Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pain is both a sensory and an emotional experience. When unrecognized and undertreated, pain extracts a significant physiologic, biochemical, and psychological toll on both the child and the family. Many disease processes and most interventional diagnostic or treatment procedures in pediatrics are associated with pain. Similarly, traumatic, developmental, cognitive, psychological, and social experiences can also trigger and maintain chronic pain.
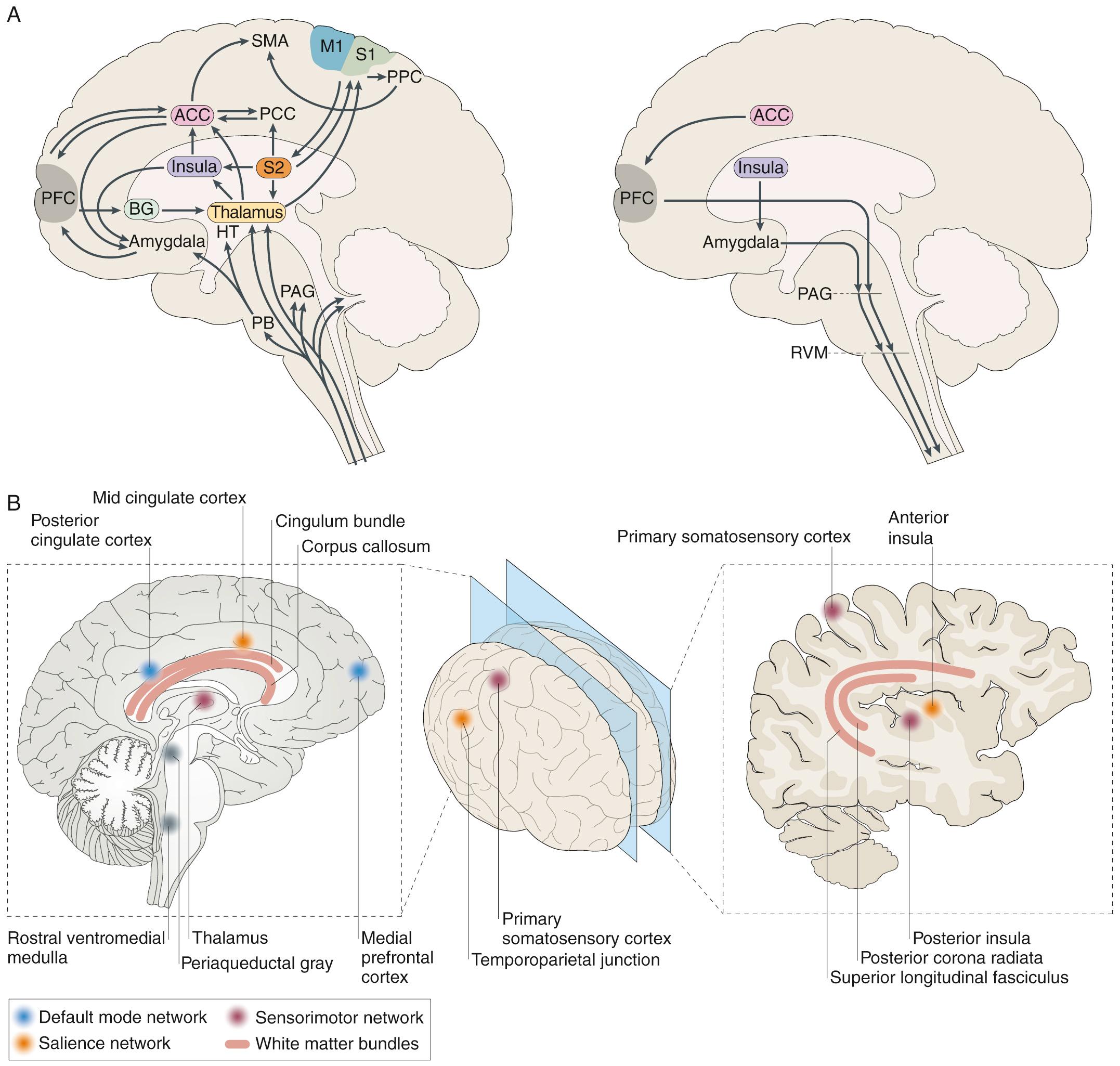
The International Association for the Study of Pain (IASP) defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.” The important elements to emphasize in this definition are (1) pain encompasses both peripheral physiologic and central neural components and (2) pain may or may not be associated with ongoing tissue damage. The experience of pain lies primarily in the strength and patterning of central neural connectivity ( Fig. 76.1 ). While immediate upstream neural activation can originate from inflammatory, structural, or biochemical events, processes not only in the periphery of the body but also in the spinal cord and the brain influence the intensity and duration of pain. Similarly, central neural processes in the brain are associated with the location, intensity, and distress associated with pain. Chronic pain can develop when the upstream neural signaling continues to activate central neural circuits, such as with continued peripheral inflammatory or structural pain-associated processes.
Often, however, pediatricians face the most difficult problems when either acute pain becomes chronic or chronic pain develops and is maintained without a definable infectious, inflammatory, metabolic, or structural cause. When no “cause” can be found, patients are often referred to mental health specialists, or the cause for the pain is labeled as “stress.” Children read this message as, “The doctor thinks I am faking pain or am crazy.” Parents see their child suffering and often seek care elsewhere, with the child undergoing numerous tests, procedures, medication trials, and visits and many physicians looking for the cause of the pain so it can be “fixed.” Meanwhile, the child may be missing school, social, and physical activities and developing poor sleep habits with increasing fatigue.
It is recognized that chronic pain, in the absence of a specific identified structural, biochemical, or inflammatory cause, develops through the initiation, maintenance, and strength of central neural connectivity patterns, the connectome of the child's brain (see Chapter 147 ). That is, what is now called “centrally mediated pain” derives from neural connectivity patterns in the brain that include centers involved in autonomic nervous system control, memory, and other cognitive centers, as well as emotional centers of the brain. In pediatrics, birth history and child development overlay these central patterns that contribute to the development of chronic pain. A child with high-functioning autism spectrum disorder (ASD) may perseverate on a pain symptom (e.g., headache) in the same way the child might perseverate on an idea or point of view (e.g., the parents may never “win” an argument with their child). Parents may understand the concept of a “sticky nervous system” as the perpetuator of the continued pain in such a child. This model of brain connectivity patterns or “top-down” mediators of chronic pain is important, since it explains how psychological and other nonpharmacologic interventions work to reduce pain and suffering. Science has come a long way since the model of pain as “psychological” or “physical.” The current model of pain also includes the impact of the gut microbiome in altering central neural processes in relation to the development and maintenance of pain.
