Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
I am indebted to Professor Hector Wong for his assistance with the prior iteration of this chapter.
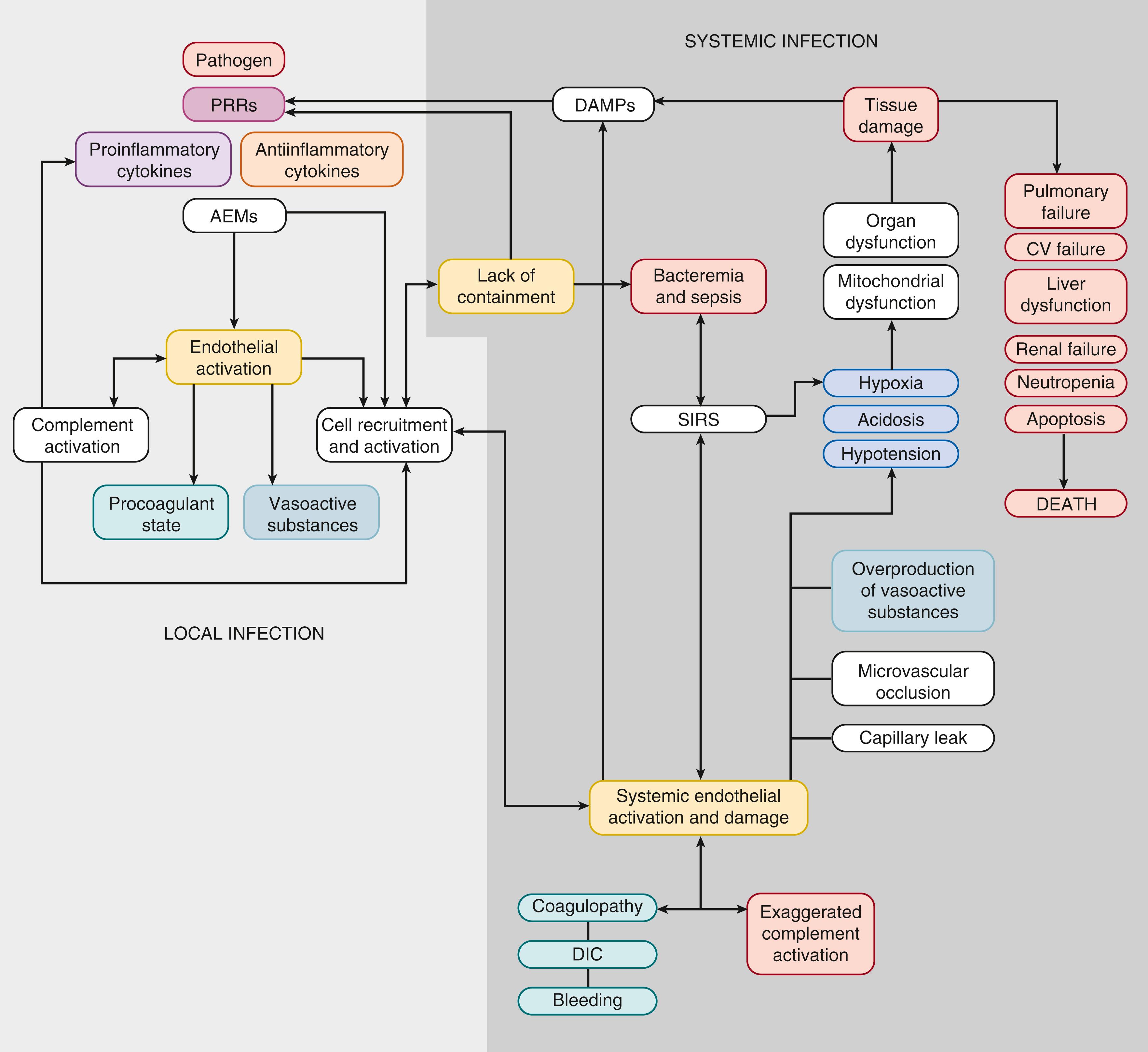
A successful immune response is critically necessary to eradicate infectious challenges and prevent dissemination of the infection throughout the host. However, if inflammation is not limited and becomes generalized, it can result in the constellation of signs and symptoms of a systemic inflammatory response syndrome (SIRS). If the infection is not contained, the spread of the pathogen from its local origin through the blood may result in systemic endothelial activation and precipitate sepsis or septic shock. Progression of sepsis to shock may lead to multi-organ dysfunction syndrome (MODS) and ultimately to death.
Host immunity is divided into innate and adaptive immune systems for purposes of discussion and teaching, but there is a great deal of interaction between the two systems. Innate immunity is rapid, largely nonspecific, and is composed of barriers, phagocytic cells, the complement system, and other soluble components of inflammation. Following breech of physical barriers, cellular elements of the innate immune response are the first line of defense against the development and progression of infection. Adaptive immunity, which is antigen-specific, long-lived, and often takes several days to develop, provides immunologic specificity and memory. These systems work together to protect the host from pathogenic challenge but may also precipitate host injury through aberrant responses. The outcome of infection is dependent on at least five major factors: (1) the pathogen, (2) the pathogen load, (3) the site of infection, (4) the host response, and (5) the underlying health status of the host. For a number of reasons, less is known about the host response in neonates compared to adults—the principal reason being the highly variable definition of sepsis.
