Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The respiratory system comprises an organ for gas exchange (the lung) and structures that move air into the lung (the respiratory pump). The lung includes the airways, alveoli, and blood vessels. The respiratory pump contains the respiratory muscles, the central nervous system and peripheral chemoreceptors that modulate pump output, and the structural tissues of the chest wall, including the ribs, cartilage, spine, and abdominal wall. Successful gas exchange requires the output of the respiratory pump to equal or exceed the load placed upon it by the lung and chest wall. Thus, respiratory failure occurs when the pump, the lung itself, or both become dysfunctional. When respiratory pump output is insufficient for the imposed load, the primary gas exchange abnormality will be hypercapnia. However, if the partial pressure of arterial carbon dioxide (Pa co 2 ) becomes high enough, hypoxemia will also occur. Alternatively, when the major problem involves the lung parenchyma, the resulting blood gas abnormality typically is hypoxemia.
For purposes of understanding the forces involved in breathing, the respiratory system can be modeled as an elastic element (lung parenchyma) and a resistive element (airways) in series with each other. This model can be likened to a balloon attached to a straw ( Fig. 160.1 ). A tissue’s compliance (ΔV/ΔP) describes how easily it can be stretched for a given pressure change. The reciprocal of compliance, or elastance (E), describes how much a tissue withstands being stretched. Greater pressure must be applied to a balloon with high elastance compared with one with lower elastance to expand it, and the applied pressure will also be proportional to the desired volume change above the resting volume. Pressure must also be applied to the system to move air through the straw. The amount of pressure necessary to generate flow will be directly related both to how quickly air flows through the straw and to the resistance of the straw itself.
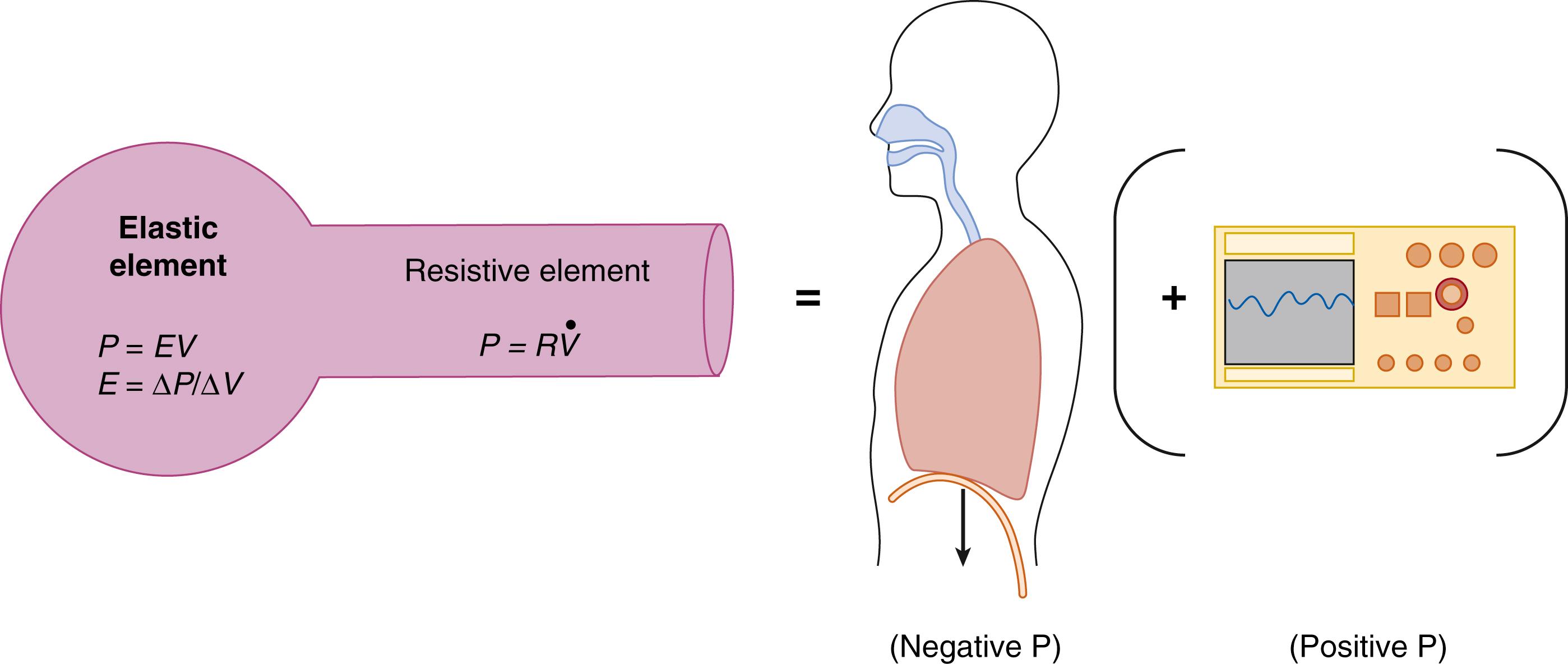
In the lung, therefore, the pressures required to move air into the alveoli include the pressure necessary to stretch the lung and chest wall above their resting volumes, described by the respiratory system’s elastance, and the pressure necessary to overcome the resistance forces associated with flow through the airways. A third pressure that must be overcome relates to the cost of accelerating the gas through the airways (inertance), but at normal respiratory rates this value is quite small and can be ignored. However, under conditions of high-frequency ventilation, the inertance pressure will predominate. When breathing at respiratory rates of less than 100 breaths/min, the forces involved with breathing can be described by a simplified equation of motion of the respiratory system:
where Pmus is the total pressure that must be developed by the respiratory muscles, E is the respiratory system elastance (or 1/compliance), V is the volume to be moved, R is the airway resistance, and