Table 76.1 specifies important pain categories typically treated (somatic, visceral, and neuropathic) and defines the elements and characteristics of nociception, the peripheral physiologic aspect of pain perception. Nociception refers to how specialized fibers (largely but not exclusively the small, unmyelinated A-delta and C fibers) in the peripheral nervous system transmit nerve impulses (usually transmitting signals originating from peripheral mechano- and chemoreceptors) through synapses in the spinal cord's dorsal horn through (but not exclusively through) the spinothalamic tracts to the brain's higher centers, where the development of neural connectivity patterns creates the experience of pain.
| PAIN CATEGORY | DEFINITION AND EXAMPLES | CHARACTERISTICS |
|---|---|---|
| Somatic | Pain resulting from injury to or inflammation of tissues (e.g., skin, muscle, tendons, bone, joints, fascia, vasculature) Examples: burns, lacerations, fractures, infections, inflammatory conditions |
In skin and superficial structures: sharp, pulsatile, well localized In deep somatic structures: dull, aching, pulsatile, not well localized |
| Visceral | Pain resulting from injury to or inflammation of viscera Examples: angina, hepatitic distention, bowel distention or hypermobility, pancreatitis |
Aching and cramping; nonpulsatile; poorly localized (e.g., appendiceal pain perceived around umbilicus) or referred to distant locations (e.g., angina perceived in shoulder) |
| Neuropathic | Pain resulting from injury to, inflammation of, or dysfunction of the peripheral or central nervous system Examples: complex regional pain syndrome (CRPS), phantom limb pain, Guillain-Barré syndrome, sciatica |
Spontaneous; burning; lancinating or shooting; dysesthesias (pins and needles, electrical sensations); hyperalgesia (amplification of noxious stimuli); hyperpathia (widespread pain in response to a discrete noxious stimulus); allodynia (pain in response to nonpainful stimulation); pain may be perceived distal or proximal to site of injury, usually corresponding to innervation pathways (e.g., sciatica) |
Assessing pain entails much more than merely quantifying it. Whenever feasible, the physician should ask the patient about the character, location, quality, duration, frequency, and intensity of the pain. Some children may not report pain because of fears (often well founded) of talking to strangers, disappointing or bothering others, receiving an injection if they report pain, returning to the hospital if they admit to pain, and other negative reactions. For infants and nonverbal children, their parents, pediatricians, nurses, and other caregivers are constantly challenged to interpret whether the child's distressed behaviors represent pain, fear, hunger, or a range of other perceptions or emotions. Similarly, lack of normal interest in play without behavioral distress signals can be manifestations of pain. Therapeutic trials of comfort measures (cuddling, feeding) and analgesic medication may be helpful in clarifying the triggers of the behaviors.
Behavior and physiologic signs are useful but can be misleading. A toddler may scream and grimace during an ear examination because of fear rather than pain. Conversely, children with inadequately relieved persistent pain from cancer, sickle cell disease, trauma, or surgery may withdraw from their surroundings and appear very quiet, leading observers to conclude falsely that these children are comfortable or sedated or, for adolescent patients, are “drug seeking.” In these situations, increased dosing of analgesics may make the child become more, not less, interactive and alert. Similarly, neonates and young infants may close their eyes, furrow their brows, and clench their fists in response to pain. Adequate analgesia is often associated with eye opening and increased involvement in surroundings. A child who is experiencing significant chronic pain may play normally as a way to distract attention away from pain. This coping behavior is sometimes misinterpreted as evidence of the child's “faking” or exaggerating pain at other times.
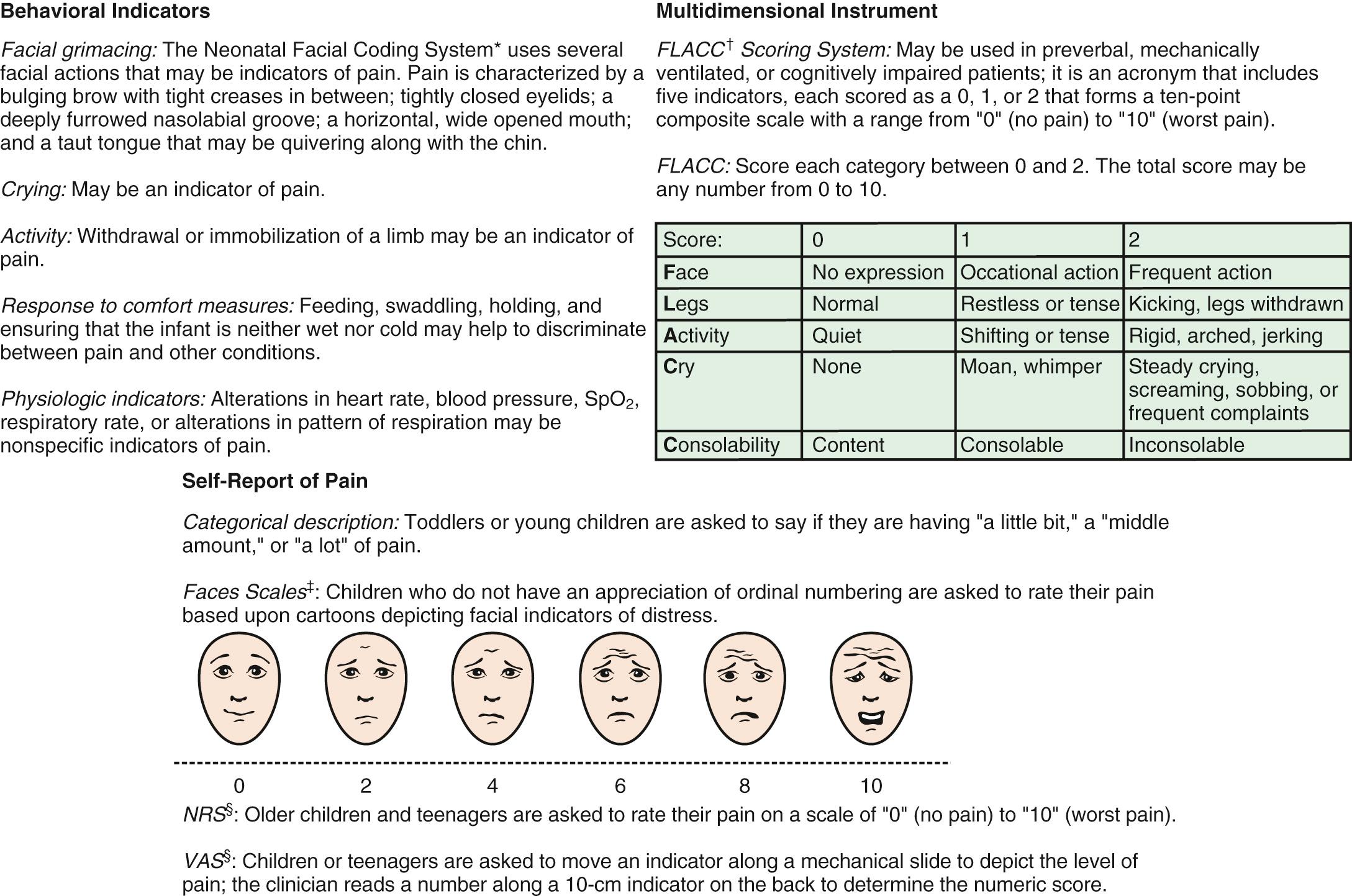
Because infants, young children, and nonverbal children cannot express the quantity of pain they experience, several pain scales have been devised in an attempt to quantify pain in these populations ( Fig. 76.2 and Table 76.2 ).
