypotelorism, upward slanting of the eyelids laterally, and a triangular shape to the forehead and supraorbital ridge, both of which are retrusive. Although typically isolated, 8% to 15% of children affected with trigonocephaly will have associated anomalies involving the extremities or the central nervous, cardiac, or genitourinary system.
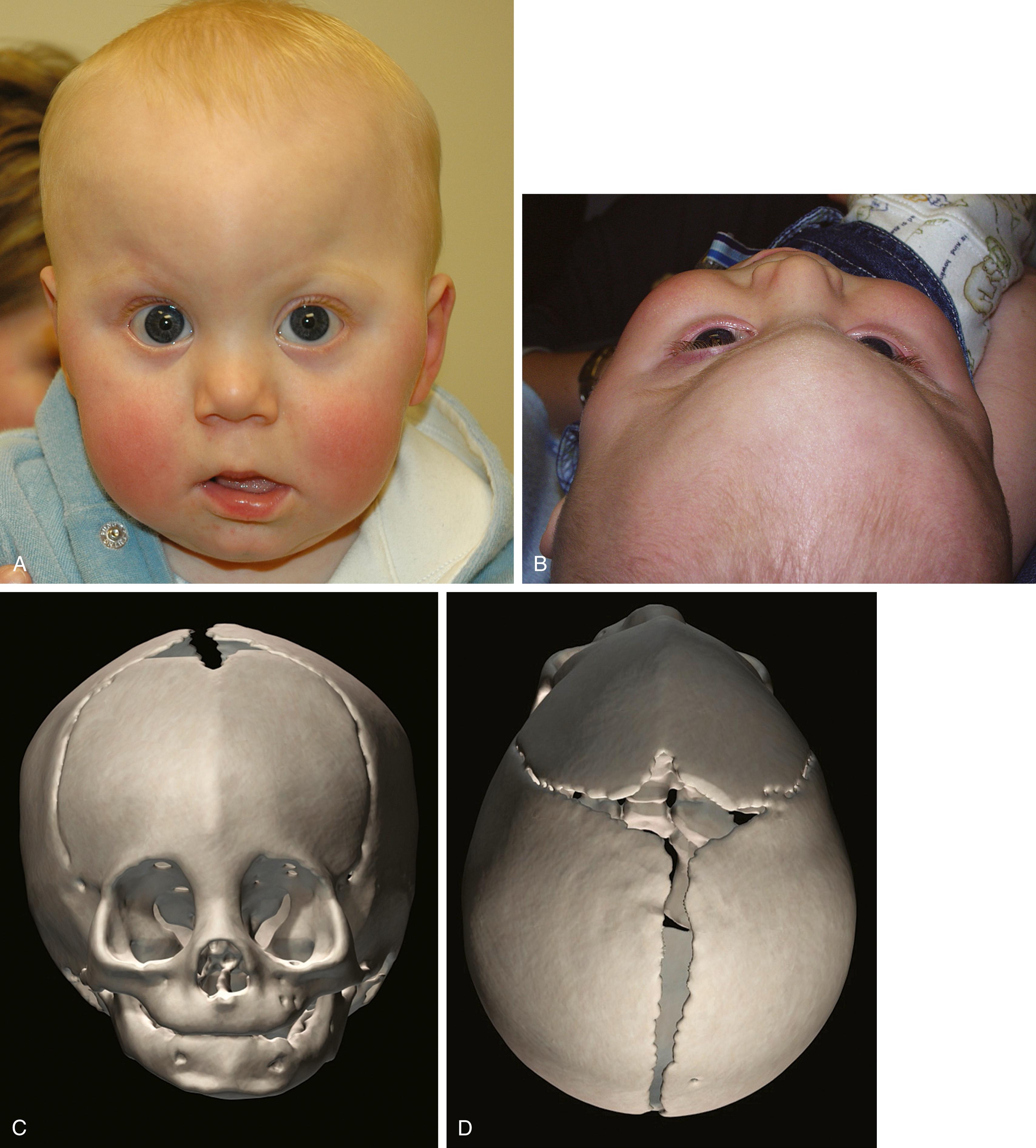
The severity of the deformity indicates the need for surgery. For instance, in some families the infant has slightly premature metopic fusion at 5 or 6 months old, resulting in a prominent forehead without significant keel-shape or hypotelorism, and no surgery is indicated. Severe shape changes can be corrected by anterior cranial vault remodeling with reshaping of the triangular fronto-orbital rim.
