Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neurosurgical lesions in infants and children have distinct manifestations and management issues. Age-related differences in the surgical lesions, anatomy and physiological responses to surgery and anesthesia underlie the clinically relevant differences between pediatric patients and their adult counterparts. Technical advances in neurosurgery and subspecialization in pediatric neurosurgery, anesthesiology and critical care have dramatically improved the outcome in pediatric patients with surgical lesions of the central nervous system (CNS). The perioperative management should be based on the developmental stage of the patient with the caveat that neonates may be vulnerable to iatrogenic CNS injury. The aim of this chapter is to highlight these age- dependent differences and their effects on the management of the pediatric neurosurgical patient during the perioperative period.
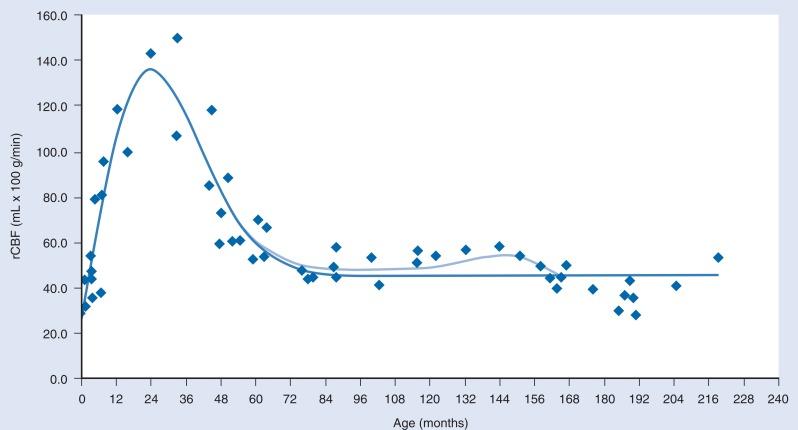
Differences in cerebrovascular physiology and cranial bone development distinguish infants and children from adults. Cerebral blood flow (CBF) is coupled tightly to metabolic demand, and both increase proportionally immediately after birth. CBF varies with the age of the patient. Computed tomography perfusion scans show that CBF peaks between 2 and 4 years and settles at 7–8 years ( Fig. 20.1 ). These changes mirror those in neuroanatomical development. The idealized autoregulatory range of blood pressure in a normal newborn is between 20 and 60 mmHg, which reflects the relatively low cerebral metabolic requirements and blood pressure during the perinatal period. More importantly, the slope of the autoregulatory curve drops and rises significantly at the lower and upper limits of the curve, respectively ( Fig. 20.2 ).
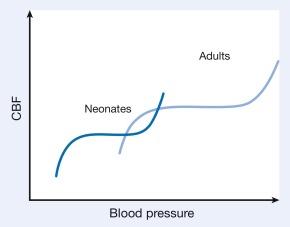
Cerebral autoregulation is intact in healthy full-term neonates. However, critically ill premature neonates have a linear correlation between CBF and systemic blood pressure. This pattern of CBF pressure-passivity occurs in premature neonates with low gestational age and birth weight, and systemic hypotension. It should be noted that systolic arterial blood pressure is a poor surrogate of cerebral perfusion pressure in premature infants. An assessment of the diastolic blood pressure and CBF velocity may be a better marker of cerebral perfusion pressure in this population. Therefore, tight blood pressure control is essential in the management of neonates to minimize both cerebral ischemia and intraventricular hemorrhage.
Transcranial Doppler studies demonstrated that the lower limit of cerebral autoregulation was equivalent among older and younger children. These observations suggest that children younger than 2 years may have lower autoregulatory reserve because of their relatively low baseline mean arterial pressures and may be at greater risk of cerebral ischemia. Multimodal analysis of cerebral perfusion in infants and children undergoing cardiopulmonary bypass surgery reveal a wide range of lower limits of autoregulation. This observation demonstrates variability in pediatric patients and highlights the limitations of current monitors to optimize cerebral perfusion.