Our understanding of the pathophysiology of sepsis largely owes to investigations in adult populations among both humans and animals. There is clear evidence from both preclinical models of sepsis that neonates manifest different host immune responses compared to adults. Even among children, neonates manifest a unique host immune response to sepsis and septic shock. , Thus, neonatal-specific clinical investigations, particularly in the very preterm infant, are required to improve both survival and long-term outcomes for these populations. By parallel processing of observational human studies with mechanistic investigations that leverage preclinical sepsis modeling, we can tap the power of transgenic approaches to tease out which elements are critical for survival and more clearly describe the pathophysiology. , A better understanding of the pathophysiology will uncover new opportunities for interventional studies ultimately aimed at improving outcomes. To this end, in this chapter, we explore the pathophysiology of sepsis in the neonate with special attention to the immunobiology of sepsis.
A discussion of the pathophysiology of sepsis must begin with a definition of sepsis. Adult and pediatric intensivists currently use consensus definitions for sepsis for goal-based therapeutic interventions. In 2016, a third iteration for the consensus definition of sepsis in adults was established and supported by 31 professional societies. The definition of sepsis is “life-threatening organ dysfunction caused by a dysregulated host response to infection . ” Organ dysfunction indicates a pathobiology more complex than simple infection plus an accompanying inflammatory response. Neonatal sepsis has been variably defined based on a number of clinical and laboratory criteria that make the study of this condition and the description of the pathophysiology very difficult. Diagnostic challenges and uncertain disease epidemiology necessarily result from a variable definition of disease. The distinction of infection from sepsis is not widely recognized in the neonatal intensive care unit (NICU). By contrast to the definitions of sepsis in adults and children, the definitions of sepsis commonly used in neonatology are not only variable but also heavily predicated on the isolation of pathogens from blood and/or the associated length of prescribed antimicrobial treatment. Meningitis, soft-tissue (omphalitis), osteomyelitis, pneumonia, and perforated bowel (spontaneous intestinal perforation [SIP] or necrotizing enterocolitis [NEC]) are frequently associated with negative blood cultures in all populations. ,
The presence of life-threatening organ dysfunction is demonstrated using a sequential organ failure assessment (SOFA) to determine risk of intensive care unit (ICU) admission or mortality. Because admission to a NICU for preterm infants is not optional, ICU admission is not applicable in this population. To define sepsis in neonates therefore requires an operational definition of organ dysfunction applicable specifically to this population (neonatal SOFA [nSOFA]) that predicts mortality in the setting of presumed infection. The progression of organ failure in neonates with lethal late-onset sepsis (LOS) was shown in a large retrospective cohort. The need for mechanical ventilation, oxygen requirement, requirement for cardiovascular support in the form of vasoactive drugs, and the prevalence of thrombocytopenia all significantly increased during the progression to mortality with LOS. Guided by those data, an objective, electronic health record (EHR)-automated, nSOFA scoring system was developed and tested that predicted LOS mortality in premature very low-birth-weight infants. ,
Sepsis or serious infection within the first 4 weeks of life kills in excess of 1 million newborns worldwide annually. , The incidence of neonatal sepsis is variable (from <1% to >35% of live births) based on gestational age and time of onset (early onset sepsis [EOS], <72 hours after birth or late onset sepsis [LOS], ≥72 hours after birth). Preterm neonates suffer the greatest sepsis incidence and mortality among all age groups ( Fig. 151.1 ).
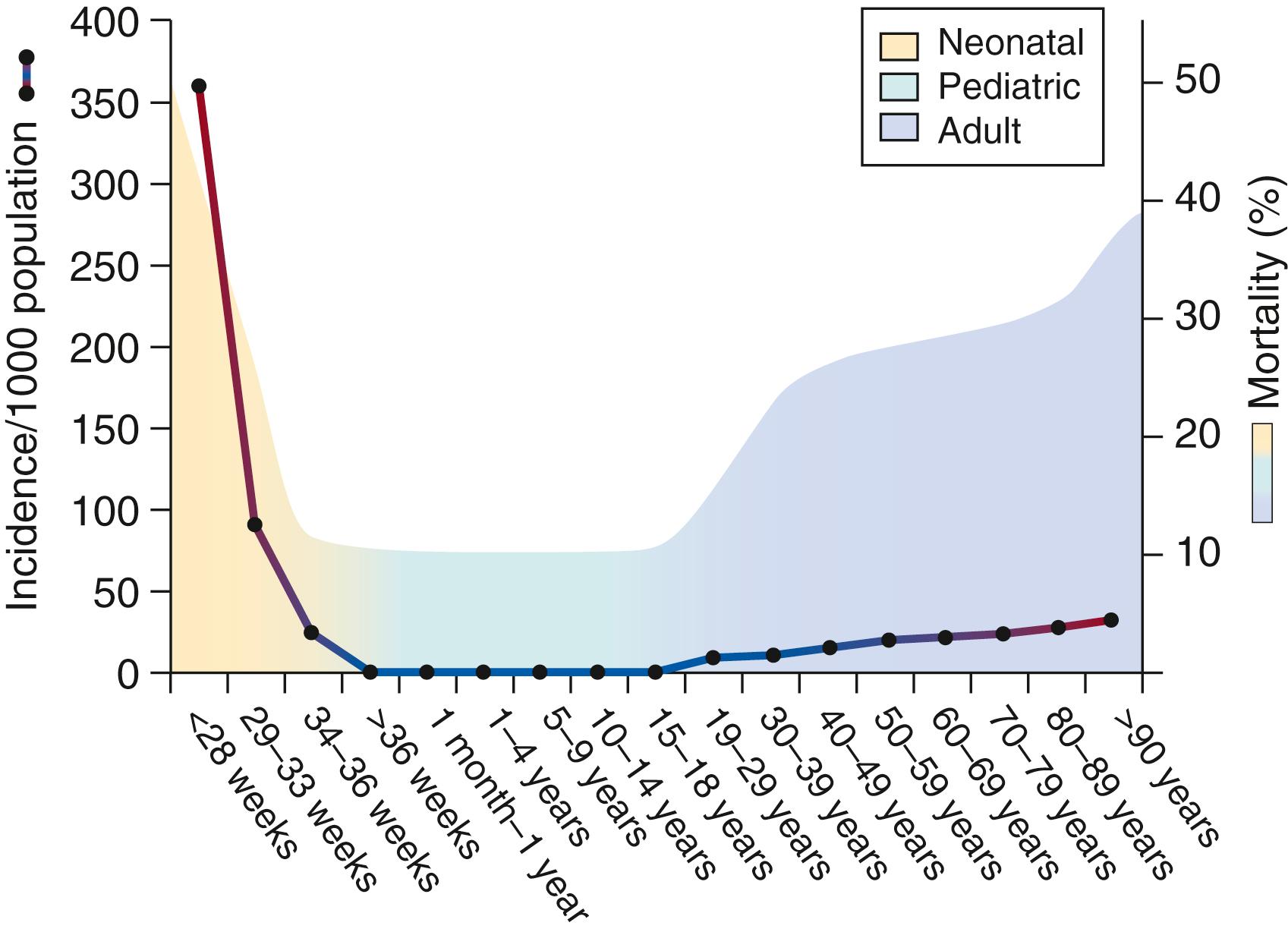
