Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Clinical presentations of endocrine disease can vary widely. Alterations in hormone balance can result in distinct phenotypes affecting linear growth, timing of puberty, and body composition as well as metabolic functions. The steady stream of discoveries of new genes, novel mutations, and alterations in gene expression has provided insight and will continue to elucidate the functional genomics of the clinical presentations associated with insufficient or excessive hormone secretion, as well as altered hormone receptor activity. Despite the explosion of new information, the clinical presentations of children with endocrine disorders remain constant. The recognition of physical signs associated with these various states assists in the diagnosis and treatment of imbalances of the endocrine system.
Hormones are chemical signals that are synthesized and secreted by many cells and are primarily transported in the bloodstream to their respective target organs. Many hormones are transported by hormone specific binding globulins. Some hormones act on neighboring cells to elicit paracrine actions and others act within cells in an autocrine action. Hormones achieve their actions by binding to their cognate receptors in specific target cells. There are several types of receptor families. Many glycoprotein hormones bind to the extracellular domain of G-protein coupled 7-transmembrane domain receptors. Insulin and insulin-like growth factor 1 (IGF-1) bind to tyrosine kinase transmembrane receptors. Steroid hormones are lipophilic and cross the cell membrane to bind to cytoplasmic nuclear receptors. Prolactin, growth hormone (GH), and leptin bind to cytokine receptors. Generally, circulating hormone concentrations are measured in peripheral blood samples. At any time point, the plasma concentration reflects the balance between hormone synthesis, secretion, and clearance. Urine and saliva can also be used to measure hormones. Techniques for hormone determination include radioimmunoassays, fluorometric assays, and liquid chromatography–tandem mass spectrometry (LC-MS/MS). Concentrations of many hormones change with age and pubertal stage. The following sections highlight the physical signs associated with normal endocrine function, as well as those due to hyposecretion and hypersecretion.
Knowing the parameters of normal growth is the essential first step to recognizing aberrations in growth and development. Clinicians who care for children need to be well acquainted with normal patterns of growth and development. Many factors influence growth, including heredity, sex, overall health, and environmental factors, such as nutrition. Linear growth is the fastest during the second trimester in utero. Rapid growth continues during the first year of life and then slows between 1 and 2 years of age ( Table 9.1 ). After 2 years of age, linear growth continues to decline slowly, averaging a growth rate of approximately 2.0 inches (5 cm) per year during childhood until it reaches a nadir just before the initiation of the pubertal growth spurt. This nadir is also known as the prepubertal dip or the prepubertal growth deceleration. A small transient increase in linear growth, named mid-childhood growth spurt, may be observed in children around 7 years of age. The increase in lean body mass and onset of adrenarche coincides with mid-childhood growth spurt. The “pubertal growth spurt” occurs during puberty. There are noticeable differences in the growth pattern of girls and boys during puberty. Girls generally start puberty at a younger chronologic age than boys do, and their pubertal growth spurt happens at an earlier pubertal stage. Peak growth velocity typically occurs at Tanner stage III in girls and at Tanner stage IV in boys. The pubertal growth spurt is also shorter in duration and displays a lower peak growth velocity in girls compared with boys. This sexual dimorphism is responsible for the difference in mature adult heights between males and females. The average mature height of males is 13 cm greater than that of females.
| Growth Rate (Per Year) | ||
|---|---|---|
| Age | Inches | Centimeters |
| Birth to 1 year old | 7–10 | 18–25 |
| 1 to 2 years old | 4–5 | 10–13 |
| 2 years old to puberty | 2–2.5 | 5–6 |
| Pubertal growth spurt: Girls | 2.5–4.5 | 6–11 |
| Pubertal growth spurt: Boys | 3–5 | 7–13 |
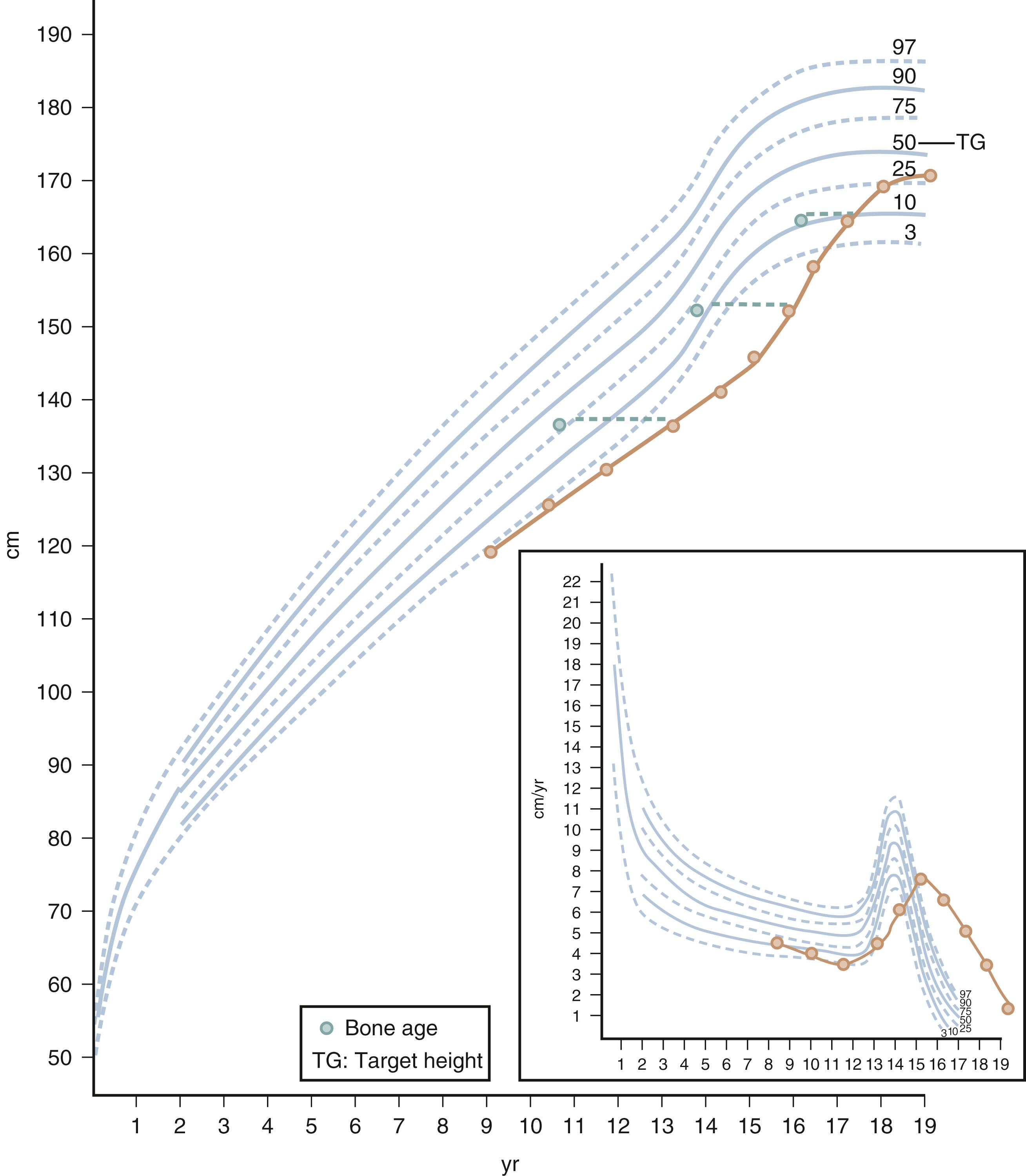
Because stature varies among healthy children, the incremental growth rate is one of the most important elements used to assess health in a child. Healthy children grow at a predictable rate. Growth velocity charts are available for both girls and boys. Growth velocity is a variable derived from height measurement at two different times and represents the increase in height during a fixed period expressed in centimeters per year. Growth velocity charts show the typical growth pattern seen at different ages and in relation to pubertal development (see Fig. 9.1 , right lower panel). Subnormal growth velocity can indicate both endocrine and nonendocrine disorders. The most critical and valuable tool to evaluate normal and pathologic growth is the growth chart. In May of 2000, the Centers for Disease Control and Prevention (CDC) released updated growth charts based on a broad population sample combining many different growth studies ( Fig. 9.2 ). These charts are readily available online at http://www.cdc.gov/growthcharts . The charts not only define the 3rd and 97th percentiles for height and weight, but they also characterize standards for head circumference and body mass index (BMI) defined as weight (kg)/height 2 (m). In contrast to adults for whom specific BMI values are used to define overweight and obesity, age- and gender-specific BMI values are used to classify children as overweight or underweight. Health care professionals can use established percentile cutoff points to identify underweight and overweight children ( Table 9.2 ). Accurate growth measurements using standardized equipment and technique should be obtained and plotted on the appropriate growth charts to identify abnormalities in growth patterns.
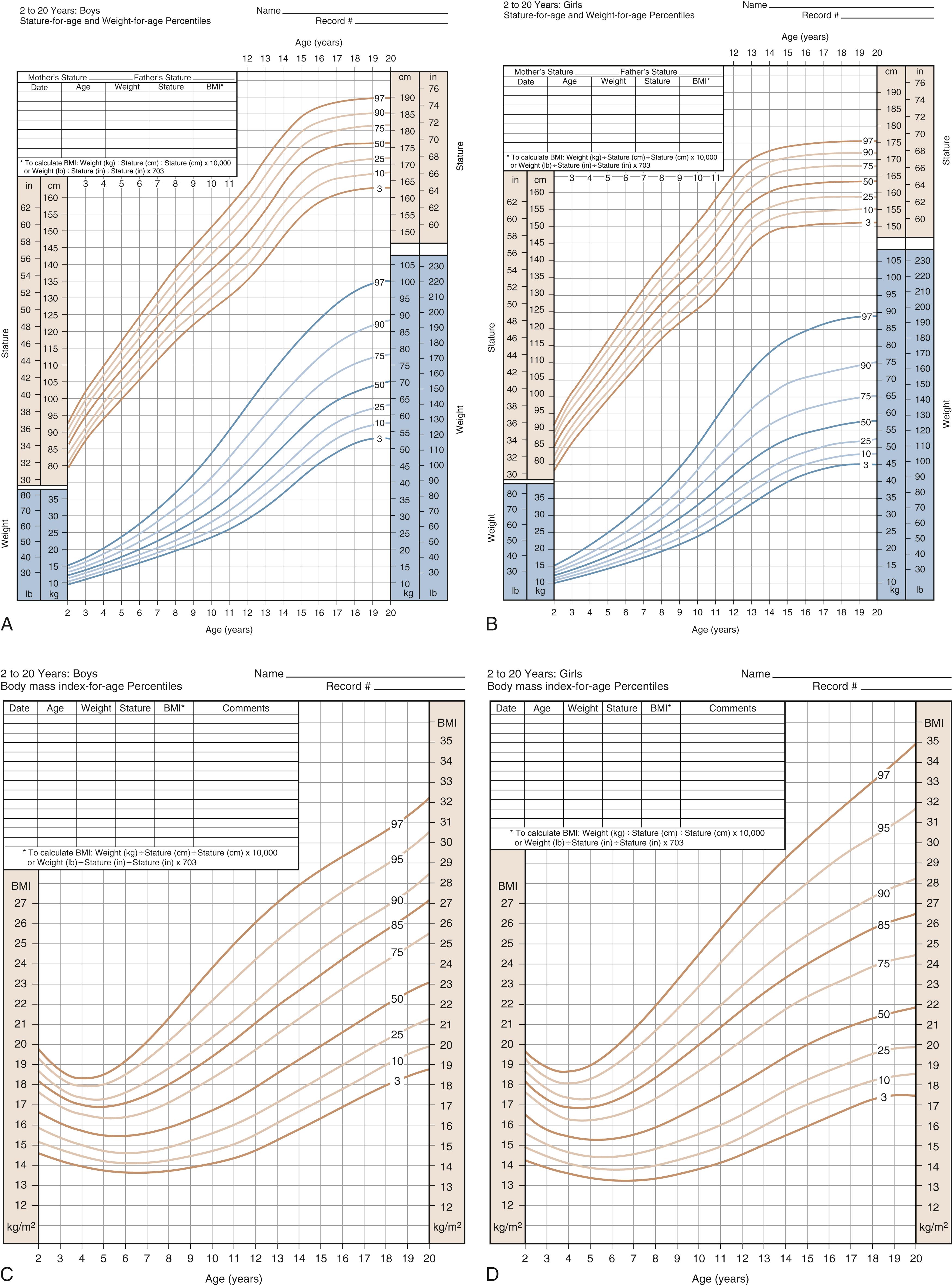
| Category | Body Mass Index for Age (Range) |
|---|---|
| Underweight | <5th percentile |
| Normal | 5th percentile to <85th percentile |
| At risk for overweight | 85th percentile to <95th percentile |
| Overweight | ≥95th percentile |
Although it can be normal for a child to change growth percentiles between birth and 18 months old, after this age children usually follow their growth curves closely. When a child crosses growth percentiles in a relatively short period, he or she should be carefully evaluated through a detailed investigation regarding the etiology of the abnormal growth pattern. Between 4 years old and adolescence, a growth rate below 4 cm per year for girls and boys should raise concerns and prompt evaluation. Adolescence is the only time during which the rapid growth rate of infancy is replicated. For girls, a sharp increase in growth velocity is the harbinger of puberty.
An important aspect of the evaluation of growth is the distinction between short stature and growth failure. Short stature is defined as a height that is more than two standard deviations (SDs) below the mean for age and sex, whereas growth failure is defined as a subnormal linear growth velocity for age and sex that, if persistent, eventually results in short stature. Accuracy in the measurements of length and standing height is critical for proper diagnosis and management of growth disorders. Inaccurate measurements will lead to erroneous calculations of growth velocity leading to parental anxiety, unnecessary referrals, and uncomfortable decision making. Measurement of recumbent length (from birth to 2 years old) and standing height (from 2 to 20 years old) require proper technique and equipment. Two people and an infantometer are needed for measurement of accurate recumbent length in an infant. A calibrated stadiometer is ideal for the measurement of standing height in an older child. In both cases, the occiput, shoulder blades, buttocks, calves, and heels should be in the same plane, which in turn should be perpendicular to the plane of the soles. The head should be positioned in the Frankfurt plane where a line from the outer canthus of the eye to the external auditory meatus should be perpendicular to the trunk plane. The delta of length or height divided by the delta of time between two measurements defines growth velocity, which is usually expressed in centimeters per year. Inherent measurement error, which should be minimized as much as possible with proper technique, has an impact in the calculation of growth velocity, especially if the two measurements are obtained in close temporal proximity. Intervals of 4 to 6 months between measurements are reasonable. Growth velocity can be plotted in sex-specific charts that display the age in the X axis and the growth velocity in the Y axis ( Fig. 9.1 , right lower panel). However, a comparison of the slope between two measurements to the slope of the standard percentiles in the growth chart provides a visual and easy assessment of the adequacy or inadequacy of the growth rate.
Children with short stature but normal growth velocity can be classified as likely having normal variants of short stature, including familial short stature and constitutional delay of growth. On the other hand, subnormal growth velocity, e.g., growth failure, should elicit concerns of an underlying pathology. Genetic syndromes, i.e., Turner, Noonan, Russell-Silver, and Down, are examples of chromosomal/genetic abnormalities associated with short stature.
Calculation of the target height provides an estimate of a child’s genetic potential. The target height, also referred to as mid-parental height (MPH), is the midpoint between the heights of parents, correcting for the 13-cm difference between male and female adult mature heights. For boys, the MPH is the midpoint between the father’s height and the corrected mother’s height (i.e., mother’s height plus 13 cm). This is easily calculated by adding 6.5 cm (2.5 inches) to the mean of the parents’ heights. For girls, the MPH is the midpoint between the mother’s height and the corrected father’s height (i.e., father’s height minus 13 cm). MPH is calculated by subtracting 6.5 cm (2.5 inches) from the mean of the parents’ heights. Ideally, this calculation should be based on measured parental heights, given that reported heights are frequently overestimated. The SD of the target has been calculated as 5 cm in boys and 4.5 cm in girls. Therefore, the target height range (MPH ± 2 SD) is MPH ± 10 cm in boys and MPH ± 9 cm in girls. Information regarding parental heights should be obtained during the initial evaluation, especially for children being evaluated with concerns of tall or short stature. As an example, calculation of the MPH of a boy whose father’s height is 179 cm and mother’s height is 160 cm would be as follows: 179 + 160 ÷ 2 + 6.5 cm = 176 cm. The target height range would be 166 to 186 cm. These calculations assume that each parent contributes 50% of the genetic makeup of the individual child, which may not be completely true. One or both parents could also have been adversely affected by environmental factors during their childhood that can impact MPH.