| NAME | FEATURES | AGE RANGE | ADVANTAGES | VALIDATION AND USES | LIMITATIONS |
|---|---|---|---|---|---|
| Visual Analog Scale (VAS) | Horizontal 10-cm line; subject marks a spot on the line between anchors of “no pain” (or neutral face) and “most pain imaginable” (or sad face) | 6-8 yr and older | Good psychometric properties; validated for research purposes | Acute pain Surgical pain Chronic pain |
Cannot be used in younger children or in those with cognitive limitations Requires language skills and numerical processing; upper anchor of “most pain” requires an experiential reference point that is lacking in many children. |
| Likert Scale | Integers from 0-10, inclusive, corresponding to a range from no pain to most pain | 6-8 yr and older | Good psychometric properties; validated for research purposes | Acute pain Surgical pain Chronic pain |
Same as for VAS. |
| Faces Scales (e.g., FACES-R, Wong-Baker, Oucher, Bieri, McGrath scales) | Subjects rate their pain by identifying with line drawings of faces, or photos of children | 4 yr and older | Can be used at younger ages than VAS and Likert | Acute pain Surgical pain |
Choice of “no pain” face affects responses (neutral vs smiling); not culturally universal. |
| Behavioral or combined behavioral-physiologic scales (e.g., FLACC, N-PASS, CHEOPS, OPS, FACS, NIPS) | Scoring of observed behaviors (e.g., facial expression, limb movement) ± heart rate and blood pressure | Some work for any ages; some work for specific age-groups, including preterm infants | May be used in both infants and nonverbal children | FLACC, N-PASS: Acute pain Surgical pain |
Nonspecific; overrates pain in toddlers and preschool children; underrates persistent pain; some measures are convenient, but others require videotaping and complex processing; vital sign changes unrelated to pain can occur and may affect total score. |
| Autonomic measures (e.g., heart rate, blood pressure, heart rate spectral analyses) | Scores changes in heart rate, blood pressure, or measures of heart rate variability (e.g., “vagal tone”) | All ages | Can be used at all ages; useful for patients receiving mechanical ventilation | Nonspecific; vital sign changes unrelated to pain may occur, and may artifactually increase or decrease score. | |
| Hormonal-metabolic measures | Plasma or salivary sampling of “stress” hormones (e.g., cortisol, epinephrine) | All ages | Can be used at all ages | Nonspecific; changes unrelated to pain can occur; inconvenient; cannot provide “real-time” information; standard normal values not available for every age bracket. |
There are several behavioral distress scales for the infant and young child, mostly emphasizing the patient's facial expressions, crying, and body movement. Facial expression measures appear most useful and specific in neonates. Autonomic and vital signs can indicate pain, but because they are nonspecific, they may reflect other processes, including fever, hypoxemia, and cardiac or renal dysfunction ( Table 76.3 ).
Increase in heart rate, respiratory rate, blood pressure, muscle tone
Oxygen desaturation
Sweating
Flushing
Pallor
Change in facial expression (grimacing, furrowing of the brow, nasal flaring, deep nasolabial groove, curving of the tongue, quivering of the chin)
Finger clenching
Thrashing of limbs
Writhing
Back arching
Head banging
Poor feeding
Sleep disturbance
Pseudoparalysis
Children age 3-7 yr become increasingly articulate in describing the intensity, location, and quality of pain. Pain is occasionally referred to adjacent areas; referral of hip pain to the thigh or area above the knee is common in this age range. Self-report measures for children this age include using drawings, pictures of faces, or graded color intensities. Children ≥8 yr can usually use verbal numerical rating scales or visual analog pain scales (VAS) accurately ( Fig. 76.2 ). Verbal numerical ratings are preferred and considered the gold standard; valid and reliable ratings can be obtained from children ≥8 yr. The numerical rating scale (NRS) consists of numbers from 0-10, in which 0 represents no pain and 10 represents very severe pain. There is debate about the label for the highest pain rating, but the current agreement is not to use the phrase “worst pain possible,” because children can always imagine a greater pain. In the United States, regularly documented pain assessments are required for hospitalized children and children attending outpatient hospital clinics and emergency departments (EDs). Pain scores do not always correlate with changes in heart rate or blood pressure.
Measuring pain in cognitively impaired children remains a challenge. Understanding pain expression and experience in this population is important, because behaviors may be misinterpreted as indicating that cognitively impaired children are more insensitive to pain than cognitively competent children. Children with trisomy 21 may express pain less precisely and more slowly than the general population. Pain in children with ASD may be difficult to assess because these children may be both hyposensitive and hypersensitive to many different types of sensory stimuli, and they may have limited communication abilities. Although self-reports of pain can be elicited from some children who are cognitively impaired, observational measures have better validation among these children. The Noncommunicating Child's Pain Checklist—Postoperative Version is recommended for children up to 18 yr. Maladaptive behaviors and reduction in function may also indicate pain. Children with severe cognitive impairments experience pain frequently, mostly not because of accidental injury. Children with the fewest abilities experience the most pain.
A number of models have been developed to understand the various factors that influence children's pain. Many of these theories focus on factors that explain the interindividual variability in pain perception and the chronicity and impairment experienced with pain. Central to these models are interrelationships among biologic, cognitive, affective, and social factors that influence children's pain and disability, commonly referred to as “biopsychosocial models” of pain. Biologic factors include the child's physical health, central nervous system (CNS) factors (pain processing), sex, pubertal status, and genetic factors. Individual child cognitive and affective factors related to perception of pain are anxiety, fear, negative affect, pain behaviors, and functional disability, whereas social factors include such areas as culture, socioeconomic status, school environment, social and peer interactions, and parental and family factors. For children, developmental factors need to be considered, such as cognitive and motor development, birth history, and epigenetic factors (the interaction in development between genetic and environmental factors).
A framework that considers the interplay of biologic, psychological, and social factors is useful for understanding pediatric pain and to guide pain assessment and the delivery of pain prevention and management. Many simple interventions designed to promote relaxation and patient control can work either alone or synergistically with pain medications for relief of pain and related distress. Moreover, psychological interventions are often coupled with physical therapy interventions to assist in the management of disabling chronic pain.