is flow.
When the equation of motion is applied to patients supported by mechanical ventilation, special considerations must be recognized. The pressure generated to create adequate movement of gas for ventilation now represents a combination of pressures from the muscles (Pmus) that lower intrapleural pressure and positive pressure from the ventilator (Pvent) applied directly to the airway:
Although both pressures work on the respiratory system to expand the lungs, they do so in opposite directions. Thus to measure pressure exerted during mechanical ventilation based on the readings from a ventilator accurately, Pmus must equal zero; that is, the patient should be relaxed or paralyzed and not contribute to the breathing effort. In addition, the changes in pressure, flow, and volume considered are referenced to end-expiratory conditions (e.g., not to atmospheric pressure but to positive end-expiratory pressure [PEEP]). When the only pressure applied to the respiratory system comes from the ventilator, then inferences about respiratory system mechanics can be made using data from the ventilator’s pressure and flow sensors. It is also important to recognize that resistance of the endotracheal tube contributes to the resistance of the airways. If the endotracheal tube is small relative to the infant’s natural airway, it can become the predominant resistance in the system.
The two points used to calculate the pressure cost of breathing reflect only those structures between them. For instance, when an esophageal balloon is inserted to estimate pleural pressure and the pressure difference is measured between airway opening pressure and pleural pressure, the derived pressure is the transpulmonary pressure . This includes pressure applied to the airways and lung parenchyma, and any mechanics measurements derived from these pressures will reflect the properties of the lung (i.e., lung compliance [ C L ] or resistance [ R L ]). If, however, the pressures are measured at the airway opening and at the body surface (as is usually done for patients supported by positive pressure ventilation), the intervening structures will include the chest wall in addition to the airways (including the artificial airway) and lung parenchyma, so that the resulting applied pressures will relate to respiratory system mechanics (i.e., respiratory system compliance [ C RS ] or resistance [ R RS ]). Because the chest wall is normally much more compliant than the lung in neonates, its addition to these calculations does not greatly alter the necessary applied pressure. However, that assumption is obviated if the chest wall becomes abnormally stiff, as it does in infants with anasarca or marked abdominal distension. If the pressure transducer of the ventilator, which is used to measure airway opening pressure, is housed within the body of the ventilator instead of at the patient’s Y-piece, then the compliance and resistance of the ventilator circuit will also become a part of the measurements.
