Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
At birth the circulation undergoes a fundamental change as blood oxygenation occurs through the lungs rather than the placenta. This transition places some newborns at risk of sudden increases in pulmonary artery pressure with resultant shunting of blood past the lungs through a patent foramen ovale or the ductus arteriosus. This may be triggered by hypoxia, hypercapnia, acidosis, and infection.
The reduced cellular mass of the neonatal heart devoted to contractility results in less compliant ventricles. This leads to a sensitivity to excessive intravascular volume, poor tolerance to increases in afterload (i.e., the development of biventricular failure), and compared to older children, a relatively rate-dependent cardiac output. In addition, the reduced cardiac calcium stores produce increased susceptibility to myocardial depression by potent anesthetics and also make neonates dependent on exogenous (i.e., blood-ionized) calcium values and vulnerable to the negative inotropic effects of ionized hypocalcemia.
The neonatal airway differs from the adult airway in four ways: the larynx is located higher in the neck, the glottis is shaped differently and angled over the laryngeal inlet, the vocal cords are angled with the narrowest portion in the subglottic region at the level of the cricoid cartilage.
Neonates have relatively larger volumes of distribution and lower clearances for most drugs. Thus loading doses generally have to be relatively larger whereas continuous infusion rates or dose intervals tend to be longer. Allometric scaling (e.g., body mass) can predict the dose requirement for most drugs in children better than simple mg/kg calculations.
The minimum alveolar concentration (MAC) of volatile anesthetic agents is higher in children compared to adults. However, for most agents the MAC in neonates is lower than for older children. Infants achieve more rapid equilibration of inspired-to-tissue concentrations of volatile agents compared to older agents, and hence relative overdose is a risk if higher concentrations are used for prolonged periods of time.
Neonates and infants are at greater risk of anesthesia-related cardiac arrest compared to older children. The etiology is most commonly related to cardiac or respiratory effects.
Former preterm infants are at risk for postoperative apnea. The use of regional anesthesia in these children may reduce the incidence of immediate postanesthesia apnea, but ongoing monitoring of the preterm infant is critically important.
Neonates and infants require adequate analgesia for painful procedures. The optimal dose of general anesthetics to achieve adequate analgesia is unclear in this population. Adult-derived electroencephalograph (EEG) algorithms such as bispectral index (BIS) cannot be used in this age group to guide anesthesia.
Temperature regulation is especially important for neonates and infants. Because of the large body surface-to-weight ratio, they are vulnerable to intraoperative hypothermia. Efforts to maintain a warm surgical unit through the use of warming devices such as hot air mattresses, application of warm surgical skin preparation solutions, and transport of the neonate or infant in an appropriate transport device, as well as keeping the infant covered during transport, all help prevent hypothermia.
Compared to adults, children are more susceptible to iatrogenic hyponatremia and subsequent significant morbidity. To minimize this risk, perioperative fluid therapy should consist of an isotonic solution. The classic 4-2-1 rule of Holliday and Segar overestimates the replacement requirement.
Preschool children are at risk of postoperative delirium and/or agitation. Agitation may be due to many factors including pain, fear, and hunger. Delirium may also cause agitation. Children manifest delirium by becoming inconsolable and not interacting with their parents or caregivers. Many strategies are known to reduce the risk of delirium. A number of approaches have been used to minimize postoperative delirium. Children receiving propofol anesthesia are at lower risk for delirium than those receiving volatile anesthetics.
Most general anesthetics cause morphologic changes to the developing brain, based on animal studies. Accelerated neuronal apoptosis is the most widely described change. Some human studies have found an association between exposure to anesthesia and surgery in early childhood and subsequent neurodevelopmental issues. This may be explained by a number of confounding factors. At the same time, increasing evidence suggests that an hour of anesthesia in infancy does not have a lasting impact on cognition and a range of other psychometric outcomes.
Pediatric anesthesia requires appropriate pediatric equipment in a range of sizes. Neonates require ventilators that are designed to meet their needs.
Preoperative anxiety is common in children. Distraction techniques, premedication with midazolam or α <ce:inf>2</ce:inf> agonists, and parental presence at induction have all been shown to reduce anxiety.
The editors and publisher would like to thank Dr. Charles J. Cote for contributing a chapter on this topic in the prior edition of this work. It has served as the foundation for the current chapter.
During development there are substantial changes in a child’s physiology and anatomy. Understanding these is key to providing safe pediatric anesthesia care. The most substantial changes occur at birth and in early infancy; however, many systems continue to develop throughout childhood.
Intrauterine development extends from conception to birth. This prenatal period is characterized by increased vulnerability to a large variety of genetic and external factors that can induce permanent organ dysfunction of variable severity ( Table 77.1 ). Identification of these prenatal risk factors is of utmost importance since they can have a major impact on perioperative management. Prenatal development is usually divided into three stages: (1) the germinal, (2) the embryonic, and (3) the fetal stage. The germinal stage starts with conception and ends approximately 2 weeks later with the implantation of the embryo into the uterine wall. One key feature of this period is the formation of the placenta. Factors, either genetic or environmental, that interfere with the implantation process lead to the termination of pregnancy. The embryonic stage comprises the period between the third and eighth weeks of pregnancy and is characterized by intense cell proliferation, migration, and differentiation leading to the establishment of all major organs. Increased vulnerability to a wide variety of substrates, commonly called teratogens, during this period can induce major developmental defects, many of them incompatible with life. The fetal stage lasts from the ninth week of pregnancy to birth and is characterized by the growth and functional differentiation of organs formed during the embryonic period. Numerous exogenous factors, such as environmental toxins, ionizing radiation, and maternal infections as well as a multitude of drugs can interfere with the physiological patterns of organ development throughout the fetal period which, in turn, will result in organ dysfunction of variable severity. Careful evaluation of prenatal history is therefore an important part of preoperative assessment and can guide further investigations prior to perioperative management.
| Gestation | Relative Weight | Neonatal Problems at Increased Incidence |
|---|---|---|
| Preterm (<37 weeks) | SGA | Respiratory distress syndrome Apnea Perinatal depression Hypoglycemia Polycythemia Hypocalcemia Hypomagnesemia Hyperbilirubinemia Viral infection Thrombocytopenia Congenital anomalies Maternal drug addiction Fetal alcohol syndrome |
| AGA | Respiratory distress syndrome | |
| Apnea | ||
| Hypoglycemia | ||
| Hypocalcemia | ||
| Hypomagnesemia | ||
| Hyperbilirubinemia | ||
| LGA | Respiratory distress syndrome | |
| Hypoglycemia; infant of diabetic mother | ||
| Apnea | ||
| Hypoglycemia | ||
| Hypocalcemia | ||
| Hyperbilirubinemia | ||
| Normal (37-42 weeks) | SGA | Congenital anomalies Viral infection Thrombocytopenia Fetal alcohol syndrome Perinatal depression Hypoglycemia |
| AGA | — | |
| LGA | Birth trauma | |
| Hyperbilirubinemia | ||
| Hypoglycemia; infant of diabetic mother | ||
| Postmature (>42 weeks) | SGA | Meconium aspiration syndrome Congenital anomalies Viral infection Thrombocytopenia Maternal drug addiction Perinatal depression Aspiration pneumonia Hypoglycemia |
| AGA | — | |
| LGA | Birth trauma | |
| Hyperbilirubinemia | ||
| Hypoglycemia; infant of diabetic mother |
While pregnancy is considered to reach full term between the completion of the 37th and the 42nd weeks of gestation, fetuses reach an age of viability that may be considered, under tight medical support, as compatible with extrauterine life between the 22nd and 26th weeks after conception. Prematurity is stratified into mild preterm (32-37 weeks), very preterm (28-31 weeks) and extremely preterm (<28 weeks) periods with increasing neonatal morbidity and mortality based on degree of prematurity ( Fig. 77.1 ).