The pharmacokinetics and pharmacodynamics of analgesics vary with age; drug responses in infants and young children differ from those in older children and adults. The elimination half-life of most analgesics is prolonged in neonates and young infants because of their immature hepatic enzyme systems and glomerular filtration. Clearance of analgesics may also be variable in young infants and children. Renal blood flow, glomerular filtration, and tubular secretion increase dramatically in the 1st few weeks, approaching adult values by 3-5 mo of age. Renal clearance of analgesics is often greater in toddlers and preschool-aged children than in adults, whereas in premature infants, clearance is reduced. Age-related differences in body composition and protein binding also exist. Total body water as a fraction of body weight is greater in neonates than in children or adults. Tissues with high perfusion, such as the brain and heart, account for a larger proportion of body mass in neonates than do other tissues, such as muscle and fat. Because of decreased serum concentrations of albumin and α 1 -acid glycoprotein, neonates have reduced protein binding of some drugs, resulting in higher amounts of free, unbound, pharmacologically active drug.
Acetaminophen and nonsteroidal antiinflammatory drugs (NSAIDs) have replaced aspirin as the most commonly used antipyretics and oral, nonopioid analgesics ( Table 76.4 ).
| MEDICATION | DOSAGE | COMMENT(S) |
|---|---|---|
| Acetaminophen | 10-15 mg/kg PO q4h 10 mg/kg IV q4h 15 mg/kg IV q6h 10 mg/kg IV q6h (<2 yr) 20-30 mg/kg/PR q4h 40 mg/kg/PR q6-8h Maximum daily dosing: 90 mg/kg/24 hr (children) 60 mg/kg/24 hr (<2 yr) 30-45 mg/kg/24 hr (neonates) |
Minimal antiinflammatory action; no antiplatelet or adverse gastric effects; overdosing can produce fulminant hepatic failure. |
| Aspirin | 10-15 mg/kg PO q4h Maximum daily dosing: 120 mg/kg/24 hr (children) |
Antiinflammatory; prolonged antiplatelet effects; may cause gastritis; associated with Reye syndrome. |
| Ibuprofen | 8-10 mg/kg PO q6h 10 mg/kg IV q4-6h to maximum of 400 mg Maximum daily dose: 2400 mg |
Antiinflammatory; transient antiplatelet effects; may cause gastritis; extensive pediatric safety experience. |
| Naproxen | 5-7 mg/kg PO q8-12h | Antiinflammatory; transient antiplatelet effects; may cause gastritis; more prolonged duration than that of ibuprofen. |
| Ketorolac | Loading dose 0.5 mg/kg, then 0.25-0.3 mg/kg IV q6h to a maximum of 5 days; maximum dose 60 mg loading with maximum dosing of 30 mg q6h | Antiinflammatory; reversible antiplatelet effects; may cause gastritis; useful for short-term situations in which oral dosing is not feasible. |
| Diclofenac sodium | 2-3 mg/kg/day divided in 2 or 3 doses | Antiinflammatory; reversible antiplatelet effects; lower risk of gastritis and ulceration compared with other NSAIDs. |
| Choline magnesium salicylate | 10-20 mg/kg PO q8-12h | Weak antiinflammatory; lower risk of bleeding and gastritis than with conventional NSAIDs. |
| Celecoxib | 3-6 mg/kg PO q12-24h | Antiinflammatory; no or minimal antiplatelet or gastric effects; cross-reactivity with sulfa allergies. |
| Nortriptyline, amitriptyline, desipramine | 0.1-0.5 mg/kg PO qhs Larger doses may be divided bid. |
For neuropathic pain; facilitates sleep; may enhance opioid effect; may be useful in sickle cell pain; risk of dysrhythmia in prolonged QTc syndrome; may cause fatal dysrhythmia in overdose; FDA states agents may enhance suicidal ideation; little or no antidepression or antianxiety effects at lower dosages. |
| Gabapentin | 100 mg bid or tid titrated to up to 3600 mg/24 hr | For neuropathic pain; associated with sedation, dizziness, ataxia, headache, and behavioral changes. |
| Quetiapine, risperidone, chlorpromazine, haloperidol | Quetiapine: 6.25 or 12.5 mg PO qd (hs); may use q6hr prn acute agitation with pain. Escalate dose to 25 mg/dose if needed. Risperidone: useful for PDD spectrum or tic disorder and chronic pain; 0.25-1 mg (in 0.25 mg increments) qd or bid; see PDR for other dosing. |
Useful when arousal is amplifying pain; often used when patient first starting SSRI and then weaned after at least 2 wk; check for normal QTc before initiating; side effects include extrapyramidal reactions (diphenhydramine may be used to treat) and sedation; in high doses, can lower the seizure threshold. |
| Venlafaxine, duloxetine | Venlafaxine: start 37.5 mg daily as the XR formulation and titrate up monthly to effective dose, 2-4 mg/kg. Duloxetine: start 20 mg daily and titrate upward to effective dose, 1-1.5 mg/kg. |
SNRIs with both clinically significant antidepression and antianxiety effects as well as analgesic effects. |
| Fluoxetine | 10-20 mg PO qd (usually in morning) | SSRI for children with anxiety disorders in which arousal amplifies sensory signaling; useful in PDD spectrum disorders in very low doses; best to use in conjunction with psychiatric evaluation. |
| Sucrose solution via pacifier or gloved finger | Preterm infants (gestational age): 28 wk: 0.2 mL swabbed into mouth 28-32 wk: 0.2-2 mL, depending on suck/swallow >32 wk: 2 mL Term infants: 1.5-2 mL PO over 2 min |
Allow 2 min before starting procedure; analgesia may last up to 8 min; the dose may be repeated once. |
Acetaminophen , a generally safe, nonopioid analgesic and antipyretic, has the advantage of intravenous (IV), rectal, and oral routes of administration. Acetaminophen is not associated with the gastrointestinal (GI) or antiplatelet effects of aspirin and NSAIDs, making it a particularly useful drug in patients with cancer. Unlike aspirin and NSAIDs, acetaminophen has only mild antiinflammatory action.
Acetaminophen toxicity can result from a large single dose or cumulative excessive dosing over days or weeks (see Chapter 77 ). A single massive overdose overwhelms the normal glucuronidation and sulfation metabolic pathways in the liver, whereas long-term overdosing exhausts supplies of the sulfhydryl donor glutathione, leading to alternative cytochrome P-450 (CYP)–catalyzed oxidative metabolism and the production of the hepatotoxic metabolite N -acetyl- p -benzoquinone imine (NAPQI). Toxicity manifests as fulminant hepatic necrosis and failure in infants, children, and adults. Drug biotransformation processes are immature in neonates, very active in young children, and somewhat less active in adults. Young children are more resistant to acetaminophen-induced hepatotoxicity than are adults as a result of metabolic differences; sulfation predominates over glucuronidation in young children, leading to a reduction in NAPQI production.