Radiographic determination of epiphyseal maturation, or bone age, is often helpful in evaluating children with short stature. By convention, a radiograph of the left hand and wrist is compared with established standard radiographs to determine the bone age or skeletal age. The most common method to assess bone maturation is the Greulich and Pyle method in which the x-ray of the hand and wrist is compared to an atlas of x-rays. This atlas was largely compiled using x-rays of White children of European ancestry obtained from the 1930s to the 1960s. Despite this limitation, bone age x-rays are a valuable tool to assess growth and pubertal development. Before 24 months of life, epiphyseal development is better estimated by radiographic examination of the hemiskeleton.
Children who have a familial or genetically determined short stature generally have a bone age equivalent to their chronologic age and display normal growth velocity. Their predicted adult height (PAH) falls within the MPH range. Constitutional delay of growth refers to children who have a later onset of puberty. Typically, the bone age of children with constitutional delay is delayed, being more consistent with the child’s height age rather than chronologic age. It is important to be aware that children with constitutional delay typically have a period of decreased linear growth within the first 3 years of life. In this variant of normal growth and pubertal development, the rates of linear growth velocity and weight gain transiently decline during these early years, often resulting in downward crossing of growth percentiles. By 2 or 3 years old, linear growth resumes at a normal rate. Subsequently, children may grow either along the lower growth percentiles or beneath the curve but parallel to it for the remainder of their prepubertal years (see Fig. 9.1 ). A family history of “late bloomers” or delayed puberty is common.
Measurement of body proportions is important in the assessment of growth disorders. Most endocrine causes of short stature are associated with proportionate short stature. Congenital disorders of bone mineralization and bone growth, such as chondrodystrophies, represent an important cause of disproportionate short stature. A simple method of determining proportions consists of measuring the lower segment (symphysis pubis to floor) and subtracting this value from the total height to determine the upper segment and then calculating the upper segment–to–lower segment ratio. This ratio, along with the arm span–to-height ratio, is used to document whether the spine or limbs are more severely shortened. Arm span is usually equal to standing height. However, in children with achondroplasia or hypochondroplasia, the long bones are disproportionately shortened. Sitting height (another way of calculating the length of the upper segment), determined with a special sitting stadiometer, is generally normal in individuals with achondroplasia, whereas the standing height is short. During adolescence, hypogonadism is often associated with increased limb length. Cranio-spinal radiation for childhood cancer therapy may result in decreased growth of the spine compared to the limbs resulting in disproportionate body habitus. Measurements of other body segments can also be performed following specific guidelines, compared to age- and sex-specific standards available elsewhere, and used in detail by dysmorphologists.
For the child with short stature and subnormal linear growth velocity, the evaluation should be comprehensive with consideration of endocrine and nonendocrine disorders. Pubertal development should always be an integral part of growth evaluation. Seemingly normal “prepubertal” growth velocity in a child in puberty may be an indication for further evaluation of growth failure. On the other hand, perceived “poor linear growth rate” in adolescents with advanced stages of puberty may be simply due to completion of most of the linear growth.
Puberty is the process through which reproductive competence is achieved and is initiated by reactivation of the hypothalamic-pituitary-gonadal axis (gonadarche). In humans and several nonhuman primates, adrenal pubertal maturation (adrenarche), indicated by increased adrenal dehydroepiandrosterone sulfate (DHEAS) secretion, occurs in close temporal proximity to gonadarche. Clinical studies have demonstrated that gonadarche and adrenarche are regulated through different molecular mechanisms. The typical sequence of pubertal events for boys and girls is generally predictable ( Fig. 9.3 ). For both boys and girls, the mean ages for the onset of puberty vary among different ethnic groups and represent the combined influences of genetic and environmental factors. Pubertal timing is influenced by parental age at puberty.
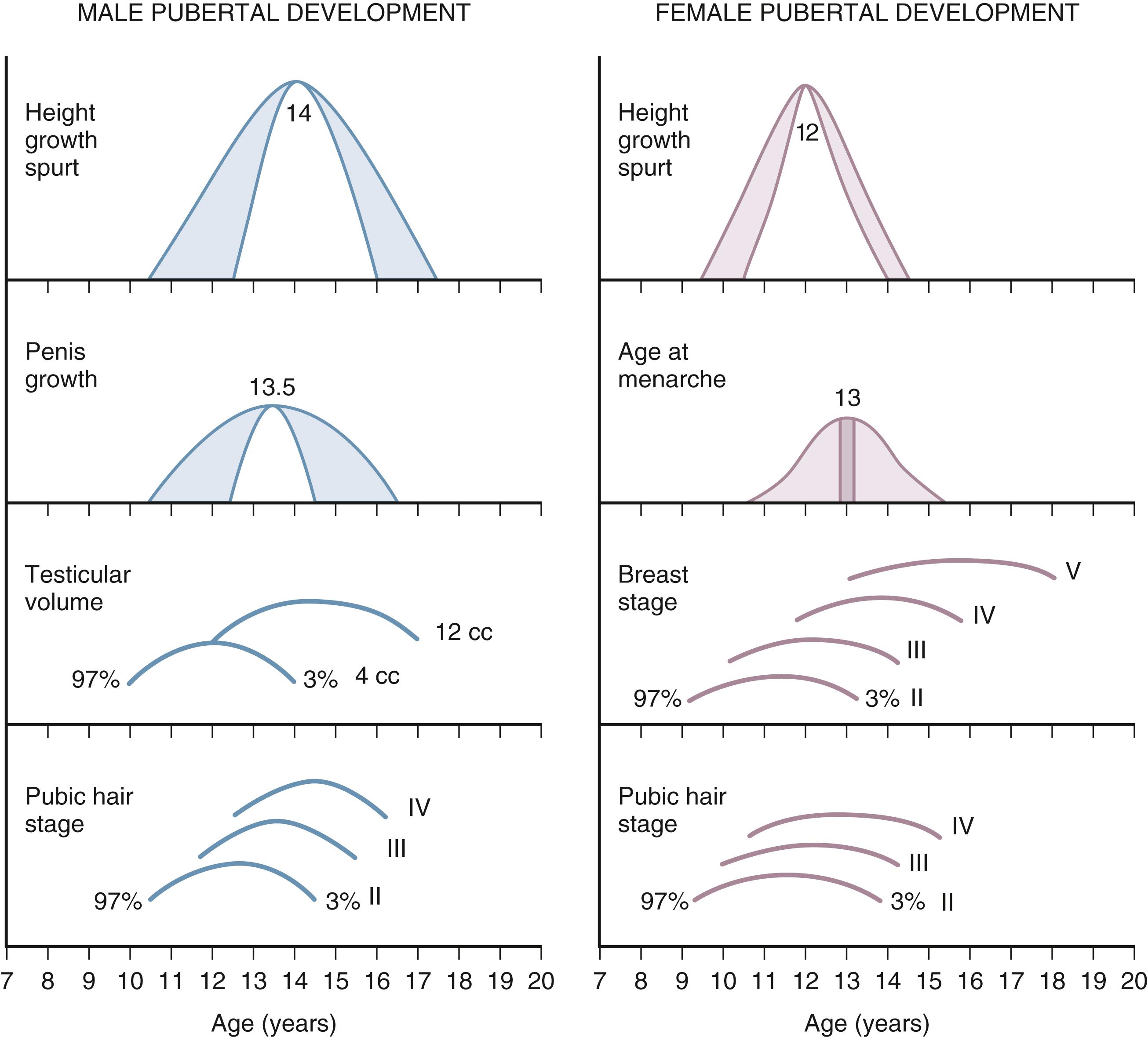
In boys, puberty is first evidenced by testicular enlargement, and usually begins between 9 and 14 years of age. Initial stages of puberty in boys may be difficult to appreciate unless testicular volume is measured using an orchidometer. Sexual hair (axillary and pubic hair) development perceived as signs of puberty may not be present in the early stages of puberty. Growth spurt and voice changes are late signs of pubertal development in boys and are typically seen during mid-puberty, approximately 2 years after the onset of testicular enlargement. Significant facial hair develops toward the end of male pubertal development when most of the linear growth is completed.
In girls, onset of puberty is evidenced by breast development (thelarche), which usually begins between 8 and 12 years of age. Among white girls, the mean age of onset of breast development and pubic hair growth occurs at approximately 10 years of age, with onset of menstrual periods (menarche) occurring around age 12 to 12 ½ years. African American females tend to enter puberty at an earlier age. Growth velocity increases at the onset of puberty in girls. Most of linear growth is completed by mid-puberty at the time of menarche.
To describe the onset and progression of pubertal changes ( Fig. 9.4 ), boys and girls are rated on five-point scales according to Tanner staging. Boys are rated for both genital development and pubic hair growth, and girls are rated for breast development and pubic hair growth. In general, for healthy children, Tanner stages of puberty for genital development and pubic hair growth are concordant. Differences in pubertal staging may be useful to formulate the differential diagnosis for children with either precocious or delayed puberty. Tanner staging of genital/breast and pubic hair development should be recorded especially for the child with precocious or delayed puberty. Inconsistent staging with age, abnormal “tempo” of progression, or abnormal sequence of pubertal signs assists with the initial differential diagnosis.
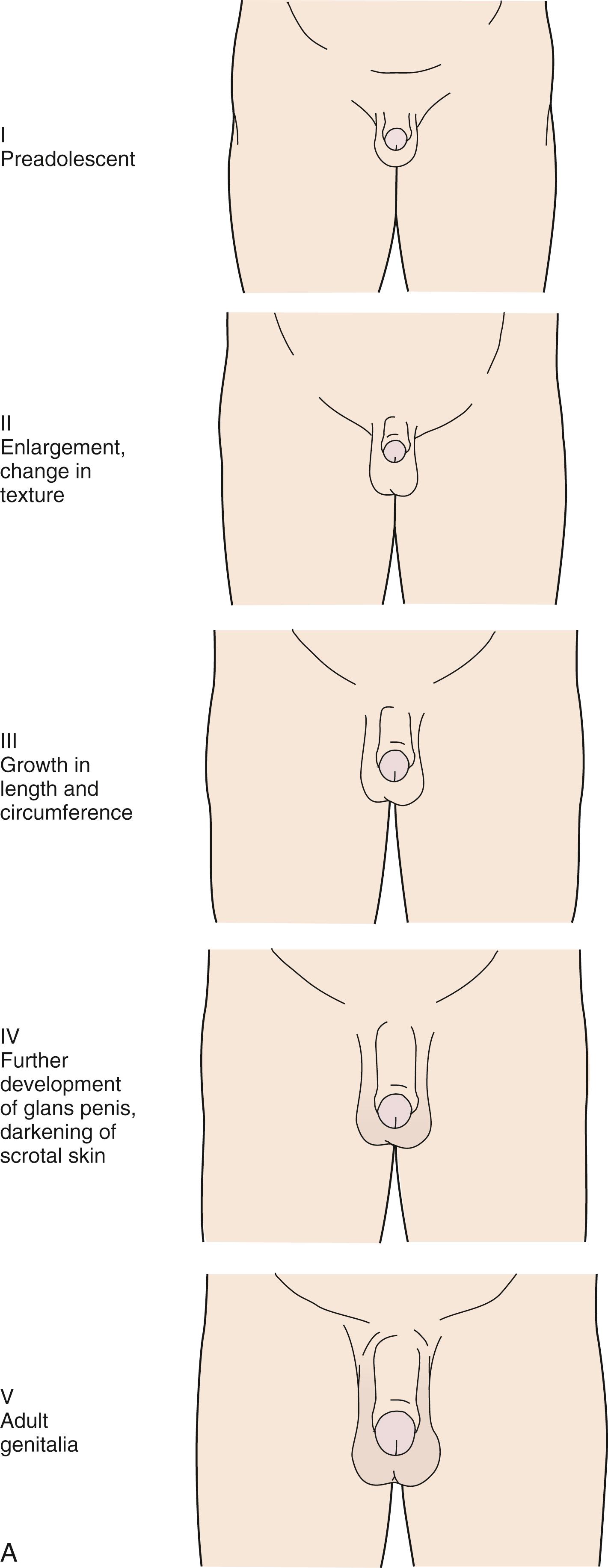
The stages for male genital development are as follows (see Fig. 9.4A ):
Stage I (preadolescent): The testes, scrotal sac, and penis have a size and proportion similar to those seen in early childhood.
Stage II: There is enlargement of the scrotum and testes and a change in the texture of the scrotal skin. The scrotal skin is “thinner” and may also be reddened.
Stage III: Further growth of the penis has occurred, initially in length with some increase in circumference. There is also increased growth of the testes and scrotum. Spermaturia (appearance of spermatozoa in early morning urine specimens) occurs during genital stage III. Transient gynecomastia occurs in approximately 50% of boys.
Stage IV: The penis is significantly enlarged in length and circumference, with further development of the glans penis. The testes and scrotum continue to enlarge, and there is distinct darkening of the scrotal skin.
Stage V: The genitalia are adult in size and shape.
The stages in male pubic hair development are as follows (see Fig. 9.4B ):
Stage I (preadolescent): Vellus hair appears over the pubes with a degree of development like that over the abdominal wall. There is no androgen-sensitive pubic hair.
Stage II: There is development of long pigmented downy hair, which is only slightly curled or straight. The hair is sparse and seen mainly at the base of the penis.
Stage III: The pubic hair is considerably darker, coarser, and curlier. The distribution of hair has now spread over the junction of the pubes.
Stage IV: The hair distribution is now adult in type but still is considerably less than that seen in adults. There is no spread to the medial surface of the thighs.
Stage V: Hair distribution is adult in quantity and type and is described as an inverse triangle. Typically, there is spread to the medial surface of the thighs.
The stages in female breast development are as follows (see Fig. 9.4C ):
Stage I (preadolescent): Only the papilla is elevated above the level of the chest wall.
Stage II (breast budding): Elevation of the breasts and papillae may occur as small mounds along with some increased diameter of the areolae. There is palpable, firm glandular tissue, “breast bud” under the areola.
Stage III: The breasts and areolae continue to enlarge, although they show no separation of contour. Palpable glandular tissue extends beyond the areolae.
Stage IV: The areolae and papillae elevate above the level of the breasts and form secondary mounds with further development of the overall breast tissue.
Stage V: Mature female breasts have developed. The papillae may extend slightly above the contour of the breast as the result of recession of the areolae.
Pubic hair growth in females is staged as follows (see Fig. 9.4B ):
Stage I (preadolescent): Vellus hair develops over the pubes. There is no sexual hair.
Stage II: Sparse, long, pigmented, downy hair, which is straight or only slightly curled, appears mainly along the labia majora.
Stage III: Considerably darker, coarser, and curlier sexual hair appears. The hair has now spread sparsely over the junction of the pubes.
Stage IV: The hair distribution is adult in type but decreased in total quantity. There is no spread to the medial surface of the thighs.
Stage V: Hair is “adult” in quantity and type and appears in an inverse triangle of the classically feminine type. There is spread to the medial surface of the thighs but not above the base of the inverse triangle.
Although not usually mentioned in the Tanner staging of male genital development, the testicular volume is a particularly important aspect. Testicular volume is best assessed by careful palpation (simple inspection frequently leads to overestimation) of the testes in comparison to a set of standards. The most common set of standards is the Prader orchidometer ( Fig. 9.5 ), which is a series of plastic or wooden ovoid shaped pieces attached by a string representing different testicular volumes (1 mL, 2 mL, 3 mL, 4 mL, 5 mL, 6 mL, 8 mL, 10 mL, 12 mL, 15 mL, 20 mL, and 25 mL). This device may not be available to all practitioners. Testicular volume of 4 mL that corresponds to the initiation of gonadarche (male genital Tanner stage II) measures 1 inch (2.5 cm) in the long axis, allowing the practitioner to determine whether a boy shows evidence of activation of the hypothalamic-pituitary-gonadal axis by a simple measurement performed with a ruler (see Fig. 9.5 ). Identifying abnormal pubertal development by performing complete physical examination and referring to the norms for age and population is critical in identifying pathology that may have significant consequences both medically and socially in a child’s life.