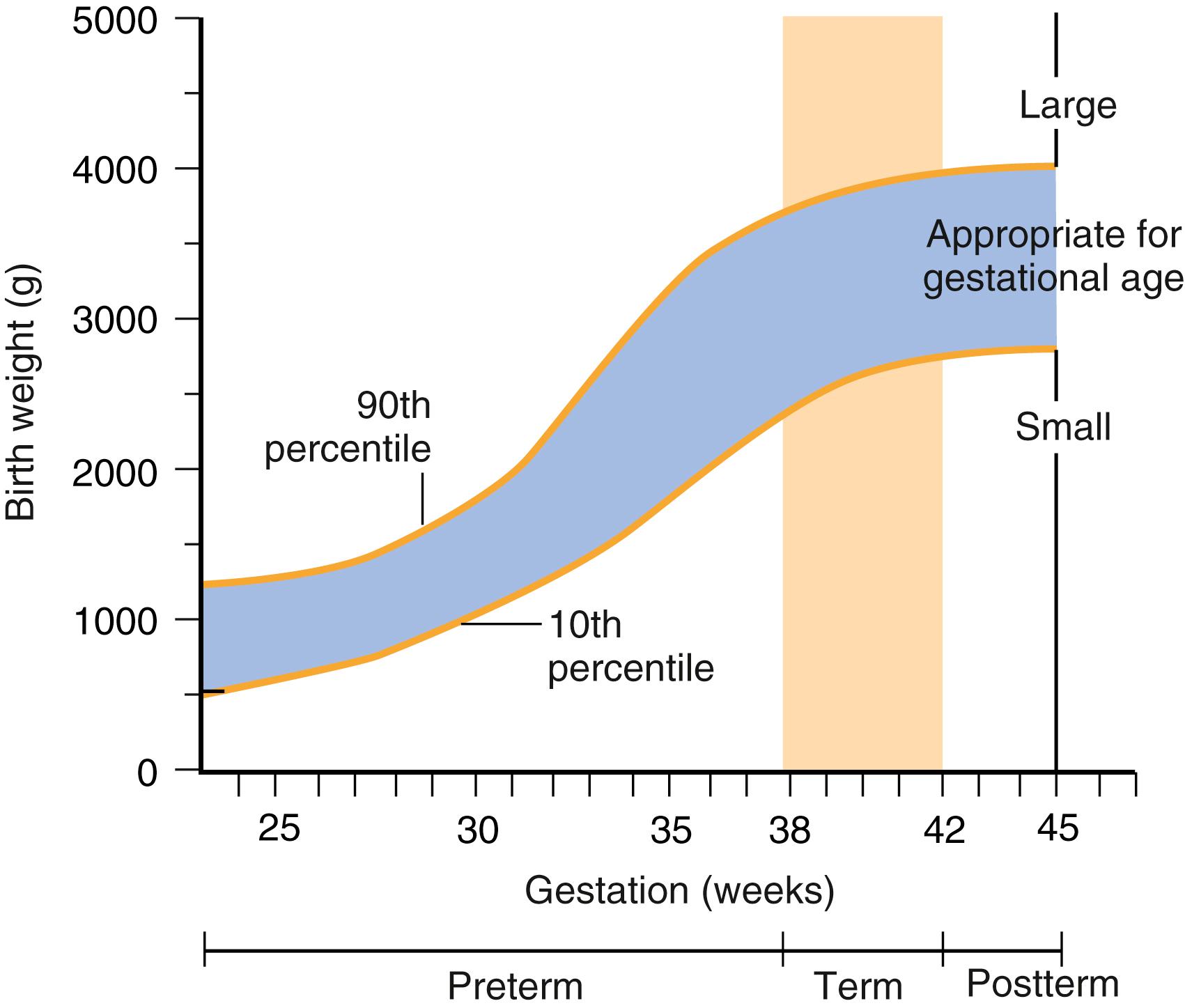
Normal birth weight at term is 2500 g to 4200 g. Infants weighing less than these norms can be classified as low birth weight (<2500 g), very low birth weight (<1500 g), and extremely low birth weight (<1000 g). Plotting weight against gestational age allows further classification into three additional categories: small for gestational age, appropriate for gestational age, or large for gestational age (see Fig. 77.1 ). Infants who are small or large for gestational age often have developmental problems or difficulties associated with maternal disease which can directly affect perioperative care (see Table 77.1 ).
The physiology of fetal life is fundamentally different from that of the neonate. The transition from intra- to extrauterine life is rapid and involves a complex and well-orchestrated series of events aimed to ensure neonatal viability. A clinically useful measure to assess the condition of the newborn infant immediately after birth is the Apgar score ( Table 77.2 ) This score is reported at 1 minutes and 5 minutes after birth for all infants and can be extended thereafter to follow fetal to neonatal transition. Apgar scores between 7 and 10 are considered reassuring, a score of 4 to 6 as moderately abnormal, while scores 3 and below are usually indicative of poor outcome. It is, nevertheless, important to note that the Apgar score has its limitations and cannot be used alone to diagnose neonatal asphyxia.
| Score | 0 Points | 1 Point | 2 Points |
|---|---|---|---|
| Appearance (skin color) | Cyanotic/pale all over | Peripheral cyanosis only | Pink |
| Pulse (heart rate) | 0 | <100 | 100-140 |
| Grimace (reflex irritability) | No response to stimulation | Grimace (facial movement)/weak cry when stimulated | Cry when stimulated |
| Activity (tone) | Floppy | Some flexion | Well flexed and resisting extension |
| Respiration | Apneic | Slow, regular breathing | Strong cry |
The cardiovascular system undergoes dramatic physiologic and maturational changes during the first year of life. In utero, most of the cardiac output is directed from the placenta across the foramen ovale into the ascending aorta (oxygenated blood), whereas superior vena cava blood (deoxygenated) is directed to both the pulmonary artery and the ductus arteriosus (see also Chapter 78 ). This pattern of circulation results in minimal intrauterine pulmonary blood flow. At birth, a number of events change hemodynamic interactions such that the fetal circulation adapts to the postuterine environment. Specifically, the placenta is removed from the circulation; portal blood pressure falls, which causes the ductus venosus to close; and blood becomes oxygenated through the lungs. Exposure of the ductus arteriosus to oxygenated blood induces ductal closure. As a result of the combined effects of lung expansion, exposure of blood to oxygen, and loss of low resistance through placental blood flow, pulmonary vascular resistance decreases while peripheral vascular resistance rapidly rises. The decrease in pulmonary vascular resistance occurs on the first day of life and continues to decrease gradually during the next several years as the architecture of the pulmonary vessels changes. An increase in pressure on the left side of the heart (caused by the increase in peripheral vascular resistance) induces mechanical closure of the foramen ovale. As a result, all three connections between the right and left sides of the circulation close. Although closure of the ductus arteriosus probably occurs primarily from an increase in arterial oxygen concentration, successful completion requires arterial muscular tissue; that such tissue is less prevalent in preterm infants may partly account for the frequent incidence of patent ductus arteriosus in preterm infants. True mechanical closure of the ductus by fibrosis does not occur until 2 to 3 weeks of age.
During this critical period, the infant can readily revert from the adult type of circulation to a fetal type of circulation; this state is called transitional circulation . Many factors (e.g., hypoxia, hypercapnia, anesthesia-induced changes in peripheral or pulmonary vascular tone) can affect this precarious balance and result in a sudden return to the fetal circulation. When such a flip-flop occurs, pulmonary artery pressure increases to systemic levels, blood is shunted past the lungs via the patent foramen ovale, and the ductus arteriosus may reopen and allow blood to shunt at the ductal level. A rapid downhill spiral may occur and lead to severe hypoxemia. In this situation, the hypoxemia may be prolonged, despite adequate pulmonary ventilation with 100% oxygen. In most cases, simple hyperventilation with resultant reduction in arterial partial pressure of carbon dioxide (Pa CO 2 ) will cause the pulmonary artery pressure to return to normal.
Risk factors increasing the likelihood of prolonged transitional circulation include prematurity, infection, acidosis, pulmonary disease resulting in hypercapnia or hypoxemia (aspiration of meconium), hypothermia, and congenital heart disease. Care must be directed to keeping the infant warm, maintaining normal arterial oxygen and carbon dioxide tensions, and minimizing the effects of anesthetic-induced myocardial depression for those newborns requiring anesthesia.
The myocardial structure of the heart, particularly the volume of cellular mass devoted to contractility, is significantly less developed in neonates than in adults. This difference, as well as developmental changes in contractile proteins, produce a leftward displacement of the cardiac function curve and less compliant ventricles. As a result of these differences, cardiac output is strongly dependent on heart rate; bradycardia is poorly tolerated because the infant cannot easily compensate for the decreased heart rate by increasing stroke volume to maintain normal cardiac output.
The most frequently encountered arrhythmia in pediatric populations is hypoxia-induced bradycardia that can lead to asystole, if not appropriately handled. Ventricular fibrillation is extremely rare in infants and children.
Generally, myocardial function is usually adequate in most infants and children including those with congenital heart disease. Rare exceptions from this rule are individuals with congenital neuromuscular and metabolic diseases where the myocardium can be seriously compromised. In neonates and infants, cardiac calcium stores are reduced because of the immaturity of the sarcoplasmic reticulum; consequently, these populations have a greater dependence on exogenous (blood-ionized) calcium and probably increased susceptibility to myocardial depression by volatile anesthetics that have calcium channel–blocking activity.