Aspirin is indicated for certain rheumatologic conditions and for inhibition of platelet adhesiveness, as in the treatment of Kawasaki disease. Concerns about Reye syndrome have resulted in a substantial decline in pediatric aspirin use.
The NSAIDs are used widely to treat pain and fever in children. NSAIDs are nonselective cyclooxygenase (COX) inhibitors, that is, drugs that nonselectively block the activity of both COX-1 (found in gastric mucosa and platelets) and COX-2 (active in inflammatory pathways and cortical renal blood flow regulation) enzymes that synthesize prostaglandins. In children with juvenile idiopathic arthritis, ibuprofen and aspirin are equally effective, but ibuprofen is associated with fewer side effects and better drug adherence. NSAIDs and coxibs used adjunctively in surgical patients reduce opioid requirements (and therefore opioid side effects) by as much as 35–40%. Although NSAIDs can be useful postoperatively, they should be used as an adjunct to, not as a substitute for, opioids in patients with acute, moderate to severe pain.
Ketorolac, an IV NSAID, is useful in treating moderate to severe acute pain in patients who are unable or unwilling to swallow oral NSAIDs. U.S. Food and Drug Administration (FDA) recommendations limit ketorolac to 5 consecutive days of administration. IV ibuprofen (Caldolor) is FDA approved for the management of pain and fever in infants and children >6 mo of age. Adverse effects of NSAIDs are uncommon but may be serious when they occur, including inhibition of bone growth and healing; gastritis with pain and bleeding; decreased renal blood flow that may reduce glomerular filtration and enhance sodium reabsorption, in some cases leading to tubular necrosis; hepatic dysfunction and liver failure; inhibition of platelet function; and an increased incidence of cardiovascular events in patients predisposed to stroke and myocardial infarction. Although the overall incidence of bleeding is very low, gastric bleeding is the most common cause of mortality related to this class of analgesics. NSAIDs should not be used in the child with a bleeding diathesis or at risk for bleeding or when surgical hemostasis is a concern, such as after tonsillectomy. The drug class is usually not used in the setting of bone healing, except perhaps in the 1st few days after surgery. Renal injury from short-term use of ibuprofen in euvolemic children is quite rare; the risk is increased by hypovolemia or cardiac dysfunction. The safety of both ibuprofen and acetaminophen for short-term use is well established (see Table 76.4 ).
Coxib drugs available in the United States are limited to oral celecoxib, whereas in Europe and elsewhere parenteral parecoxib and oral rofecoxib are available. Parecoxib was not FDA approved, whereas rofecoxib was approved and withdrawn from marketing due to concern of enhanced risk of heart attack and stroke in high-risk adults, which has subsequently been found to be associated with all the coxibs and all the NSAID drugs as well. The coxib drugs are selective COX-2 enzyme inhibitors; therefore they are effective antiinflammatory and analgesic molecules that generally do not result in platelet inhibition or bleeding, or in gastric inflammation or ulceration, findings that may be seen with the nonselective COX inhibitors in the NSAID class. However, coxib drugs do inhibit regulation of cortical renal blood flow, and therefore carry the same risk of renal toxicity and acute tubular necrosis, particularly in the setting of low cardiac output states or dehydration. Celecoxib is therefore an appropriate primary or adjunctive analgesic to use in children after surgery, children with gastric mucosal pathology, or oncology patients in whom concern for hemostasis contraindicates conventional NSAIDs.
Opioids are analgesic substances either derived from the opium poppy ( opiates ) or synthesized to have a similar chemical structure and mechanism of action ( opioids ). The older, pejorative term “narcotics” (narcotic analgesics) should not be used for these agents because it connotes criminality and lacks pharmacologic descriptive specificity. Opioids are administered for moderate and severe pain, such as acute postoperative pain, sickle cell crisis pain, and cancer pain. Opioids can be administered by the oral, rectal, oral transmucosal, transdermal, intranasal, IV, epidural, intrathecal, subcutaneous (SC), or intramuscular (IM) route. Regardless of route of administration, the site of action is at mu (µ) opioid receptors in the peripheral nervous system, spinal cord, brainstem, and higher CNS centers. Historically, infants and young children have been underdosed with opioids because of concern about significant respiratory side effects. Once thought to represent infants' particular sensitivity, the opioids' respiratory depressant effects are now known to result from infants' lower metabolic clearance of opioids and higher blood levels with frequent dosing. With proper understanding of the pharmacokinetic and pharmacodynamics of opioids, children can receive effective relief of pain and suffering with a good margin of safety, regardless of pharmacokinetic maturity, age, or size ( Tables 76.5 to 76.8 ).
Morphine, hydromorphone, or fentanyl is regarded as 1st choice for severe pain.
Dosing should be titrated and individualized. There is no “right” dose for everyone.
The right dose is the dose that relieves pain with a good margin of safety.
Dosing should be more cautious in infants, in patients with coexisting diseases that increase risk or impair drug clearance, and with concomitant administration of sedatives.
Hydromorphone is metabolized by CYP2D6 and fentanyl by CYP3A4, and to some extent 2D6; drugs that compete for 2D6 enzyme will raise blood levels and increase risk of respiratory depression.
Morphine is metabolized by glucuronidation to an active metabolite, morphine-6-glucuronide, which accumulates and causes CNS toxicity in renal impairment.
Anticipate and treat peripheral side effects, including constipation, nausea, and itching.
Give doses at sufficient frequency to prevent the return of severe pain before the next dose.
Use a drug delivery method, such as patient-controlled anesthesia or continuous infusions, that avoids the need for “prn” decision-making.
With opioid dosing for >1 wk, taper gradually to avoid abstinence syndrome.
When converting between parenteral and oral opioid doses, use appropriate potency ratios (see Table 76.6 ).
Tolerance refers to decreasing drug effect with continued administration of a drug. Over time a patient will need higher dosing to achieve the same clinical effect; however, tolerance to sedation and respiratory depression develop more rapidly than tolerance to analgesia. Thus, with higher doses, patients do not experience oversedation or respiratory depression.
Dependence refers to the need for continued drug dosing to prevent abstinence syndrome when a drug is abruptly discontinued, or its dose reduced. Abstinence syndrome is characterized by irritability, agitation, autonomic arousal, nasal congestion, piloerection, diarrhea, jitteriness, and yawning; it is produced by administration of potent opioids for >5-7 days.