Risk factors for developing sepsis in neonates, particularly the very premature, have been well described. , , Maternal factors that contribute to the risk of neonatal sepsis have been identified. Prematurity, low birth weight (<2500 g, LBW), a positive maternal vaginal culture for group B streptococcus (GBS), prolonged rupture of membranes, maternal intrapartum fever, and chorioamnionitis (intraamniotic infection) are strongly associated with GBS EOS. Chorioamnionitis is associated with the greatest risk of subsequent clinically or culture-proven sepsis. Studies demonstrate the risk of sepsis in clinical chorioamnionitis-exposed newborns is strongly dependent on gestational age, with minimal risk in neonates 35 weeks and older and greater risk with increasing degrees of prematurity. The risk of neonatal sepsis conferred by maternal GBS colonization is significantly reduced with adequate intrapartum antibiotic prophylaxis. Despite the efficacy of this intervention, the incidence of invasive GBS disease in African American neonates is rising as is the incidence of Escherichia coli sepsis in preterm and term neonates. Vaginal delivery in the presence of maternal active primary herpes simplex virus (HSV) significantly increases the risk for neonatal HSV, which often has a fulminant course and high mortality. Preexisting maternal immunodeficiency or sepsis also increases the risk for sepsis in the neonate. Factors immediately after delivery and in the postnatal period influence the risk for the development of sepsis. Nonmodifiable risk factors such as gender (male), extremely low birth weight (ELBW) less than 1000 g and gestational age less than 30 weeks increase risk for the development of sepsis. In contrast, risk is increased by clinical interventions such as parenteral alimentation, intubation and mechanical ventilation, and central venous access. ,
Metabolic demands may represent a substantial risk factor for the development of infection and sepsis in neonates. , The neonatal immune response is consistent with disease tolerance (minimizing harm from immunopathology), whereas a disease resistance strategy (minimizing harm from pathogens) predominates in adults. Increased resistance to disease requires substantial energy, and thus host metabolism is inexorably connected to host inflammatory responses. Accordingly, immunometabolism is an area of intense research across multiple disciplines and disease states including cancer, sepsis, and autoimmunity. Neonates, particularly those born extremely preterm, have daily caloric needs that are at least fivefold greater than adults (150 kcal/kg vs. 25 kcal/kg). In conjunction with a lack of functional fat stores, the critical differences in metabolic demand and substrate utilization between neonates and adults severely restrict the capacity for a preterm neonate to mount a disease resistance response. Importantly, although a state of “metabolic bankruptcy” may be reached early after infectious challenge in the neonate, adults with chronic infection/critical illness may also demonstrate similar metabolic deficits that contribute to a persistent immune-catabolism syndrome (PICS).
Because sepsis definitions in neonates are largely predicated on the isolation of a pathogen from blood rather than the presence of life-threatening organ dysfunction, studies that describe the primary source of infection in the absence of bacteremia are frequently considered as separate clinical entities, e.g., meningitis, pneumonia, peritonitis (SIP/NEC), urinary tract infection (UTI), soft-tissue/skin. For example, among 1961 adults with severe sepsis, the most common primary sites in order were lung, abdominal, genitourinary, and skin/soft tissue. Among patients aged greater than 55 years admitted to an surgical ICU with a diagnosis of severe sepsis or septic shock, the most common primary sites in order were abdominal, lung, necrotizing soft tissue, and UTI. A prevalence study of 569 children with severe sepsis in pediatric ICUs worldwide revealed the order of primary site infections was lung (40%), blood (19%), abdominal (8%), central nervous system (CNS) (4%), genitourinary (4%), skin (4%), and unknown (16%) (18). Among 429 neonates with late-onset infection, the causes were blood (primary [38%] and central line-associated bloodstream infection [29%]), urine (8%), lung (6%), CNS (6%), peritonitis (6%), and other (ear, nose, and throat infection, bone and joint infection, or skin and soft tissue infection [7%]). An understanding of the primary sources for sepsis across populations is necessary to inform relevant and effective strategies for intervention.
