Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The term chronic critical limb ischemia (CLI) was first coined in 1982 and was intended to describe limb-threatening arterial insufficiency in the absence of diabetes mellitus (DM). Since the initial description of CLI, the incidence and prevalence of DM has markedly increased with a current worldwide prevalence of 422 million, which is a fourfold increase from 1980 figures. Since ischemia accompanies DM in approximately half the cases, the concept of CLI has undergone a frameshift to recognize the changing pattern of lower extremity limb-threatening ischemia, with an increased contribution of DM to the disease phenotype. An improved understanding of the changing patterns of diabetic vascular disease with respect to pathophysiology, arteriographic patterns, wounds, and infection has enhanced the recognition, management, and outcomes of diabetic vascular disease. This chapter reviews diabetic vascular disease with respect to patterns of disease, distributions of pedal wounds, and infection as well as the common presentation, management strategies, and costs.
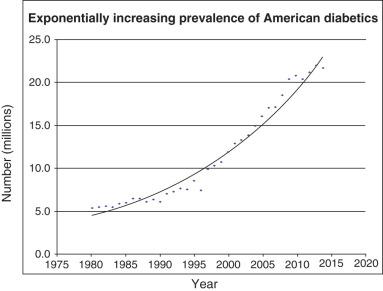
Diabetes now affects 9% to 10% of the US adult population, with type 2 DM responsible for over 90% of cases ( Fig. 12.1 ). There is also a rising childhood prevalence of type 2 DM, which now comprises approximately half of all childhood DM cases. The increase in type 2 DM parallels the childhood obesity epidemic and especially affects American ethnic minorities. Type 2 diabetes comprises 90% to 95% of all cases of DM. Worldwide figures mirror those in the United States, with a worldwide prevalence of 9%, and DM is more common in males than in females. Most countries have experienced a dramatic increase in the rates of type 2 DM, with no country having experienced a statistically significant decrease in the prevalence of DM since 1980. Five countries contain approximately half of the world's diabetics: China, India, the United States, Brazil, and Indonesia. Peripheral artery disease (PAD) has also increased in worldwide prevalence, with an estimated 202 million people living with PAD globally as of 2010. With respect to the CLI subpopulation, recent estimates of CLI have also increased, to a current prevalence of 0.2% to 0.4% cited within the US Medicare database. The increases in both PAD and CLI, while modest relative to the increased prevalence of DM, remain important due to the synergistic effect of ischemia and DM on major amputation rates.
The prevalence of PAD among adult Americans with diabetes is twofold higher compared with a cohort of similarly aged Americans without diabetes. The 4-year estimate of amputation-free survival (AFS) is approximately 45% among those presenting with diabetic foot ulceration and PAD. This rate is significantly lower than that found in comparable populations of patients with only PAD. Patients with PAD and DM have a 1.5- to 5-fold higher risk of major amputation than comparable patients with either PAD or DM alone. These risks amplify the baseline risk, which is already elevated in patients with DM alone and is 10 to 30 times greater than that in similarly aged nondiabetics. These risks are apparent in the United States, with DM having accounted for approximately 60% of nontraumatic lower-limb amputations in patients aged 20 years or older. DM was responsible for approximately 73,000 lower limb amputations in the United States in 2010. Although the age-adjusted rate of major amputation in diabetics is decreasing, the explosion in the number of diabetics is such that the overall rate of amputations as a percentage of the population is unchanged. Worldwide there are approximately 1 million lower extremity diabetic amputations each year. Extrapolating from this statistic, a diabetic lower extremity amputation occurs approximately every 20 seconds worldwide. Survival at 4 years is also worse for patients with diabetic foot ulcerations only compared with those with diabetic foot ulcerations and PAD. Although caregivers often focus on limb salvage, these data suggest that practitioners must also focus on efforts to improve mortality in this particularly high-risk cohort.