The same balloon-and-straw model used for the entire respiratory system can be used to describe a single alveolus and the airway that supplies it. The compliance of the balloon and resistance of the associated airway influence how quickly the unit fills and empties with pressure changes. The time required for such a unit to fill or empty following a step-change in pressure is described by its time constant ( Fig. 160.2 ). During exhalation, for example, one time constant represents the time it takes for a unit to empty by 63%; a compartment will empty 95% in three time constants and 99% in five time constants. The time constant of a lung unit can be represented by the equation:
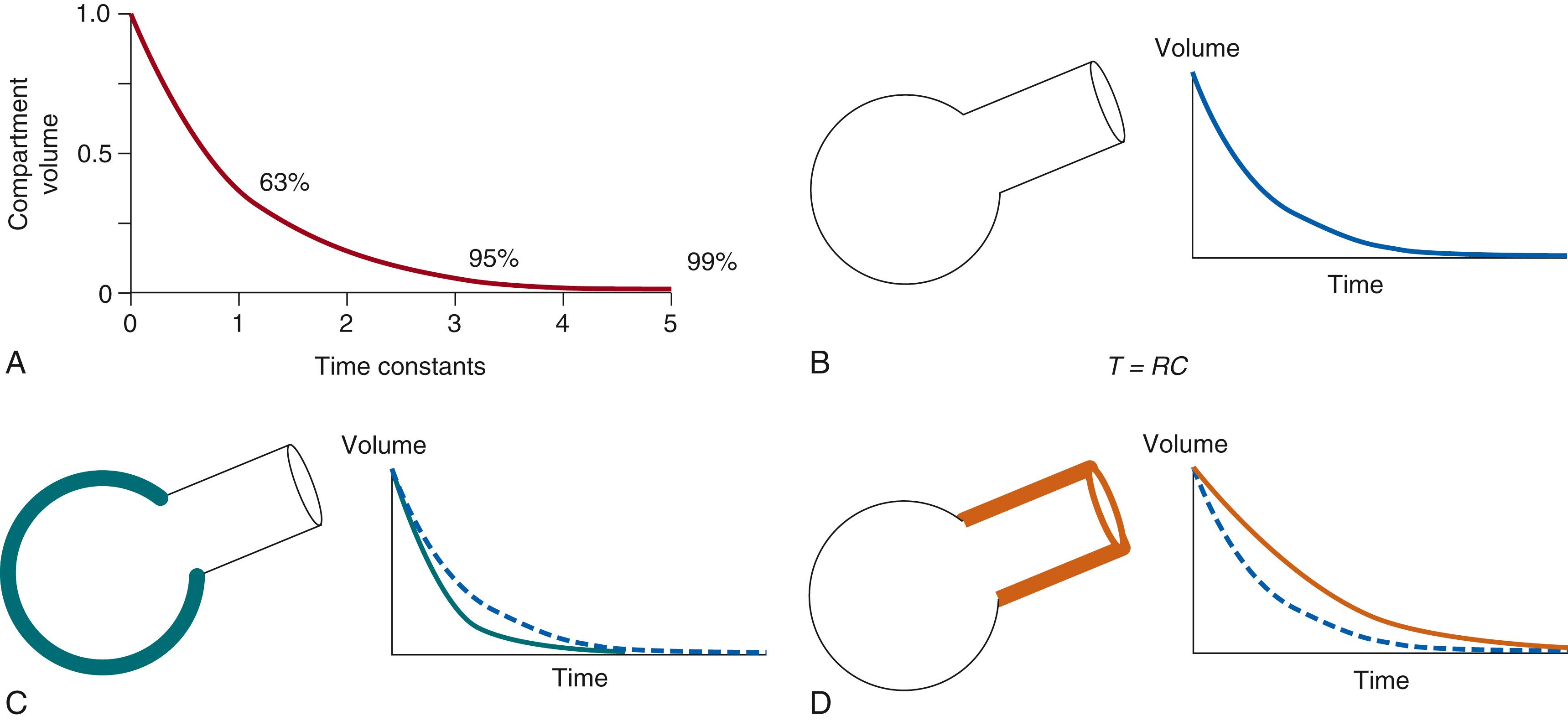
where τ is the time constant, R is the resistance of the airway, and C is the compliance of the alveolus. When the characteristics of the lung are regionally uniform, the entire lung can be described by a single τ. When used to describe a phenomenon associated with mechanical ventilation, τ refers to the time course response of the passive respiratory system for either emptying or filling after a step change in pressure. Because τ = RC, the effects of altering resistance or compliance can be anticipated (see Fig. 160.2 ). If the balloon is made stiffer (compliance decreased, as in fibrosis), the elastic recoil will be greater, and τ will be shorter. However, if the straw is narrowed, the resistance will be greater and τ will be longer.
In conditions in which there are compartments with widely differing time constants, lung compliance will decrease as the respiratory rate increases. This frequency dependence of compliance occurs because those units with longer time constants fill and empty at slower rates, and as the frequency increases, they become progressively more distended. In turn, they will compress areas with shorter time constants, making it harder for them to fill. The sum result is a decrease in overall compliance.
