Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
children who require neurosurgical procedures present unique challenges to pediatric anesthesiologists. In addition to addressing problems common to general pediatric anesthesia practice, anesthesiologists must consider the effects of anesthesia on the developing central nervous system (CNS) of children with neurologic disease. This chapter reviews the age-dependent physiology of the CNS of children undergoing neurosurgical procedures requiring anesthesia and addresses some of the unique situations that anesthesiologists must face when anesthetizing children for neurosurgical procedures.
The skull can be compared to a rigid container with almost incompressible contents. Under normal conditions, the intracranial space is occupied by the brain and its interstitial fluid (80%), cerebrospinal fluid (CSF, 10%), and blood (10%). In pathologic states, space-occupying lesions such as edema, tumors, hematomas, or abscesses alter these proportions. The Monro-Kellie hypothesis, elaborated in the 19th century, states that the sum of all intracranial volumes is constant. An increase in the volume of one compartment must be accompanied by an approximately equal decrease in the volume of the other compartments, except when the cranium can expand to accommodate a larger volume. Gradual increases in intracranial volumes, such as a slow-growing tumor or hydrocephalus, can be compensated by the compliant nature of open fontanelles and sutures in young children; increasing head circumference can result. However, herniation can occur even in children with open fontanelles if large increases in intracranial pressure (ICP) develop acutely. In the nonacute situation, the brain can compensate for pathologic increases in intracranial volume by intracellular dehydration and reduction of interstitial fluid.
Under normal conditions, CSF exists in dynamic equilibrium, with absorption balancing production. The rate of CSF production in adults is approximately 0.35 mL/minute or 500 mL/day. The average adult has 100 to 150 mL of CSF distributed throughout the brain and subarachnoid space. Children have correspondingly smaller volumes of CSF, but the rate of CSF production is similar to that of adults.
Production of CSF is only slightly affected by alterations of ICP and is usually unchanged in children with hydrocephalus. Some drugs, including acetazolamide, furosemide, and corticosteroids, are mildly effective in transiently decreasing CSF production. There is an inverse relationship between the rate of CSF production and serum osmolality; an increase in serum osmolality causes a decrease in CSF production. Choroid plexus papillomas causing overproduction of CSF are rare but are more likely to occur during childhood.
Absorption of CSF is not well understood, but the arachnoid villi appear to be important sites for reabsorption of CSF into the venous system. One-way valves between the subarachnoid space and the sagittal sinus appear to open at a gradient of about 5 mm Hg. Some resorption may occur from the spinal subarachnoid space and from the ependymal lining of the ventricles. Resorption increases with an increase in ICP. However, CSF absorption is decreased by pathologic processes that obstruct arachnoid villi or interfere with CSF flow, such as intracranial hemorrhage, infection, tumor, and congenital malformations.
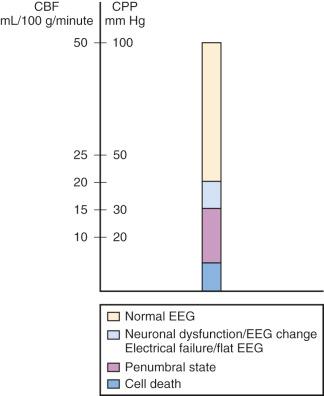
Increased ICP causes secondary brain injury by producing cerebral ischemia and ultimately causing herniation. Ischemia occurs when ICP increases and cerebral perfusion pressure (CPP) decreases. As cerebral blood flow (CBF) and the supply of nutrients are curtailed, cell damage and death occur, leading to increased intracellular and extracellular water and further increases in ICP. When ICP increases, CPP decreases, the brain becomes ischemic, and cell death can ensue ( Fig. 26.1 ).
Several herniation syndromes exist. The most common is transtentorial herniation, in which the uncus of the temporal lobe is displaced from the supratentorial to the infratentorial space. Compression of the third cranial nerve and brainstem results in pathognomonic signs of pupillary dilatation, hemiparesis, and loss of consciousness. If this compression is not promptly relieved, apnea, bradycardia, and death occur.
In cerebellar herniation, the cerebellar tonsils herniate through the foramen magnum from the posterior fossa to the cervical spinal space. This can lead to obstruction of CSF circulation and ultimately to hydrocephalus. Compression of the brainstem results in cardiorespiratory failure and death.