For girls, increased BMI may be associated with premature adrenarche and earlier pubertal onset. Large-scale population studies have identified a secular trend for slightly earlier (by 2 ½ to 4 months) ages at menarche throughout the early 20th century. However, the age of menarche has been relatively stable since the 1960s. Boys with rapid weight gain during childhood tend to have a later onset of puberty. The physiologic basis for this intriguing discordant effect of obesity on pubertal timing in boys versus girls is unexplained. These shifts, however, have not generally altered clinical practice guidelines regarding evaluation of early or late puberty.
Short stature and growth failure are the most common reasons for referral of children for a pediatric endocrine evaluation. Less commonly, children are referred to endocrinology for evaluation of tall stature and accelerated growth. The child’s past medical history, family history, and other aspects of physical examination are relevant for a thorough evaluation.
A practical approach for the evaluation of growth disorders can incorporate the elements described above including accurate measurements of height and weight, growth velocity, and skeletal maturation along with assessment of body proportions, genetic height potential (MPH), and stage of pubertal development.
Laboratory evaluation of short children includes targeted investigation of suspected etiologies. Growth hormone deficiency (GHD) is not a common cause of short stature. GH secretion occurs throughout the day in a pulsatile fashion mostly during the night at the onset of the first slow wave sleep (sleep stages III and IV). Determination of random GH levels is not useful except during the first few weeks of life. Beyond the first few weeks of life, the adequacy of GH secretion needs to be assessed through provocative GH stimulation tests or at the time of hypoglycemia. GH secreted from the anterior pituitary gland stimulates secretion of IGF-1, a peptide that mediates most of the anabolic and mitogenic action of GH. Random serum levels of IGF-1 are more reliable as surrogate marker of GH sufficiency in normal weight children given that its secretion is not pulsatile. Nutritional state is a key factor that can alter the IGF-1 serum levels. Underweight children may have a low IGF-1 level with no GH deficiency. Obesity can mask GH deficiency with normal random IGF-1 levels. GH also has IGF-1 independent action on the growth plate such as differentiation of prechondrocytes. More information on GH physiology can be found in the section hypothalamic-pituitary-IGF axis.
Children with proportionate short stature can be classified into two groups depending on their skeletal maturation: (1) those with short stature and concordant bone age, and (2) those with short stature and delayed bone age. In turn, each one of these two groups can be divided into those growing at a normal rate and those growing at a decreased rate. Therefore, four groups result from this classification ( Fig. 9.6 ). Those with adequate growth velocity likely have one of the normal variants of short stature: familial short stature (concordant bone age) and constitutional delay of growth (delayed bone age). The genetic height potential of a child is heavily influenced by the growth achieved by both parents and their relatives. The heritability of height has been estimated to be 0.7 to 0.8, rising to as much as 0.9 between identical twins.
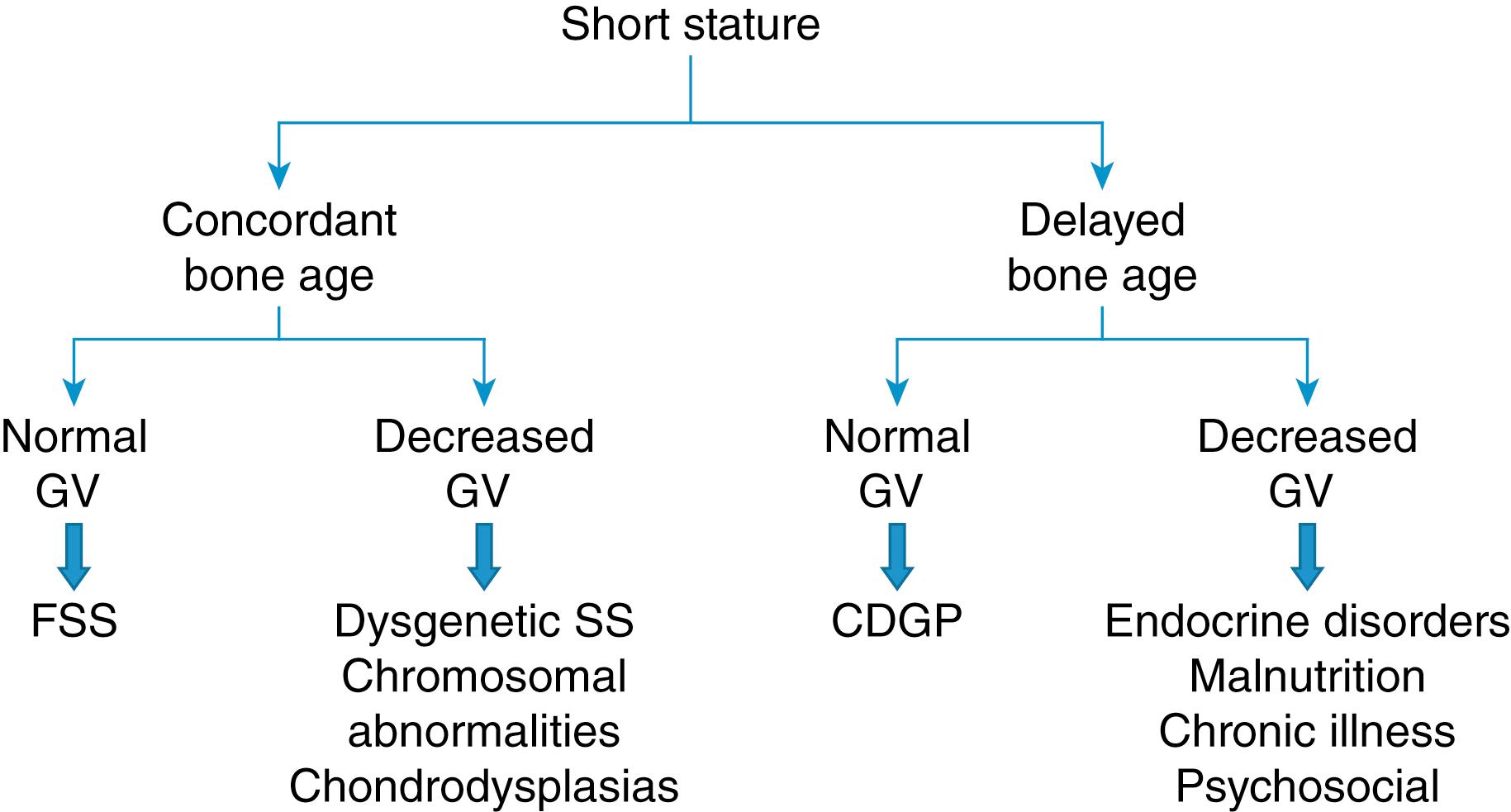
On the other hand, children with decreased growth velocity (growth failure by definition) are more likely to have a pathological reason for their abnormal growth pattern. Endocrine disorders (e.g., GH deficiency, GH insensitivity, hypothyroidism, and Cushing syndrome) and nonendocrine causes (e.g., malnutrition, chronic illness, and psychosocial deprivation) are likely associated with growth failure accompanied by delayed skeletal maturation, whereas dysgenetic (e.g., chromosomal disorders, some specific genetic syndromes, and chondrodysplasias) are more likely to be associated with growth failure and concordant skeletal maturation.
Although this algorithm is useful in the assessment of short stature, two caveats need to be emphasized: (1) this approach is not guaranteed because exceptions may occur, and (2) a short child does not necessarily fall into one single category but may have components of two or more categories. An example of the first caveat is the short stature associated with pseudohypoparathyroidism type 1a (Albright hereditary osteodystrophy). These individuals have both a dysgenetic as well as an endocrine component to their short stature, yet their skeletal maturation is frequently described as advanced. Another example involves girls with Turner syndrome, a dysgenetic cause of short stature, who may have a delay in skeletal maturation at the usual time of puberty due to low estrogen production and gonadal dysgenesis. The finding of abnormal body proportions in a child with short stature should elicit suspicion for chondrodysplasias and rickets. Typical body proportions are usually preserved in other causes of short stature.
Endocrine causes of short stature including GH deficiency, GH insensitivity (GH receptor gene variants or defects in the GH postreceptor cascade leading to deficient IGF-1), hypothyroidism, or sustained hypercortisolism (Cushing syndrome) either from endogenous or exogenous sources are characterized by linear growth deceleration, whereas weight gain is either not affected or even increased. This is a useful distinguishing feature of endocrine disorders in comparison to the other three causes of short stature in the last category of the algorithm (malnutrition, chronic illness, and psychosocial deprivation) where the weight is usually more affected than the height.
Nonendocrine causes of pathologic short stature include chronic illness (e.g., renal tubular acidosis, celiac disease, cystic fibrosis, inflammatory bowel disease, and other chronic disorders), adverse psychosocial milieu, undernutrition, and genetic disorders. Common nonendocrine and endocrine causes of short stature are listed in Boxes 9.1 and 9.2 , respectively.
Familial short stature (genetic)
Constitutional delay of growth and development
Malnutrition
Inadequate caloric intake
Pharmacologic, such as medications for ADHD
Anorexia nervosa
Pharmacologic steroid treatment
Idiopathic short stature
Intrauterine growth retardation
Systemic disease
Pulmonary
Cystic fibrosis
Asthma
Cardiac and circulatory
Congenital heart disease (cyanotic)
Acquired heart disease
Renal
Renal insufficiency
Pyelonephritis (chronic)
Renal tubular acidosis
Nephropathic cystinosis
Gastrointestinal and hepatic
Malabsorption
Celiac disease
Inflammatory bowel disease
Hepatic insufficiency
Neurologic
Mental retardation with growth delay
Musculoskeletal and connective tissue
Storage diseases
Hypotonia
Skeletal dysplasias
Immunologic
Immune deficiencies
HIV
Mucopolysaccharidosis
Syndromes associated with short stature
Chromosomal abnormalities
Trisomy 21
Trisomy 13
Trisomy 18
Turner syndrome
Noonan syndrome
Prader-Willi syndrome
Progeria
Russell-Silver syndrome
Cockayne syndrome
Seckel syndrome
CHARGE syndrome
18q deletion
Disproportionate short stature
Achondroplasia
Hypochondroplasia
Spina bifida
Radiation to spine
Pituitary development
GH deficiency
Septo-optic dysplasia
Mutations in HESX1, SOX2, SOX3, GLI2, LHX3, LHX4, PROP1, and POU1F1 genes
Craniopharyngioma
Transection of pituitary stalk due to trauma
Radiation damage to hypothalamus/pituitary
GH gene mutation
Laron dwarfism (GH receptor mutation)
IGF-1 deficiency
IGF-1 gene mutation
IGF-1 receptor gene mutation
STAT5 mutation
Thyroid hormone
Congenital hypothyroidism
Acquired hypothyroidism
Thyroid hormone resistance
Consumptive hypothyroidism
Cortisol hypersecretion
Cushing syndrome
Cushing disease
Diabetes insipidus
Congenital ADH deficiency
Nephrogenic diabetes insipidus
Wolfram syndrome
Acquired
Postoperative
Neoplasms
Infiltrative (Langerhans cell histiocytosis)
Defects in aldosterone synthase (CYP11B2)
Bone and mineral metabolism
Vitamin D deficiency rickets
Hypophosphatemic rickets
Pseudohypoparathyroidism
Pseudopseudohypoparathyroidism (hereditary Albright osteodystrophy)
Poorly controlled diabetes mellitus (Mauriac syndrome)
Tall stature is an uncommon concern in children except in very tall girls (above 6 feet). Rapid growth with tall stature during early childhood on the other hand is not an uncommon cause for referral to endocrinology. Endocrine causes of tall stature include gigantism associated with excessive GH secretion from a pituitary adenoma. Some genetic disorders, i.e., neurofibromatosis type 1, McCune-Albright syndrome, Carney complex, and multiple endocrine neoplasia type 1 [MEN 1], can be associated with excessive pituitary GH secretion. Other overgrowth syndromes may have tall stature as part of their dysmorphology. Soto syndrome is characterized by tall stature, macrocephaly, and a nonprogressive neurological disorder with mental retardation. Soto syndrome has been associated with haploinsufficiency of the nuclear receptor binding SET domain protein 1 (NSD1) gene located on chromosome 5q35.2-35.3 and with heterozygous mutations in the nuclear factor 1, X-type (NFIX) gene on chromosome 19p13.3. Klinefelter syndrome, associated with 47,XXY karyotype, is a relatively common sex chromosome aneuploidy presenting with tall stature. Marfan syndrome is an autosomal dominant disorder associated with tall stature and linked to mutations in the fibrillin-1 (FBN1) gene. Homocystinuria is an autosomal recessive disorder like Marfan syndrome including tall stature and lens subluxation. But unlike Marfan syndrome, intellectual disability is common in homocystinuria.
Patients with precocious puberty and virilizing congenital adrenal hyperplasias (CAHs) may present with rapid growth and tall stature but may ultimately be short adults due to premature fusion of growth plates if unrecognized and not adequately treated in a timely manner. Unrecognized hyperthyroidism may lead to rapid linear growth but, similarly to precocious puberty, may lead to advanced skeletal maturation with consequent attenuation of growth. Overnutrition causes excessive weight gain and accelerated linear growth. In this group exogenous obesity precedes increased height velocity. Skeletal maturation is often advanced in obese children with likely early epiphyseal fusion and normal final height.
Gonadotropins are glycoproteins secreted by the anterior pituitary gland. These glycoprotein hormones include follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Pulsatile gonadotropin-releasing hormone (GnRH) secretion by the hypothalamic GnRH neurons, the so-called GnRH pulse generator, regulates pituitary secretion of LH and FSH. The GnRH neurons originally develop in the olfactory placode and migrate during early fetal development from the nose through the forebrain to the hypothalamus. The embryologic origin of the GnRH neurons results in the association of hypogonadotropic hypogonadism and anosmia. Pulsatile release of GnRH is important in the secretion of FSH and LH from the anterior pituitary gland. Continuous secretion of GnRH inhibits the release of FSH and LH.
The amplitude and frequency of GnRH pulses varies during the newborn period, infancy, childhood, puberty, and adulthood. After active secretion in late gestation and in the early neonatal period during the so-called “mini-puberty” of infancy, the GnRH pulse generator becomes quiescent until the onset of puberty. “Mini puberty” of infancy is a transient period of gonadal activation starting in the second week and ending by 4 to 6 months of life. Gonadarche is characterized by the resumption of GnRH secretion. The first sign of reactivation of the GnRH pulse generator, and onset of gonadarche, is increased nocturnal LH secretion. Hypogonadotropic hypogonadism is characterized by low gonadotropin concentrations. In primary gonadal failure, deficiency of sex steroids interferes with negative feedback inhibition, resulting in elevated gonadotropin secretion during early infancy, puberty, and adulthood ( Fig. 9.7 ). Measurement of random levels of FSH and LH from late infancy to the onset of puberty is typically not helpful because the GnRH pulse generator is quiescent.