Common potential consequences of infection in very low-birth-weight (VLBW) infants (<1500 g) include prolonged hospital stay, need for intubation and surfactant therapy, prolonged mechanical ventilation, chronic lung disease, severe intraventricular hemorrhage, delayed initiation of enteral feedings and prolonged parenteral nutrition, extended need for central venous lines, need for umbilical, or peripheral arterial access, and patent ductus arteriosus (PDA). , , , ,
Four out of 10 neonates that develop sepsis die or experience major morbidity including neurodevelopmental impairment (NDI) as well as impairment of hearing and vision. Studies of children at 9 and 10 years of age after preterm birth and LOS reveal persistently increased risks for motor impairment, attention-deficit hyperactivity disorder, and intellectual impairments compared to their preterm peers without sepsis. , Sepsis-associated NDI was associated with a higher prevalence of white matter abnormality compared to nonseptic controls. Seventy-three percent of ELBW infants that develop Candidiasis suffer death or NDI including retinopathy. Outcomes are worse following neonatal septic shock. The composite of death or severe sequelae (cerebral palsy, severe developmental delay, hearing impairment, blindness, or short bowel syndrome) occurred in 52% of all neonates with septic shock, and only 28% of all neonates were alive and considered normal at 18 months of age. Septic shock mortality was greatest in ELBW infants (71%).
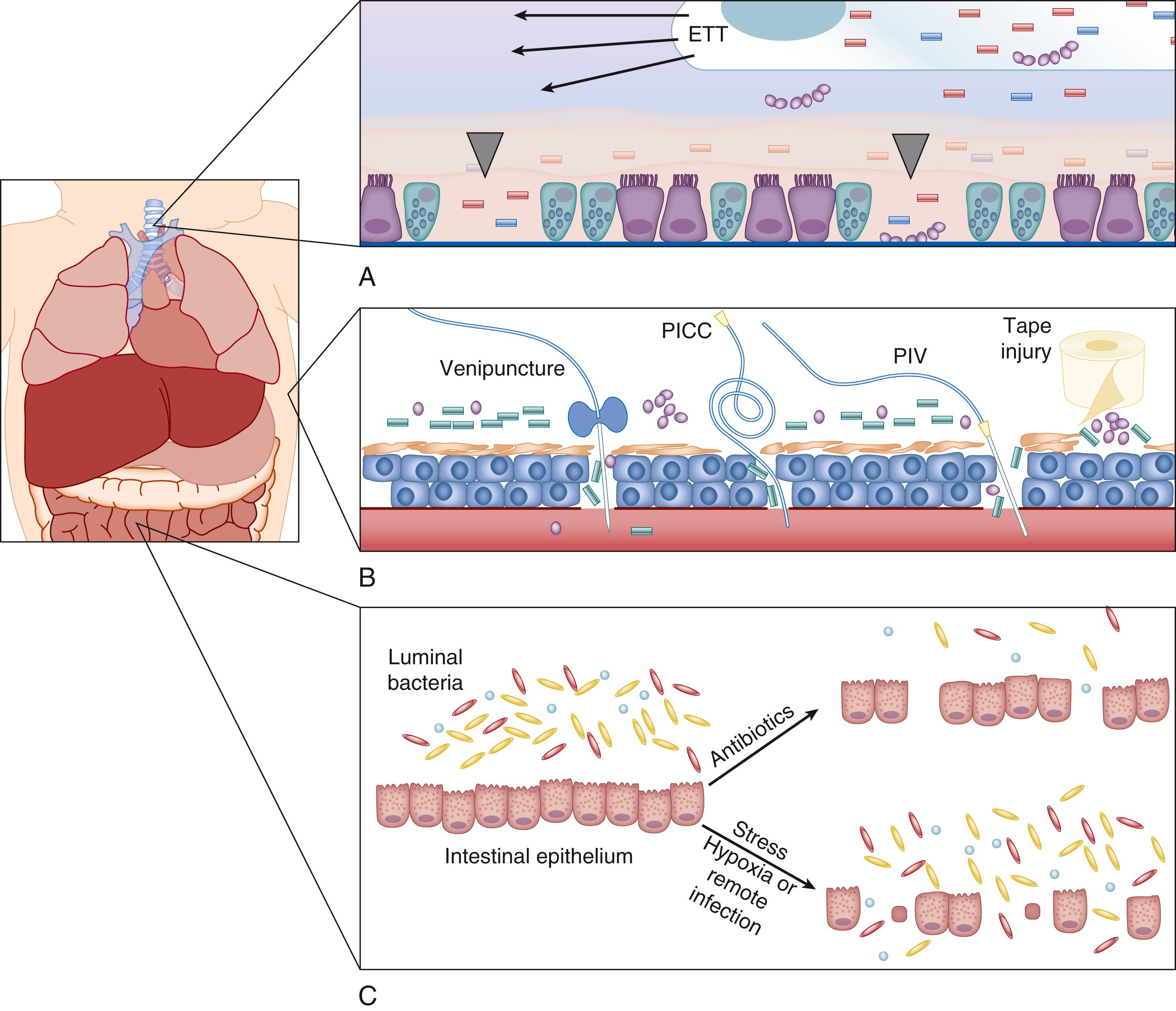
Once the local barrier function (skin and mucosal surfaces) has been compromised ( Fig. 151.2 ), pathogen recognition by local immune sentinel cells is the first step toward development of an immune response ( Fig. 151.3 ). Elegant sensing mechanisms have evolved to facilitate detection of potentially pathogenic microorganisms. Since Dr. Charles Janeway predicted their existence, multiple classes of pathogen recognition receptors (PRRs) have been discovered that serve as detectors of pathogen-associated molecular