Addiction, a psychiatric pathology, refers to psychological craving, compulsive drug-seeking behavior, and drug use despite medical harm. Addiction has strong genetic and environmental determinants. Opioid therapy will not lead to addiction in nonsusceptible individuals, and opioid underdosing does not prevent addiction; it may in fact increase drug-seeking behavior for relief of pain (e.g., watching the clock), referred to as “pseudoaddiction.”
| DRUG | EQUI-ANALGESIC DOSES | PARENTERAL DOSING | IV:PO DOSE RATIO | ORAL DOSING | COMMENTS | |||
|---|---|---|---|---|---|---|---|---|
| IV | Oral | <50 kg | >50 kg | <50 kg | >50 kg | |||
| Fentanyl | 10 µg | 100 µg | 0.5-1 µg/kg q1-2h 0.5-1.5 µg/kg/hr |
0.5-1 µg/kg q1-2h 0.5-1.5 µg/kg/hr |
Oral transmucosal: 1 : 10 Transdermal: 1 : 1 |
Oral transmucosal: 10 µg/kg Transdermal: 12.5-50 µg/hr |
Transdermal patches available; patch reaches steady state at 24 hr and should be changed q72h | 70-100 times as potent as morphine with rapid onset and shorter duration. With high doses and rapid administration, can cause chest-wall rigidity. Useful for short procedures; transdermal form should be used only in opioid-tolerant patients with chronic pain. |
| Hydrocodone | N/A | 1.5 mg | N/A | N/A | N/A | 0.15 mg/kg | 10 mg | Weak opioid; only available in form with acetaminophen. |
| Hydromorphone | 0.2 mg | 0.6 mg | 0.01 mg q2-4h 0.002 mg/kg/hr |
0.01 mg q2-4h 0.002 mg/kg/hr |
1 : 3 | 0.04-0.08 mg/kg q3-4h | 2-4 mg q3-4h | 5 times the potency of morphine; no histamine release and fewer adverse events than morphine. |
| Meperidine | 10 mg | 30 mg | 0.5 mg/kg q2-4h | 0.5 mg/kg q2-4h | 1 : 4 | 2-3 mg/kg q3-4h | 100-150 mg q3-4h | Primary use in low doses is for treatment of rigors and shivering after anesthesia or with amphotericin or blood products. Not appropriate for repeated dosing. |
| Methadone | 1 mg | 2 mg | 0.1 mg/kg q8-24h | 0.1 mg/kg q8-24h | 1 : 2 | 0.2 mg/kg q8-12h PO; available as liquid or tablet | 2.5 mg TID | Duration 12-24 hr; useful in certain types of chronic pain; requires additional vigilance because it will accumulate over 72 hr and produce delayed sedation. When patients tolerant of opioids are switched to methadone, they show incomplete cross-tolerance and improved efficacy. Since methadone is associated with prolonged QTc, monitoring is needed for children receiving high and extended dosing. |
| Morphine | 1 mg | 3 mg | 0.05 mg/kg q2-4h 0.01-0.03 mg/kg/hr |
Bolus: 5-8 mg q2-4h | 1 : 3 | Immediate release: 0.3 mg/kg q3-4h Sustained release : 20-35 kg: 10-15 mg q8-12h 35-50 kg: 15-30 mg q8-12h |
Immediate release: 15-20 mg q3-4h Sustained release: 30-90 mg q8-12h |
Potent opioid for moderate/severe pain; may cause histamine release. Sustained-release form must be swallowed whole; if crushed, becomes immediate acting, leading to acute overdose. |
| Oxycodone | N/A | 3 mg | N/A | N/A | N/A | 0.1-0.2 mg q3-4h; available in liquid (1 mg/mL) | Immediate release: 5-10 mg q4h Sustained release: 10-120 mg q8-12h |
Strong opioid only available as an oral agent in North America; more potent than and preferable to hydrocodone. Sustained-release form must be swallowed whole; if crushed, becomes immediate acting, leading to acute overdose. |
| Respiratory depression |
|
| Excessive sedation without evidence of respiratory depression |
|
| Nausea and vomiting |
|
| Pruritus |
|
| Constipation |
|
| Urinary retention | Straight catheterization, indwelling catheter. |
* Avoid in patients taking monoamine oxidase inhibitors.
† May be associated with extrapyramidal side effects, which may be more often seen in children than in adults.
| OPIOID | IM/IV DOSE (mg) | ORAL DOSE (mg) | T 1/2β (hr) |
|---|---|---|---|
| Morphine | 10 | 30 | 2-3 |
| Meperidine | 100 | 400 | 3-4 |
| Oxycodone | 15 | 20-30 | 2-3 |
| Fentanyl | 0.15-0.2 | — | 3-5 |
| Alfentanil | 0.75-1.5 | — | 1-2 |
| Sufentanil | 0.02 | — | 2-3 |
| Diamorphine | 5 | 60 | 0.5 * |
| Methadone | 10 | 10-15 | 15-40 |
| Hydromorphone | 1.5 | 7.5 | 3-4 |
| Tramadol † | 100 | 100 | 5-7 |
| Buprenorphine | 0.4 | 0.8 (sublingual) | 3-5 |
| Pentazocine | 60 | 150 | 3-5 |
| Nalbuphine | 10-20 | — | 2-4 |
| Butorphanol | 2 | — | 2-3 |
Published reports vary in the suggested doses considered to be equianalgesic to morphine. Therefore, titration to clinical response in each patient is necessary.
Suggested doses are the results of single-dose studies only. Therefore, use of the data to calculate total daily dose requirements and repeated or continuous doses may not be appropriate.
There may be incomplete cross-tolerance between these drugs. In patients who have been receiving one opioid for a prolonged period, it is usually necessary to use a dose lower than the expected equianalgesic dose when changing to another opioid, and to titrate to effect.
* Rapidly hydrolyzed to morphine.
† Only part of its analgesic action results from action on µ-opioid receptors.
Opioids act by mimicking the actions of endogenous opioid peptides, binding to receptors in the brain, brainstem, spinal cord, and to a lesser extent in the peripheral nervous system, and thus leading to inhibition of nociception. Opioids also bind to µ receptors in the pleasure centers of the midbrain, particularly in genetically susceptible individuals, a factor responsible for the euphoric effect in some individuals as well as the predilection to psychological dependence and addictive behavior. Opioids also have dose-dependent respiratory depressant effects when interacting with the µ-opioid receptors in the respiratory centers of the brainstem, depressing ventilator drive and blunting ventilatory responses to both hypoxia and hypercarbia. These respiratory depressant effects are increased with co-administration of other sedating drugs, particularly benzodiazepines or barbiturates.
Optimal use of opioids requires proactive and anticipatory management of side effects (see Table 76.7 ). Common side effects include sedation, constipation, nausea, vomiting, urinary retention, and pruritus. Tolerance usually develops to the side effect of nausea , which typically subsides with long-term dosing, but nausea may require treatment with antiemetics, such as a phenothiazine, butyrophenones, antihistamines, or a serotonin receptor antagonist such as ondansetron or granisetron. Pruritus and other complications during patient-controlled analgesia with opioids may be effectively managed by low-dose IV naloxone.