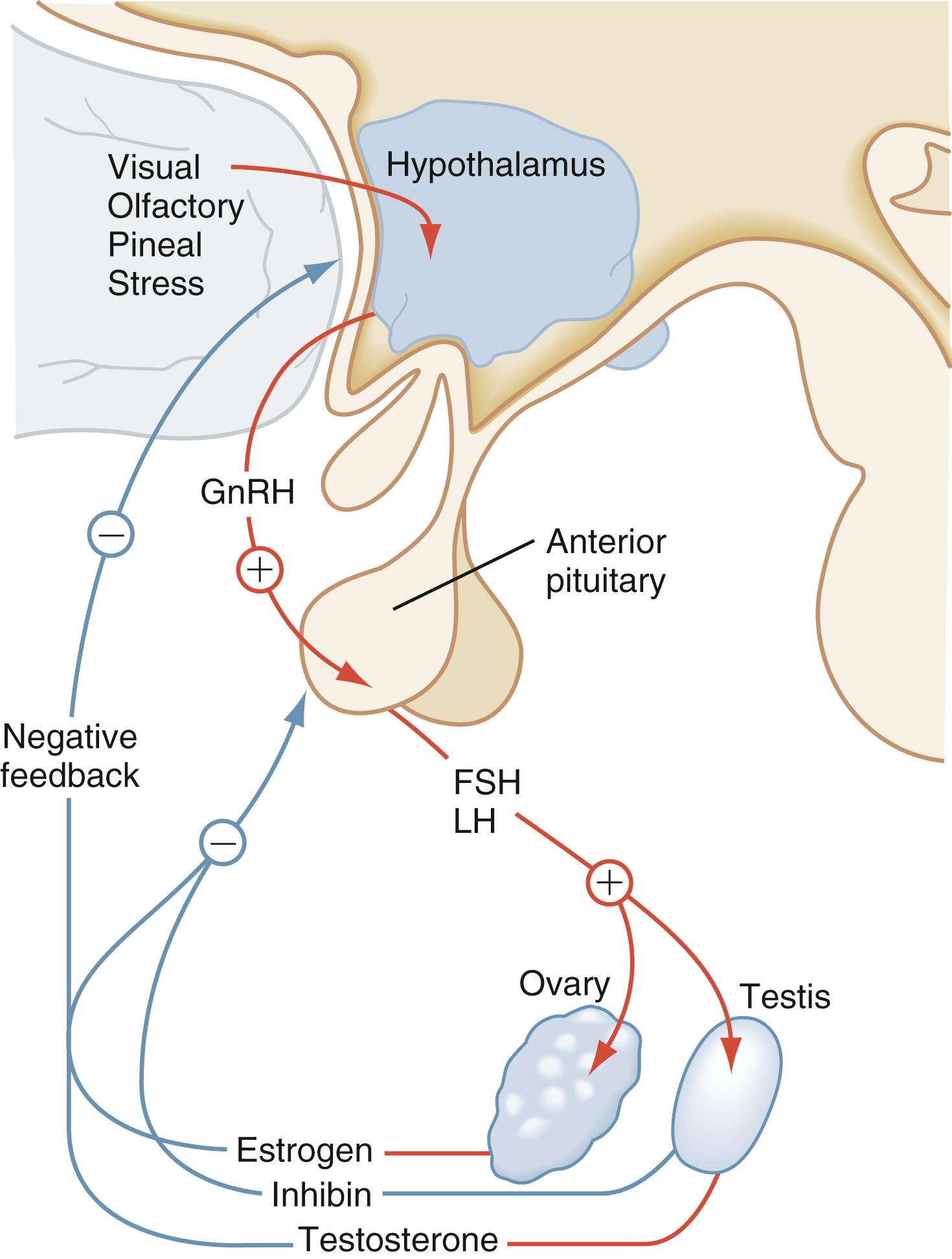
The secretory activity of GnRH neurons represents the integrated response to many hormones, neurotransmitters, and environmental influences. Input signals include sex steroids, glucocorticoids, leptin, insulin, IGF-1, ghrelin, FGF21, orexigenic peptides, and anorexigenic peptides with the net effect being signal integration to govern pubertal maturation and ongoing reproductive function.
In males, LH binds to the LH receptor on Leydig cells to directly stimulate testosterone synthesis and secretion. In the testes, FSH supports Sertoli cell development and spermatogenesis. Sertoli cells secrete anti-Müllerian hormone (AMH) with elevated levels during fetal life, which decline with the onset of puberty. AMH provides a functional marker of immature Sertoli cells. AMH levels may be low in males with diminished Sertoli cell function such as anorchia, testicular failure secondary to chemotherapy, radiation therapy. At the onset of puberty, testosterone secreted by the Leydig cells suppresses AMH secretion by the maturing Sertoli cells. In the testis Sertoli cells and Leydig cells function in parallel. In Klinefelter syndrome, as an example, Leydig cell function with normal testosterone production persists despite diminishing Sertoli cell function; this leads to relatively normal LH concentrations and elevated FSH concentrations.
In females, the ovary is characterized by a two-cell model (in series) for steroidogenesis. LH stimulates ovarian theca cells to synthesize androstenedione, which serves as the precursor for estradiol synthesis. Subsequently, in the ovarian granulosa cells, FSH increases expression of aromatase, the enzyme that converts androgens to estrogens. Granulosa cells do not secrete AMH in fetal life. With the onset of puberty, granulosa cells of developing follicles increase AMH secretion. In females, AMH appear to be a marker of ovarian reserve by reflecting the number of small growing follicles. AMH concentrations are typically low in girls with Turner syndrome and may be elevated in women with polycystic ovary syndrome (PCOS).
Gonadotropin secretion, especially in females, is influenced by the metabolic state and by the degree of adiposity. Very athletic and lean girls experience delayed puberty with low gonadotropin levels. Girls with eating disorders of the restrictive type experience amenorrhea with suppressed gonadotropins. Pubertal development may be slow among boys who restrict food intake to maintain a low weight for wrestling. Leptin, a hormone produced by the adipose tissue, exerts a significant effect on the hypothalamus and is a major link between energy balance and gonadotropin secretion.
Delayed puberty is defined in girls by the absence of breast development by 12 years of age and in boys by the lack of testicular enlargement by 14 years of age. Disorders of puberty can be classified as central (hypothalamic or pituitary) or peripheral (gonadal) in origin. Hypothalamic or pituitary disorders can be classified as permanent or transient hypogonadotropic hypogonadism. Primary gonadal failure is classified as hypergonadotropic hypogonadism.
A common cause of delayed puberty, especially in boys, is constitutional delay, which refers to a normal variant of the timing of puberty. There is usually a history of “late bloomers” in the family. Children are otherwise healthy without any symptoms or signs of underlying chronic medical problems. The bone age is typically delayed with good linear growth potential; the predicted final height is appropriate for the family. However, delayed or stalled puberty may be one of the first signs of many underlying medical conditions. Therefore, careful evaluation and follow up of children with presumed constitutional delay in puberty is required. In other words, constitutional delay of growth and puberty is a diagnosis of exclusion.
Hypogonadotropic hypogonadism may be suspected in boys with micropenis in the newborn period. Many genetic and acquired conditions are associated with hypogonadotropic hypogonadism. Kallmann syndrome refers to hypothalamic hypogonadism associated with anosmia due to impaired migration of GnRH and olfactory neurons; this form is often due to mutations in the ANOS1/KAL1 gene located at Xp22.31. Hypothalamic hypogonadism can also occur with a normal sense of smell. As noted earlier, the number of genes associated with delayed puberty has greatly expanded ( Box 9.3 ). The function of these genes ranges from the development and migration of the GnRH neurons during fetal life to genes regulating GnRH and gonadotropin secretion. Inheritance patterns include autosomal recessive, autosomal dominant, and X-linked. In some instances, variations in several different genes, oligogenicity, is associated with the phenotype of delayed puberty. For some genetic variants, the phenotype includes additional clinical features. CHARGE syndrome (coloboma of the eye, heart defects, atresia of the nasal choanae, retardation of growth and/or development, genital and/or urinary abnormalities, and ear abnormalities and deafness) due to mutations in the chromodomain helicase DNA binding protein 7 (CHD7) gene is associated with delayed puberty. Other disorders associated with delayed puberty include Prader-Willi, Noonan, and Bardet-Biedl syndromes.
Acquired gonadotropin deficiency can be due to trauma, neoplasms, radiation therapy, infiltrative disorders, hyperprolactinemia, or chronic illness. Undernutrition and chronic diseases, such as anorexia nervosa, inflammatory bowel disease, celiac disease, cystic fibrosis, hypothyroidism, glucocorticoid excess, poorly controlled type 1 diabetes mellitus, and sickle cell anemia can be associated with delayed or arrested puberty.
Hypergonadotropic hypogonadism is due to ovarian insufficiency/failure in girls and testicular failure in boys. Gonadotropin levels are typically elevated due to the absence of negative feedback because gonadal sex steroid (estrogen or testosterone) secretion is low. Delayed puberty due to hypergonadotropic hypogonadism can be congenital due to genetic conditions or acquired due to underlying causes such as autoimmune, vascular accidents, trauma, chemotherapy (alkylating agents have the highest risk), radiation to abdomen, pelvis, gonads, or total body radiation.
Several genetic conditions are associated with hypergonadotropic hypogonadism. Some genetic conditions are relatively common whereas others are rare and may be difficult to diagnose without extensive workup such as whole exome sequencing. Turner syndrome and Klinefelter syndrome are sex chromosome aneuploidies associated with gonadal insufficiency/failure and hypergonadotropic hypogonadism. Fragile X premutations and congenital disorders of glycosylation are other genetic conditions associated with hypergonadotropic hypogonadism and delayed puberty. Non-immune premature ovarian failure has been associated with galactosemia and with mutations in the forkhead box L2 (FOXL2) , newborn ovary homeobox (NOBOX) , factor in germline alpha (FIGLA) , and bone morphogenetic protein 15 (BMP15) genes. Premature ovarian insufficiency can be due to an autoimmune process—either isolated or associated with other autoimmune disorders such as adrenal and thyroid autoimmune disorders.
Complete androgen insensitivity syndrome can present as delayed/stalled puberty in phenotypic females with primary amenorrhea. Androgen insensitivity occurs secondary to mutations in the androgen receptor gene, which is located at Xq11-q12. Rarely, androgen insensitivity is associated with variants in other genes associated with androgen signaling. In utero , androgen action is essential for retention of wolffian duct derivatives, development of the prostate, and differentiation of male external genitalia. Common clinical presenting features of complete androgen insensitivity syndrome (CAIS) include inguinal or labial masses in an otherwise normal appearing female infant. The adolescent girl may present with breast development and primary amenorrhea. Breast development occurs because gonadotropin secretion at the time of puberty stimulates increased testosterone production, which is then aromatized to estradiol. Due to normal Sertoli cell function and AMH secretion, the uterus, cervix, and upper portion of the vagina are absent, and there is a blind ending vaginal pouch. In all instances, the karyotype is 46,XY. LH concentrations are generally elevated with normal FSH concentrations. The clinical features for patients with partial androgen insensitivity syndrome (PAIS) vary depending on the extent of receptor function. The phenotype can vary from female external genital appearance with mild clitoromegaly to a minimally undervirilized male with gynecomastia.
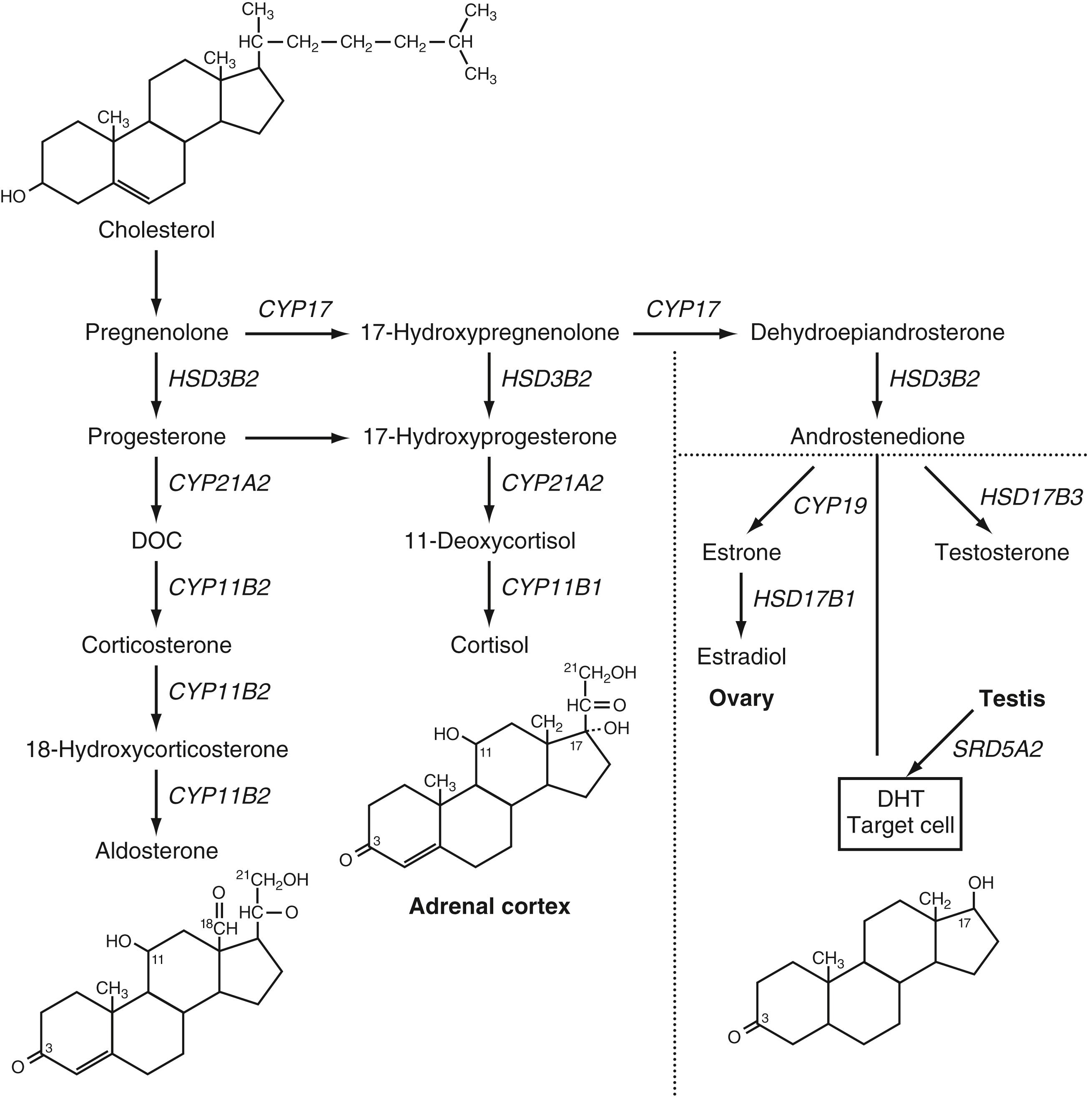
Some individuals with disorders of steroidogenesis or aberrant gonadal differentiation present with delayed puberty. Patients with 17-hydroxylase deficiency have mutations in the 17α-hydroxylase/17,20-lyase (CYP17A1) gene that encodes the P450c17 enzyme. This enzyme is essential to produce cortisol and sex steroids ( Fig. 9.8 ). In classic 17-hydroxylase deficiency, patients with both XX and XY karyotypes are phenotypic females and both present with lack of secondary sexual development. Females with P450-oxidoreductase deficiency due to POR mutations have been reported to have delayed puberty and ovarian cysts. Individuals with 17-hydroxysteroid dehydrogenase type 3 deficiency due to HSD17B3 variants or 5α-reductase deficiency due to SRD5A2 variants have 46,XY karyotype, genital ambiguity at birth, and typically virilize with the increased gonadotropin secretion of puberty.
Patients with XX or XY gonadal dysgenesis may present with delayed puberty. With the presence of a Y chromosome, patients with XY gonadal dysgenesis have an increased risk for gonadoblastoma or dysgerminoma in the dysgenetic gonad(s). Testicular regression syndrome, also known as vanishing testes or congenital anorchia, is typically diagnosed in infancy or early childhood, but it can present in peripubertal boys with nonpalpable testicles or delayed puberty. External genitalia are typically normal male but can vary from undervirilized male to normal male depending on the timing of in utero testicular loss; this disorder is presumed to be secondary to an in utero vascular event that disrupts testicular blood flow. Mutations in the DEAH-Box RNA helicase DHX37 gene have been identified in affected boys.
Another disorder that may present with primary amenorrhea is dysgenesis of the Müllerian ducts and uterus. Müllerian duct aplasia–renal agenesis–cervicothoracic somite dysplasia (MURCS) syndrome is an uncommon developmental disorder characterized by uterine hypoplasia or aplasia, renal agenesis or ectopy, vertebral anomalies, and short stature. Ultrasound studies to assess for the presence of the uterus should include renal ultrasounds. Isolated Müllerian duct agenesis without the other associations in 46, XX females is called Mayer-Rokitansky-Kuster-Hauser syndrome (MRKHS).