The clinical signs of increased ICP vary in children. Papilledema, pupillary dilation, hypertension, and bradycardia may be absent despite intracranial hypertension, or these signs may occur with normal ICP. When associated with increased ICP, they are usually late and dangerous signs. Chronic increases in ICP are often manifested by complaints of headache, irritability, and vomiting, particularly in the morning. Papilledema may not be present even in children dying as a result of intracranial hypertension. A diminished level of consciousness and abnormal motor responses to painful stimuli are frequently associated with an increased ICP. Computed tomography (CT) or magnetic resonance imaging (MRI) can reveal small or obliterated ventricles or basilar cisterns, hydrocephalus, intracranial masses, and midline shifts. Diffuse cerebral edema is a common finding when increased ICP is associated with closed-head injury, encephalopathy, or encephalitis.
Techniques to monitor ICP in adults have been successfully used in children. Noninvasive techniques seem to be less accurate than invasive methods. Ventricular catheters are generally accepted as the most accurate and reliable means of measurement, permitting removal of CSF for diagnostic or therapeutic indications. The major risks of intraventricular catheters are infection and hemorrhage; although rare, they can lead to devastating complications. These catheters may be difficult to insert precisely when they are needed most, as in a child with severe cerebral edema and small ventricles. Compared with intraventricular catheters, subarachnoid bolts can be placed even when the ventricles are obliterated. This procedure minimizes trauma to brain tissue and poses less risk of serious infection and hemorrhage. The major disadvantages are that subarachnoid bolts may underestimate ICP, particularly in areas distant from their insertion site, and they are difficult to stabilize in infants with thin calvarias.
Epidural monitors that do not require a fluid interface can be implanted outside the dura, avoiding the risks of CSF contamination and the limitations of fluid-dependent systems. Most epidural systems correlate well with intraventricular measurements, but they cannot be recalibrated after insertion. Epidural monitors have also been secured noninvasively to the open anterior fontanelle of infants and appear to reflect changes in ICP. Fiber optic catheters with self-contained transducers can also be used to measure ICP from intraventricular, subarachnoid, or intraparenchymal sites. These monitors avoid some of the problems of external fluid-filled transducers, but like epidural transducers, they cannot be recalibrated after insertion.
The normal ICP in children is less than 15 mm Hg. In term neonates, normal ICP is 2 to 6 mm Hg; it is probably even less in preterm infants. Children with intracranial pathology but normal ICP values occasionally exhibit pressure waves, which are considered abnormal. In children with open fontanelles, the ICP may remain normal despite a significant intracranial pathologic process; increasing head circumference may be the first clinical sign. Bulging fontanelles may not develop, especially when the process evolves slowly.
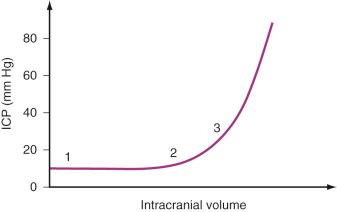
The absolute value of ICP does not indicate how much compensation is possible. If the ICP increases significantly, compensatory mechanisms have failed. However, pathologic states may be present despite an ICP within the normal range. Intracranial compliance (i.e., the change in pressure relative to a change in volume) is a valuable concept. Fig. 26.2 is a schematic diagram of the relationship between the addition of volume to intracranial compartments and ICP. The shape of the curve depends on the time over which the volume increases and the relative size of the compartments. At normal intracranial volumes (point 1 in the figure), ICP is low, but compliance is high and remains so despite small increases in volume. If volume increases rapidly, compensatory abilities are surpassed, and further increases in volume are reflected as increases in pressure. This can occur when the ICP is still within normal limits but the compliance is low (point 2). If the ICP is already increased, further volume expansion causes a rapid increase in ICP (point 3). In clinical practice, compliance can be evaluated with a ventriculostomy catheter or by observing the response of ICP to external stimulation (e.g., tracheal suction, coughing, agitation).
Several physiologic and mechanical factors such as a greater percentage of brain water content, less CSF volume, and a greater percentage of brain content to intracranial capacity contribute to a relatively decreased intracranial compliance in children compared with adults. Children may be at increased risk for herniation compared with adults when similar relative increases in ICP have occurred. However, infants faced with a slowly increasing ICP may have a greater compliance because of their open fontanelles and sutures.