An isolated metopic ridge is a feature of normal metopic suture closure. Such patients do not demonstrate any of the characteristics of trigonocephaly, such as supraorbital retrusion or hypotelorism. Families should be counseled that a metopic ridge is nonpathological and should be expected to become less prominent with time.
The coronal sutures may be affected either unilaterally or, less commonly, bilaterally, resulting in synostotic anterior plagiocephaly or brachycephaly (“short skull” in the anteroposterior dimension that is wider and taller), respectively. Bicoronal synostosis is more often associated with syndromic diagnoses. Nonsyndromic unilateral coronal synostosis has an estimated incidence of 1 in 2500 live births and accounts for 15% to 30% of cases of craniosynostosis. Its etiology is unknown, but proposed causes include fetal head constraint, thyrotoxicosis, and certain vitamin deficiencies.
Synostosis of a single coronal suture results in a widened ipsilateral palpebral fissure, an elevated and anteriorly positioned ipsilateral ear, nasal root deviation toward the affected suture, chin deviation away from the affected suture, and a superiorly and posteriorly displaced supraorbital rim and eyebrow known as the harlequin eye deformity ( Fig. 23.8 ). Bilateral craniosynostosis restricts anteroposterior growth and causes retrusion of the fronto-orbital region, leading to compensatory widening and raised height of the anterior cranium; the result is described as brachycephaly, acrocephaly, oxycephaly, or turricephaly (see Table 23.2 ). In bilateral craniosynostosis, there is increased incidence of ICH.
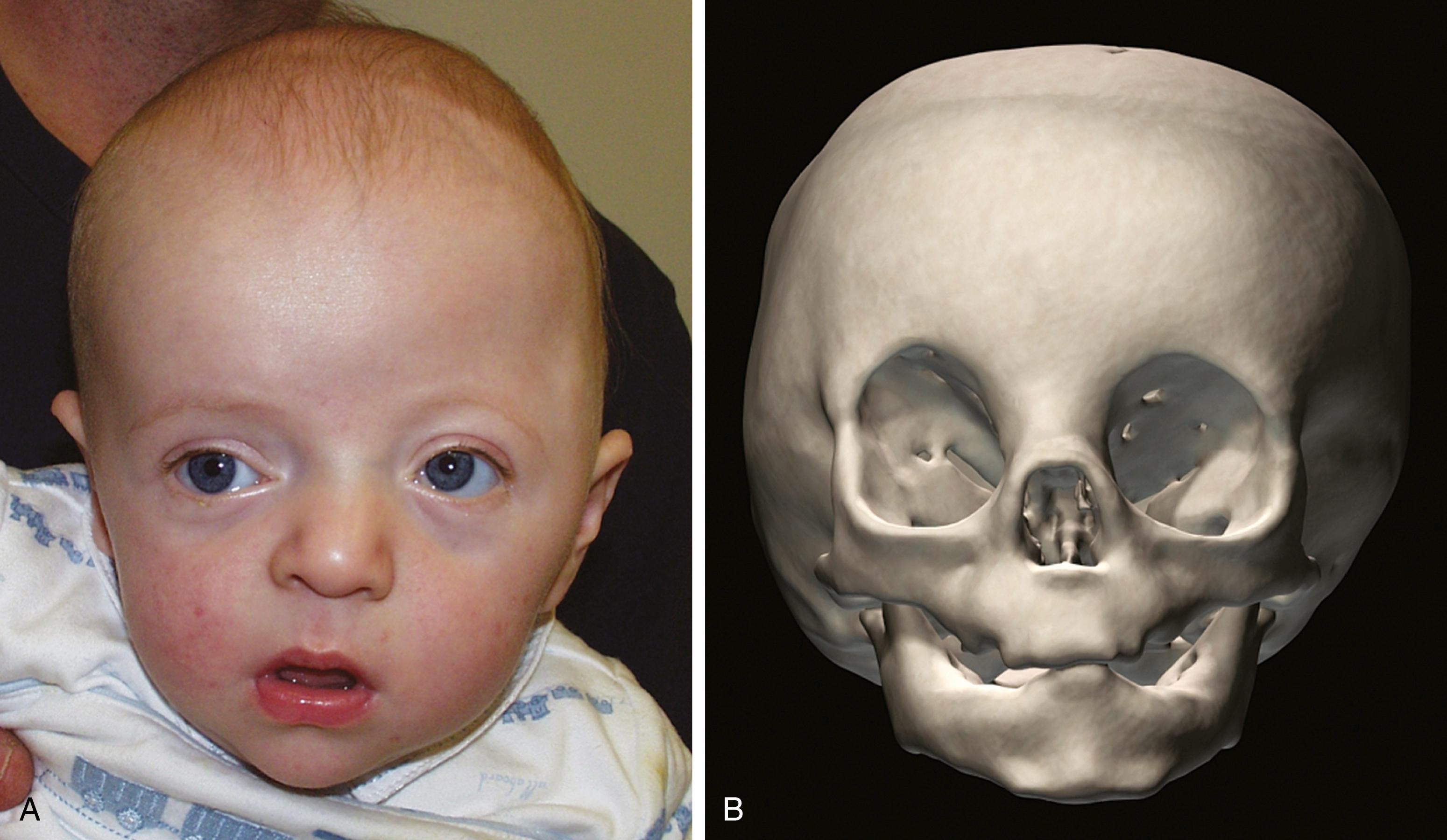
Treatment of coronal synostosis includes anterior cranial expansion with vault remodeling and fronto-orbital advancement. Some centers perform suture craniectomy with helmet therapy, especially for the unicoronal coronal synostosis, where symmetry is difficult to achieve.
Lambdoid synostosis may involve one or both of the lambdoid sutures and is the least common of the craniosynostoses. When it occurs, it is usually unilateral and causes a synostotic posterior plagiocephaly. A raised ridge may be palpable over the involved suture, and there is often a mastoid bulge of the affected side of the occiput with contralateral parietal bossing ( Fig. 23.9 ). From the vertex view (see Fig. 23.5 ), a trapezoid head shape is seen, with contralateral frontal bossing, posterior-inferior displacement of the ipsilateral ear, and ipsilateral occipitomastoid bossing. This view best distinguishes rare true lambdoid suture synostosis from common deformational plagiocephaly. True lambdoid craniosynostosis requires cranial vault remodeling, whereas deformational plagiocephaly is treated by positioning or helmet therapy.