Puberty reflects activation of the hypothalamic-pituitary-gonadal (HPG) axis. The physical manifestation of puberty is “gonadarche” characterized by breast development in girls and testicular enlargement in boys. During this process, ovarian and testicular volumes increase. The diagnosis of precocious or early development is considered when the physical signs of puberty develop prior to age 8 years in girls and 9 years in boys. Children presenting with early onset of puberty should be evaluated regarding the age at pubertal onset and the sequence of pubertal changes. Medical history should focus on the temporal sequence of pubertal changes. Clinical features of androgen and estrogen effects should be noted during the physical examination. Growth acceleration in girls is present at the onset of puberty and is a very sensitive sign of precocious puberty. Gonadotropin-dependent precocious puberty, also known as central precocious puberty or GnRH-dependent precocious puberty, is marked by a premature activation of the HPG axis and follows the same sequence as normal puberty. In girls, the causes of isosexual precocity (development along lines of the same sex) are related to alterations in gonadal or central nervous system (CNS) function. Gonadotropin-dependent precocious puberty follows an early onset of pulsatile LH and FSH secretion and the subsequent response of the ovary. Hypothalamic hamartomas are composed of structurally abnormal nervous system tissue and may be associated with GnRH-dependent precocious puberty ( Fig. 9.9 ). Gelastic seizures can be associated with hypothalamic hamartomas and precocious puberty. Intracranial neoplasms are more commonly associated with precocious puberty in boys as compared with girls. Low-dose radiation therapy for CNS tumors can result in hypothalamic dysfunction and present with precocious puberty. Obtaining family history of pubertal development can be helpful. Paternally inherited loss of function mutations in the makorin ring finger 3 (MKRN3) and delta homolog 1 (DLK1) genes are associated with precocious puberty. Available data suggest that the makorin ring finger 3 protein inhibits GnRH secretion; declining circulating levels are noted with the onset of puberty.
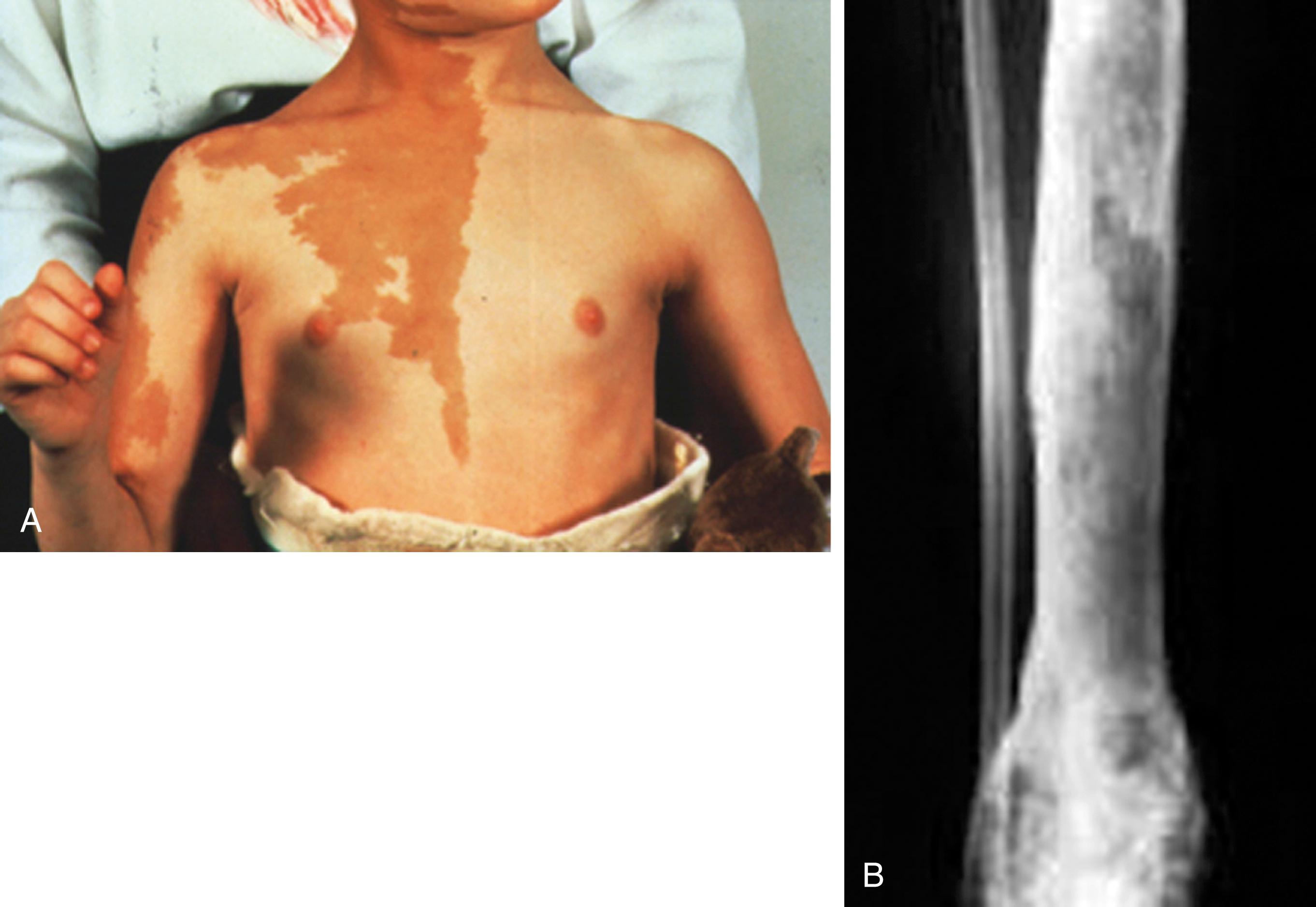
In GnRH-independent forms of precocious puberty, there is an increased production of gonadal steroids. This leads to the physical changes of puberty in the absence of activation of the HPG axis. McCune-Albright syndrome, characterized by irregular café-au-lait spots ( Fig. 9.10A ), polyostotic fibrous dysplasia (see Fig. 9.10B ), and sexual precocity, is one example of a GnRH-independent cause of precocious puberty. Physical changes may not follow the normal sequence of pubertal development seen in central precocious puberty. McCune-Albright syndrome is due to a somatic activating mutation of the gene encoding the α-subunit of G protein coupled receptor, which is located at chromosome 20q13.2. Other endocrine manifestations may include gigantism, Cushing syndrome, hyperprolactinemia, hyperthyroidism, and hyperparathyroidism.
Gonadal or adrenal neoplasms secreting sex steroids, such as Leydig cell tumors in boys, are rare causes of precocious puberty. The sequence of pubertal changes may differ from typical because rapid increase of sex steroids can cause rapid progression of pubertal signs. In boys, hCG-secreting tumors can also be a cause of isosexual precocity due to LH-like actions of hCG. Typically, hCG tumors do not cause precocious puberty in girls because estradiol is not produced in the absence of FSH stimulated aromatase expression. Familial male-limited precocious puberty or testotoxicosis is characterized by premature testicular testosterone production, testicular enlargement, and precocious puberty. It is due to constitutive activating mutations of the LH receptor (LHR) gene.
Long-standing and severe primary hypothyroidism in girls may be associated with isosexual precocity because of the partial structural homology between thyrotropin (TSH) and the gonadotropins (LH, FSH). In addition to signs of hypothyroidism, girls may have breast development and vaginal bleeding. This is also known as the Van Wyk-Grumbach syndrome. Boys with severe hypothyroidism may have enlargement of the testes due to the same mechanism. In these boys, testicular enlargement is often not associated with other secondary sexual characteristics such as pubic hair development and genital enlargement.
Exposure to exogenous hormones such as estrogens, estrogen-like substances including phytoestrogens, and testosterone may cause the development of secondary sexual characteristics and mimic precocious puberty.
Premature adrenarche describes the early onset of the normal increase in adrenal androgen production. Adrenarche is considered early when it occurs before 8 years of age in girls and 9 years of age in boys. Clinical features of adrenarche include acne, adult type body odor, axillary hair, and pubic hair development (pubarche). Children with premature adrenarche are characterized by a premature increase in DHEAS concentrations. Premature isolated (benign) adrenarche is not associated with pubertal signs such as breast development in girls and testicular enlargement in boys. Premature adrenarche progresses slowly; other pubertal signs do not develop early. Bone age is typically normal but may rarely be advanced. Height prediction is normal for genetic potential. Differential diagnostic considerations include late onset CAH and androgen-secreting tumors. Androgen-secreting adrenal or gonadal tumors are extremely rare. In children with benign premature adrenarche, adrenal steroid hormone concentrations may be elevated for chronologic age but are normal for the degree of pubic hair development. Although advanced skeletal maturation is more common in CAH, ACTH stimulation tests may be necessary to distinguish late-onset CAH from benign premature adrenarche. To diagnose late-onset CAH, the ACTH-stimulated 17-OH-Progesterone level is typically greater than 1500 ng/dL.
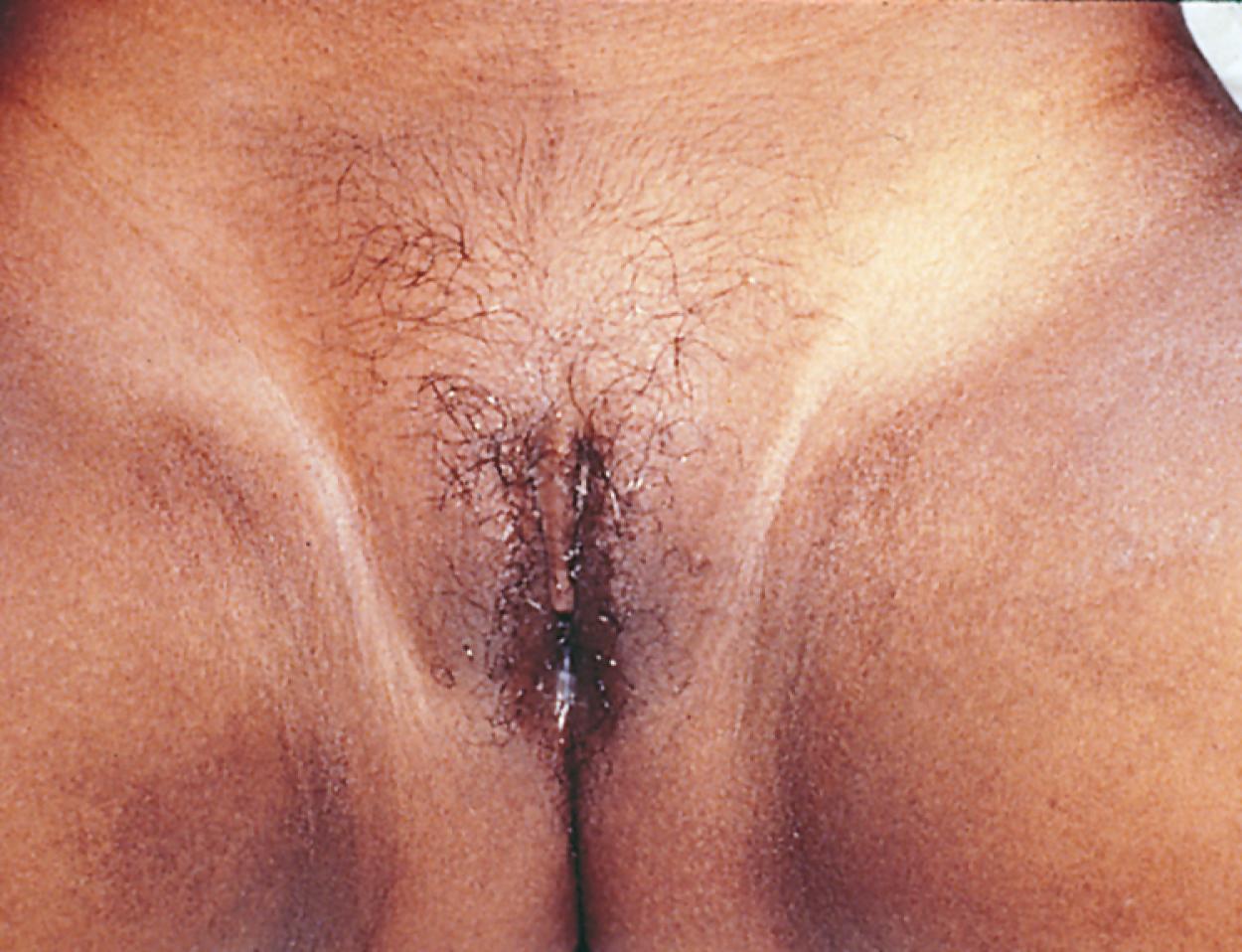
Premature pubarche refers to the development of pubic hair (pubarche) before 8 years of age in girls and 9 years of age in boys ( Fig. 9.11 ). Associated findings may include axillary hair, adult-type body odor, and acne. Typically, DHEAS concentrations are normal for age. Premature pubarche is often a normal variant. Rapid progression of pubarche should prompt evaluation for other disorders such as adrenal or gonadal androgen secreting tumors, disorders of steroidogenesis, or environmental exposures. On occasion, young infants may present with coarse hairs in the genital area (typically the scrotum in boys and the inner aspect of the labia majora in girls) which disappear spontaneously in late infancy, suggesting that this is a benign phenomenon. Progressive increase of hair in these areas should alert the clinician to the possibility of pathologic causes.
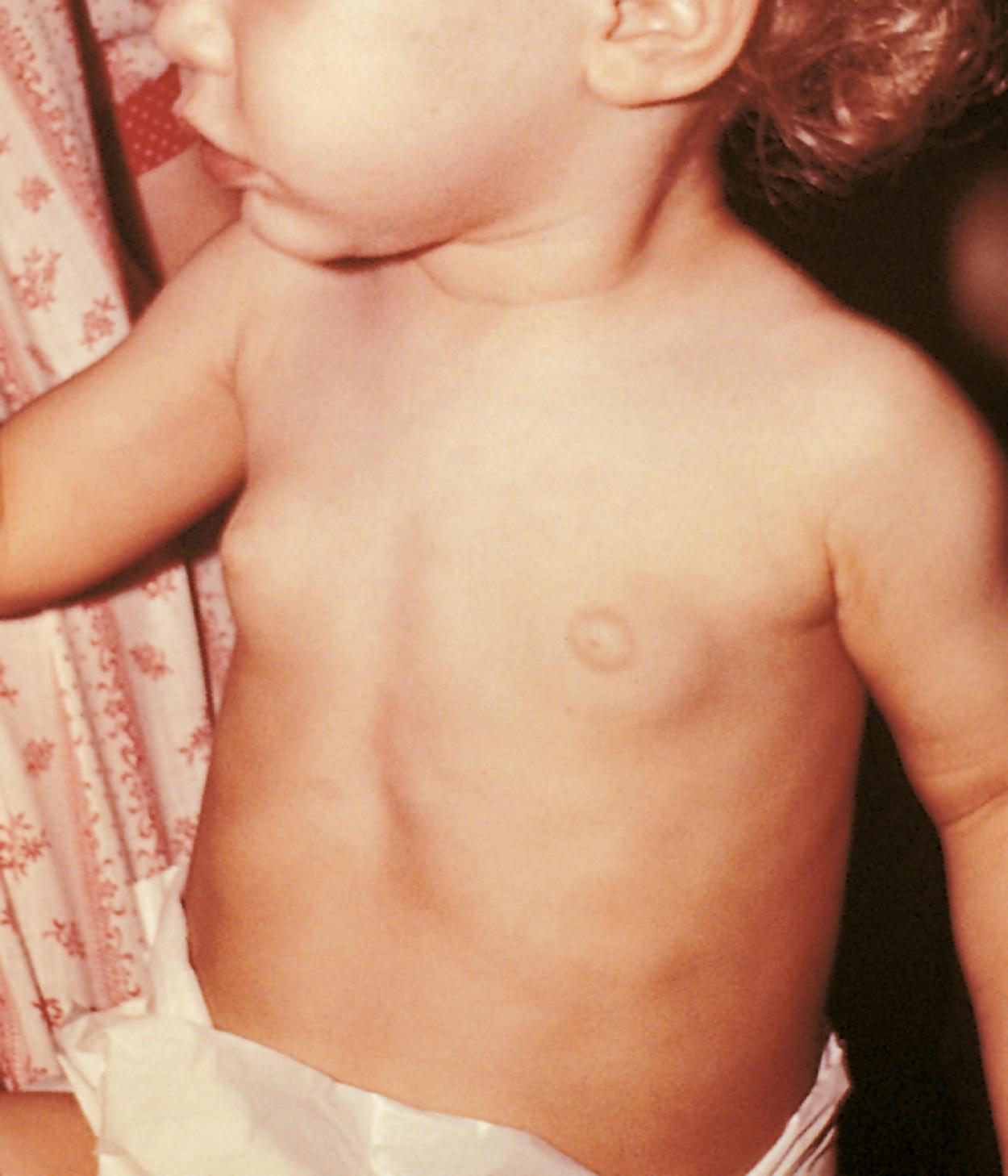
Premature thelarche is a transient and self-limited condition defined by the early onset of isolated breast development. Typically, premature thelarche develops during the first or second year of life without any other signs of puberty. The breast tissue may appear unilaterally or bilaterally and may regress or persist ( Fig. 9.12 ). Typically, there is a waxing and waning pattern to breast enlargement. Other features of pubertal development, including growth acceleration and pubic hair development, are absent. In most cases, breast enlargement does not progress beyond Tanner stage III. Hence, premature thelarche is a diagnosis of exclusion and cannot be made in the presence or progression of other pubertal signs. The ingestion of exogenous estrogens, such as oral contraceptives, should always be suspected in cases of breast enlargement. One should query regarding exposure to phytoestrogens such as lavender and tea tree oils. Premature thelarche must be differentiated from other forms of progressive precocious puberty. Breast enlargement secondary to maternal hormones may occur in either sex in the immediate neonatal period. The etiology of premature thelarche is unclear. In some instances, activating mutations of the GNAS1 gene, encoding the α-subunit of the Gs protein have been described in the absence of other elements of McCune-Albright syndrome. Some young girls develop significant estradiol secretion and exaggerated thelarche that does not progress to complete puberty. These girls do not show the typical activation of pituitary-ovarian axis seen in central precocious puberty. Close follow up is recommended. Transient ovarian cysts in young girls can secrete estradiol and present with transient stimulation of breast tissue which resolves spontaneously.