The pulmonary system is not capable of sustaining life until both the lungs and the vascular system have sufficiently matured to allow the exchange of oxygen from air to the bloodstream across the pulmonary alveolar-vascular bed. The lung bud septates from the foregut during the first trimester and the gas exchanging portions of the airway are formed during the second trimester. Alveolar ductal development starts at gestation week 24 while the septation of the air sacs begins around gestational week 36. Alveoli then increase in number and size until a child is approximately 8 years old. Further growth is manifested as an increase in size of the alveoli and airways. At term, complete development of surface-active proteins helps maintain patency of the airways. If a child is prematurely born and these proteins are insufficient, then respiratory failure (e.g., respiratory distress syndrome) may occur.
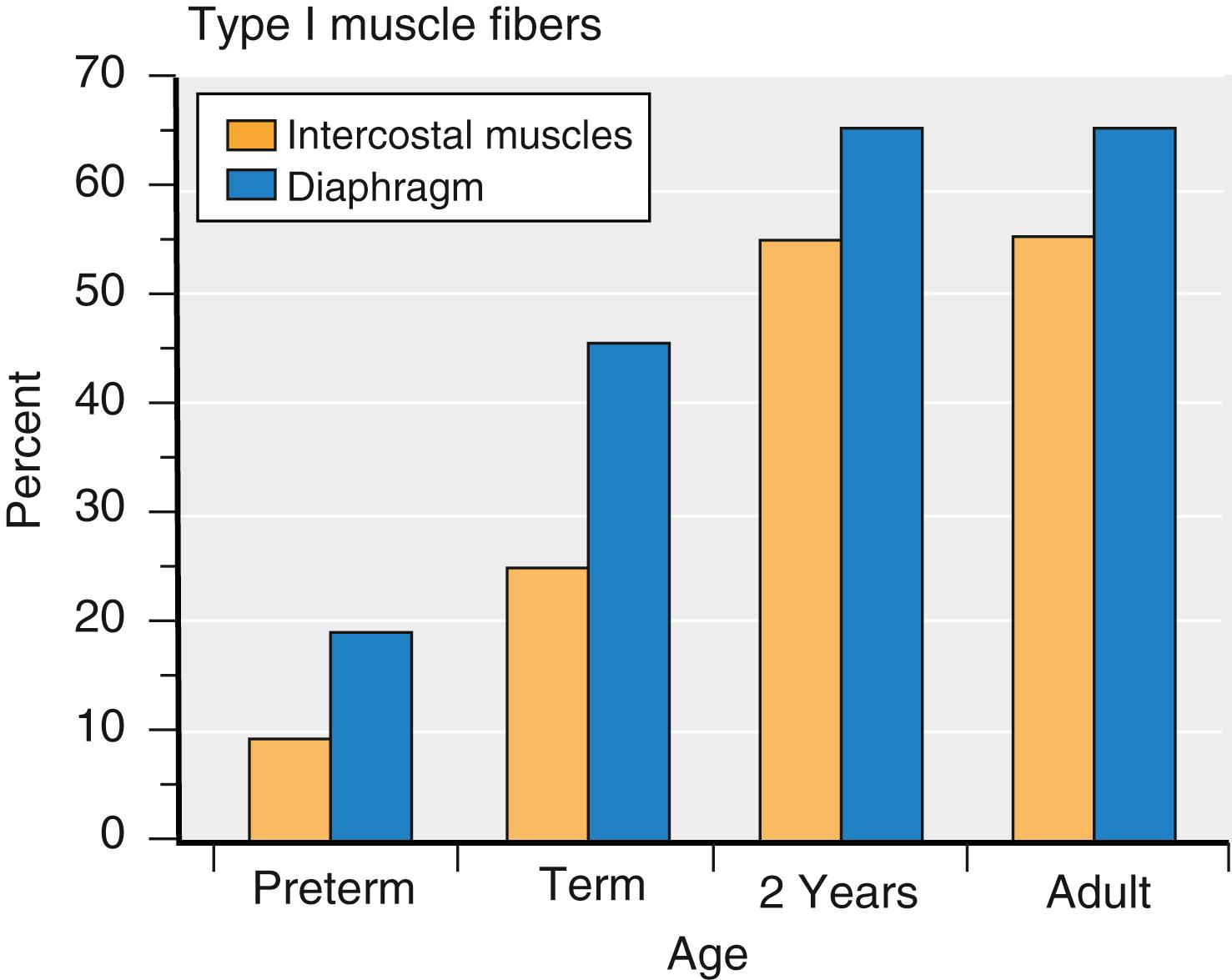
Respiration is less efficient in infants than adults. The airway of infants is highly compliant and poorly supported by the surrounding structures. The chest wall is also highly compliant; therefore the ribs provide little support for the lungs; that is, negative intrathoracic pressure is poorly maintained. The small diameter of the airways increases resistance to airflow. Thus functional airway closure accompanies each breath. Dead space ventilation is proportionally similar to that in adults; however, oxygen consumption is two to three times higher. In preterm infants, the work of breathing is approximately three times that of adults. This increased work of breathing can increase significantly by cold stress (i.e., increased metabolic demand for oxygen) or any degree of airway obstruction. Another important factor is the composition of the diaphragmatic and intercostal muscles. These muscles do not achieve the adult configuration of type I muscle fibers until the child is approximately 2 years old ( Fig. 77.2 ). Because type I muscle fibers provide the ability to perform repeated exercise, any factor that increases the work of breathing contributes to early fatigue of the respiratory muscles of infants; this partially explains why the infant’s respiratory rate and hemoglobin desaturation is so rapid, and their propensity to develop fatigue and apnea with airway obstruction.
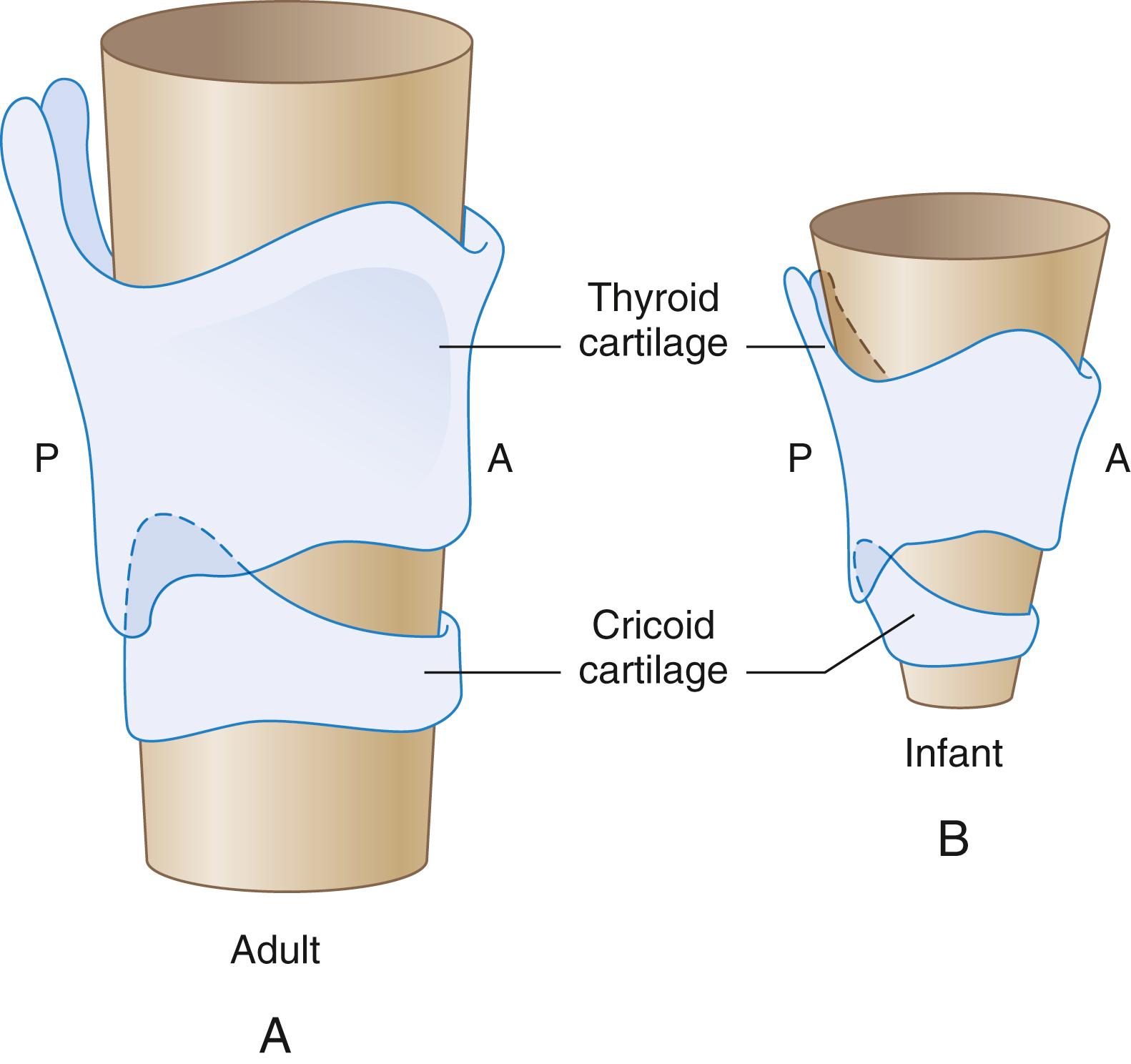
Differences in airway anatomy explain the more likely potential for technical airway difficulties in infants than in teenagers or adults. Typically, the airway of infants differs from adults in five ways : (1) The relatively large size of the infant’s tongue, in relation to the oropharynx, suggests that the infant is more likely to sustain airway obstruction and technical difficulties during induction of anesthesia and laryngoscopy. Recently, however, magnetic resonance imaging (MRI) studies have called this into question by showing that soft tissues surrounding the upper airway grow proportionally to the skeletal structures during childhood. (2) Other anatomic differences may account for some of the airway management challenges in children. The larynx is located higher (more cephalic) in the neck, thus making straight blades more useful than curved blades. (3) The epiglottis is shaped differently, being short, stubby, omega shaped, and angled over the laryngeal inlet. Control with the laryngoscope blade is therefore more difficult. (4) The vocal cords are angled; consequently, a blindly passed tracheal tube may easily lodge in the anterior commissure rather than slide into the trachea. (5) Finally, the infant larynx is funnel shaped, the narrowest portion occurring at the cricoid cartilage ( Fig. 77.3 ). While classic teaching is that the adult larynx is cylindrical and the infant larynx is funnel shaped, it is now recognized that the narrowest portion of the airway in approximately 70% of adults is also in the same subglottic region at the level of the cricoid cartilage as it is in children. Nonetheless, the challenges of tracheal tube placement in children are different than they are in adults. For adult patients, the airway size is much larger, so the commonly used tracheal tubes are usually easy to advance past the glottic opening. In infants or young children, a tracheal tube that easily passes the vocal cords may be tight in the subglottic region because of the relatively greater proportional narrowing at the level of the cricoid cartilage.