In addition to CSF, cerebral blood volume (CBV) represents another compartment in which compensatory mechanisms influence ICP. Although the CBV occupies only 10% of the intracranial space, changes related to dynamic blood volume occur, often initiated by anesthesia or intensive care procedures. As with other vascular beds, most intracranial blood is contained in the low-pressure, high-capacitance venous system. Increases in intracranial volume are initially met by decreases in CBV. This response is apparent in hydrocephalic infants, in whom venous blood shifts from intracranial to extracranial vessels, producing distended scalp veins.
In the normal adult, CBF is approximately 55 mL/100 g of brain tissue per minute. This represents almost 15% of the cardiac output for an organ that accounts for only 2% of body weight. Estimates of CBF are less uniform for children. Normal CBF in healthy awake children is approximately 100 mL/100 g of brain tissue per minute, which represents up to 25% of cardiac output. CBF in neonates and preterm infants (approximately 40 mL/100 g of brain tissue per minute) is less than in children and adults. In infants, CBF is subject to modification by sleep states and feeding.
Understanding CBF in neonates, infants, and other children is fundamental to being able to safely care for these most vulnerable patients during procedures requiring sedation and general anesthesia. Recent work has focused on delineating the factors that contribute to adequate CBF in children undergoing general anesthesia. Classically, teaching has focused on a variety of factors that underlie maintenance and autoregulation of CBF—mean arterial pressure (MAP), Pa CO2 , and so on. While these concepts remain critical underpinnings of neurophysiology, it is also incumbent upon anesthesia providers to have a broader understanding and approach to the idea of CBF. Regulation of CBF is best understood as the nexus of different physiologic systems. These systems include the respiratory, cardiovascular autonomic, nervous, and endocrine systems, metabolic processes, and the intracranial environment itself. In light of this approach, CBF is regulated by integrative processes that involve respiratory gas exchange, hemodynamic parameters and their consequent effects on cerebrovascular resistance. In adults, the cerebral metabolic rate for oxygen consumption (CMR o 2 ) is 3.5 to 4.5 mL O 2 /100 g per minute; in children, it is greater. General anesthesia reduces CMR o 2 by as much as 50%. Coupling of CBF and CMR o 2 is probably mediated by the effect of the local hydrogen ion concentration on cerebral vessels. Conditions that cause acidosis (e.g., hypoxemia, hypercarbia, ischemia) dilate the cerebral vasculature, which augments CBF and CBV. A reduction in brain metabolism (i.e., CMR o 2 ) similarly reduces CBF and CBV. When autoregulation is impaired, CBF is determined by factors other than metabolic demand. If the CBF exceeds metabolic requirements, luxury perfusion or hyperemia exists. Many pharmacologic agents act directly on the cerebral vasculature to alter CBF and CBV.
CPP is a useful and practical estimate of the adequacy of the cerebral circulation, because CBF is neither easily nor widely measured. Defined as the pressure gradient across the brain, CPP is the difference between the systemic MAP at the entrance to the brain and the mean exit pressure (i.e., central venous pressure [CVP]). When ICP exceeds CVP, it replaces CVP in the calculation of CPP. In supine children, the mean CPP is the difference between the MAP and the mean ICP (CPP = MAP − ICP). If the brain and heart are positioned at different heights, all pressures should be referenced at the level of the head (e.g., external auditory meatus).
Alterations in cerebral perfusion under general anesthesia make anesthetizing neonates and infants a particularly challenging endeavor. Neonates already have an increased perioperative morbidity and mortality. Recent concerns for the risks of adverse neurocognitive outcomes from exposure to common anesthetic drugs have evolved into greater efforts in optimizing the physiologic management of neonates undergoing general anesthesia (see Chapter 25 ). It comes as no great surprise that part of this renewed effort to improve the care of neonates has focused on understanding the role of an adequate cerebral perfusion. Problems include difficulties with accurate blood pressure measurement, knowledge of appropriate hemodynamic goals, maintaining said normal hemodynamic ranges and hemodynamic goals while avoiding hypocapnia.