Lipomastia/adipomastia refers to the presence of adipose tissue in the pectoral area that may resemble breast development. This is frequently seen in obesity, both in boys and girls. True breast tissue is palpable underneath the nipple/areola, whereas in pure lipomastia there is no palpable glandular tissue under the nipple, giving the impression of a “donut” with a hollow center surrounded by a rim of adipose tissue.
The first vaginal bleeding, known as “menarche,” typically occurs 2 years after the onset of breast development. It coincides with Tanner stages IV and V breast development. Isolated vaginal bleeding in prepubertal girls does not follow this typical sequence and is abnormal. It should raise suspicion for foreign body in the genital tract and warrants thorough evaluation. The possibility of sexual abuse needs to be excluded. Isolated benign ovarian cysts are another common cause of vaginal bleeding in prepubertal girls. This can be seen in normal girls as a benign self-limiting condition or if it is recurrent, it can be a manifestation of McCune-Albright syndrome. Rapidly growing, estrogen-producing adrenal or ovarian tumors may present with vaginal bleeding; often other signs of puberty are present.
Gynecomastia means female–like breasts seen in males. It should not be used to describe breast development in females, which is “thelarche.” Gynecomastia is common in adolescent males. Up to 70% of adolescent males may develop gynecomastia. Typically, gynecomastia is benign and regresses during the later portion of puberty. True gynecomastia should be differentiated from lipomastia, which is seen in overweight individuals. Estrogens stimulate and testosterone inhibits the proliferation of breast tissue. Estrogen-to-testosterone ratio is more important than the absolute levels of estrogen in gynecomastia. Phytoestrogens, lavender oil, and tea tree oil mimic the action of estrogens and can stimulate the development of breast tissue. Good history and examination should differentiate the benign conditions from the less common nonbenign causes of gynecomastia.
Physiological neonatal gynecomastia is common in newborns due to the transfer of maternal estrogen through the placenta. It can be associated with milk secretion, the so called “witch’s milk.” Stagnation of milk and superadded infection can result in mastitis and breast abscess, which may require surgical drainage. Neonatal gynecomastia is usually self-remitting as the effects of maternal estrogen wear off. Minimal manipulation is recommended to prevent further stimulation of breast tissue and the introduction of infection.
Pubertal gynecomastia results from relative estrogen excess due to variable amounts of aromatization of adrenal and testicular androgens to estrogens in peripheral tissues, particularly adipose tissue, and liver, while testosterone levels are not yet robust. Physiological pubertal gynecomastia can be unilateral and usually resolves spontaneously over 1 to 2 years ( Fig. 9.13 ). It might persist longer in obese boys or never resolve due to the higher estrogen levels from aromatization of androgens in peripheral fat tissue and permanent glandular breast tissue formation. Decreased androgen production in boys with testicular hypogonadism such as seen in boys with Klinefelter syndrome can cause progressive gynecomastia. Decreased androgen effect due to androgen receptor mutations seen in androgen insensitivity syndrome results in high testosterone which is aromatized to estradiol and results in stimulation of breast development. Typically, the phenotype is female in CAIS. Other conditions that can cause decreased androgen synthesis and gynecomastia are 5α reductase deficiency, 17β hydroxysteroid dehydrogenase type 3 deficiency. Increased estrogen production from adrenal or testicular tumors, hCG-secreting tumors, and familial aromatase excess syndrome are rare causes of gynecomastia. Obesity, malnutrition, chronic liver disease, hyperthyroidism, and breast cancer (rare) are other nonbenign causes of gynecomastia. Drug-induced gynecomastia should be considered in boys who are taking chronic medication or taking substances such as marijuana.
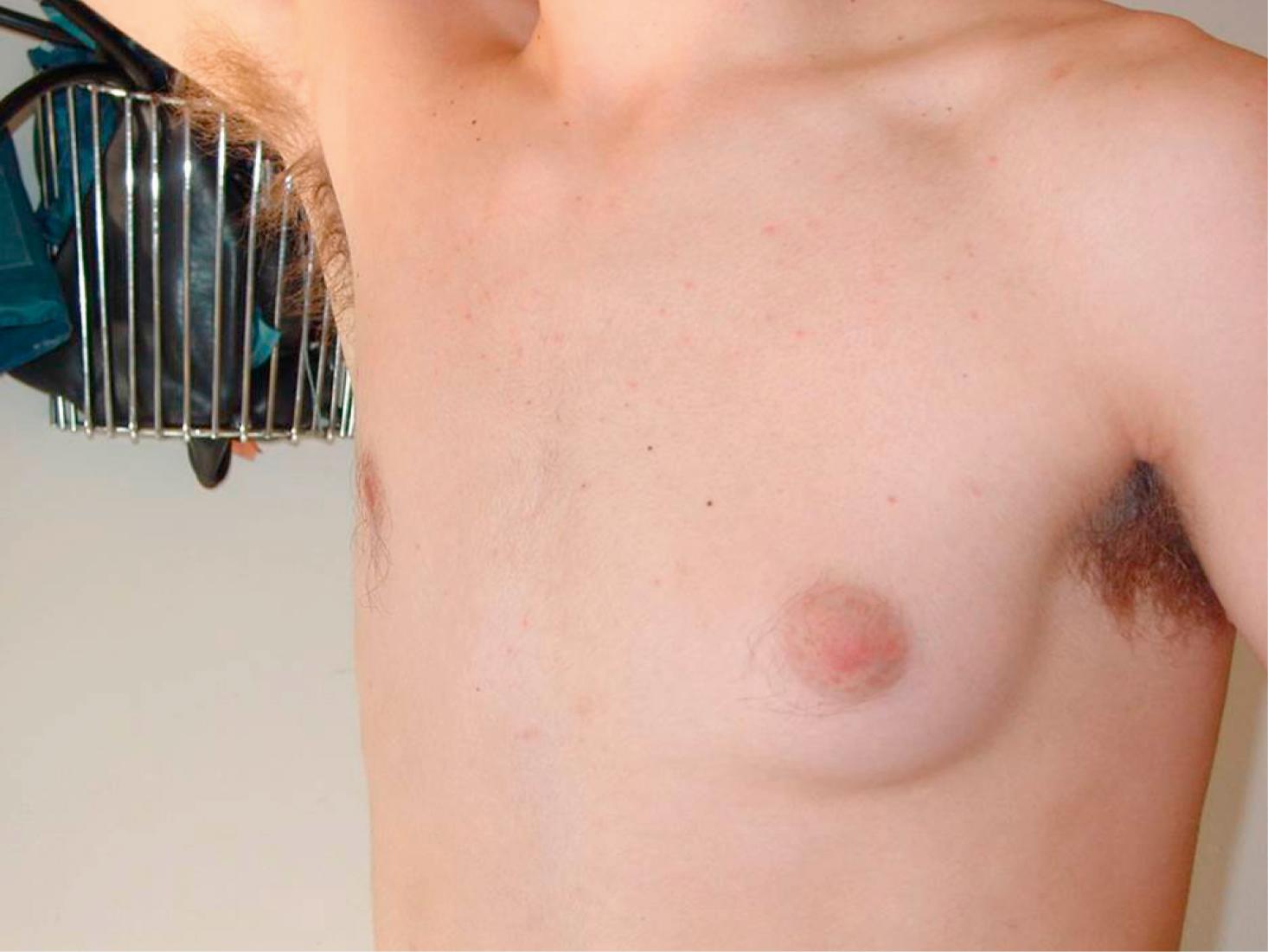
Breast asymmetry is the finding of mild-moderate difference in breast size, shape, and position on a clinical examination. It is common in pubertal adolescent girls and can be a feature of physiological pubertal gynecomastia in boys (see Fig. 9.13 ). It is mostly due to the fluctuating response of breast tissue to the hormonal environment during puberty. In most instances where the asymmetry is due to hormonal fluctuations, it resolves as breast development is completed. Other causes such as breast mass or abscess are rare. Breast asymmetry is a feature of Poland syndrome, which is an uncommon congenital malformation affecting the chest wall, shoulder, arm, and hand.
Galactorrhea means “milk flow,” derived from the Greek word “galakt” meaning milk and “rhoia” meaning flow. Galactorrhea describes the milk production outside of pregnancy or breastfeeding in females. Galactorrhea can occur in males as well. Prolactin secreted from the anterior pituitary lactotrophs is the key hormone in milk production. The hypothalamus secretes dopamine, which inhibits pituitary prolactin production. Several hypothalamic releasing factors increase prolactin secretion. Vasoactive intestinal polypeptide (VIP) secreted by the hypothalamus has a stimulatory effect on lactotrophs. Thyrotropin releasing hormone (TRH), which stimulates the anterior pituitary to secrete thyroid stimulating hormone (TSH), has a minor stimulating effect on the prolactin secretion. Estrogens directly stimulate pituitary lactotrophs and prolactin secretion. Physiologic causes of galactorrhea include nipple stimulation and placental transfer of maternal hormones in newborns. Pathologic causes include CNS lesions that interfere with the hypothalamic inhibitory function on prolactin synthesis and secretion such as tumors in the hypothalamic pituitary area (craniopharyngioma, meningioma, germinoma) or prolactin-secreting adenomas of the pituitary gland (prolactinomas). Local lesions of the breast such as herpes zoster, burns, trauma, and systemic disease such as renal failure and hypothyroidism may cause galactorrhea. Antipsychotics such as risperidone, gastrointestinal motility promoting agents (metoclopramide), and antihypertensive medication (verapamil, methyldopa) can cause galactorrhea as a side effect.
Gigantomastia is a rare condition characterized by the diffuse enlargement of both breasts. It occurs mostly in peripubertal girls or in the postpartum period. It can be physically and psychologically disabling. Often, etiology is unknown. Most cases occur during significant changes in endogenous hormones such as during puberty and pregnancy. It is thought to be due to abnormal stimulation of breast tissue from circulating physiologic levels of hormones or due to hormonal excess. Drug-induced gigantomastia has been described with penicillamine and cyclosporine.
The pituitary gland is considered “the master gland” regulating diverse endocrine functions. It develops as a fusion of cells from two distinct embryonic origins. One is an outgrowth of ectodermal cells from the roof of the primitive pharynx (known as Rathke pouch) which forms the anterior lobe (adenohypophysis), and the other, a downgrowth of neural tissue (neural ectoderm) from the floor of the forebrain, forms the posterior lobe (neurohypophysis). The anterior pituitary gland is regulated largely by small peptide hormones secreted by cells located in the hypothalamus. The posterior pituitary gland has direct neuronal connections to the hypothalamus. The oral ectoderm and neuroectoderm interact as they oppose each other to form the pituitary gland. This apposition and interaction are critical for the anterior pituitary cell differentiation. Aberrant development of the cells forming Rathke pouch can lead to anterior pituitary hormone deficiencies.
The hypothalamus is derived from neuroectodermal tissue of the diencephalon and surrounds the inferior aspect of the third ventricle. Its basilar portion consists of the median eminence and pituitary stalk, which provide the common route for hypothalamic small peptide hormones to reach the pituitary gland. Various regions in the hypothalamus secrete small peptide hormones that use this pathway through the pituitary portal system to regulate anterior pituitary hormone secretion. The neurohypophysis, consisting of unmyelinated axons and axon terminals, extends from the median eminence to the posterior pituitary gland.
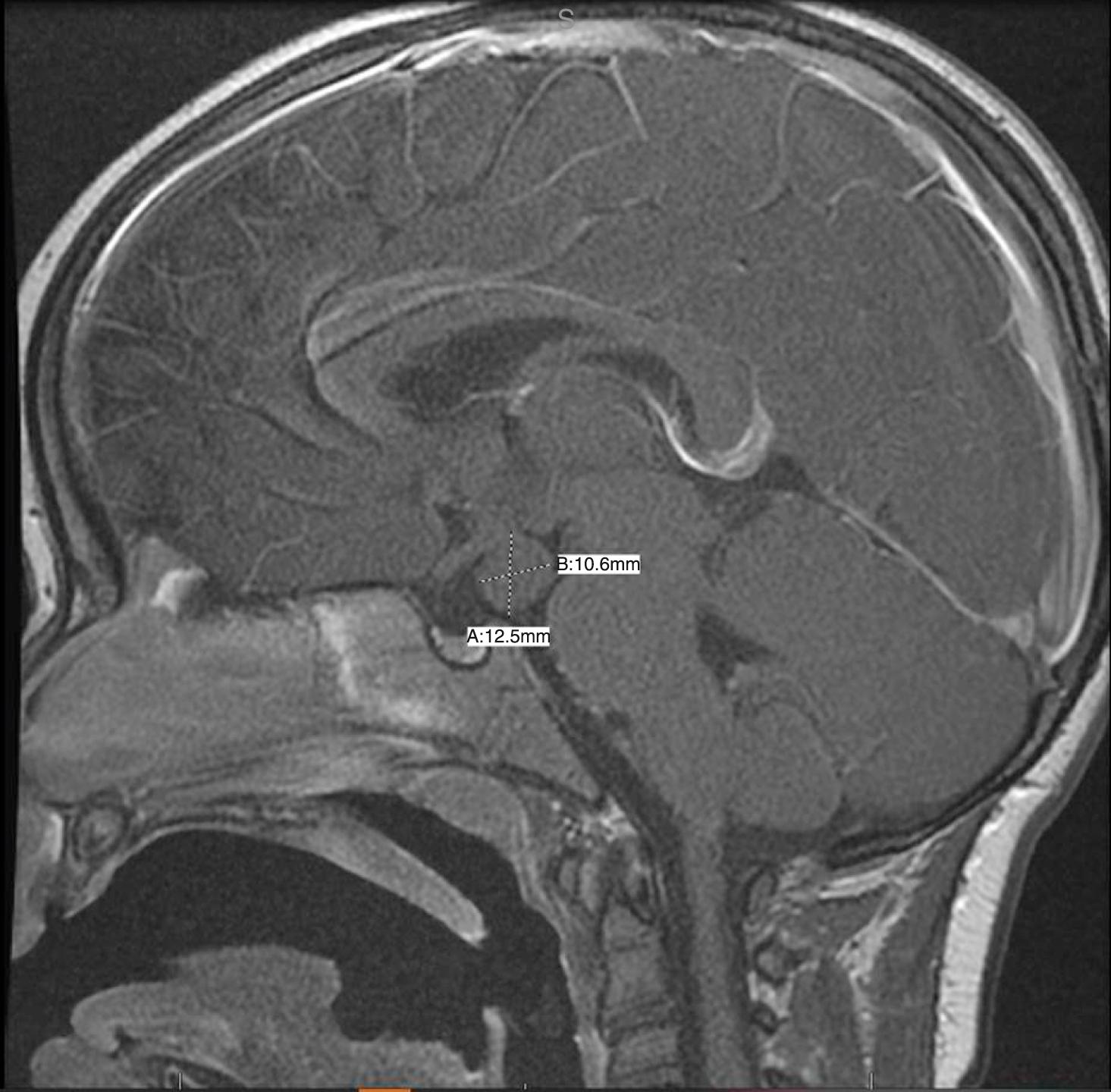
Both congenital and acquired abnormalities of pituitary function occur. Aberrant development of the pituitary may be associated with an ectopic location of the posterior pituitary at the base of the hypothalamus. Ectopic posterior pituitary is often associated with absence of the pituitary stalk. Affected individuals manifest varying anterior pituitary hormone deficiencies in the absence of diabetes insipidus since the function of the ectopic posterior pituitary is preserved. The abnormal location of the posterior pituitary is often visible as the posterior pituitary bright spot on magnetic resonance imaging (MRI) ( Fig. 9.14 ).