Although neonates and infants are considered as obligate nasal breathers, they can also utilize the oral airway to maintain ventilation both spontaneously and in response to complete nasal obstruction. Even in preterm infants, the prevalence of spontaneous oral breathing has been reported to be as high as 50% during sleep, and oral breathing could be consistently initiated in this population upon nasal obstruction.
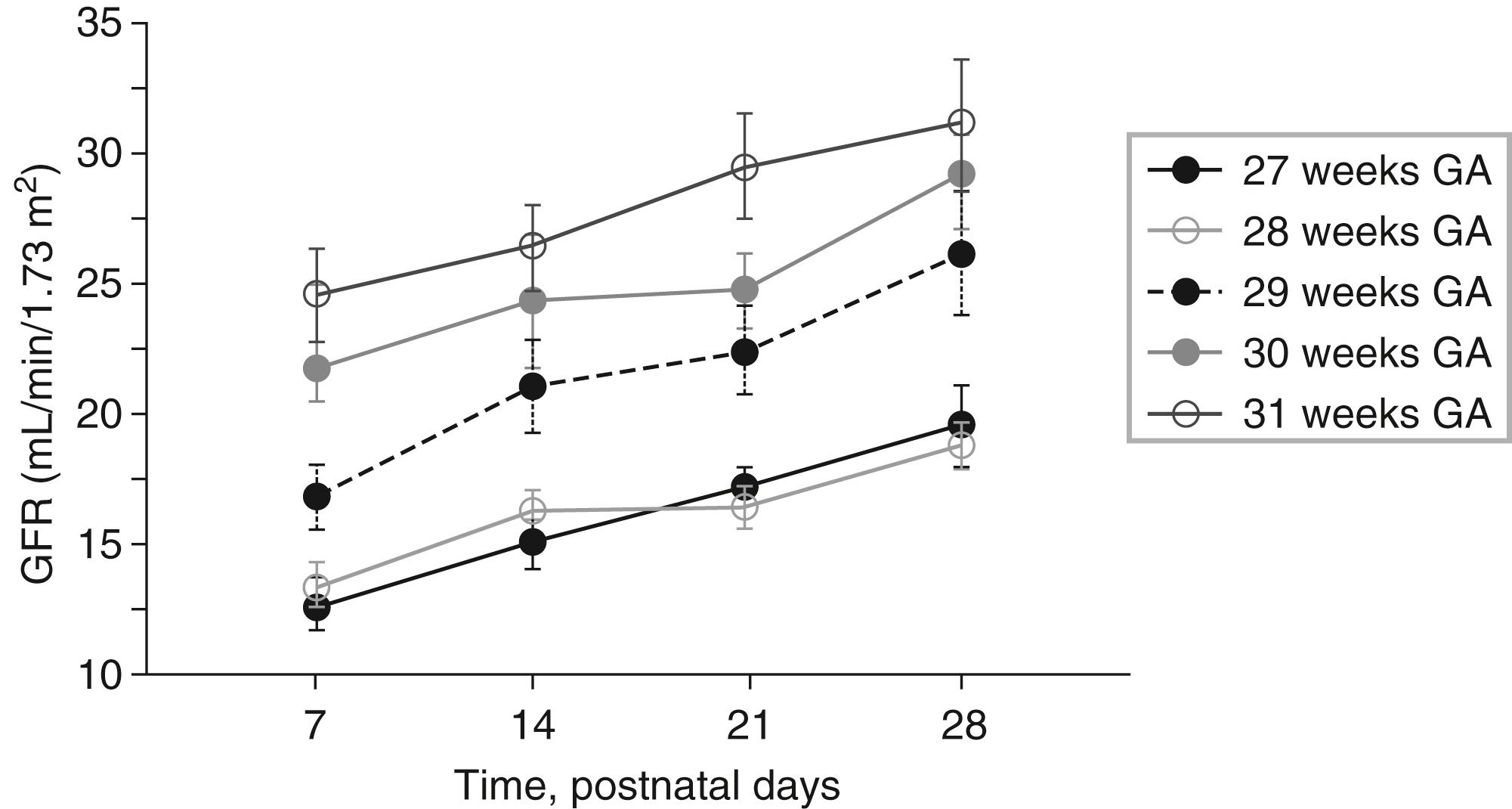
Renal function is diminished in neonates with even less function in preterm infants as a result of lower renal perfusion pressures and immature glomerular and tubular function ( Table 77.3 ) ( Fig. 77.4 ). In full-term infants, maturation of glomerular filtration and tubular function is nearly complete by approximately 20 weeks after birth, although delayed in preterm infants. Complete maturation of renal function occurs at approximately 2 years of age. As a result of the delayed development, newborns have reduced ability to excrete free water and solute loads; the half-life of medications excreted by means of glomerular filtration will be prolonged (e.g., antibiotics). Dosing intervals should be longer in neonates.
| Age | Glomerular Filtration Rate (mL/min/1.73 m 2 mean) | Range |
|---|---|---|
| 1 day | 24 | 3-38 |
| 2-8 days | 38 | 17-60 |
| 10-22 days | 50 | 32-68 |
| 37-95 days | 58 | 30-86 |
| 1-2 years | 115 | 95-135 |
At term, the functional maturity of the liver is incomplete. Most enzyme systems for drug metabolism are developed, but not yet induced (stimulated) by the material that they metabolize. As the infant grows, the ability to metabolize medications rapidly increases for two reasons: (1) hepatic blood flow increases and hence more drug is delivered to the liver, and (2) the enzyme systems develop and are induced. The cytochrome P450 system is responsible for phase I drug metabolism of lipophilic compounds. This system reaches approximately 50% of adult levels at birth. The capacity for drug metabolism (e.g., caffeine) is reduced. However, this is not true for all lipophilic medications. The ability of neonates to metabolize some drugs is dependent on specific individual drug cytochromes. CYP3A (cytochrome P450, family 3, subfamily A) is generally present at adult values at birth, whereas other cytochromes are absent or reduced. Phase II reactions involve conjugation that makes the drug more water-soluble to facilitate renal excretion. These reactions are often impaired in neonates and result in jaundice (decreased bilirubin breakdown) and long drug (and their active metabolites) half-lives (e.g., the half-life of morphine and benzodiazepines is several days). Some of these reactions do not achieve adult activity until after 1 year of age.