patterns (PAMPs) including cell wall and membrane components, flagellum, nucleic acids, and carbohydrates. A litany of PRR classes have been discovered including the Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), retinoic-acid-inducible protein I (RIG-I)-like receptors (RLRs), peptidoglycan recognition proteins (PGRPs), β 2 -integrins, and C-type lectin receptors (CLRs). The TLRs, β 2 -integrins, and CLRs detect pathogens on the cell surface and in the endosome, whereas RLRs and NLRs detect pathogens intracellularly. The discovery that TLR4 was integral for a robust lipopolysaccharide (LPS)-mediated inflammatory response that occurs with gram-negative sepsis may be why TLRs have been more thoroughly investigated in the setting of sepsis than have other PRRs. Each of the 10 known TLRs in humans, present on and within multiple cell types, recognizes extracellular and intracellular pathogens via specific PAMPs. , Multiple TLRs may be activated in concert by intact or partial microorganisms and activate multiple second messenger pathways simultaneously. ,
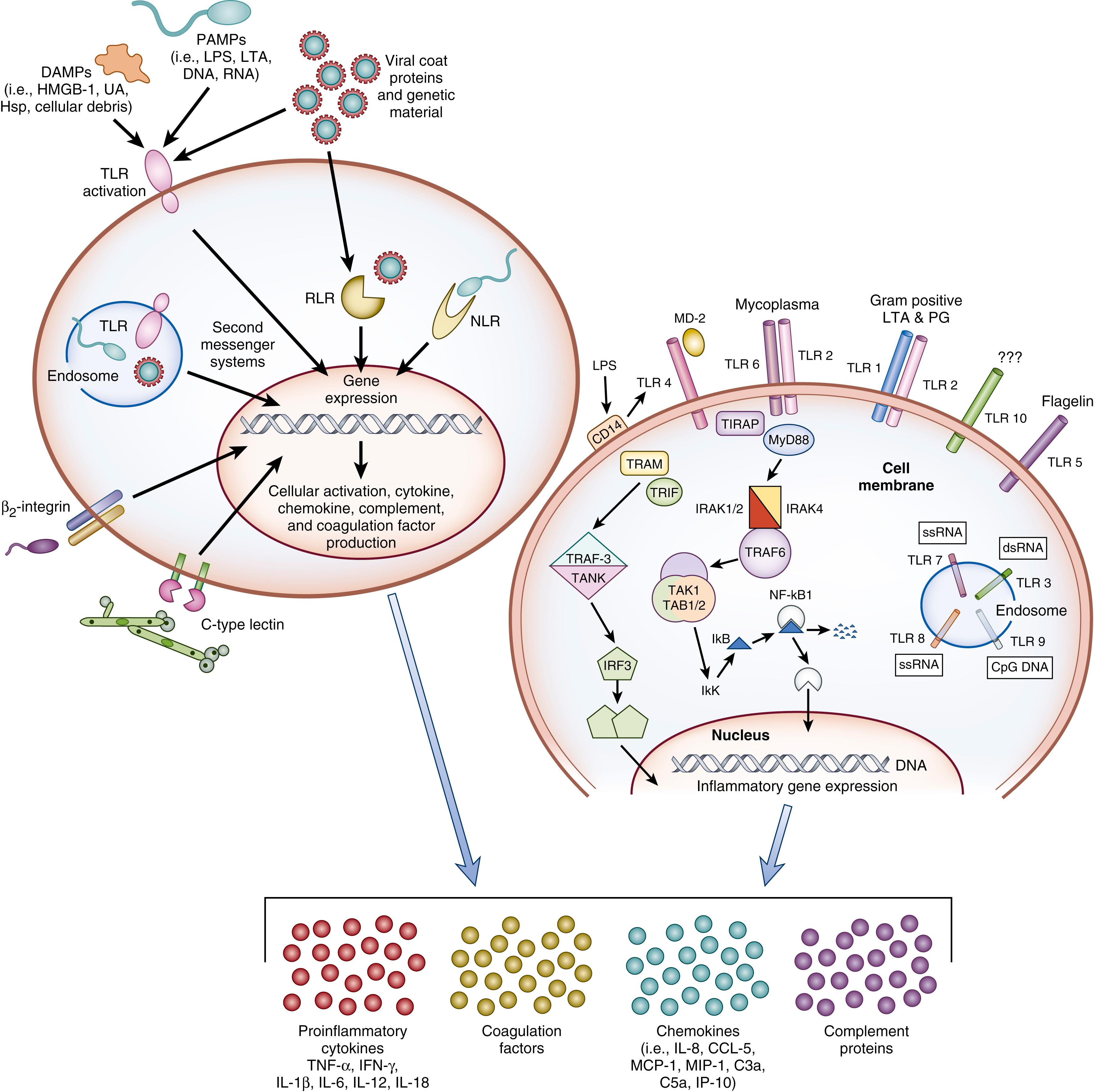
LPS is the prototypic mediator of systemic inflammation and generates many of the clinical findings of sepsis and septic shock including MODS and death. LPS signals through TLR4 in conjunction with the adaptor proteins CD14 and myeloid differentiation factor-2 (MD-2). A study in adults demonstrated a reduction in mortality and improvement in hemodynamics when serum LPS was reduced. LPS is elevated in blood from septic neonates and those with NEC even in the absence of gram-negative bacteremia. High levels of circulating endotoxin found during sepsis and NEC are associated with multiple organ failure, thrombocytopenia, neutropenia, and death. Administration of anti-LPS antibodies to small numbers of neonates with sepsis ( n = 16) and endotoxemia reduced the time to recovery, but not mortality compared to placebo-treated neonates. Reduction of serum LPS by exchange transfusion in infected neonates ( n = 10) may be associated with improved survival.