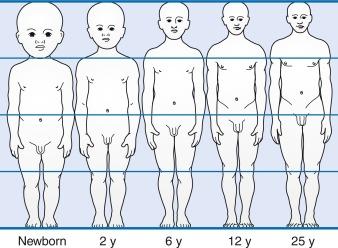
Adults and infants differ in the percentage of cardiac output directed to the brain. CBF is 10–20% of the cardiac output during the first 6 months and peaks at 55% between the second and fourth years. CBF settles to the adult levels of 15% by 7–8 years. The head of the infant and child also accounts for a large percentage of the body surface area and blood volume ( Fig. 20.3 ). This feature places the young child at risk for significant hemodynamic instability during neurosurgical procedures.
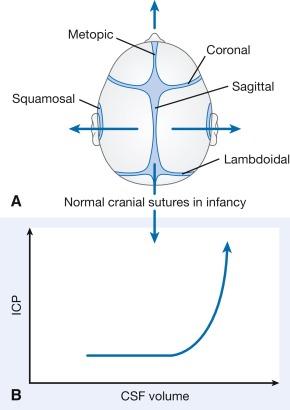
The infant cranial vault is also in a state of flux. Open fontanels and cranial sutures result in a compliant intracranial space ( Fig. 20.4 ). The mass effect of a slow-growing tumor or insidious hemorrhage is often masked in an infant by a compensatory distended fontanel and widening of the cranial sutures. However, acute increases in cranial volume due to massive hemorrhage or an obstructed ventricular system cannot be attenuated by expansion of the immature cranial vault and often result in life-threatening intracranial hypertension.
Neonates and infants have functionally immature organ systems. The neonatal renal system is characterized by a decreased glomerular filtration rate and concentrating ability. These changes result in diminished excretion of saline and water, and limit the neonate’s ability to compensate for fluctuations in fluid and solute loads. Drugs that are renally excreted may have a prolonged half-life. Hepatic function is also diminished in neonates, and the metabolism of drugs may be delayed owing to decreased activity of hepatic enzymes. Total body water drops from 85% in premature infants to 65% in adults, whereas body fat content increases from less than 1% in premature infants to 15% in term infants and 35% in adults. The total protein level follows a similar trend. Therefore, hydrophilic drugs have more binding sites, and hydrophobic drugs fewer, in infants. The constellation of these factors should prompt the clinician generally to decrease the weight-adjusted dose and frequency of administration of drugs given to the newborn.
Given the systemic effects of general anesthesia and the physiologic stress of surgery, an organ system review is essential for identifying coexisting disease, and anticipating potential physiologic derangements that increase the risk of perioperative complications. General perioperative concerns in infants and children are listed in Table 20.1 . Preoperative laboratory tests should be tailored to the proposed neurosurgical procedure. Given the risk of significant blood loss associated with surgery, the hematocrit, prothrombin time, and partial thromboplastin time should be measured to uncover any insidious hematologic disorder. Patients with suprasellar pathology should undergo an endocrinology evaluation. Table 20.2 matches special concerns in pediatric patients with neurologic problems.