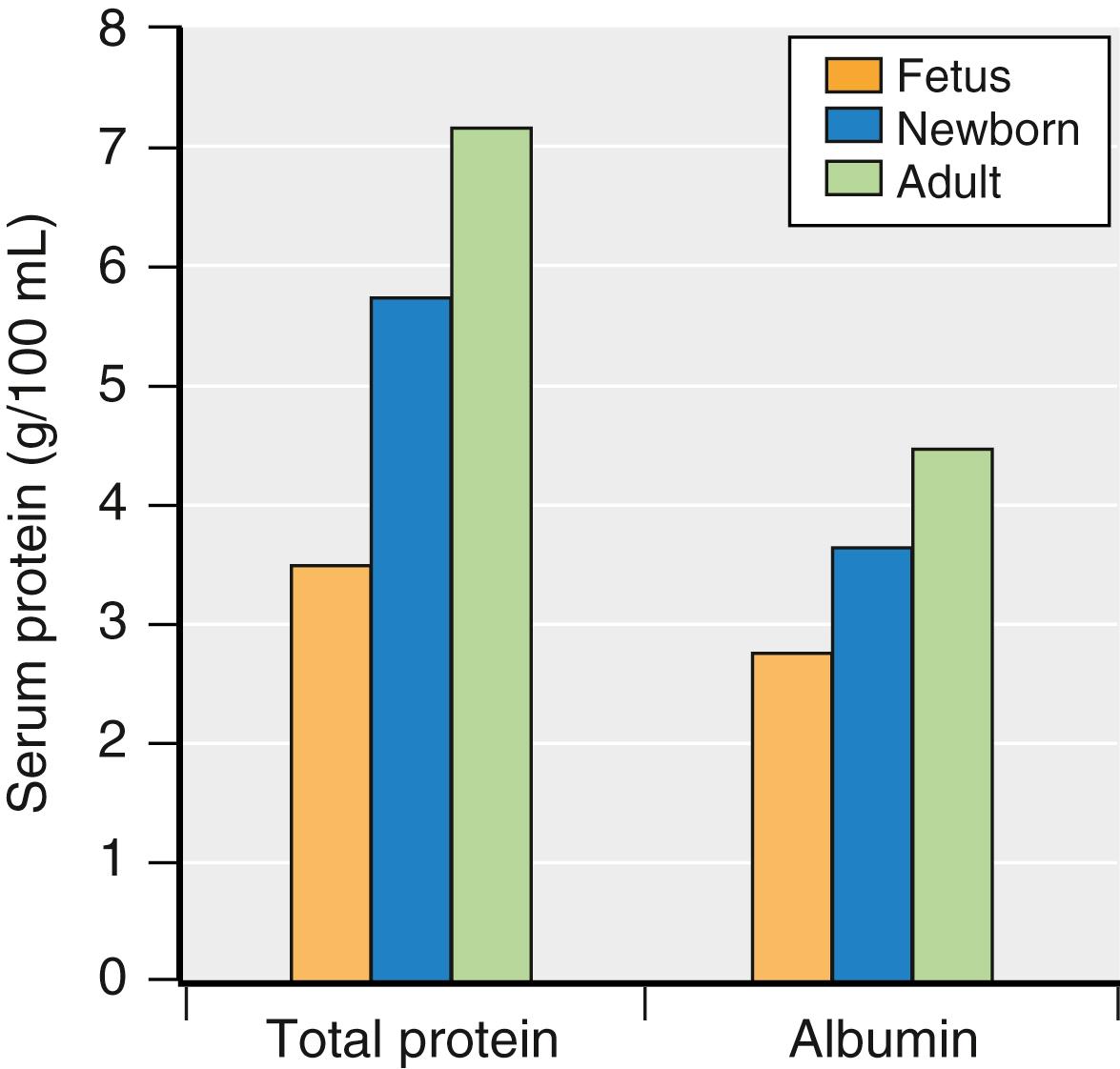
A preterm infant’s liver has minimal glycogen stores and is unable to manage large protein loads. These differences account for the neonate’s tendency toward hypoglycemia and acidemia and for the failure to gain weight when the diet contains too much protein. Additionally, plasma levels of albumin and other proteins necessary for the binding of drugs are lower in full-term newborns (and are even lower in preterm infants) than in older infants ( Fig. 77.5 ). This condition has clinical implications regarding neonatal coagulopathy (e.g., need for vitamin K at birth), as well as for drug binding and its pharmacodynamic effects; the lower the albumin value, the less protein binding of some drugs with resultant greater levels of unbound drug (i.e., unbound drug is the portion available to cross biologic membranes). In addition, the binding of some drugs to albumin may be altered in the presence of hyperbilirubinemia in the neonatal period; this effect is more important for drugs with high protein binding because a greater fraction of unbound drug will occur.
At birth, gastric pH is alkalotic; by the second day of life, pH is in the normal physiologic range for older children. The ability to coordinate swallowing with respiration does not fully mature until infants are 4 to 5 months of age, resulting in a high incidence of gastroesophageal reflux, particularly in preterm newborns. If a developmental problem exists within the gastrointestinal system, then symptoms will generally occur within 24 to 36 hours of life. Upper intestinal abnormalities are exhibited as vomiting and regurgitation, whereas lower intestinal abnormalities produce abdominal distention and a failure to pass meconium.
The fetus uses two compensatory mechanisms to assure adequate oxygen delivery in the relatively hypoxemic in utero environment. One of them is the increased red blood cell production resulting from increased fetal renal erythropoietin secretion in response to hypoxemia. The other compensatory mechanism is the production of fetal hemoglobin. Fetal hemoglobin has a high affinity for oxygen, causing a leftward shift in the oxyhemoglobin dissociation curve, increasing oxygen uptake at the lower oxygenated placental vascular bed. Hemoglobin levels are high at birth (160-240 g/L) but rapidly decrease during the first 3 months of life because of decreased renal erythropoietin production in the normoxic ex utero environment. Fetal hemoglobin will be progressively replaced by adult hemoglobin during the first 6 months of postnatal life. The extent of this physiologic anemia is more important in premature infants and may contribute to the need for perioperative blood transfusion.
The hemostatic system of the neonate and infant has many unique features compared to adults. At birth, levels of vitamin K-dependent coagulation factors are low. They reach adult levels by 6 months of age. Fibrinogen levels are comparable between newborns and adults. However, fibrinogen polymerization does not reach its full capacity during the first few postnatal months, thereby leading to prolonged thrombin time. Platelet number at birth is also comparable to adults, but platelet function is impaired during early life. Despite these apparent deficits, the postnatal period represents a hypercoagulable state, since inhibitors of coagulation are also decreased by 30% to 50% in the newborn. Antithrombin III and protein S levels reach maturity by 3 months of age whereas protein C and plasminogen levels reach adult levels after 6 months of life. The overall results of this hypercoagulable state are higher risks of thrombotic complications in neonates and infants. Children between 1 and 16 years of age have a 25% lower ability to form thrombin compared to adults, and the incidence of venous thrombosis in this population is estimated to be very low (0.05%-0.08%). At adolescence, the physiology of the coagulation system matures. In the adolescent population, additional factors such as smoking, obesity, pregnancy, and use of oral contraceptives become relevant. As a result of some of these factors, recent guidelines recommend considering thromboprophylaxis in postpubertal adolescents.
In utero, the immune system of the fetus remains tolerant to maternal alloantigens. After birth, exposure to myriads of environmental antigens, including those derived from intestinal bacteria, leads to a rapid development of the immune system. However, full maturation of both the innate and adaptive immune systems is achieved after several years of life. Therefore young children are at increased risk from many pathogenic viruses, bacteria, fungi, and parasites when compared to adults.
In humans, the neural tube is formed between the third and fourth week of pregnancy and is followed by an active phase of cell proliferation and migration during the second trimester. With particular relevance to neonatal and pediatric perioperative care, the most intense phase of brain development takes place between the beginning of the third trimester of pregnancy and the first few years of postnatal life. During this period, also called the brain growth spurt, the nervous system undergoes important differentiation, including the formation of myriads of synaptic contacts between neurons. Neural activity plays a preponderant role in these events especially during critical periods of development when the nervous system is particularly sensitive to and relies on external stimuli to drive differentiation of neuronal networks. Pharmacologic interference with physiologic activity patterns during this period may lead to impaired brain development.
Both premature and term newborns show strong pain behavior that is more diffuse and untuned when compared to older children and adults. The first functional and reflex responses to tactile and noxious stimuli are aimed to protect the individual from tissue damage and can trigger a range of physiologic responses throughout the whole organism. The onset of pain awareness or “feelings” in humans remains undefined and largely debated. Nevertheless, there is evidence that early painful experiences, even if nonconscious, might alter subsequent central nervous system (CNS) function and that adequate pain relief can improve outcome.