Bacterial cell wall components (e.g., lipoteichoic acid) signal primarily through TLR1/2/6, flagellin through TLR5, and CpG double-stranded DNA through TLR9. Common viral PAMPs such as double-stranded RNA or single-stranded RNA signal through TLR3 and TLR7/8, respectively (see Fig. 151.3 ). Agonist-TLR binding results in a signaling cascade of intracellular second messenger proteins ultimately leading to production of cytokines and chemokines as well as activation of other antimicrobial effector mechanisms. Key intracellular messengers critical for effective TLR signaling include myeloid differentiation factor 88 (MyD88), Toll/IL-1 receptor domain (TIR) containing adaptor protein (TIRAP), TIR domain containing adapter-inducing interferonβ (TRIF), TRIF-related adaptor molecule (TRAM), IL-1 receptor-associated kinase (IRAK)-1 and IRAK-4, and nuclear factor of κ light polypeptide gene enhancer in B cells 1 (NF-κB). Signaling through MyD88 typically leads to the production of NF-κB-dependent inflammatory cytokines/chemokines, whereas signaling through TRIF induces production of type I interferons (IFN) as well as NF-κB-related inflammatory cytokines. Upregulation of TLR2 and TLR4 mRNA in neonates occurs during gram-positive and gram-negative infection, respectively, across gestational ages. Dysregulation or overexpression of TLR4 is involved in the development of NEC in experimental animal models, implicating the importance of TLRs in the initial immune response to pathogens and their role in neonatal sepsis.
Beyond TLRs, other important intracellular PRRs include NLRs and RLRs. For NLRs, multiple cytosolic proteins are able to act as PAMP sensors (e.g., NOD leucine rich repeat and pyrin domain containing [NLRP]1, NLRP3) and coalesce with adaptor proteins and pro-caspase-1 (CASP1) to form a multimeric protein complex termed the inflammasome . The formation of the inflammasome activates CASP1 that cleaves the inactive precursor proteins of IL-1β and IL-18 to their active forms. Small molecular NLR inhibitors are available and could be beneficial if aberrant NLR signaling was shown to contribute to neonatal sepsis outcomes. RLRs are cytoplasmic RNA helicases that, like TLR3, sense double-stranded RNA of viral origin and induce type I IFN production and NF-κB activation. To date, the impact of RLR and NLR signaling has not been specifically examined in neonates with sepsis.
In addition to its roles in leukocyte adhesion, phagocytosis, migration and activation, and complement binding, the β 2 -integrin complement receptor 3 (CR3), also known as MAC-1 and CD11b/CD18 , functions as a pathogen sensor on the surface of phagocytes. CR3 binds LPS as well as a broad range of other microbial products, in cooperation with or independent of CD14, leading to upregulation of inducible nitric oxide (NO) synthase (iNOS) and NO production. Diminished expression of L-selectin and CR3 on stimulated neonatal neutrophils (PMN) impairs neonatal PMN and monocyte activation and accumulation at sites of inflammation. Decreased expression of L-selectin and CR3 persists for at least the first month of life in term infants, possibly contributing to an increased risk for infection. The expression of CR3 (CD11b) may be reduced further in preterm neonates compared to term neonates. In umbilical cord blood from neonates less than 30 weeks’ gestation, PMN CR3 content was similar to levels found in patients with type 1 leukocyte adhesion deficiency (failure to express CD18). , Thus, decreased leukocyte CR3 surface expression increases the likelihood of suboptimal pathogen detection and cellular activation, particularly in the preterm neonate.
CLRs are PRRs that recognize bacterial, viral, fungal, and parasitic carbohydrate moieties. CLRs may be expressed on the cell surface (e.g., macrophage mannose receptor, Mincle [CLR], dectin-1/2 [transmembrane PRRs]) or secreted as soluble proteins (e.g., mannose-binding lectin [MBL]) as one of the acute phase reactants (APRs). Once bound to its carbohydrate ligand, MBL initiates activation of complement via the lectin pathway to promote opsonization and phagocytic clearance of pathogens. Plasma MBL concentrations are low at birth (especially in preterm infants), but rise steadily throughout infancy and childhood. Low levels of MBL are associated with the increased incidence of sepsis in neonates. In addition to decreased concentrations at birth, certain genetic polymorphisms of MBL, namely MBL2, have also been associated with an increased risk of infection. However, this association has not been found in all studies. M-ficolin activates the complement system in a manner similar to MBL and is elevated in neonates with sepsis.