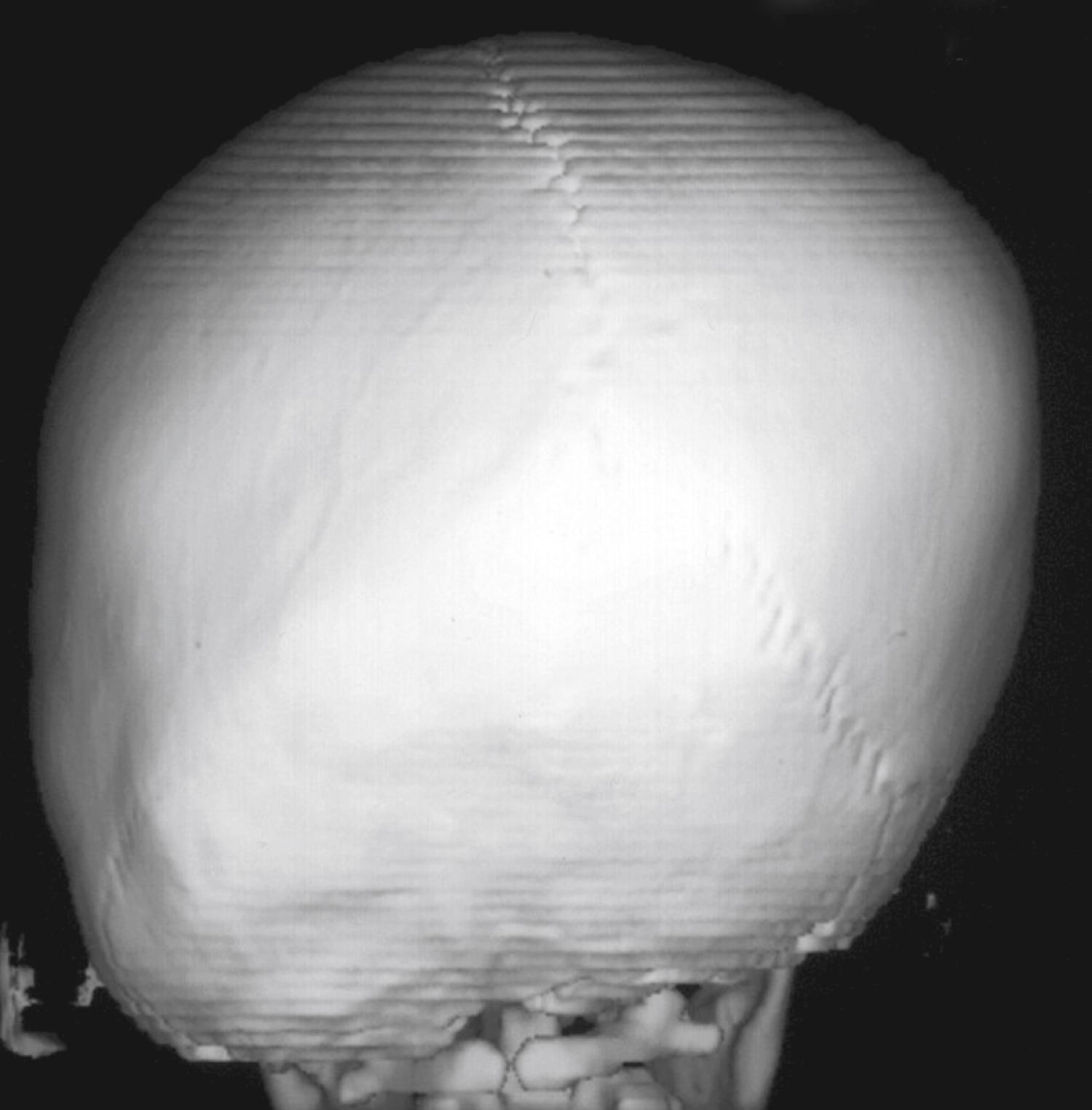
The most severe skull dysmorphology and highest risk of ICH result from multiple suture fusion. When the coronal and lambdoid sutures fuse bilaterally with fusion of the anterior metopic suture, compensatory bulging at the remaining open sagittal and squamosal suture leads to a superior and lateral bulging, which is described as the cloverleaf deformity, or kleeblattschädel. Although multiple suture synostoses can be sporadic, they are much more likely to be associated with syndromes.