Infants are especially vulnerable to hypothermia because of the large ratio of body surface area to weight, the thinness of the skin, and a limited ability to cope with cold stress. Cold stress causes increased oxygen consumption and a metabolic acidosis, particularly in preterm infants because of even thinner skin and limited fat stores. The infant compensates by shivering and nonshivering (cellular) thermogenesis (metabolism of brown fat); however, the minimal ability to shiver during the first 3 months of life makes cellular thermogenesis the principal method of heat production. As a result of these issues, managing heat loss is vital for newborns undergoing anesthesia and surgery. Placing the baby on a warming mattress and warming the surgical unit (80°F or warmer) will reduce heat lost by conduction. Keeping the infant in an incubator and covered with blankets minimizes heat lost through convection. The head should also be covered, since heat loss from the scalp is significant. Heat lost from radiation is decreased with the use of a double-shelled isolette during transport. Heat lost through evaporation is lessened by humidification of inspired gases, the use of plastic wrap to decrease water loss through the skin, and warming of skin disinfectant solutions. Hot air blankets are the most effective means of warming children; at the same time, especially in neonates, overheating must be avoided. Anesthetics also impact thermoregulation, particularly nonshivering thermogenesis in neonates.
For nearly all drugs used in anesthesia, the dose required in children and adults differs. These differences are due to factors such as growth, maturation, and differing profiles of concurrent morbidity. A thorough understanding of developmental pharmacology may reduce drug error in children. Size alone cannot predict the differences between adults and children. In adults, many drugs are given on a per kilogram basis. This assumes clearance and volume of distribution remain fixed relative to weight. This assumption is not valid for children. Pharmacokinetics in children varies with body composition, renal and hepatic function, and with altered protein binding. Renal and hepatic function in turn changes with age as relative blood flow and organ maturity change with age. The pharmacodynamics of anesthesia drugs may also differ substantially in children. The changes in pharmacokinetics and pharmacodynamics are most pronounced in neonates. It is important to note that for many drugs, knowledge is limited regarding drug pharmacology in children in general and infants and neonates in particular. The evidence upon which to guide practice is limited; as a result, many anesthesia drugs are used “off label” in small children.
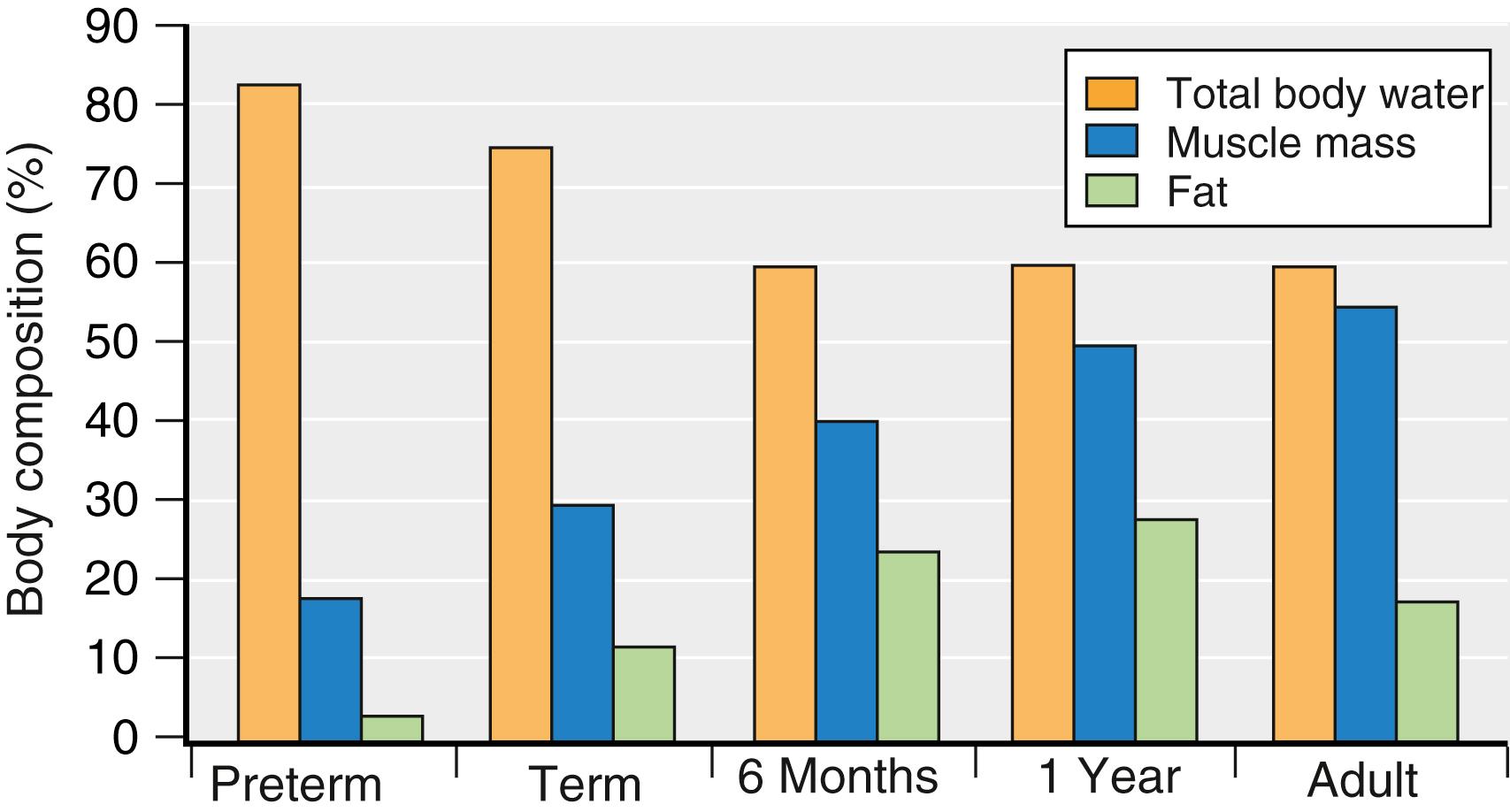
The body compartments (e.g., fat, muscle, water) change with age ( Fig. 77.6 ). Total body water content is significantly higher in preterm infants than in term infants and in term infants than in 2-year-olds. Neonates and infants have a substantially greater extracellular fluid volume compared to intracellular fluid volume. Fat and muscle content increases with age. These alterations in body composition have several clinical implications for neonates. First, a drug that is water soluble has a large volume of distribution and usually requires a large initial dose (mg/kg) to achieve the desired blood level (e.g., most antibiotics, succinylcholine). Second, because the neonate has less fat and muscle, a drug that depends on the redistribution into fat or muscle for the termination of its action will have a long clinical effect (e.g., fentanyl, propofol, and thiopental).
Neonates have reduced total plasma protein levels, including lower levels of albumin (which binds acidic drugs such as diazepam and barbiturates) and α 1 acid glycoprotein (which binds lidocaine and alfentanil). Reduced protein levels mean that drugs that are highly protein bound will have a higher free fraction and hence a greater drug effect; however it is important to note that this is only clinically relevant for drugs that have a very high degree of protein binding, a high extraction ratio, and a narrow therapeutic index (such as lidocaine). Some drugs, such as caffeine and ceftriaxone, may also displace bilirubin from plasma proteins increasing the risk of kernicterus in sick neonates.
Clearance is the fundamental parameter in predicting drug elimination. It is also an important characteristic for determining duration of effect, dosing interval, and infusion rate. Drugs are cleared through a combination of metabolism and excretion. Clearance changes with age in a complex manner. Clearance (expressed as L/h/kg) is greater in a toddler than it is for an older child. The difference is related to a nonlinear relationship between many aspects of organ function and size. This nonlinear relationship is not related to organ maturity and is surprisingly constant across different aspects of organ function, age, and species. It is known as allometry and can be expressed as
Using body surface instead of body mass results in an allometric exponent of approximately 2/3 and is a reasonable predictor of clearance. Other pediatric pharmacologists argue that an allometric exponent of 3/4 better reflects actual function.
Allometry alone, however, does not explain changes to clearance in the infant and neonate.
For these infants, organ maturity has a substantial impact. A sigmoid hyperbolic or Hill model, in addition to allometry, is needed to predict clearance in this age group. Using postmenstrual age provides a better fit than chronologic age, consistent with organ maturity being on a continuum from fetal to postnatal life. The time to full maturation of renal and hepatic clearance varies between drugs. In general the slope is steepest in the neonatal period and full maturity is achieved by 2 years of age ( Fig. 77.7 ).