Respiratory failure is a common problem for neonates admitted to the neonatal intensive care unit (NICU). Preterm infants often demonstrate abnormalities of parenchymal function because of surfactant deficiency or structural problems such as lung hypoplasia. Term infants can also experience lung parenchymal respiratory failure in the setting of pneumonia, aspiration syndromes, or pulmonary hemorrhage. The neonatal respiratory pump has a limited ability to compensate for an increased load because of its structural and compositional immaturity. When respiratory failure ensues, mechanical ventilation can be life-saving, but often the very modalities used to support adequate gas exchange impose additional damage to airways and lung parenchyma. A brief review of the maturational aspects of the respiratory system is helpful in understanding the neonate’s predisposition to respiratory failure and the expected mechanical alterations of the injured lung. This in turn provides a basis for determining the most appropriate type of mechanical ventilatory support to be used.
There are important maturational differences in the structure and function of the neonatal respiratory pump compared with that of the older child or adult. The passive properties of the chest wall, expressed as its compliance, are determined by the stiffness of the rib cage and the tissue-elastic properties of the intercostal and ventral abdominal muscles. An inverse relationship exists between age and chest wall compliance, with a gradual reduction of compliance of the chest wall between birth and 3.5 years of age. Furthermore, a similar relationship exists between gestational age and chest wall compliance, so that the younger the gestational age, the greater the chest wall compliance. Chest wall compliance has been calculated to be three times greater than lung compliance at term, and it remains greater than lung compliance in healthy children beyond the first year of age. This relationship results in a reduction in lung volume at the passive end-expiration lung volume and favors alveolar collapse. When positive pressure is applied to the lung during assisted ventilation, the more compliant chest wall is also less able to protect alveoli from overdistension and injury than a stiff chest wall can. ,
Parenchymal diseases that result in an additional reduction in lung compliance or increase in airway resistance exacerbate the disparity between chest wall and lung compliance. The clinical manifestation of this imbalance is chest wall distortion with sternal retractions and paradoxical movement of the thoracic cage and abdomen. The movement of the thoracic and abdominal compartments relative to each other can be described in terms of their phase angle. When an infant’s chest wall and abdomen move in the same direction simultaneously, breathing is synchronous; a phase angle of 0 degrees reflects this synchrony, whereas a phase angle of 180 degrees reflects complete paradoxical motion or “see-saw” breathing. Term infants demonstrate fairly synchronous breathing, whereas preterm infants demonstrate moderate thoracoabdominal asynchrony. To illustrate, a recent study of tidal breathing patterns in preterm and term infants showed that the 18 term infants had a phase angle of 14.0 ± 6.8 degrees compared with a phase angle of 79.1 ± 45.6 degrees among 537 preterm infants born at 26.9 ± 1.4 weeks gestation and studied at 37.5 ± 2 weeks postmenstrual age (PMA). Among the group of preterm infants, there was no difference in phase angles between those who went on to develop bronchopulmonary dysplasia (BPD) and those who did not. In contrast, thoracoabdominal asynchrony was worse in a group of 10 infants with established BPD studied at 49 ± 3.2 weeks PMA, in whom the phase angle was 102 ± 16 degrees, with some infants demonstrating frank paradox. Furthermore, the degree of thoracoabdominal asynchrony correlated directly with pulmonary resistance and inversely with pulmonary compliance. The inward movement of the chest wall during inspiration in such infants results in loss of thoracic volume during each breath, thereby making breathing less efficient. This imposes additional work on the diaphragm: diaphragm displacement is doubled in preterm neonates recovering from respiratory distress syndrome (RDS) in order for them to maintain an adequate tidal volume in the face of a reduction in thoracic volume. Similarly, electrical activity of the diaphragm, which correlates with work of breathing, increases in preterm infants being weaned from nasal continuous positive airway pressure (CPAP) to low flow nasal cannula therapy, and the amplitude of diaphragmatic electromyogram activity is higher in those infants failing such a wean in therapy compared with those who succeed. If chest wall distortion is excessive, hypoventilation and eventual respiratory failure can ensue. Preterm infants subjected to inspiratory resistive loading demonstrate greater thoracoabdominal asynchrony than term infants, and they are unable to maintain tidal volume and minute ventilation under the imposed load.