PRR stimulation results in rapid inflammatory mediator transcription and translation directed at cellular activation and clearance of pathogenic organisms (see Fig. 151.3 ). Elevations of pro-inflammatory cytokines during sepsis and septic shock, including IL-1β, IL-6, IL-8 (CXCL8), IL-12, IL-18, IFN-γ, and tumor necrosis factor-α (TNF-α), have been identified. Compared to adults with sepsis, neonates with sepsis produce less IL-1β, TNF-α, IFN-γ, and IL-12. The decreased cytokine production is due in part to decreased production of important intracellular mediators of TLR signaling including MyD88, interferon regulatory factor 5 (IRF5), and p38, which exhibit gestational age-specific diminution. Studies have demonstrated impaired inflammasome activation and mature IL-1β production by neonatal mononuclear cells. , In a comprehensive study (>140 analytes) of serum from neonates evaluated for LOS, IL-18 emerged as a predictive biomarker to differentiate infected from noninfected neonates, similar to data from adults with sepsis. IL-18 reduces PMN apoptosis, drives IFN-γ production, and induces production of TNF-α, IL-1β, and CXCL8. IL-18 primes PMNs for degranulation with production of reactive oxygen intermediates (ROI) on subsequent stimulation. Dysregulation of many of these functions linked to IL-18 are seen in sepsis and septic shock. Increased IL-18 has been demonstrated in premature neonates with brain injury and also an experimental model of NEC, highlighting a common pathway activated with states of ischemia and inflammation. IL-18-null neonatal mice are highly protected from polymicrobial sepsis, whereas replenishing IL-18 increased lethality of sepsis or endotoxemia. IL-18-augmented sepsis mortality depended on IL-1R1 signaling but not adaptive immunity. IL-18 administration in sepsis increased IL-17A production by murine intestinal γδT cells as well as Ly6G + myeloid cells, and blocking IL-17A reduced IL-18-potentiated mortality to both neonatal sepsis and endotoxemia. Excessive levels of IL-1β, TNF-α, IL-6, CXCL8, IL-10, and IL-18, such as those seen with advanced stage NEC, severe sepsis or septic shock, correlate with poor survival. , Altered cytokine levels (increased IL-10 and IL-6, and decreased CCL5) may identify those neonates at highest risk for the development of sepsis-associated disseminated intravascular coagulation (DIC).
Pro-inflammatory cytokine production leads to activation of endothelial cells including increased expression of cellular adhesion molecules (CAMs) that facilitate leukocyte recruitment and diapedesis ( Fig. 151.4 ). Upregulation of CAMs (soluble intercellular CAM [sICAM], vascular CAM [VCAM], L-, P-, and E-selectins, and CD11b/CD18) during sepsis facilitates rolling and extravascular migration of leukocytes. Decreased neonatal PMN and monocyte L-selectin and MAC-1 expression impairs PMN accumulation at sites of inflammation. ,
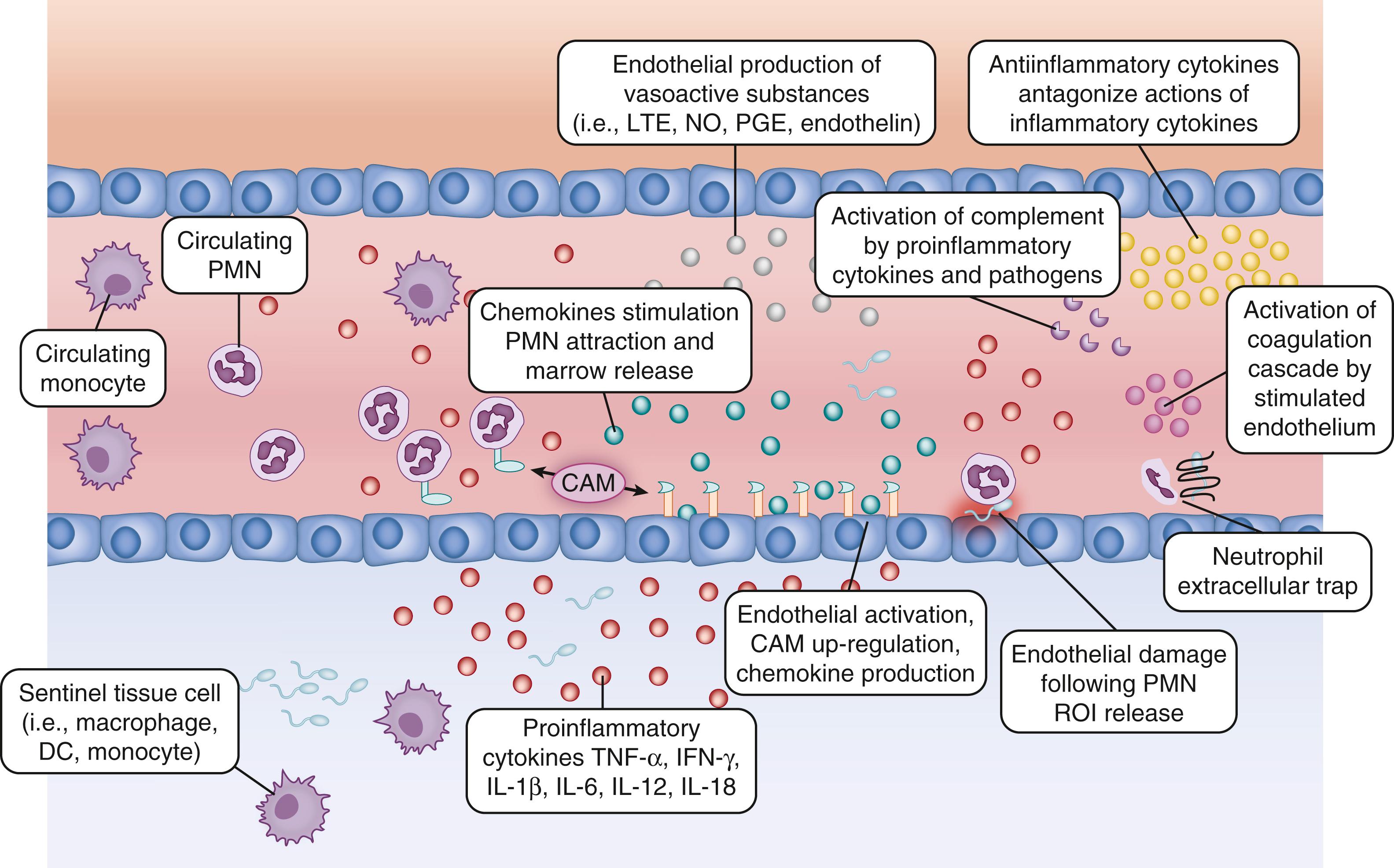
Chemokine gradients produced by endothelial cells and local macrophages are necessary in addition to CAM interactions for effective and specific leukocyte attraction and accumulation (see Fig. 151.4 ). Without adequate leukocyte recruitment, there is increased risk for propagation from a local to a systemic infection. Although poor cellular chemotaxis in the neonate has been observed, it is not likely a result of reduced serum concentrations of chemokines, as baseline levels are similar in preterm and term neonates compared to adults. Suboptimal cellular chemotaxis may be related to other mechanisms such as poor CR upregulation following stimulation, deficiencies in another downstream signaling process, or inhibition by bacterial products. A wide variety of chemokines are increased during sepsis, including CCL2, CCL3, CCL5, CXCL8, and CXCL10. Other chemo-attractive molecules also increase with sepsis, including complement proteins C3a and C5a, antimicrobial proteins and peptides (APPs), including cathelicidins and defensins, as well as components of invading bacteria themselves. , The importance of chemo-attractive substances in the pathogenesis of severe sepsis is highlighted by studies showing that CXCL8 can be used as a stratifying factor for survival in children, and C5a is implicated in sepsis-associated organ dysfunction in adults. Chemokine investigations in septic neonates revealed that CXCL10 is a sensitive early marker of infection, and low CCL5 levels may predict development of DIC.