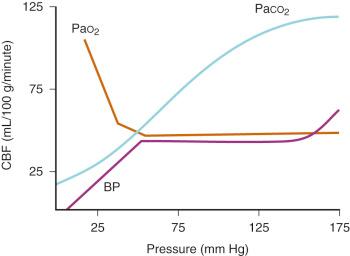
Classical teaching has maintained that, in adults, CBF remains relatively constant within an MAP range of 50 to 150 mm Hg ( Fig. 26.3 ). There is growing evidence that this concept that blood flow is constant over this MAP range is not necessarily correct. Interpretation of a constant flow is the result of limitations to this research that include methods of measuring and the assumptions made by such methods (e.g., transcranial Doppler [TCD] and the assumption that the diameter of the measured vessel remains constant), the manipulation of CBF pharmacologically (the influence on cerebrovascular tone is not well understood and controversial), and the confounding effect of the arterial partial pressure of carbon dioxide (Pa co 2 ) alterations during pharmacologic manipulations of blood pressure. Despite these limited data, as clinicians we still depend on published evidence to optimize the care of our patients under anesthesia. It is useful to understand these new concepts but try to not simply disregard older, probably too basic, ideas of maintenance of CBF in our patients under anesthesia.
We can view autoregulation as a mechanism that enables brain perfusion to remain relatively stable despite changes in MAP or ICP. There is recent evidence that this relationship between MAP and CBF is likely more pressure passive than originally thought. In fact, it seems as if stricter autoregulation occurs at greater values of MAP than smaller values. Autoregulation is mediated by autonomic and myogenic control of vascular resistance, although there remains considerable debate regarding the exact mechanism and exact location of this modulation. When CPP decreases, cerebral vessels dilate to maintain CBF, thereby increasing CBV. When CPP increases, cerebral vasoconstriction occurs, maintaining the CBF with a reduced CBV. When ICP and CVP are low, MAP normally approximates CPP. Beyond the range of autoregulation, CBF becomes more pressure-dependent. In children with chronic hypertension, the upper and lower limits of autoregulation are increased. Cerebral autoregulation can be abolished by acidosis, medications, tumor, cerebral edema, and vascular malformations, even at sites far removed from a discrete lesion.
The limits of autoregulation for normal infants and children are unknown, but autoregulation probably occurs at lower absolute values than in adults. Although the lower limit of autoregulation in adults is approximately 50 mm Hg MAP, this blood pressure may be beyond that of the neonate. Intact autoregulatory mechanisms have been demonstrated within lower blood pressure ranges in newborn animals compared with mature animals. Children younger than 6 months during sevoflurane anesthesia had a decrease in CBF velocity that was not present until the MAP was 38 mm Hg. That study has not been repeated, so results should perhaps be taken with a degree of skepticism. Cerebral autoregulation may even be abolished in critically ill humans.
CBF is constant over a wide range of oxygen tensions. When the partial pressure of arterial O 2 (Pa o 2 ) decreases to less than 50 mm Hg, CBF increases exponentially in adults; for example, at a Pa o 2 of 15 mm Hg, CBF is doubled compared with normal (see Fig. 26.3 ). The resulting increase in CBV increases ICP when intracranial compliance is low; the lower limit for Pa o 2 is probably less in neonates. Oxygen delivery is more important than the actual Pa o 2 . Evidence suggests that hyperoxia decreases CBF. Kety and Schmidt demonstrated a 10% decrease in CBF in adults breathing 100% O 2 , although decreases of 33% have been reported in neonates. However, a decrease in MAP with sevoflurane anesthesia has also been reported to increase regional brain oxygenation as measured by near-infrared spectroscopy (NIRS). This lends further credence to the idea that various physiologic systems have complex interactions that lead to modulation of CBF.
The relationship between the arterial partial pressure of carbon dioxide (Pa co 2 ) and CBF typically is linear (see Fig. 26.3 ). In adults, a 1-mm Hg increase in Pa co 2 increases CBF by approximately 2 mL/100 g per minute. The direct effect of changes in Pa co 2 on CBF and the consequent effect on CBV are the basis for the fact that hyperventilation reduces ICP. Likewise, increases in Pa co 2 increase the CBF, although the limits at which this occurs in neonates differ from those in adults. In lambs and monkeys, CBF does not seem to change in response to decreased Pa co 2 . There are no data to suggest the lower limits of Pa co 2 effect on CBF in human infants and children. Similarly, there is little information about the extent and duration of cerebrovascular responsiveness to hyperventilation in brain-injured and critically ill children. Moderate hyperventilation has been used to rapidly reduce ICP, but several reports have demonstrated worsening cerebral ischemia in children with compromised cerebral perfusion.