Glucose reacts nonenzymatically with the amino groups of proteins to form Schiff bases, followed by Amadori rearrangement. Nonenzymatic glycosylation is more likely when patients are hyperglycemic, resulting in early glycation products. These become irreversible reactions with dehydration, condensation, and cross-linking, resulting ultimately in advanced glycation end products (AGEs). AGEs dissipate only with protein turnover; hence they accumulate in proteins with long half-lives, such as collagen and elastin. AGEs then bind to the receptor for advanced glycation end products (RAGEs), which mediates a variety of processes to accelerate atherosclerosis. RAGE activation results in increased extracellular matrix production and type IV collagen deposition, increased monocyte migration, and increased expression of cell adhesion molecules, namely vascular cell adhesion molecule-1 (VCAM-1). RAGE's also bind to S-100 proteins, which are highly expressed in atherosclerotic plaque in the context of DM. They likewise increase expression of nuclear transcription factor κB (NFκB), resulting in an increase in a variety of inflammatory cytokines such as tumor necrosis factor α (TNFα), interleukin-1β (IL-1β), and transforming growth factor-β (TGFβ). The confluence of these changes is an increase in the development of atherosclerotic plaques in diabetics.
One of the prototypical final manifestations of diabetic vascular disease is calcification of the media in medium-sized arteries. AGEs have been associated with the development of medial calcification by several in vitro studies. AGEs bind RAGEs in vascular smooth muscle cells (VSMCs), inducing a change into an osteogenic phenotype. Ultimately it appears that the altered osteogenic VSMCs are responsible for hydroxyapatite deposition within the media of a diabetic's arteries. Although the precise pathway of this phenotypic change is unclear, the VSMC exposure to AGEs mediated by RAGEs appears critical to the development of medial calcification. Medial calcification is more frequently associated with DM, with intimal calcification found mostly in nondiabetic-associated atherosclerosis.
DM alters the angiographic pattern of atherosclerotic obstructive disease, compared with that which occurs in nondiabetics. There is an increased predilection for disease in the infrapopliteal vessels, especially among elderly males. This tendency to the infrapopliteal location may significantly increase major amputation rates among diabetics, especially those whose occlusions are primarily located in the popliteal or infrapopliteal vessels. Graziani et al. retrospectively reviewed 417 patients with diabetes and CLI to characterize the angiographic patterns of disease among diabetics; 2893 lesions were discovered, and 74% were located in the infrapopliteal vessels. Moreover, most of the occlusions were more extensive (>10 cm) and were more prevalent in the infrapopliteal vessels than in the vessels above the knee. The anterior tibial and posterior tibial arteries were most frequently affected, with relative sparing of the peroneal artery. This pattern of disease had been previously observed, but the reasons for this pattern remain unexplained.
The most frequent pattern of disease includes an occlusion of the femoropopliteal artery and concomitant occlusion of one or more of the tibial vessels. The next most frequent pattern involves occlusion of one of the tibial vessels with diffuse disease in the remaining vessels, and occlusion of all crural vessels was found in 28% of patients. The pedal vasculature is frequently spared, however, with 88% of patients having at least one patent pedal vessel. The significance of the arteriographic pattern of atherosclerotic disease in patients with DM is that revascularization of the tibial and pedal vasculature is becoming increasingly relevant.
The microvasculature has been traditionally thought to be occluded in diabetic vascular disease and was viewed as a major contributor to major limb amputation in patients with DM, although this concept is incorrect. Prior researchers extrapolated data from patients with diabetic retinopathy having higher major amputation rates. It was widely assumed that the microvasculature of the foot would behave similarly to that of other microvascular beds in patients with DM. However, the association between diabetic retinopathy and nephropathy may simply be a reflection of poorer long-term glycemic control, with the outcomes reflective of the complications of prolonged hyperglycemia rather than obliterative occlusions of the foot microvasculature. Indeed, subjects with DM do not have extensive occlusions of the microvasculature, as has been traditionally taught. Moreover, the endothelial layer does not appear to have increased proliferative lesions that could obstruct flow at the level of the arterioles.
Arteriolar patency does not signify that the microvasculature is entirely normal in diabetic vascular disease. The microvasculature appears to have increased permeability. This increased permeability may result in a greater deposition of plasma proteins and fluid in the extracellular space, and this process can theoretically decrease oxygen and nutrient diffusion to the tissues, thereby decreasing clearance of infectious or necrotic debris in the soft tissue of the foot. Endothelial function also appears to be abnormal, with reduced availability of nitric oxide, due to increased destruction via free radical overproduction. These changes may decrease the ability of diabetics to augment capillary blood flow to areas of relative ischemia; further study is required to quantify the microvascular dysfunction in diabetic feet and understand the significance of microvascular dysfunction in DM.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here