Craniosynostoses involving more than one suture are much less common, tend to be syndromic, and are usually caused by sporadic mutations, which are inherited through autosomal dominant transmission ( Table 23.3 ). The best characterized are the acrocephalosyndactyly syndromes, which share the features of multisutural craniosynostosis (bicoronal and or bilambdoid), midface hypoplasia, abnormal facies, and limb abnormalities. The genetic abnormality usually inactivates the fibroblast growth factor receptor (FGFR) gene family that is involved in regulating bone growth, leading to hypertrophic bone development. FGFR1, FGFR2, and FGFR3 are differentially involved; and although each syndrome is phenotypically distinct, patients will all demonstrate varying degrees of supraorbital rim retrusion and midface hypoplasia with weak infraorbital support, resulting in shallow orbits and high risk of exposure keratopathy that can lead to blindness. Craniofacial anomalies such as cleft palate, mandibular growth problems, stylohyoid calcification, and other cranial suture involvement are common. Such patients are at high risk of ICH and may also have variable hydrocephalus; neurocognitive impairment; and cranial base, cervical spine, and other vertebral abnormalities. Inner ear problems (such as otitis media requiring myringotomy tubes) and inner nose and airway issues (such as choanal, tracheal, and laryngeal abnormalities) require consultation with an otolaryngologist. Cardiac, renal, and other visceral anomalies are also common.
| Gene/Protein | Syndrome | Chromosome |
|---|---|---|
| FGFR1 | Pfeiffer | 8 |
| FGFR2 | Apert | 10 |
| Crouzon | 10 | |
| Pfeiffer | 10 | |
| Jackson-Weiss | 10 | |
| Beare-Stevenson | 10 | |
| FGFR3 | Muenke | 4 |
| Crouzon with acanthosis nigricans | 4 | |
| TWIST | Saethre-Chotzen | 7 |
| Treacle | Treacher Collins | 5 |
Apert syndrome is autosomal dominant with incomplete penetrance and a birth prevalence of 5.5 to 16 per 1 million live births. The majority of cases are sporadic; more than 98% of patients with Apert syndrome have identifiable mutations in the FGFR2 gene, resulting in multiple anomalies in all systems ( Fig. 23.10 ; Box 23.1 ). Two distinguishing features include symmetric complex syndactylies of the hands and feet, and cognitive defects.