| Condition | Anesthetic Implications |
|---|---|
| Congenital heart disease | Hypoxia, arrhythmias, and cardiovascular instability; paradoxical air emboli |
| Prematurity | Postoperative apnea |
| Gastrointestinal reflux | Aspiration pneumonia |
| Upper respiratory tract infection | Laryngospasm, bronchospasm, hypoxia, pneumonia |
| Craniofacial abnormality | Difficulty with airway management |
| Condition | Anesthetic Implications |
|---|---|
| Denervation injuries | Hyperkalemia after succinylcholine Resistance to nondepolarizing muscle relaxants, abnormal response to nerve stimulation |
| Long-term anticonvulsant therapy | Hepatic and hematologic abnormalities Increased metabolism of anesthetic agents |
| Arteriovenous malformation | Potential congestive heart failure |
| Neuromuscular disease | Malignant hyperthermia Respiratory failure Sudden cardiac death |
| Chiari malformation | Apnea Aspiration pneumonia |
| Hypothalamic or pituitary lesions | Diabetes insipidus Hypothyroidism Adrenal insufficiency |
Closed-claim analysis studies have revealed that neonates and infants are at higher risk for perioperative morbidity and mortality than other age groups. , Respiratory and cardiac-related events account for a majority of these complications. Given the urgent nature of many pediatric neurosurgical procedures, a thorough preoperative evaluation may be difficult. A complete airway examination is essential, since some craniofacial anomalies may require specialized techniques to secure the airway. Congenital heart disease may not be apparent immediately after birth and may complicate the perioperative course of the neonate undergoing an emergency neurosurgical procedure. Therefore, echocardiography can be helpful in the assessment of the heart, especially in the neonate, and a pediatric cardiologist should evaluate a patient with suspected cardiac problems in order to optimize cardiac function prior to surgery.
Perioperative anxiety plays a significant role in the care of the pediatric neurosurgical patient. These issues are related to the cognitive development and age of the child ( Table 20.3 ). Preoperative sedatives given prior to the induction of anesthesia can ease the transition from the preoperative holding area to the operating room. Midazolam administered orally is particularly effective in relieving anxiety and producing amnesia. If an indwelling intravenous catheter is in place, midazolam can be slowly titrated to achieve sedation.
| Age Group | Concerns |
|---|---|
| Infants (0–9 months) | None; will separate easily from parents |
| Preschoolers (9 months–5 year) | Stranger anxiety; difficulty with separation from parents |
| Grade schoolers (6–12 years) | Fear of needles/pain |
| Adolescence (> 12 year) | Anxiety about surgery and self-image |
Preoperative fasting regimens have dramatically evolved over the years and vary according to local preferences. The purpose of limiting oral intake is to minimize the risk of pulmonary aspiration of gastric contents. However, prolonged fasting periods can potentially result in both hypovolemia and hypoglycemia, which in turn can lead to hemodynamic and metabolic instability during anesthesia. Although the scientific validity of many recommendations has not been investigated, a common guideline is provided in Table 20.4 .
| Fasting Time (Hours) | Substance to be Withheld |
|---|---|
| 2 | Clear liquids |
| 4 | Breast milk |
| 6 | Formula |
| 8 | Solid food |
The patient’s preoperative status dictates the appropriate technique and drugs for induction of anesthesia. General anesthesia can be induced with sevoflurane and nitrous oxide with oxygen. A nondepolarizing muscle relaxant is then administered after intravenous (IV) access has been established to facilitate intubation of the trachea. If the patient already has an IV catheter, anesthesia can be induced with a sedative-hypnotic drug such as propofol (2–4 mg/kg). It should be noted that neonates are vulnerable to propofol-induced hypotension that can persist for 30 minutes, especially when there is no surgical stimulation. Patients who are vomiting or have recently ingested food or fluids are at risk for aspiration pneumonitis and so should undergo rapid-sequence induction of anesthesia with propofol, followed immediately with a rapid-acting muscle relaxant and cricoid pressure.