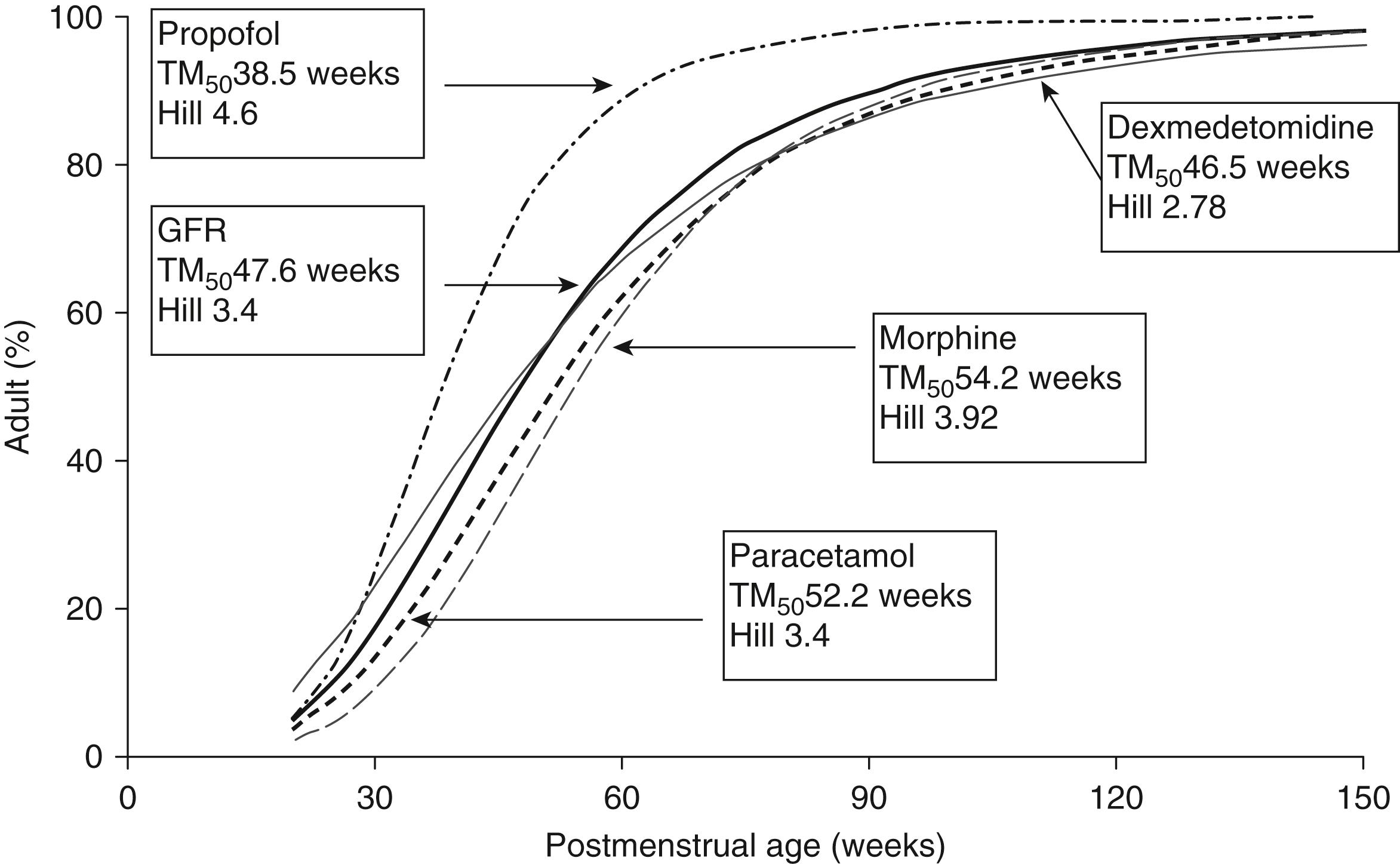
Many drugs such as succinylcholine, atracurium, and remifentanil undergo clearance independent of the liver or kidney. The nonspecific esterases that metabolize remifentanil are mature at birth. The clearances for these drugs do not require an adjustment for maturation and can be predicted largely through allometry alone.
For drugs that require hepatic or renal clearance, neonates and infants will have a lower clearance resulting in longer elimination half-times, and hence infrequent dosing and lower infusion rates at steady state. In older children, elimination half-times may appear to be shorter, but this difference tends to disappear with allometric (body mass) scaling.
In addition to the differences in drug pharmacokinetics in neonates, other factors will have influence on drug dosing and clearance. Some of the critical factors include sepsis, congestive heart failure, and increases in intraabdominal pressure affecting renal and hepatic function.
In older children pharmacodynamic properties of most anesthetic agents are probably similar to those in adults, albeit with some notable exceptions such as anticoagulants. In infants and neonates much less is known about the pharmacodynamics of anesthetic agents. The lack of data are partly due to the lack of robust and validated measures of various aspects of anesthetic effect in the infant and neonatal population. For example, fundamental anesthesia endpoints such as pain, memory, and even unconsciousness can be difficult to assess in infants. Surrogate measures of anesthesia effect, such as the EEG, are also unreliable in infants. With increasing understanding of the developmental neurobiology of pain and consciousness it is likely that we will identify other clinically significant pharmacodynamic differences in infants.
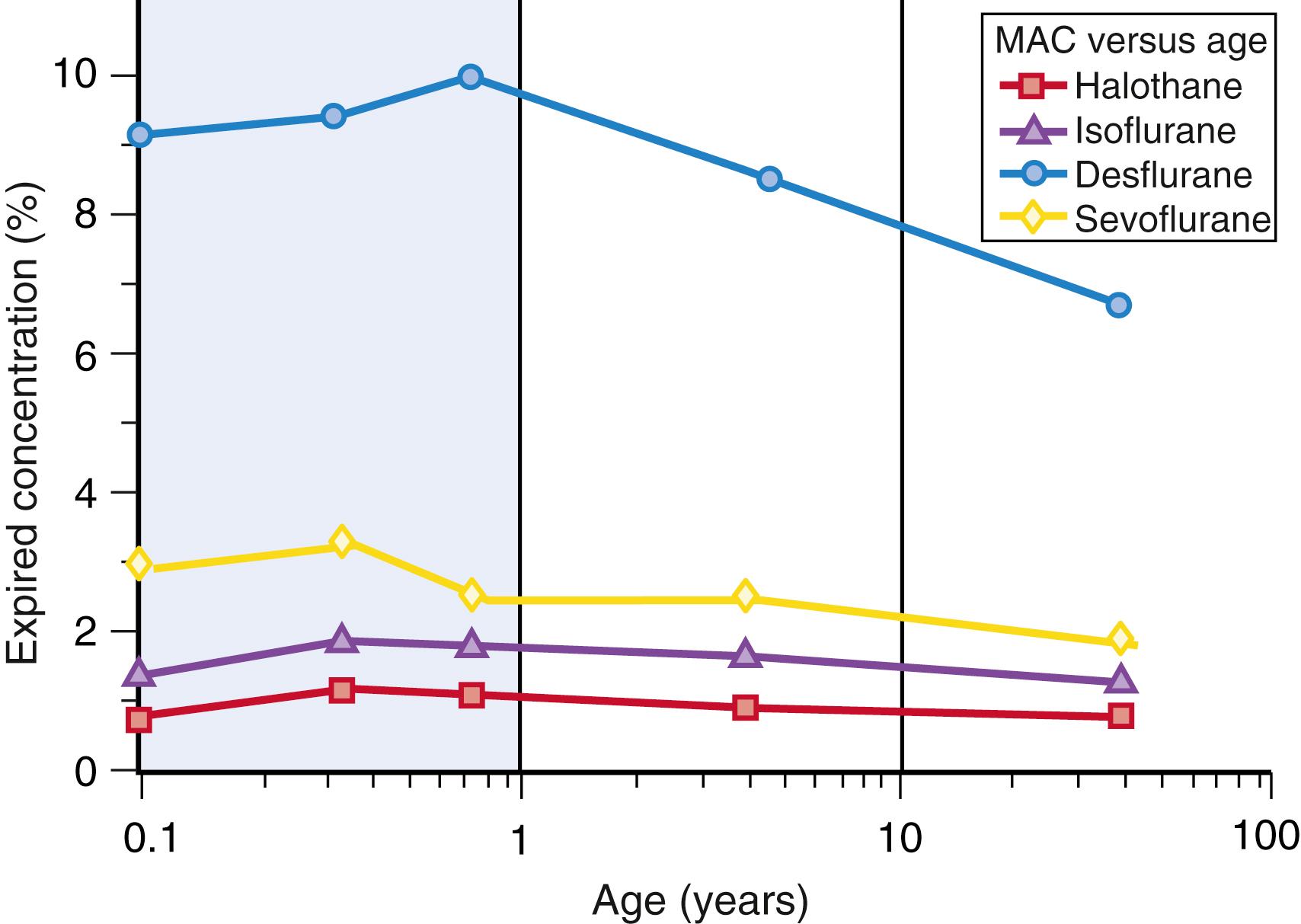
The expired minimum alveolar concentration (MAC) of an inhaled anesthetic required in children changes with age ( Fig. 77.8 ). Anesthetic requirement is smaller for preterm than for term neonates and smaller for term neonates than for 3-month olds. Infants have a higher MAC than that of older children or adults; the reasons for these age-related differences in MAC are not known. When considering the impact of age on MAC, it must be noted that the evidence is limited; the number of studies and the number of children in each study are small.