The most common, troubling, but treatable side effect is constipation . Patients who take opioids for chronic pain for long periods predictably develop tolerance to the sedative and analgesic effects of opioids over time, but tolerance to constipation does not occur, and constipation remains a troublesome and distressing problem in almost all patients with long-term opioid administration. Stool softeners and stimulant laxatives should be administered to most patients receiving opioids for more than a few days. Osmotic and bulk laxatives are less effective, usually producing more distention and discomfort. A peripherally acting opiate µ-receptor antagonist, methylnaltrexone, promptly and effectively reverses opioid-induced constipation in patients with chronic pain who are receiving opioids daily. Methylnaltrexone is approved for use as either an injectable or oral formulation, but only the SC injection is commercially available, which most children will object to receiving. Naldemedine and naloxegol are other agents with actions similar to methylnaltrexone. A novel laxative, lubiprostone , is a colonic chloride channel inhibitor that impairs water reabsorption in the colon and is very effective for opioid-induced constipation.
Media and government attention the “opioid epidemic” has reasonably led to scrutiny of the prescription of opioids to children, and recent FDA approval of opioid formulations for children has raised alarm and criticism by some vocal critics of the use of opioids for medical purposes. Thus, one of the potent barriers to effective management of pain with opioids is the fear of addiction held by many prescribing pediatricians and parents alike. Pediatricians should understand the phenomena of tolerance, dependence, withdrawal, and addiction (see Table 76.5 ). Opioid addiction is the result of the complex interplay of genetic predisposition, psychiatric pathology, and social forces, including poverty, joblessness, hopelessness, and despair. The dramatic increase in the amount of opioid abuse and overdoses and opioid-related deaths since 2001 has been largely restricted to the adult white population age 30-55 yr, not in children or adolescents. A longitudinal study of children and adolescents treated for medical reasons with opioids found that there was no increased risk of the development of substance abuse, at least until their mid-20s. Other epidemiologic studies have shown a negligible increase in opioid overdoses and deaths in the black and Latino populations, but rather a relationship to the unemployment rate. Thus the rational short- or even long-term use of opioids in children does not lead to a predilection for or risk of addiction in a child not otherwise at risk because of genetic background, race, or social milieu.
It is equally important for pediatricians to realize that even patients with recognized substance abuse diagnoses are entitled to effective analgesic management, which often includes the use of opioids. If legitimate concerns exist about addiction in a patient, safe effective opioid pain management is often best managed by specialists in pain management and addiction medicine. Table 76.9 outlines the U.S. Centers for Disease Control and Prevention (CDC) opioid recommendations for chronic pain (primarily in adults).
Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient. If opioids are used, they should be combined with nonpharmacologic therapy and nonopioid pharmacologic therapy, as appropriate.
Before starting opioid therapy for chronic pain, clinicians should establish treatment goals with all patients, including realistic goals for pain and function, and should consider how therapy will be discontinued if benefits do not outweigh risks. Clinicians should continue opioid therapy only if there is clinically meaningful improvement in pain and function that outweighs risks to patient safety.
Before starting and periodically during opioid therapy, clinicians should discuss with patients known risks and realistic benefits of opioid therapy and patient and clinician responsibilities for managing therapy.
When starting opioid therapy for chronic pain, clinicians should prescribe immediate-release opioids instead of extended-release/long-acting (ER/LA) opioids.
When opioids are started, clinicians should prescribe the lowest effective dosage. Clinicians should use caution when prescribing opioids at any dosage, should carefully reassess evidence of individual benefits and risks when increasing dosage to ≥50 morphine milligram equivalents (MME)/day, and should avoid increasing dosage to ≥90 MME/day or carefully justify a decision to titrate dosage to ≥90 MME/day.
Long-term opioid use often begins with treatment of acute pain. When opioids are used for acute pain, clinicians should prescribe the lowest effective dose of immediate-release opioids and should prescribe no greater quantity than needed for the expected duration of pain severe enough to require opioids. Three days or less will often be sufficient; more than seven days will rarely be needed.
Clinicians should evaluate benefits and harms with patients within 1 to 4 weeks of starting opioid therapy for chronic pain or of dose escalation. Clinicians should evaluate benefits and harms of continued therapy with patients every 3 months or more frequently. If benefits do not outweigh harms of continued opioid therapy, clinicians should optimize other therapies and work with patients to taper opioids to lower dosages or to taper and discontinue opioids.
Before starting and periodically during continuation of opioid therapy, clinicians should evaluate risk factors for opioid-related harms. Clinicians should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose, such as history of overdose, history of substance use disorder, higher opioid dosages (≥50 MME/day), or concurrent benzodiazepine use, are present.
Clinicians should review the patient's history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving opioid dosages or dangerous combinations that put him or her at high risk for overdose. Clinicians should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
When prescribing opioids for chronic pain, clinicians should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications as well as other controlled prescription drugs and illicit drugs.
Clinicians should avoid prescribing opioid pain medication and benzodiazepines concurrently whenever possible.
Clinicians should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
All recommendations are category A (apply to all patients outside of active cancer treatment, palliative care, and end-of-life care) except recommendation 10 (designated category B, with individual decision making required); see full guideline for evidence ratings.