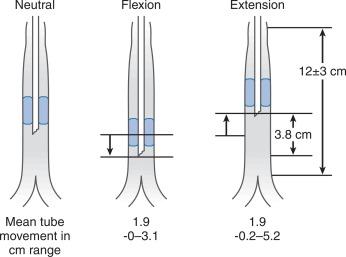
Developmental changes in airway anatomy have a significant effect on the management of the pediatric airway. The infant’s larynx is funnel-shaped, being narrowest at the level of the cricoid ring. This feature puts the infant at risk for subglottic obstruction secondary to mucosal swelling after prolonged endotracheal intubation with a tight-fitting endotracheal tube. Cuffed endotracheal tubes can be used, but the cuff pressure should be checked frequently and adjusted to minimize tracheal injury. Since the infant’s trachea is relatively short, an endotracheal tube can easily migrate into a mainstem bronchus when the head is flexed, as it will be for a suboccipital approach to the posterior fossa or the cervical spine ( Fig. 20.5 ). Therefore, great care should be devoted to ensuring proper position of the endotracheal tube during tracheal intubation, and the anesthesiologist should auscultate both lung fields to rule out inadvertent intubation of a mainstem bronchus after final positioning of the patient. Nasotracheal tubes are best suited for the patient in the prone position, because they are easier to secure, less likely to kink at the base of the tongue when the head is a flexed and prevent pressure injury to the tongue.
Timing of tracheal extubation sometimes presents a challenge after neurosurgical procedures. Patients with Chiari malformation and brainstem surgery may exhibit intermittent postoperative apnea, vocal cord paralysis, or other irregularities before resuming a stable respiratory pattern. Significant airway edema and postoperative obstruction can complicate prone procedures or operations involving significant blood losses and large volume replacement. Preexisting pulmonary dysfunction, as in infants with bronchopulmonary dysplasia or older children with neuromuscular disease, may force delays in extubation because of respiratory insufficiency. In these cases, standard tracheal extubation criteria and the presence of an endotracheal tube air leak less than 20 cm H 2 O can assist the clinician in appropriate decision-making. Lingual or supraglottic swelling can cause airway obstruction and can be diagnosed by direct laryngoscopy. When swelling is significant, postoperative lung ventilation in the intensive care unit, head-up positioning and gentle forced diuresis lead to improved conditions within 24 hours.
The diminutive size of the neonate or infant requires careful preoperative planning to allow adequate access to the patient for both the neurosurgeon and anesthesiologist ( Table 20.5 ). Skull fixation with a Mayfield frame is standard practice in craniotomies and cervical spine surgery. However, infants and small children have thin skulls and are at increased risk for fractures and epidural hemorrhage. Therefore, age-appropriate fixation pins and tensions should be utilized. The prone position is commonly used for posterior fossa and spinal cord surgery. Although the sitting position has been used less often in pediatric patients, it may be appropriate for obese patients, who may be difficult to ventilate in the prone position. In addition to the physiologic sequelae of the prone position, a whole spectrum of compression and stretch injuries has been reported. Padding under the chest and pelvis can support the torso. It is important to ensure free abdominal wall motion because greater intra-abdominal pressure can impair ventilation, cause compression of the vena cava, and increase epidural venous pressure and bleeding. Soft rolls are generally used to elevate and support the lateral chest wall and hips to minimize the increases in abdominal and thoracic pressure. In addition, a Doppler probe can be applied to the chest without causing pressure sores.
| Position | Physiologic Effect |
|---|---|
| Head elevated | Enhanced cerebral venous drainage |
| Decreased cerebral perfusion pressure (potential cerebral blood flow decrease) | |
| Increased venous pooling in lower extremities | |
| Postural hypotension | |
| Head down | Increased cerebral venous and intracranial pressure |
| Decreased functional residual capacity (lung function) | |
| Decreased lung compliance | |
| Prone | Venous congestion of face, tongue, and neck |
| Decreased lung compliance | |
| Increased abdominal pressure can lead to compression of the vena cava | |
| Lateral decubitus | Decreased compliance of down-side lung |
Many neurosurgical procedures are performed with the head slightly elevated to facilitate venous and cerebrospinal fluid drainage from the surgical site. However, superior sagittal sinus pressures decrease with increasing head elevation and increase the likelihood of venous air embolism (VAE). Extreme head flexion can cause brainstem compression in patients with posterior fossa pathology, such as a mass lesion or Chiari malformation. Furthermore, significant rotation of the head can compress the jugular vein, which in turn impedes venous return leading to impairment of cerebral perfusion and increases in ICP and venous bleeding.