Anatomic differences related to maturation also place the respiratory pump at a mechanical disadvantage. The shape of the mature thoracic cavity in axial section is elliptical, with a smaller anterior–posterior diameter than lateral diameter. Additionally, the ribs are caudally declined. In the infant, the thorax is more circular, and the ribs are positioned more horizontally. The main function of the intercostal muscles in older children and adults is to elevate the rib cage and increase thoracic volume, whereas in the neonate, intercostal muscle contraction conserves tidal volume by stiffening the chest wall and diminishing chest wall distortion. The anatomic arrangements in the infant limit the potential for thoracic expansion by rib elevation with intercostal contractions, making the infant more reliant on diaphragm function to generate an adequate tidal volume.
There are also critical differences between infants and adults in the relationship of the diaphragm with the chest wall. In the adult, during quiet breathing the diaphragm fibers run parallel with the inner thoracic wall over approximately one-quarter to one-third of the rib cage. Contraction of the diaphragm results not only in a reduction of intrapleural pressure, but also in an increase in intraabdominal pressure through this area of apposition. The increase in abdominal pressure is applied to incompressible abdominal viscera residing within the lower rib cage, and these in turn act as a fulcrum to elevate the lower rib cage within the zone of apposition. In contrast, the infant appears to have less of an area of apposition, with insertion of the diaphragm onto the chest wall at a more acute angle. This arrangement makes the neonatal diaphragm less efficient in expanding the lower rib cage. Additionally, if air trapping occurs because of lung disease, the diaphragm is caudally displaced, and the area of apposition is further reduced: contraction of the diaphragm will reduce rather than expand the lower rib cage (Hoover sign) and compromise tidal volume.
The types of muscle fibers composing the diaphragm and intercostal muscles change throughout gestation and into infancy. , The proportion of high oxidative or fatigue-resistant muscle fibers is low in preterm infant ventilatory muscles but increases throughout late gestation and through 8 months of age. This suggests that the preterm infant is more prone to develop respiratory muscle fatigue when a load is imposed. However, other studies in nonhuman primates demonstrate that although the fibers of ventilatory muscles undergo maturational changes in a similar time course to those of humans, other fiber types are present that confer high oxidative capacity to the preterm diaphragm and intercostal muscles, making them relatively fatigue resistant. , These differences may be the result of species differences, alterations in tissue sampling among biopsy or necropsy specimens, or differences in the histochemical techniques used to identify the oxidative capacity of the muscle fibers. However, premature birth alone can interfere with the normal postnatal growth of diaphragm muscle fibers, as evidenced by a decrease in the cross-sectional area of diaphragm muscle fibers 10 days after birth in a preterm baboon model. That lack of continued growth of diaphragm fibers makes the ventilatory muscles less able to meet the demand of spontaneous breathing. In human neonates, as little as 12 days of continuous mechanical ventilation produced marked diaphragmatic atrophy. Older children undergoing mechanical ventilation for acute respiratory failure also demonstrated diaphragmatic atrophy with a 3.4% daily reduction in diaphragm thickness, and the degree of diaphragmatic atrophy was accentuated in those subjects treated with neuromuscular blockade. Thus mechanical ventilation schemes that inhibit spontaneous breathing efforts likely enhance the disruption of normal diaphragm development and promote atrophy.
Peripheral chemoreceptor responses to hypoxia, central nervous system chemoreceptor responses to carbon dioxide, and brainstem respiratory rhythmogenesis are all immature in preterm infants. Furthermore, they demonstrate developmental plasticity so that perinatal exposure to hyperoxia or hypoxia can lead to lasting changes in response well beyond the newborn period. Carotid body sensitivity to hyperoxia and hypoxia increases over the first 10 weeks of life in healthy term infants. However, preterm infants of 27 weeks gestational age (range 25 to 32 weeks) who required at least 1 week of mechanical ventilation and supplemental oxygen beyond 28 days of age demonstrated a blunted ventilatory response to hypoxia or hyperoxia at 93 ± 14 days compared with preterm infants of 30 weeks gestational age (range 28 to 36 weeks) who required no supplemental oxygen or mechanical ventilation studied at 38 ± 6 days. Animal models suggest that moderate exposure of the preterm infant to hyperoxia for days to weeks during the period of maturation of the respiratory control system can lead to long-lasting hypoventilation and diminished responses to acute hypoxia. There is also a suggestion that intermittent periods of hypoxia predispose the preterm neonate to episodes of apnea by sensitizing the carotid body chemoreceptor to hypoxia, leading to hyperventilation followed by periods of apnea and respiratory instability on subsequent exposure to hypoxic stimuli. , Among 37 of 49 preterm infants who failed a physiologic challenge involving a reduction in supplemental oxygen or augmented airflow at 36 weeks PMA, 16 (43.2%) demonstrated an increase in periodic breathing leading to sustained or severe drops in SpO 2 , reflecting unstable ventilatory control. Maturational effects on the ventilatory response to hypercapnia are not well understood. However, chronic carbon dioxide retention eventually blunts central drive to hypercapnia; this can, in turn, make the preterm infant with chronic hypercapnia more reliant on already-impaired peripheral chemoreceptors for ventilatory control. This subsequently could lead to episodes of apnea when the infant is exposed to hyperoxia.