It is also important to note that MAC measures only one aspect of anesthesia effect and reflects primarily a spinal cord reflex. Compared to adults, older children have a similar relationship between MAC and other measures of anesthetic effect. The ratio of MAC to MAC awake , MAC intubation , MAC LMA insertion , MAC extubation , and MAC BAR are similar in children to adults. The relationship between MAC and the EEG, and hence most anesthesia depth monitors, is not as consistent in children and adults. Children have a higher BIS (bispectral index) for a specific fraction of MAC. The significance of this is unclear. In infants and neonates there are no data to determine how MAC relates to other aspects of anesthetic effect. It is clear that the relationship between MAC and the EEG is substantially different in infants compared to adults, but once again the clinical significance of this is unclear.
Halothane, sevoflurane, isoflurane, and desflurane all produce a dose-dependent reduction in systemic blood pressure. It is unclear if this is a direct effect on myocardial contractility and vascular smooth muscle or an indirect effect via autonomic or neurohumoral reflexes. The myocardial depressant effect is greater in neonates compared to older children. All of these agents also have a dose-dependent effect on ventilator drive and response to carbon dioxide.
The rate of rise of inhalational anesthetic concentration depends on rate of delivery determined by the inspired concentration, minute ventilation, and ratio of minute ventilation to residual functional capacity; it also depends on the rate of uptake that is determined by the cardiac output, tissue/blood solubility, and alveolar to venous partial pressure gradient.
Attainment of steady state, where the alveolar and inspired fractions equilibrate, is faster in children than adults. This difference is due to a greater minute ventilation relative to functional residual capacity as well as a lower tissue/blood solubility. This effect in children is greater for more soluble agents such as halothane and less for sevoflurane and desflurane.
The faster attainment of steady state in neonates can increase the risk of overdose during induction of anesthesia, particularly if a high inspired concentration is used for an excessively long period. The risk may be greater for agents when greater MAC multiples can be delivered by the vaporizer; for example, a halothane vaporizer can deliver up to 5.75 MAC multiples versus 2.42 MAC multiples for a sevoflurane vaporizer ( Table 77.4 ).
| Agent | Maximum Vaporizer Output (%) | MAC (%) | Maximum Possible MAC Multiples |
|---|---|---|---|
| Halothane | 5 | 0.87 | 5.75 |
| Isoflurane | 5 | 1.20 | 4.2 |
| Sevoflurane | 8 | 3.3 | 2.42 |
| Desflurane | 18 | 9.16 | 1.96 |
Halothane is now rarely, if ever, used in the United States and many other countries; however, it is still widely used in developing countries. Halothane is a relatively potent agent, but has a greater blood solubility and slower induction and emergence if similar MAC multiples of inspired concentrations are used. It does not have a noxious smell and hence, prior to the use of sevoflurane, was the agent of choice for inhalational inductions in children. Halothane, being a polyhalogenated alkane, has subtle differences in pharmacodynamic properties compared to the other ether inhalational anesthetics. Halothane has more “analgesic” properties than the ethers and has a higher BIS at equivalent MAC multiples. The MAC of halothane is low in neonates, highest in infants, and then steadily declines with age.
Halothane is a potent myocardial depressant that can have profound effects on neonates and children. Halothane also causes sensitization of the myocardium to arrhythmias. The first Pediatric Perioperative Cardiac Arrest (POCA) registry study reported that halothane was a major cause for perioperative cardiac arrest. It was thought to be particularly dangerous with the use of controlled ventilation without reducing the inspired concentration after induction. The decline in cardiac events in subsequent POCA audits has been attributed to the decline in use of halothane in the United States. At the same time, halothane may be used safely, but care should be exercised particularly if the anesthesiologist is not experienced with its use.
Sevoflurane is a polyhalogenated ether. It has a low blood solubility facilitating a relatively rapid inhalational induction. Sevoflurane is less pungent than isoflurane and desflurane and has become the agent of choice for inhaled induction of anesthesia in children. Unlike other inhalational agents, the MAC is similar with neonates and infants, but like other agents becomes lower with age after infancy: 3.3% for neonates, 3.2% for infants 1 to 6 months old, and 2.5% for children older than 6 months. Sevoflurane is associated with a greater incidence of emergence delirium compared to halothane (see later). Sevoflurane is also reported to cause epileptiform changes in the EEG when delivered at high concentrations in children. The clinical significance of these EEG changes is not clear.
Isoflurane, a polyhalogenated ether, has a blood solubility between halothane and sevoflurane. As with sevoflurane, it has a relatively lower MAC in neonates, peaks in infancy, and then declines with age. It is more potent than sevoflurane but has a relatively more noxious smell, which makes an inhalational induction with it unacceptable for most children.
Desflurane is another polyhalogenated ether, with a blood solubility lower than isoflurane or sevoflurane. Similar to sevoflurane and isoflurane, desflurane’s MAC peaks in infancy, is lower in neonates, and declines with age after infancy. The low solubility facilitates a more rapid emergence. It is however not suitable for inhalational induction in children because of its pungent odor and an unacceptable incidence of laryngospasm (∼50%). It is however suitable for maintenance of anesthesia in children although the package insert indicates that it is not recommended for maintenance in children without tracheal tubes.
Nitrous oxide is an odorless gas that has a low solubility in blood, but is relatively nonpotent. The MAC of nitrous oxide has not been accurately determined in children. When nitrous oxide is used with an inhaled anesthetic, it reduces the concentration required for the more potent inhalational agents. It may speed the uptake of more potent anesthetics, but the underlying theory behind this “second gas” effect has been challenged. This characteristic is probably of limited clinical relevance. Nitrous oxide is a weak analgesic; it can be used alone or in conjunction with other agents for procedural sedation and analgesia for children. Since it is odorless, it is also commonly used for an inhalational induction in cooperative children. For example, breathing high concentrations for a short period can provide considerable sedation prior to adding sevoflurane. In many institutions the routine use of nitrous oxide during maintenance of anesthesia has declined since it is associated with an increased risk of postoperative nausea and vomiting in adults; however studies have shown little evidence that nitrous oxide has any impact on postoperative nausea and vomiting in children.
Xenon is another odorless anesthetic gas that has a relatively low potency. While it is currently not routinely used, it has some potential advantages over other anesthetic agents. The MAC in children is unknown. In adults it has remarkably little cardiovascular effect. It has thus been proposed as a potentially superior anesthetic for children with significant congenital heart disease but only preliminary studies have evaluated this to date. Its high cost mandates the use of either very low fresh gas flows or complex scavenging and recycling systems. This may reduce the practicality of its use in many pediatric settings.
Children can become agitated on emergence or shortly after arrival in the postanesthesia care unit (PACU). The reported incidence of agitation varies enormously, reflecting the variety of definitions of agitation and delirium used in various studies. The potential list of causes or associated factors is long. Agitation may be due to many factors including pain, cold, full bladder, presence of restrictive casts, fear, anxiety due to parental separation, or simply having a “tantrum.” Agitation is best measured with the Cravero scale. The initial management of agitation is to try to identify or rule out likely causes. In some cases agitation may be due to delirium. Delirium is characterized by reduced awareness of the environment and altered cognition or perceptual disturbances. Typically the child is disoriented and does not respond to parents or staff. There is generally no eye contact and the child cannot be consoled. If the delirium is associated with thrashing around or violent movement, this is known as “emergence delirium.” Emergence delirium occurs most frequently in preschool children. It is distressing to staff and parents. It may also lead to self injury, and dislodging dressings, drains, and intravenous lines. In most cases, emergence delirium may persist for 10 to 20 minutes but is self-limiting. Delirium may also be hypoactive; in this situation, the child has a delirium, but is inactive and not agitated, and thus is at less risk of doing harm to themselves.
The cause for delirium is not precisely known. It may relate to the mode of awakening. It is intriguingly similar to night terrors in children. It is more common when maintenance anesthesia included either sevoflurane or desflurane. It is unusual after total intravenous anesthesia (TIVA) with propofol. Many agents have been found to reduce the incidence. The most effective is using TIVA or giving 2 to 3 mg/kg of propofol before emergence. Fentanyl and α 2 agonists have also been found to be effective. Propofol, clonidine, and midazolam have all been described as useful in management. When managing delirium other causes for agitation should be considered, particularly pain. Emergence delirium may occur after painless procedures; however there is also some evidence that pain may increase the risk of emergence delirium. Other physiologic changes, including hypoxia, metabolic derangement, and hyponatremia, may also cause agitation or delirium, so must be ruled out, particularly if the delirium is prolonged.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here