Damage-associated molecular patterns (DAMPs or alarmins), such as intracellular proteins or mediators released by dying or damaged cells including IL-1α, high mobility group box (HMGB)-1, IL-33, and S100 proteins, may also activate PRRs and thus play a critical role in the amplification of the host immune response. Neonatal mice that lack IL-1 receptor 1 (IL-1R1; cognate receptor for IL-1α and IL-1β) had improved sepsis survival and attenuated plasma inflammatory mediator production compared with wild-type (WT) septic mice. The survival benefit in IL-1R1 knockout mice was not replicable with pharmacologic use of an IL-1R antagonist in WT mice and furthermore was restricted to mice that lacked IL-1α specifically. Taken together, these data highlight the importance of rapid, tissue-level DAMP-signaling in the pathophysiology of sepsis.
HMGB1 is involved in the progression of sepsis to septic shock in adults. , Macrophages or endothelial cells stimulated with LPS or TNF-α produce HMGB1, which signals through TLR2, TLR4, and receptor for advanced glycation end products (RAGE). HMGB1 results in cytokine production, activation of coagulation, and PMN recruitment. , HMGB1 mediates disruption of epithelial junctions within the gut via the induction of reactive nitrogen intermediates (RNIs), leading to increased bacterial translocation. The role of HMGB1 and RAGE signaling in human neonates with sepsis has not been well characterized, but has been shown to be involved in the pathophysiology of NEC in a preclinical model. Significantly lower soluble RAGE (sRAGE) was found in human fetuses that mounted robust inflammatory responses and HMGB1 levels correlated significantly with levels of IL-6 and S100β in fetal circulation.
S100 proteins A8/A9 are highly elevated in the plasma of human newborns for several days after birth. The heterodimer S100A8/A9 specifically programs human neonatal monocytes at birth by inducing MyD88-dependent gene programs via NF-κB and IRF5 activation, without influencing TRIF-dependent genes. Genetic deletion of S100A9 resulted in greater murine neonatal mortality and a hyperinflammatory response compared to the WT neonatal mouse. The net effect of birth-associated programming by these S100 proteins was prevention of hyperinflammation via a selective, transient microbial unresponsiveness while allowing for sufficient immunologic protection. Myeloid-derived suppressor cells (MDSCs), phenotypically similar to PMNs and monocytes, have significant immunosuppressive activity and are present in high numbers after birth in mice and humans. In mice, MDSC suppressive activity was triggered by lactoferrin and mediated by S100A8 and S100A9 as well as NO and prostaglandin (PG) E 2 . A more complete understanding of the mechanisms that underlie neonatal-specific inflammatory responses may allow for precision-medicine approaches in the future that address specific deficits in individual patients.
Other specific DAMPs, including heat shock proteins (Hsps) and uric acid, may also stimulate TLRs, regulate PMN function, and function as immune adjuvants. Hsp production in septic neonates has not been evaluated to date but polymorphisms in Hsps increase the risk for acute renal failure (ARF) in preterm neonates. Hsps are significantly elevated in septic adults and children. Elevated Hsp60 and Hsp70 measured within 24 hours of pediatric ICU admission were associated with septic shock, and there was a trend toward a significant association with death. , Uric acid is a DAMP that can increase cytokine production, PMN recruitment, and dendritic cell (DC) stimulation and may also serve as an antioxidant. Uric acid is reduced in the serum of septic neonates compared to control neonates.
In addition to facilitating leukocyte attraction, pro-inflammatory stimuli result in production of vasoactive substances that decrease or increase vascular tone and alter vascular permeability (see Fig. 151.4 ). These include platelet-activating factor (PAF), thromboxane (TBX), leukotrienes (LTE), NO, PG histamine, and bradykinin. , These substances are produced predominantly by host endothelium and mast cells. Activated PMNs produce phospholipase A2 (PLA 2 ), which is increased in the serum of neonates with sepsis and leads to generation of vasoactive substances including PGE and LTE. TBX produced by activated platelets and endothelin (ET-1) produced by activated endothelium are potent vasoconstrictors that participate in the development of pulmonary hypertension. Systemic overproduction of cytokines and vasoactive substances is associated with circulatory alterations and organ failure seen in severe sepsis and septic shock ( Fig. 151.5 ). ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here