The gas exchange region of the lung undergoes significant changes during the latter part of gestation. Respiratory mechanics of an infant born prematurely precariously balance the system between respiratory success and failure as the lungs continue to undergo changes that would otherwise occur in late gestation. Airway resistance is higher, while total lung volume is much lower in the preterm than in the term infant. Over the last trimester, saccules subdivide, causing lung volume to almost triple between 30 and 40 weeks gestation ; the growth of the airways proceeds more slowly, in a linear fashion. In a rabbit model, premature birth without any postnatal exposure to positive pressure ventilation or supplemental oxygen results in smaller pups with smaller lungs and lower alveolar surface area, as well as alterations in lung function including decreased dynamic compliance and increased tissue resistance. It is likely that the normal progression of air space development is also altered in infants who survive extremely premature birth even without the development of BPD because the factors that result in premature birth also tend to cause the lung to mature more rapidly. , The result is a preterm lung with fewer, larger alveoli, thinned air space walls, and increases in the quantity of pulmonary surfactant.
Elastin deposition provides a scaffolding for saccular development so that as the number of saccules increases, the amount of elastin in the lung also increases. The volume of elastin in the lung doubles between 22 and 30 weeks gestation and doubles again over the next 20 weeks. The lower amount of elastin in the saccules and alveolar ducts of the preterm lung predisposes them to overdistension with the application of some positive pressure ventilation strategies. The reduced elastin content also causes the preterm lung to have a lower elastic recoil pressure than the lung of a term infant or child. Elastic recoil pressure (Pel) is the transmural pressure across the alveolus, or alveolar pressure (Palv) minus pleural pressure (Ppl) as in the equation:
Lung elastic recoil, along with the outward recoil of the chest wall, provides a tethering effect on small airways that causes them to dilate at a higher lung volume. The elastin fibers within the saccule walls create a meshwork that surrounds intraparenchymal airways and creates the structure through which elastic recoil exerts its outward pull on small airways. Because alveolar multiplication is largely a postnatal event, there are fewer alveolar wall attachments on small airways in the preterm lung. Furthermore, perinatal insults can contribute to these developmental alterations; hyperoxia-exposed neonatal mice show a significant reduction in bronchiolar-alveolar attachments in adulthood compared with mice that breathed room air over the first week of life. In addition, the highly compliant neonatal chest wall adds little to the outward traction on airways. Along with the lower elastic recoil of the preterm lung, these issues cause the tethering effect to be less pronounced on preterm small airways, making them less stable and more prone towards closure at lower lung volumes. The tendency for airways to close, combined with the absence of structures like pores of Kohn or canals of Lambert to provide collateral ventilation, increases the risk for atelectasis in the infant lung.
The lung has a tendency to recoil inward, resulting from both its elastic properties and surface tension forces. The presence of surfactant in the newborn minimizes surface tension forces, but the inward tissue forces of the lung remain greater than the outward recoil forces of the highly compliant neonatal chest wall. If left to the passive balance of forces, this combination would result in an equilibrium end-expiratory lung volume that is proportionally lower in infants compared with that of older children and adults. Furthermore, if the neonate were to rely on the balance of these forces to establish the end-expiratory lung volume or functional residual capacity (FRC), airway closure would occur during normal exhalation, and gas exchange would be compromised. Instead, the infant uses several strategies to maintain FRC above the equilibrium resting lung volume. These include adduction of the laryngeal muscles (laryngeal braking), postinspiratory contraction of the inspiratory muscles to slow expiratory flow, and employment of an expiratory time that is shorter than the time it would take the respiratory system to empty. , Endotracheal intubation or tracheostomy placement negates the ability of laryngeal muscles to retard expiratory flow and promotes a decrease in resting lung volume and the development of atelectasis if PEEP is not applied to the airway opening.