Optimal intravenous access is mandatory prior to the start of surgery because access to the infant and small child during neurosurgical procedures can be limited. Typically two large-bore venous cannulas are sufficient for most craniotomies. Should initial attempts fail, central venous cannulation may be necessary. Cannulation of the femoral vein avoids the risk of pneumothorax associated with subclavian catheters and does not affect cerebral venous return. Furthermore, femoral catheters are more accessible during operations on the head. Since significant blood loss and hemodynamic instability can occur during craniotomies, an arterial catheter would provide direct blood pressure monitoring and sampling for blood gas analysis.
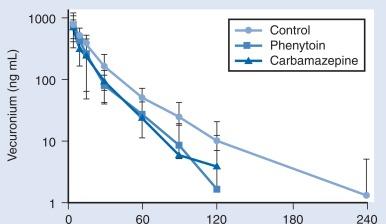
Sevoflurane is the principal anesthetic for induction of anesthesia in infants and children followed by opioid and low-dose (0.2–0.5%) isoflurane. The incidence of awareness under anesthesia has been reported to be 0.8% in children, a value higher than in adults. Deep neuromuscular blockade with a nondepolarizing muscle relaxant is maintained to avoid patient movement and minimize the amounts of anesthetic agents needed. Patients who have undergone long-term anticonvulsant therapy will require larger doses of muscle relaxants and narcotics because of the enzymatic metabolism induced by these agents ( Fig. 20.6 ). Muscle relaxants should be withheld or their effects permitted to wear off when assessment of motor function during neurosurgery is planned.
Anesthetic-induced developmental neurotoxicity has been fully substantiated in preclinical reports, but the retrospective clinical studies have been inconclusive. It is well known that extremely and very low-birth-weight survivors have poor neurological and cognitive outcomes. Cerebral injury due to cerebral hypoperfusion, metabolic derangements, coexisting disease and surgery have the potential to worsen neurological outcomes in neonates and infants. Therefore, intraoperative hypotension, hypocarbia, oxygenation, glycemia and temperature control should be aggressively managed in these vulnerable patients.
Hemodynamic stability during intracranial surgery requires careful maintenance of intravascular volume and electrolytes. Sudden blood loss or venous air embolus can rapidly deteriorate to cardiovascular collapse. Therefore, normovolemia should be maintained throughout the procedure. Estimation of the patient’s blood volume is essential in determining the amount of allowable blood loss and the time to transfuse blood. Blood volume depends on the age and size of the patient, as delineated in Table 20.6 . Normal saline is commonly used as the maintenance fluid during neurosurgery because it is mildly hyperosmolar and should minimize cerebral edema. However, rapid infusion of large quantities of normal saline (> 60 mL/kg) can be associated with hyperchloremic acidosis. Given the relatively large blood volume of the neonate or infant, the maintenance rate of fluid administration depends on the weight of the patient ( Table 20.7 ). Significant blood loss is likely in most craniotomies in infants and children, so the maximum allowable blood loss should be determined in advance so the anesthesiologist will know when blood should be transfused to the patient. However, there are no guidelines for threshold in transfusing blood and the decision to transfuse should be dictated by the type of surgery, underlying medical condition of the patient, and potential for additional blood loss, both intraoperative and postoperative. Hematocrit values of 21–25% should provide some impetus for blood transfusion. Packed red blood cells (10 mL/kg) will raise the hematocrit by 10%. Initially, blood losses should be replaced with 3 mL of normal saline for each 1 mL of lost blood or a colloid solution, such as 5% albumin, equal to the blood loss. Depending on the extent and length of the surgical procedure and exposure of vascular beds, additional fluid administration at 3–10 mL/kg/h may be necessary.