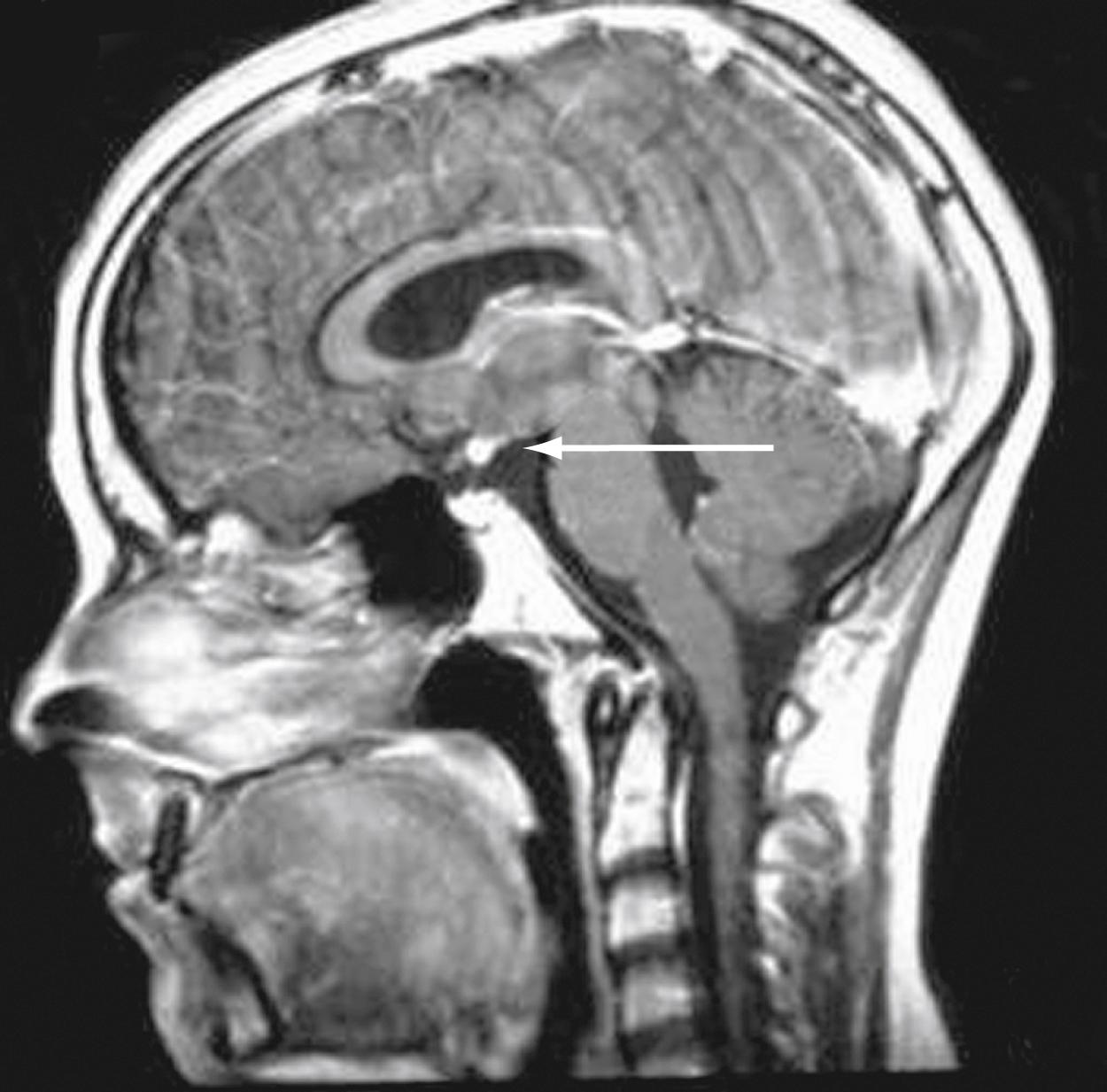
Structural abnormalities of the CNS, such as septo-optic dysplasia ( Fig. 9.15A and B) and holoprosencephaly (see Fig. 9.15C ), can interfere with both anterior and posterior pituitary function. Septo-optic dysplasia refers to a collection of anatomic findings including absence or abnormal development of the corpus callosum/septum pellucidum, optic nerve hypoplasia, and pituitary hormone deficiencies. Some patients with septo-optic dysplasia present with pituitary hormone deficiencies; some patients may develop pituitary hormone deficiencies over time. Gene defects have been increasingly identified among patients with septo-optic dysplasia. Genes associated with septo-optic dysplasia include HESX1, SOX2, SOX3, and OTX2 .
Holoprosencephaly is a developmental disorder in which there is incomplete separation of the cerebral hemispheres. Genes associated with holoprosencephaly include SHH, SIX3, ZIC2, and GLI2 . Both septo-optic dysplasia and holoprosencephaly may represent the consequences of teratogen exposure.
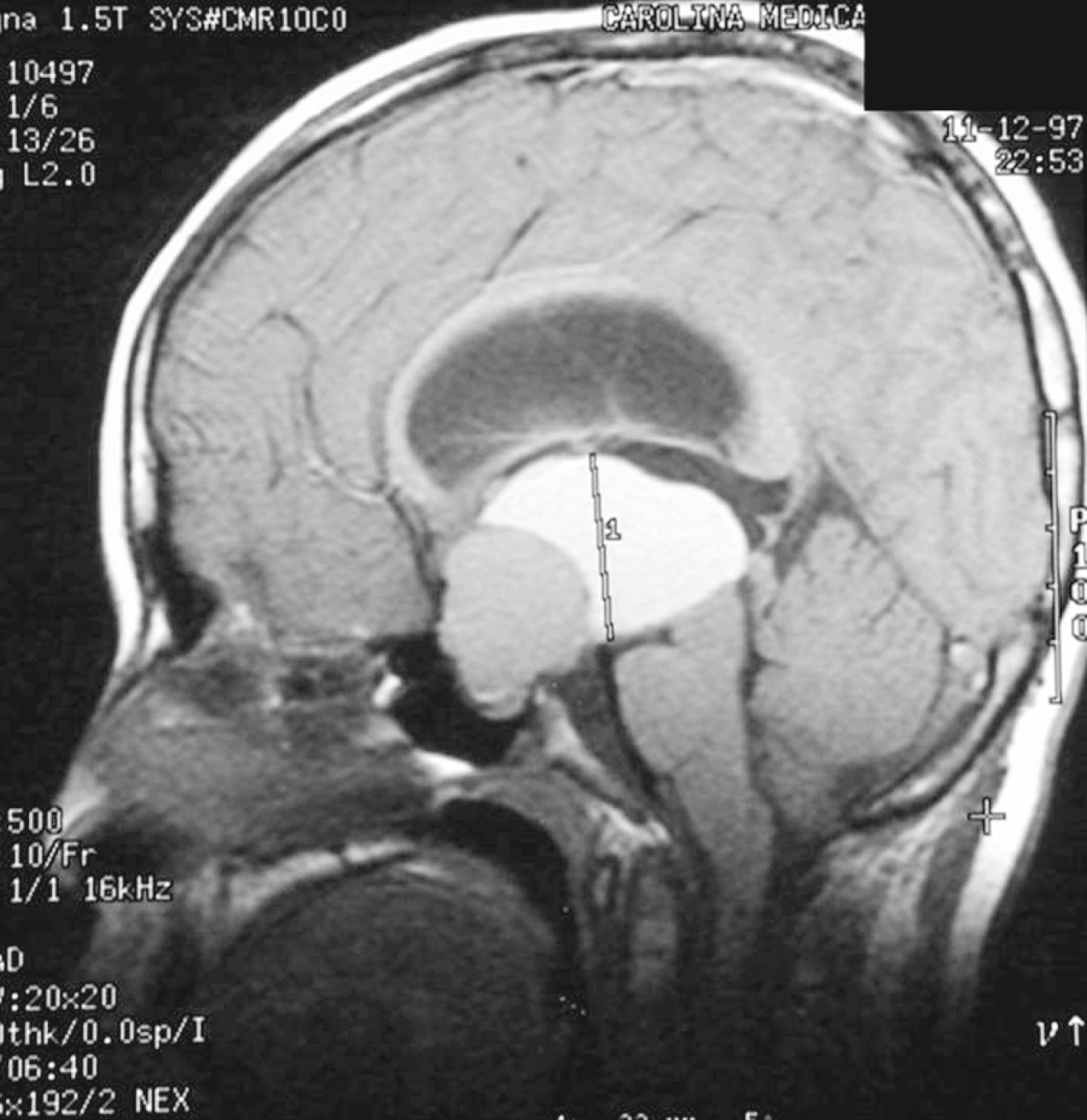
Craniopharyngiomas and CNS tumors can be associated with acquired hypopituitarism ( Fig. 9.16 ). Craniopharyngiomas are nonmalignant congenital epithelial tumors involving the sellar region. Although benign, these tumors can infiltrate into the hypothalamus, optic chiasm, and local vascular structures. There are two different subtypes of craniopharyngioma—adamantinomatous and papillary. The adamantinomatous form is the most common non-neuroepithelial intracranial lesion in children. The tumor and its treatment often result in hypopituitarism, diabetes insipidus, obesity, and visual deficits. Although craniopharyngiomas are not malignant lesions, tumor recurrence is common. Thus, these children will need to have lifelong endocrine care.
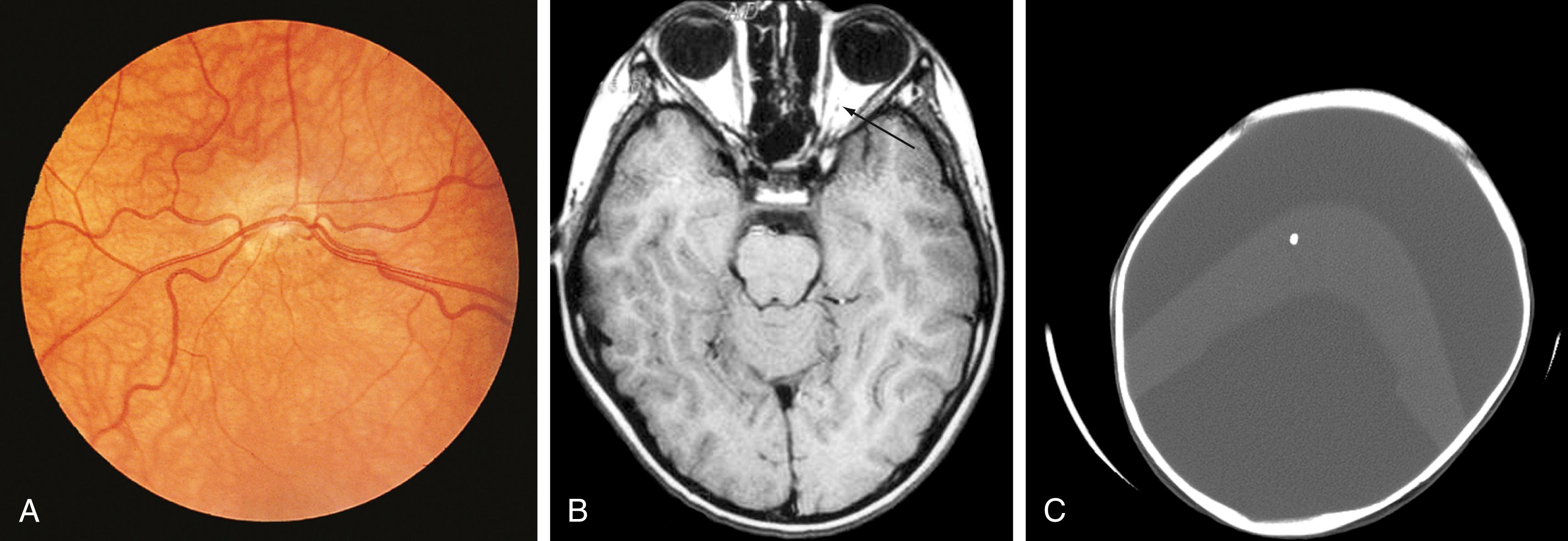
Optic pathway gliomas account for 2% to 5% of childhood brain tumors. These tumors may involve the hypothalamus and third ventricle. Clinical features include impaired visual acuity, strabismus, nystagmus, and diencephalic syndrome. Neurofibromatosis type 1 is associated with these tumors, which paradoxically can be associated with precocious puberty.
Additional causes of acquired hypopituitarism include germ cell tumors, surgical treatment, irradiation to treat CNS tumors, granulomatous infiltration (such as Langerhans cell histiocytosis), and traumatic interruption of the pituitary stalk. Diabetes insipidus can be the presenting endocrine manifestation for germ cell tumors and Langerhans cell histiocytosis.
The anterior pituitary, with its diverse cell types and hormonal secretory patterns, controls many important biologic processes. It contains cells that secrete three types of hormones: (1) corticotrophin-related peptide hormones (ACTH), (2) glycoprotein hormones (FSH, LH, TSH), and (3) somatomammotropins (GH, prolactin). These compounds have great biologic potency with tight regulation of hormone secretion governed by positive and negative feedback signals. Anterior pituitary hormone deficiencies cause subsequent hypofunction in the output of secondary endocrine glands, with substantial consequences for growth and development.