All preacinar airways are formed by 16 weeks gestation, but the airways continue to undergo structural maturation throughout gestation. Although differentiation of airway structures into muscle and cartilage occurs at an early gestational age, the tissues that comprise the airways continue to undergo changes that result in a marked reduction in airway compliance throughout the latter part of gestation. Cartilage is first identified in the trachea and main bronchi by 10 weeks gestation, and new cartilage continues to appear in more peripheral segmental airways until approximately 25 weeks gestation. The cartilage first appears as precartilage, after which it undergoes transformation to cartilage by deposition of ground substance. , Thus some of the cartilage present in the more peripheral airways at 25 weeks gestation is still in precartilage form. The consequence of this maturational process is that there is a five-fold reduction in tracheal compliance throughout the last trimester, and the airways continue to stiffen postnatally. The mechanical properties of the individual components of the airway wall (i.e., cartilage and smooth muscle) parallel the changes in tracheal compliance, becoming stiffer throughout gestation. ,
As a result of its increased compliance, the preterm trachea is easier to deform when exposed to positive pressure than is a term or adult trachea. Even brief periods of exposure to positive pressure results in a marked increase in resting volume of the extremely immature airway. Furthermore, the degree of deformation is related to the degree and duration of pressure applied. Acquired tracheomegaly has been described in infants 27 ± 0.6 weeks gestation exposed to positive pressure ventilation of 15 to 25 cm H 2 O for 25.4 ± 4.9 days. The preterm airway is also more collapsible than more mature airways, and resistance through preterm tracheal segments exposed to modest positive pressure ventilation for as little as 2 hours resulted in a significantly higher resistance through the ventilated segments than through unventilated segments. The highly compliant nature of preterm central airways translates clinically into tracheomalacia or bronchomalacia, especially in premature neonates who go on to develop BPD. Although the prevalence of tracheobronchomalacia (TBM) among infants with BPD is not well described, it was found in 36.2% of 974 neonates with BPD who were entered into a multicenter database over a 5-year period and who underwent bronchoscopy.
Therefore the physiologic properties of the extremely preterm airway that has been exposed to mechanical ventilation can affect gas exchange significantly. The increase in size of the airway after it has been deformed increases dead space, and the increased tendency for the airway to collapse on exhalation can lead to prolonged expiratory times and air trapping. Such expiratory events occasionally lead to acute episodes of hypoxemia, cyanosis, and respiratory distress (“BPD spells”) and require application or increased amounts of expiratory distending pressure and occasionally sedation or pharmacologic paralysis. The presence of significant TBM also can alter the amount of PEEP used to prevent airway collapse. , In a retrospective analysis, those BPD infants diagnosed with TBM were found to have a longer and more complicated neonatal course, were more likely to undergo tracheostomy placement, and required a longer course of mechanical ventilation than those with noncollapsible airways. Among 17 infants with BPD who underwent ultrashort echo-time magnetic resonance imaging (MRI) sequence to evaluate their central airways, those with severe BPD ( n = 11) had a significantly greater range of airway narrowing compared with those with mild/moderate BPD or controls.
However, the finding of significant central airway collapse in neonates with severe lung disease may not always be a primary abnormality of the central airways. The presence of large airway collapse represents a balance between the intrinsic stiffness of the airway wall and the magnitude of the transmural pressure exerted across the airway wall ( Fig. 160.3 ). Healthy infants, for instance, can narrow the airway by as much as 50% with crying or straining, reflecting an increase in transmural pressure. In contrast, some infants with lung disease will have abnormally compliant airway segments that collapse when transmural pressure is normal or low. However, others can have large airway collapse as a secondary manifestation of small airway obstruction. Elevated peripheral airway resistance not only will accentuate the intraluminal pressure drop from alveolus to mouth, but also will often cause the neonate to use abdominal accessory muscles to exhale forcefully to overcome the obstruction. The resulting lower intraluminal pressure within larger airways and higher pleural pressure occurring from accessory muscle use greatly increases collapsing transmural pressure. Because the neonatal airway is already a highly compliant structure, the resulting applied pressure can cause localized or diffuse central airway collapse. In adults, this phenomenon is referred to as “excessive dynamic airway collapse” (EDAC) and is distinguished from TBM because the cartilaginous rings retain their shape while there is significant invagination of the pars membranacea (posterior membrane); in contrast, TBM refers to collapse of the cartilaginous portion of the airway. However, given the highly compliant nature of neonatal airways, such a distinction can be difficult to make.