| Age | Estimated Blood Volume (mL/kg) |
|---|---|
| Preterm neonate | 100 |
| Full-term neonate | 90 |
| ≤ 1 year | 80 |
| 1–12 years | 75 |
| Adolescents and adults | 70 |
| Weight (kg) | Rate |
|---|---|
| ≤ 10 | 4 mL/kg/h |
| 10–20 | 40 mL + 2 mL/kg/h for every kg over 10 kg |
| ≥ 20 | 60 mL + 1 mL/kg/h for every kg over 20 kg |
Pediatric patients, particularly infants, are at particular risk for hypoglycemia. Small premature infants, who have limited reserves of glycogen and limited gluconeogenesis, require continuous infusions of glucose at 5–6 mg/kg/min to maintain serum levels. Surgery elicits a stress response, and children are generally able to maintain normal serum glucose levels without exogenous glucose administration. Since inadvertent hypoglycemia occurs in fasted infants and children, frequent glucose measurements are recommended. Limited evidence now suggests that tight glycemic control may reduce postoperative infections but still carry undue risk of hypoglycemia. ,
Brain edema can be managed initially with hyperventilation and elevation of the head above the heart. Should these maneuvers fail, mannitol can be given at a dose of 0.25–1.0 g/kg IV. This agent will transiently alter cerebral hemodynamics and raise serum osmolality by 10–20 mOsm/kg. However, repeated dosing of mannitol can lead to extreme hyperosmolality, renal failure, and further brain edema. Furosemide is a useful adjunct to mannitol for decreasing acute cerebral edema and has been shown in vitro to prevent the rebound swelling due to mannitol. All diuretics interfere with the ability to use urine output as a guide to intravascular volume status.
Patients undergoing major craniotomies and spine surgery are at risk of sudden hemodynamic instability due to hemorrhage, VAE, herniation syndromes, or manipulation of cranial nerves. The potential for cerebral hypoperfusion generally warrants placement of an arterial cannula for continuous blood pressure monitoring. The utility of central venous catheterization remains controversial. Cannulation of the jugular or subclavian vein with multiple-orifice catheters in adults is often preferred, particularly when VAE is anticipated. However, these multiple-orifice catheters are too large for infants and most small children, and are not used in pediatric settings. Furthermore, monitoring of the central venous pressure may not accurately reflect intravascular volume in small children. Therefore, the risks of a central venous catheter may outweigh its benefits. Even when VAE occurs, a single-orifice catheter is not often successful for aspirating air, presumably because of the high resistance of the small-gauge catheters used in these patients.
Venous air emboli have been detected during many craniotomies in infants and children, primarily because the head of a small child is large in relation to the rest of the body and rests above the heart in either the prone or supine position ( Fig. 20.7 ). Standard neurosurgical positioning often includes elevation of the patient’s head to optimize cerebral venous drainage. However, this maneuver can increase the risk for air entrainment into the venous system through open venous channels in bone and sinuses. Patients with cardiac defects and the potential for right-to-left shunting, such as patent foramen ovale and patent ductus arteriosus, are at risk for paradoxical air emboli, leading to cerebral and myocardial infarction. A precordial Doppler ultrasound device can detect minute VAE and should be routinely used in conjunction with an end-tidal carbon dioxide analyzer and arterial catheter in all craniotomies in order to detect VAE early, before significant hemodynamic instability develops. The Doppler probe is best positioned on the anterior chest, usually just over or to the right of the sternum at the fourth intercostal space (i.e., the nipple line). An alternative site on the posterior thorax can be used in infants in the prone position who weigh approximately 6 kg or less. In addition to the characteristic changes in Doppler sounds, sudden decreases in end-tidal CO 2 , dysrhythmias, ischemic changes in the electrocardiogram, or a combination of these, can occur with VAE.
Advances in neurophysiologic monitoring have enhanced the ability to safely perform more definitive neurosurgical resections in functional areas of the brain and spinal cord. However, the depressant effects of many anesthetic agents limit the utility of these monitors. A major part of preoperative planning should include a thorough discussion of the modality and type of neurophysiologic monitoring during the perioperative period.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here