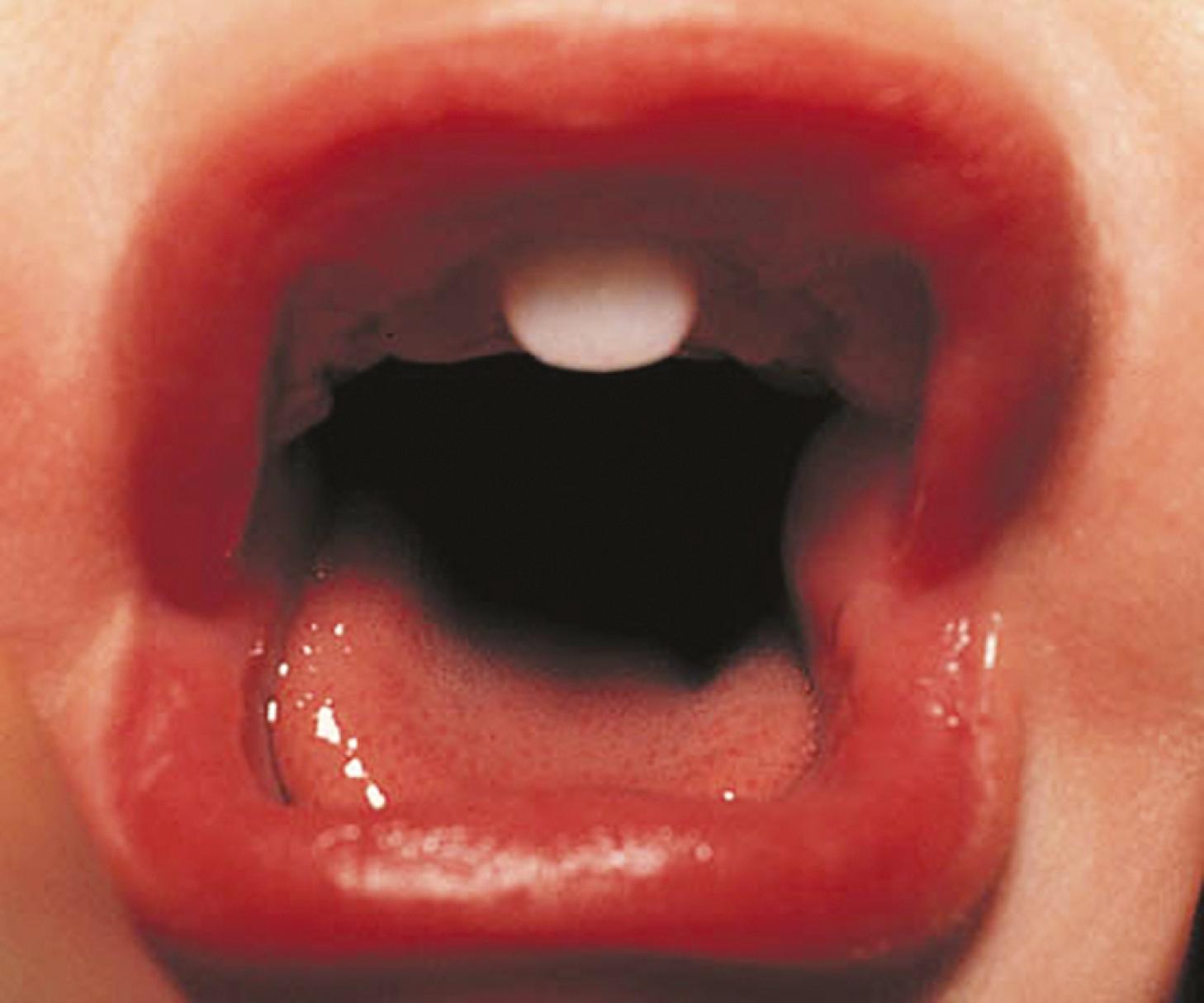
Children with midline defects have a higher incidence of hypopituitarism when compared with normal children. The child shown in Fig. 9.17 has a single central incisor, an example of a midline abnormality associated with GH deficiency. Thus, specific alterations in physical appearance should alert physicians to a possible abnormality in anterior pituitary development, potentially associated with secondary hormone deficiencies (e.g., TSH deficiency affecting thyroid function). Many molecular etiologies for autosomal recessive, autosomal dominant, and X-linked disorders affecting anterior pituitary development and function have been elucidated. Teratogen exposure at a critical time of embryogenesis can also affect the development and/or function of the anterior pituitary gland. Acquired causes such as tumors, trauma, surgery, radiation therapy, inflammation, and autoimmune hypophysitis can alter pituitary functions.
Vasopressin and oxytocin are two evolutionarily related peptides—each composed of nine amino acids. These hormones are synthesized in the hypothalamus and stored in the posterior pituitary gland. Expression of vasopressin and oxytocin genes occurs in the hypothalamic paraventricular and supraoptic nuclei. On magnetic resonance T1-weighted images, the posterior pituitary has characteristic high signal intensity. The presence of this high signal intensity adjacent to the median eminence with absence of the normal pituitary bright spot within the sella on T1-weighted images is evidence of an ectopic posterior pituitary. An ectopic posterior pituitary is often associated with anterior pituitary hormone deficiencies, but typically these patients do not have diabetes insipidus (see Fig. 9.14 ). Vasopressin, also known as arginine vasopressin (AVP) or antidiuretic hormone (ADH), is a hormone that is important in water balance. It is synthesized and carried via axonal transport to the posterior pituitary, its primary site of storage. It is then released into systemic circulation. AVP acts primarily on the kidneys at V2 receptors to aid in the reabsorption of water by affecting water permeability in the collecting duct of the kidney. At high concentrations, it also causes constriction of the arterioles through its action at V1 receptors, thereby leading to an increase in blood pressure. V3 receptors in the pituitary contribute to adrenocorticotropic hormone (ACTH) release by potentiating the action of corticotropin-releasing hormone (CRH). Osmoreceptors in the hypothalamus detect an increase in osmotic pressure in the blood, leading to increased AVP secretion and increased thirst. The combination of increased AVP secretion leading to increased renal reabsorption of free water and increased oral fluid intake will decrease osmolality. Other factors that increase AVP secretion include pain, trauma, nausea, and vomiting. Head trauma, brain tumors, encephalitis, pneumonia, and some drugs are associated with overproduction of AVP. This can lead to inappropriate water retention and hyponatremia, known as the syndrome of inappropriate antidiuretic hormone secretion (SIADH). The symptoms of SIADH include headache, apathy, nausea, vomiting, and impaired consciousness.
Underproduction of AVP results in central diabetes insipidus. Diabetes insipidus can result from pituitary tumors, head trauma, infiltrative disease processes (such as Langerhans cell histiocytosis, sarcoidosis, hemochromatosis, and autoimmune hypophysitis), or from any surgery that damages the pituitary gland and hypothalamus. Familial central diabetes insipidus, which is inherited in both recessive and dominant patterns, is rare and has its onset in infancy. Wolfram syndrome is an autosomal dominant form of central diabetes insipidus often associated with diabetes mellitus, optic atrophy, and deafness (DIDMOAD). Nephrogenic diabetes insipidus is characterized by failure of the kidney tubules to respond to ADH. Genetic causes of nephrogenic diabetes insipidus include X-linked forms due to mutations in the V2 receptor gene and autosomal forms due to mutations in the aquaporin-2 gene. Acquired nephrogenic diabetes insipidus can be caused by drugs, such as lithium. Psychogenic water drinking, hypercalcemia, hypokalemia, sickle cell anemia, and polycystic kidney disease can also impair renal concentrating ability independent of AVP production or action.
Oxytocin secretion occurs in response to nervous stimulation of the hypothalamus. This hormone causes contraction of the smooth muscle of the uterus and of the myoepithelial cells lining the duct of the mammary gland. Although some oxytocin is found in males, its function is unclear.
Growth hormone (GH) influences linear growth and modulates several complex metabolic processes. GH secretion is regulated by the relative balance between the levels of growth hormone–releasing hormone (GHRH) and somatostatin ( Fig. 9.18 ). Both GHRH and somatostatin are secreted by the hypothalamus. The GH receptor is a single transmembrane protein. After the binding of GH to its receptor, a second GH receptor dimerizes with the first receptor to initiate the signal transduction process. GH generates direct effects via the GH receptor signal transduction pathway and secondary effects by promoting an increase in IGF-1 and insulin-like growth factor–binding protein-3 (IGF-BP3).
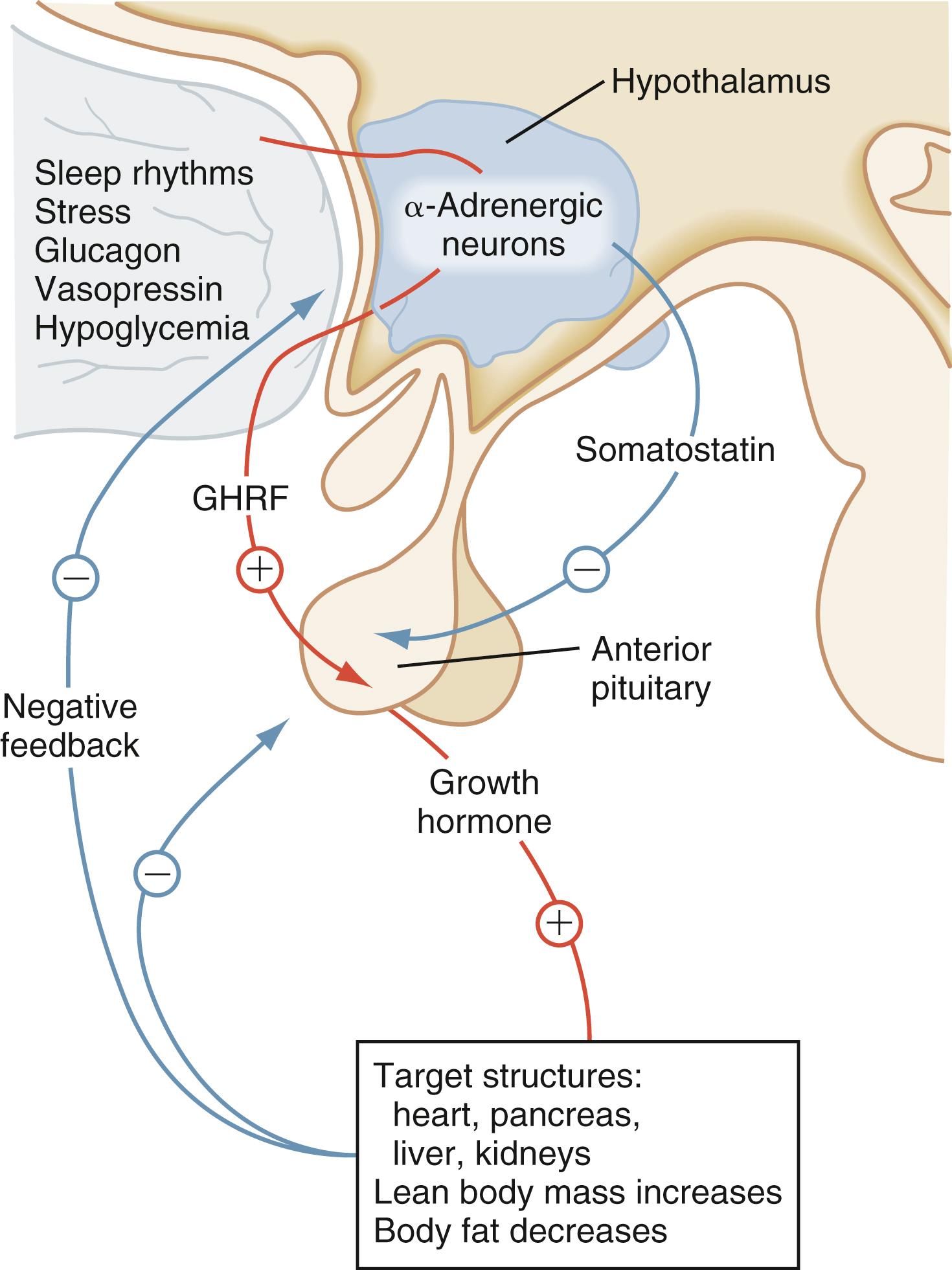
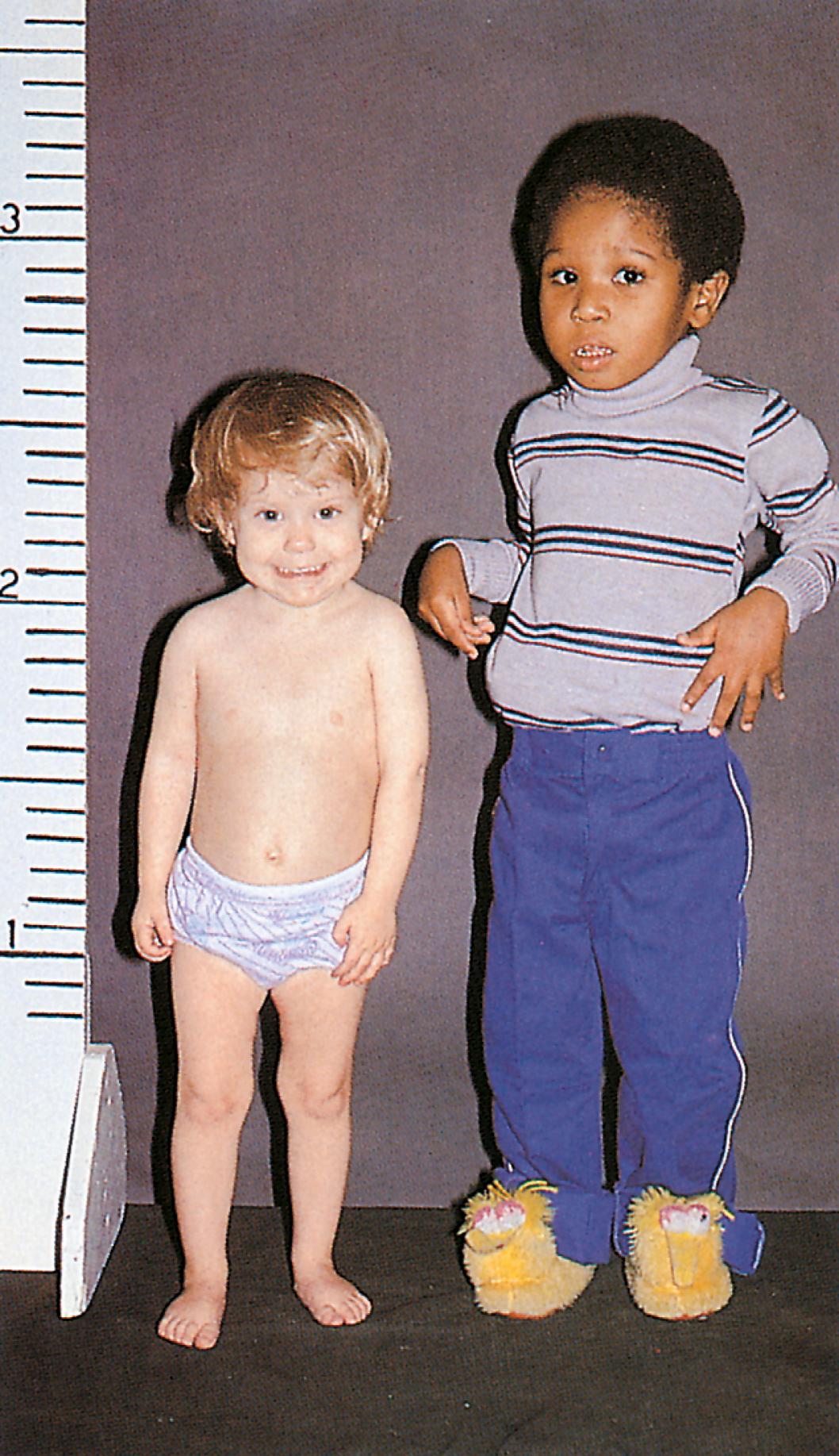
In children with hypopituitarism due to GH deficiency, GH treatment markedly improves growth velocity. GH stimulates an increase in lean body mass, as well as a marked increase in the size of the heart, pancreas, liver, and kidneys. It has positive effects on carbohydrate, fat, and protein metabolism and causes a decrease in body fat. GH inhibits carbohydrate uptake by muscle. This diabetogenic effect of GH action is a known complication of GH hypersecretion. Typically, children with GH deficiency have normal birth weights and normal growth patterns during the first year of life, after which time their growth velocities decelerate. As seen in Fig. 9.19 , GH-deficient children have a characteristic “kewpie” doll appearance. They are often described as being “cherubic” because of their short stature, excess subcutaneous fat, retarded body proportion changes, and high-pitched voices. Infants with hypopituitarism may present in the early neonatal period with hypoglycemia or prolonged jaundice. On physical examination, male infants with hypopituitarism and growth hormone deficiency may have small penis due to concomitant LH deficiency.
The pulsatile nature of GH secretion necessitates provocative stimulation tests to diagnose GH deficiency. Thus, measurements of random GH concentrations are not helpful in the evaluation of short stature. IGF-1 concentrations may be low in children with GH deficiency. Because IGF-1 concentrations also reflect nutritional status, IGF-1 concentrations may also be low in children with inadequate caloric intake. Laron syndrome is a genetic condition where mutation in growth hormone receptor results in resistance to growth hormone and inability to generate IGF-1 from the liver cells. Individuals with Laron syndrome have severe short stature (adult males 4.5 feet, adult females 4 feet) that can be treated with recombinant human IGF-1.
Excessive GH secretion is uncommon in children. If GH excess begins during childhood, gigantism with increased growth velocity ensues leading to abnormal tall stature, while GH excess in an individual with closed epiphyses will develop acromegaly manifested by soft tissue growth of the hands and feet and coarsening of facial features. Random GH, IGF-1, and IGF-BP3 concentrations are usually elevated in gigantism/acromegaly. Oral glucose tolerance test (OGTT) may be performed to diagnose growth hormone excess. Oral glucose load decreases growth hormone secretion in normal individuals but not in gigantism/acromegaly.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here