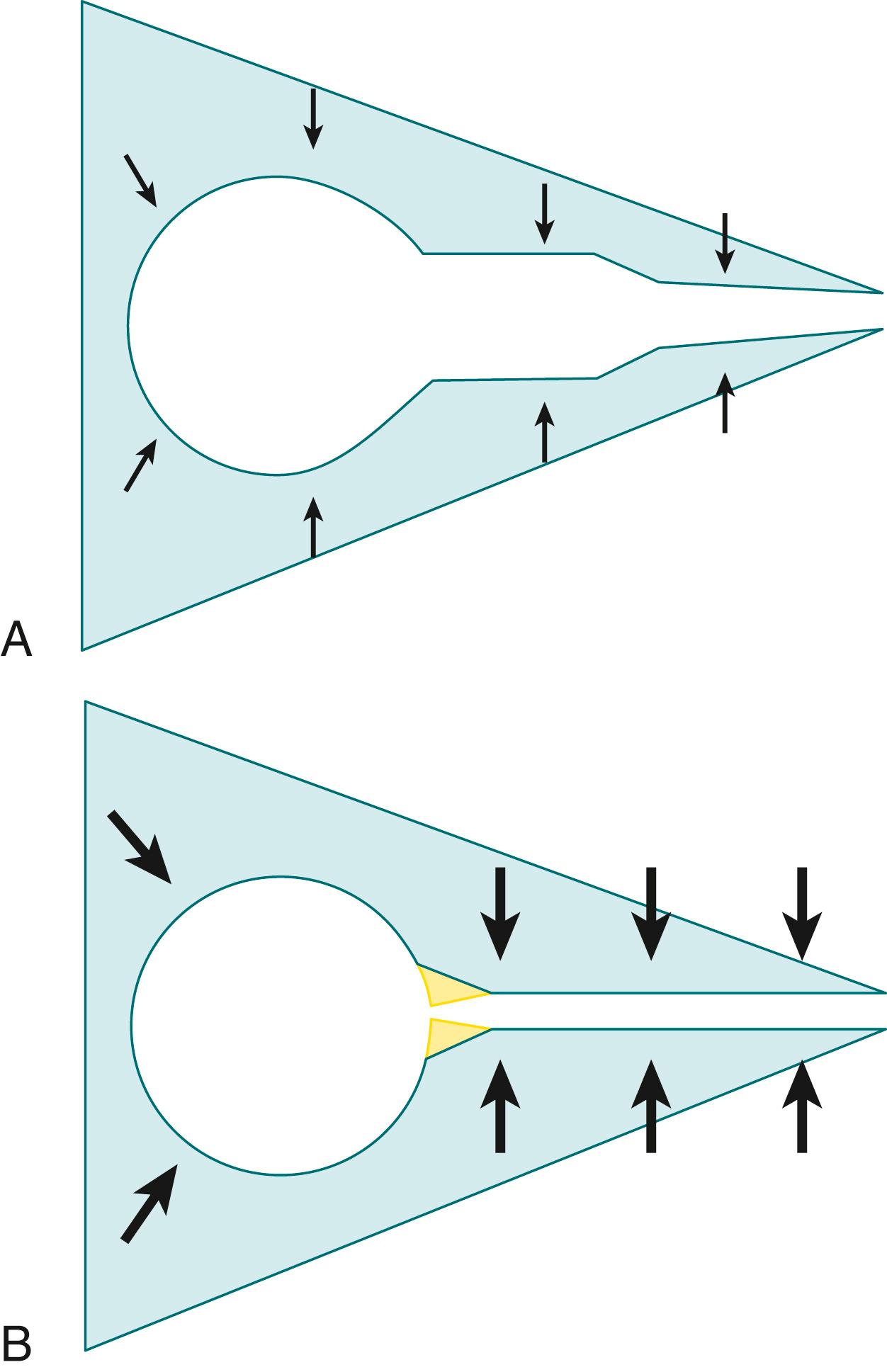
Treatment with distending pressure can reduce airway collapse by at least two mechanisms. , The pressure applied at the airway opening can redistribute the intraluminal pressure gradient from alveolus to mouth, thereby decreasing transmural pressure across the collapsible segment. Alternately, the distending pressure can raise lung volume, which in turn dilates smaller airways and reduces resistance. This would also serve to decrease transmural pressure and might decrease the need for forceful exhalation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here