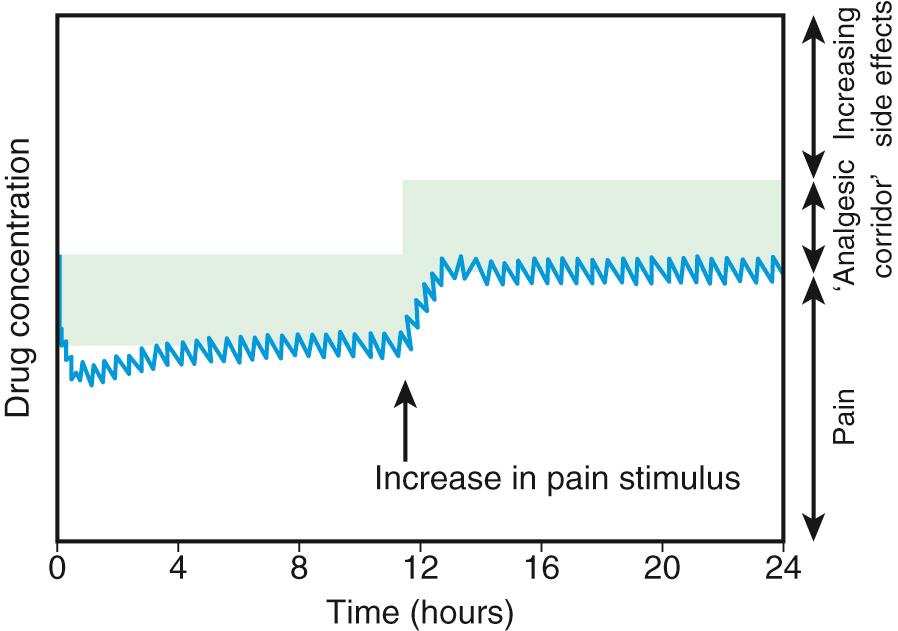
There is no longer a reason to administer opioids by IM injection. Continuous IV infusion of opioids is an effective option that permits more constant plasma concentrations and clinical effects than intermittent IV bolus dosing, without the pain associated with IM injection. The most common approach in pediatric centers is to administer a low-dose basal opioid infusion, while permitting patients to use a patient-controlled analgesia (PCA) device to titrate the dosage above the infusion ( Fig. 76.3 ) (see Chapter 74 ). Compared with children given intermittent IM morphine, children using PCA reported better pain scores. PCA has several other advantages: (1) dosing can be adjusted to account for individual pharmacokinetic and pharmacodynamic variation and for changing pain intensity during the day; (2) psychologically the patient is more in control, actively coping with the pain; (3) overall opioid consumption tends to be lower; (4) therefore fewer side effects occur; and (5) patient satisfaction is generally much higher. Children as young as 5-6 yr can effectively use PCA. The device can also be activated by parents or nurses, known as PCA-by-proxy (PCA-P), which produces analgesia in a safe, effective manner for children who cannot activate the PCA demand button themselves because they are too young or intellectually or physically impaired. PCA overdoses have occurred when well-meaning, inadequately instructed parents pushed the PCA button in medically complicated situations, with or without the use of PCA-P, highlighting the need for patient and family education, use of protocols, and adequate nursing supervision.
Because of the high risk of adverse side effects (respiratory depression), the FDA has issued contraindications for the pediatric use of codeine and tramadol ( Table 76.10 ).
Use of codeine to treat pain or cough in children <12 yr old is contraindicated.
Use of tramadol to treat pain in children <12 yr old is contraindicated.
Use of tramadol for treatment of pain after tonsillectomy or adenoidectomy in patients <18 yr old is contraindicated. (Codeine was already contraindicated in such patients).
Use of codeine or tramadol in children 12-18 yr old who are obese or who have an increased risk of serious breathing problems, such as those with obstructive sleep apnea or severe lung disease, is not recommended.
Use of codeine or tramadol in breastfeeding women should be avoided.
Local anesthetics are widely used in children for topical application, cutaneous infiltration, peripheral nerve block, neuraxial blocks (intrathecal or epidural infusions), and IV infusions ( Table 76.11 ) (see Chapter 74 ). Local anesthetics can be used with excellent safety and effectiveness. Local anesthetics interfere with neural transmission by blocking neuronal sodium channels. Excessive systemic dosing can cause seizures, CNS depression, and (by cardiac and arteriolar sodium channel blockade) hypotension, arrhythmias, cardiac depression, and cardiovascular collapse. Local anesthetics therefore require a strict maximum dosing schedule. Pediatricians should be aware of the need to calculate these doses and adhere to guidelines.
| DRUG | DOSE | NOTES |
|---|---|---|
| INTACT SKIN | ||
| Lidocaine 2⋅5% and prilocaine 2⋅5% (EMLA cream) | <3 mo old or <5 kg: 1 g 3–12 mo and >5 kg: 2 g 1–6 yr and >10 kg: 10 g 7–12 yr and >20 kg: 20 g |
60 min is needed to achieve maximum effect; cover cream with an occlusive dressing |
| Lidocaine 70 mg and tetracaine 70 mg (Synera patch) | Age ≥3 yr: apply patch | 20–30 min needed to achieve maximum effect |
| Tetracaine 4% (Ametop) | >1 mo and <5 yr: apply 1 tube of gel (1 g) >5 yr: apply up to 5 tubes of gel (5 g) |
30 min before venipuncture 45 min before intravenous cannulation |
| WOUNDS | ||
| Lidocaine, epinephrine, tetracaine (LET) solution or gel * | Age ≥1 yr: apply to wound | 20 min needed for maximum effect |
* Also referred to as ALA on the basis of alternative names for the constituents: adrenaline, lignocaine, amethocaine. These mixtures are locally made by hospital formularies, with a common formula being lidocaine 4% plus epinephrine 0⋅1% plus tetracaine 0⋅5%. The cocaine-based formulation was historically avoided on wounds of digits, ears, penis, nose, mucous membranes, close to the eye, or deep wounds involving bone, cartilage, tendon, or vessels. The lidocaine-based formulation can be used in such settings.
Topical local anesthetic preparations do not generally result in measurable systemic blood levels and can reduce pain in diverse circumstances: suturing of lacerations, placement of peripheral IV catheters, lumbar punctures, and accessing indwelling central venous ports. The application of tetracaine, epinephrine, and cocaine results in good anesthesia for suturing wounds but should not be used on mucous membranes. Combinations of tetracaine with phenylephrine and lidocaine-epinephrine-tetracaine are equally as effective, eliminating the need to use a controlled substance (cocaine). EMLA, a topical eutectic mixture of lidocaine and prilocaine used to anesthetize intact skin, is frequently applied for venipuncture, lumbar puncture, and other needle procedures. A 5% lidocaine cream (Elemax) is also effective as a topical anesthetic.
Lidocaine is the most commonly used local anesthetic for cutaneous infiltration. Maximum safe doses of lidocaine are 5 mg/kg without epinephrine and 7 mg/kg with epinephrine. Although concentrated solutions (2%) are commonly available from hospital pharmacies, more dilute solutions (0.25% and 0.5%) are equally as effective as 1–2% solutions. The diluted solutions cause less burning discomfort on injection and permit use of larger volumes without achieving toxic doses. In the surgical setting, cutaneous infiltration is more often performed with bupivacaine 0.25% or ropivacaine 0.2% because of the much longer duration of effect; maximum dosage of these long-acting amide anesthetics is 2-3 mg/kg and 3-4 mg/kg, respectively.
Neuropathic pain may respond well to the local application of a lidocaine topical patch (Lidoderm) for 12 hr/day ( Table 76.12 ). Peripheral and central neuropathic pain also may respond to IV lidocaine infusions, which may be used in hospital settings for refractory pain, complex regional pain syndromes, and pain associated with malignancies or the therapy of malignancies, such as oral mucositis following bone marrow transplantation. In these patients, 1-2 mg/kg/hr should be administered, and the infusion titrated to achieve a blood lidocaine level in the 2-5 µg/mL range, with use of twice-daily therapeutic blood monitoring. Table 76.13 outlines approaches to central neuropathic pain.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here