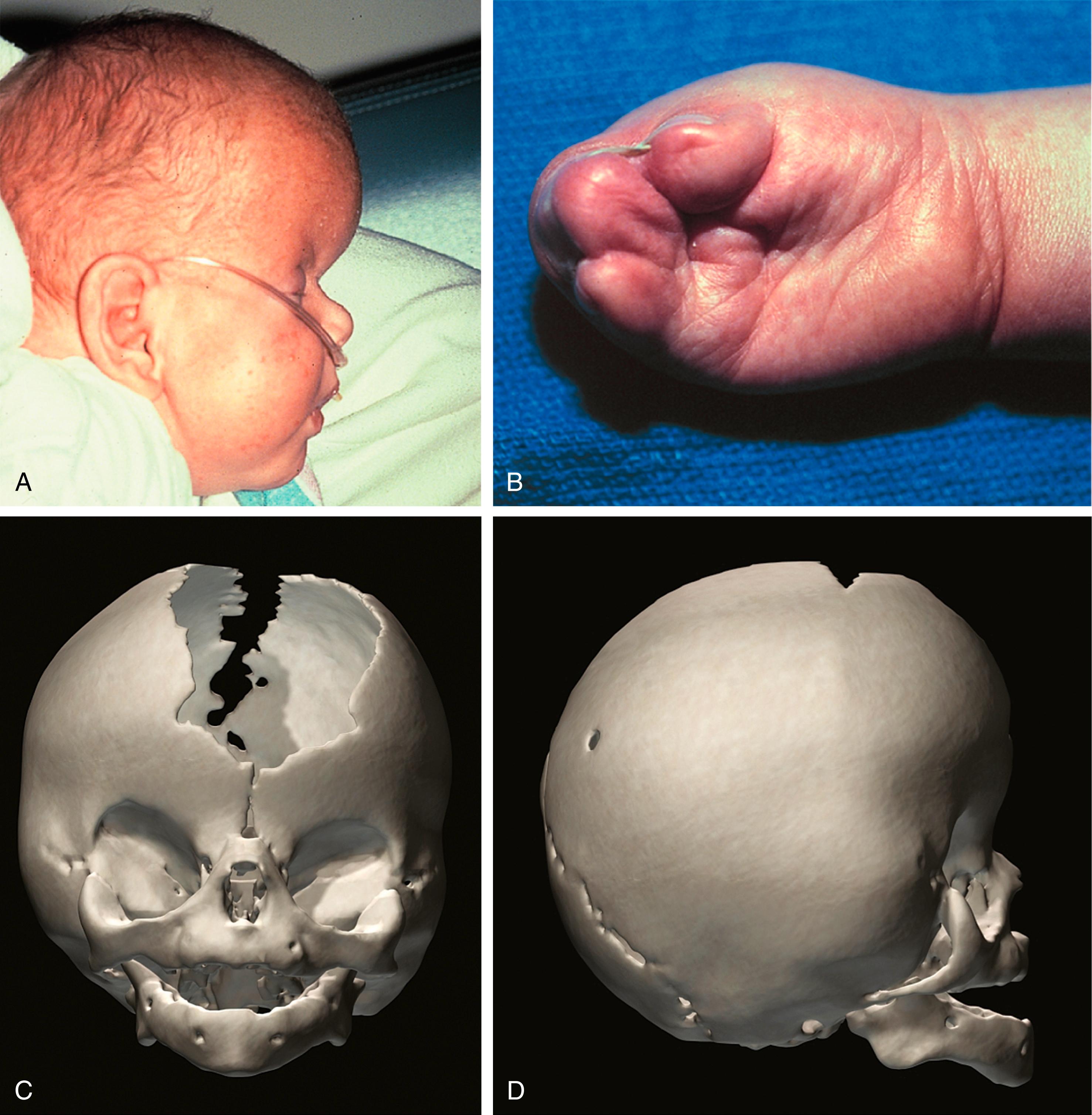
Brachycephaly due to coronal suture synostosis
Hypertelorism
Proptosis
V-pattern exotropia
Midface hypoplasia
Choanal stenosis
Cleft palate
Stylohyoid calcification
Conductive hearing deficits
Symmetrical syndactyly of the hands
Cervical spine fusion
Shortened humeri
Cardiovascular anomalies
Hydronephrosis
Cryptorchidism
Tracheal anomalies
Obstructive sleep apnea
Diffuse acne
Their bicoronal synostosis results in brachycephaly with a high cranium that is described as turricephalic. They have delayed closure of their metopic and sagittal sutures between 2 and 4 years old, resulting in hypertelorism. Their abnormal facies have more severe features, including ptosis and downward-slanting palpebral fissures. Their midface deficiency is often associated with hypoplastic or stenotic choanae, leading to airway obstruction requiring tracheostomy. A sleep study can diagnose obstructive sleep apnea requiring treatment with a continuous positive airway pressure device.
Their shallow orbits can result in proptosis, but patients with Apert syndrome also commonly have strabismus with a V -shaped pattern: exotropic upward gaze divergence with esotropic downward gaze, as well as other ophthalmic problems.
Although significant hydrocephalus is not common, the risk of neurosurgical abnormality is high and therefore baseline magnetic resonance imaging (MRI) is recommended along with the usual CT scan to evaluate the bone on a regular basis.
Seventy-two percent of children with Apert syndrome are noted to have brain anomalies, leading to developmental delays and cognitive deficits. Infants operated on before their first birthday but after 3 months old are more likely to have intelligence quotients (IQs) greater than 80. Unfortunately, children who underwent similar procedures before 3 months old trended toward lower IQs and required additional operations.
A hallmark of Apert syndrome is complex symmetric syndactyly of the hands and feet, especially of the middle three digits, leaving the first and fifth digits separate; this results in a “mitten hand” appearance (see Fig. 23.10B ). Plastic surgeons can separate the digits to produce a more functional hand.