Autoregulation of CBF is impaired in regions of damaged brain. Blood vessels in an ischemic zone are subject to hypoxemia, hypercarbia, and acidosis, which are potent stimuli for vasodilation. These vessels develop maximally reduced cerebrovascular tone or vasomotor paralysis. Small, localized lesions may impair autoregulation in areas far removed from the site of injury. The extent of autoregulatory impairment varies in children with brain damage.
Preoperative evaluation of infants and children is discussed in Chapter 4 . Children who are scheduled for neurosurgery might have been healthy until the onset of their symptoms, might have been developmentally delayed from birth, or may have impaired neuromuscular function. The anesthetic plan, including postoperative care, needs to consider the particular issues of each child and the disease state.
A history of food or drug allergies, eczema, or asthma may provide warning of an adverse reaction to contrast agents frequently used in neuroradiologic procedures. Special attention should be given to symptoms of allergy to latex products, such as lip swelling after blowing up a toy balloon or tongue swelling after insertion of a rubber dam into the mouth by a dentist, because latex anaphylaxis has been reported in some children who have undergone multiple operations, especially those with a meningomyelocele. Children with latex allergy may also report allergies to fruits (e.g., kiwi, banana, avocado, strawberry, and others).
Concurrent pediatric diseases and symptoms of neurologic lesions may influence the conduct of anesthesia. Protracted vomiting, enuresis, and anorexia related to intracranial lesions should prompt evaluation of hydration and electrolytes. Diabetes insipidus or inappropriate secretion of antidiuretic hormone are common. A history of the use of aspirin or aspirin-containing remedies for headaches or respiratory tract infections is information that is not usually forthcoming but may have important implications for operative and postoperative bleeding. Corticosteroids are often initiated at the time of diagnosis of intracranial tumors, and they should be continued and a pulse dose administered during the perioperative period. Therapeutic concentrations of anticonvulsants should be verified preoperatively and maintained perioperatively. Children receiving long-term anticonvulsants may develop toxicity, especially if seizures are difficult to control; this is frequently manifested as abnormalities in hematologic or hepatic function, or both. Children receiving long-term anticonvulsant therapy may also require increased amounts of sedatives, nondepolarizing muscle relaxants, and opioids because of enhanced metabolism of these drugs (see also Chapters 7 and 24 ).
The physical examination should encompass a brief neurologic evaluation, including level of consciousness, motor and sensory function, normal and pathologic reflexes, integrity of the cranial nerves, and signs and symptoms of intracranial hypertension. Examination of pupillary size and responsiveness can detect benign anisocoria. Preoperative respiratory assessment should include the effects of motor weakness, impaired gag and swallowing mechanisms, and evidence of active pulmonary disease, such as aspiration pneumonia. Muscle atrophy and weakness should be documented, because upregulation of acetylcholine receptors may precipitate sudden hyperkalemia after administration of succinylcholine and induce resistance to nondepolarizing muscle relaxants in the affected limbs.
In all but the most minor procedures, laboratory data should include a hematocrit determination. Blood typing and crossmatching should be performed for any major procedure. The need for additional studies, such as evaluation of coagulation parameters, serum electrolyte levels and osmolality, blood urea nitrogen and creatinine values, arterial blood gas analysis, chest radiography, or electrocardiography (ECG), is determined on an individual basis. Liver function tests and a hematologic profile should be obtained if not recently reviewed in children receiving long-term therapy with anticonvulsants. Specific neuroradiologic studies are usually obtained by the neurosurgeon and should be reviewed by the anesthesiologist. For example, the anesthesiologist should know which children with a ventriculoperitoneal shunt have “slit ventricles” because these children have special risks in the perioperative period (see “ Hydrocephalus ”). Information on the amount of sedation needed to perform radiologic studies may also be helpful in planning the induction of anesthesia. Preoperative neurophysiologic studies, including electroencephalography (EEG) and evoked potentials, may provide a baseline for comparison of intraoperative and postoperative evaluations.
Sedation is usually withheld from pediatric neurosurgical patients until they arrive in the preoperative area to allow titration of drug to desired effect while under direct supervision. Opioids are usually withheld preoperatively because they may cause nausea or respiratory depression, especially in children with increased ICP, and sedatives alone usually are adequate to relieve anxiety.
Sedatives are administered in the parents' presence to facilitate a smooth separation and induction. Midazolam (~0.5-1.0 mg/kg) may be given orally; it usually requires 10 to 20 minutes to take effect. Incremental doses of intravenous (IV) midazolam (0.05 mg/kg) may also be useful in children who tolerate IV placement.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here