Finally, the skin of patients with Apert syndrome may display excessive sweating. Patients have been shown to have an increased number of sweat glands and prominent sebaceous glands. During adolescence the skin becomes oily, and in 70% of patients, acne erupts on the face, chest, and back.
Crouzon syndrome is another autosomal dominant acrocephalosyndactyly with incomplete penetrance that presents as a sporadic new mutation 60% of the time. The birth prevalence is 15 to 16 per 1 million live births and is also associated with mutations in FGFR2. Its distinguishing features include normal extremities.
The heads of patients with Crouzon syndrome are overall brachycephalic from the bicoronal synostosis, although other sutures can be involved, leading to other skull shapes ( Fig. 23.11 ; Box 23.2 ). Their midface hypoplasia similarly leads to proptosis and also an illusion of mandibular prognathism with a severe underbite called class III malocclusion.
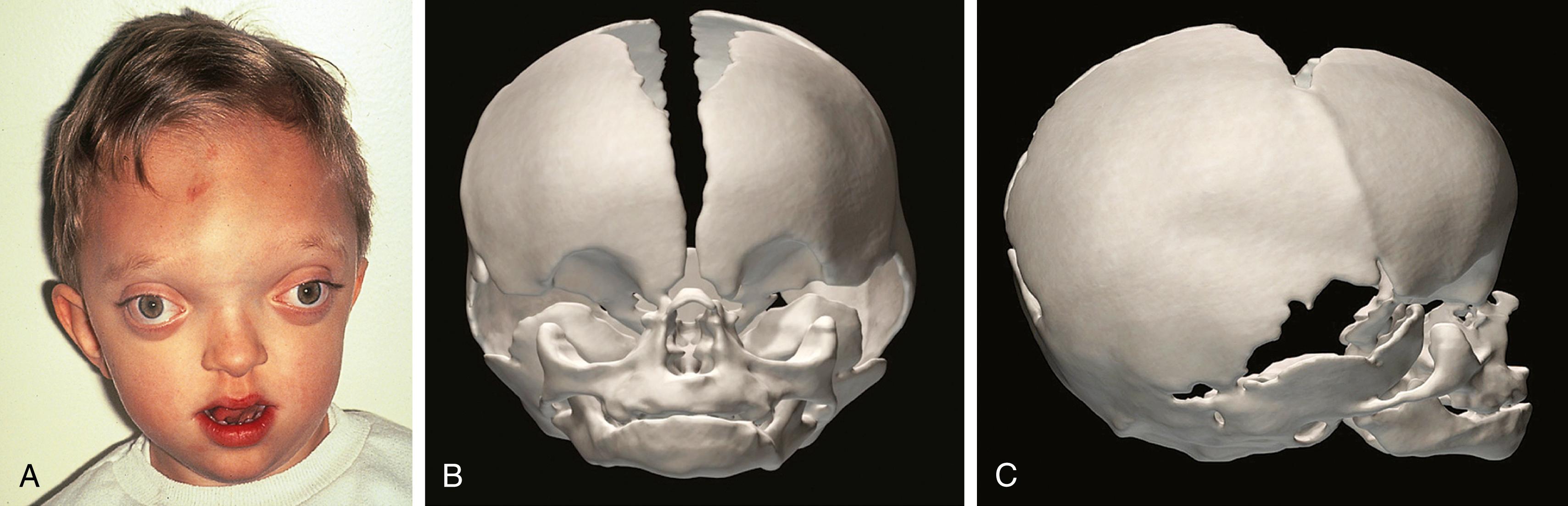
Brachycephaly due to coronal suture synostosis
Hydrocephaly with Chiari I malformation
Hypertelorism
Proptosis
Midface hypoplasia
“Beaked” nose
Cleft palate
Stylohyoid calcification
Conduction and/or neurosensory hearing deficit
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here