Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hypoglycemia in neonates has been a topic of major concern and of controversy for many decades. These arise from two competing clinical issues. On the one hand, for a small number of infants born with persistent forms of hypoglycemia, there is the serious risk of seizures and permanent brain injury if not detected early and treated adequately. For example, over half of the children with genetic forms of congenital hyperinsulinism suffer from seizures and permanent brain injury that could have been prevented if their hypoglycemia had been diagnosed and treated before discharge from the newborn nursery. On the other hand, there is a competing need to avoid overdiagnosis and unnecessary interventions, because hypoglycemia to varying degrees is common in normal infants during the first days of life and usually has no apparent consequence. For example, in normal newborns, mean plasma glucose concentrations transiently drop by 25 to 40 mg/dL from the fetal range of 70 to 100 mg/dL to a nadir of approximately 55 to 65 mg/dL immediately after birth; glucose levels then quickly rise within 1 to 2 days back again into the normal range for infants, children, and adults of 70 to 100 mg/dL. This transitional neonatal hypoglycemia in normal newborns can create confusion in recognizing cases with permanent or persistent hypoglycemia disorders. Contributing to the difficulty in balancing concerns about persistent, potentially damaging forms of hypoglycemia and the commonness of transitional hypoglycemia in normal newborns is the imbalance in our understanding of these conditions. The persistent forms of hypoglycemia that can present in the newborn have been well described and their diagnosis and treatment well defined. However, the mechanism(s) and management of transitional neonatal hypoglycemia remain poorly understood, further complicating efforts to distinguish the etiology of hypoglycemia in individual cases. The purposes of this chapter are (1) to review the pathophysiology of neonatal hypoglycemia with an emphasis on the mechanism underlying transitional neonatal hypoglycemia in normal newborns; (2) to describe the severe prolonged hyperinsulinism disorder that frequently occurs in neonates with perinatal stress such as birth asphyxia or intrauterine growth retardation; and (3) to outline the diagnosis and management of the major genetic or other persistent hypoglycemia disorders, which are most likely to be encountered in the neonatal period.
Since glucose is an obligate fuel for brain metabolism and serves as the predominant fuel for the rest of the body, plasma glucose concentrations must be controlled within narrow limits at all times. The major elements of glucose homeostasis include (1) the glucose set-point (the target glucose concentration that maintains normal physiology); (2) various glucose thresholds (levels at which individual hormonal and neural glucoregulatory signaling factors are activated or inhibited to raise or lower plasma glucose levels); and (3) glucose sensor(s) that responds to glucose level to transmit a signal to control glucoregulatory factors.
As shown in Fig. 152.1 , in children and adults, the glucose set-point is approximately 5 mmol/L, which represents the normal range of plasma glucose in both intrauterine and extrauterine life of 70 to 100 mg/dL. Plasma glucose levels are tightly maintained at this set-point by the counter-balancing glucose-lowering actions of insulin, which is suppressed below a glucose threshold of approximately 4 mM (70 mg/dL), and the glucose-raising actions of various counterregulatory factors with individual glucose thresholds ranging from 65 to 55 mg/dL, including especially glucagon and the sympathetic nervous system, as well as the more long-term effects of cortisol and growth hormone. The major glucose sensor for maintaining glucose homeostasis is glucokinase, a form of hexokinase expressed specifically in pancreatic islets and liver. Glucokinase is a low-affinity glucose-phosphorylating enzyme, which unlike the forms of hexokinase in all other tissues, becomes highly active only at glucose levels above 4 mM and thus sets the glucose threshold for insulin release by pancreatic islets. As evidence of the important role of this enzyme as the key glucose sensor for insulin secretion, glucokinase loss of function mutations result in diabetes and mutations (or drugs) that increase glucokinase activity cause hyperinsulinemic hypoglycemia.
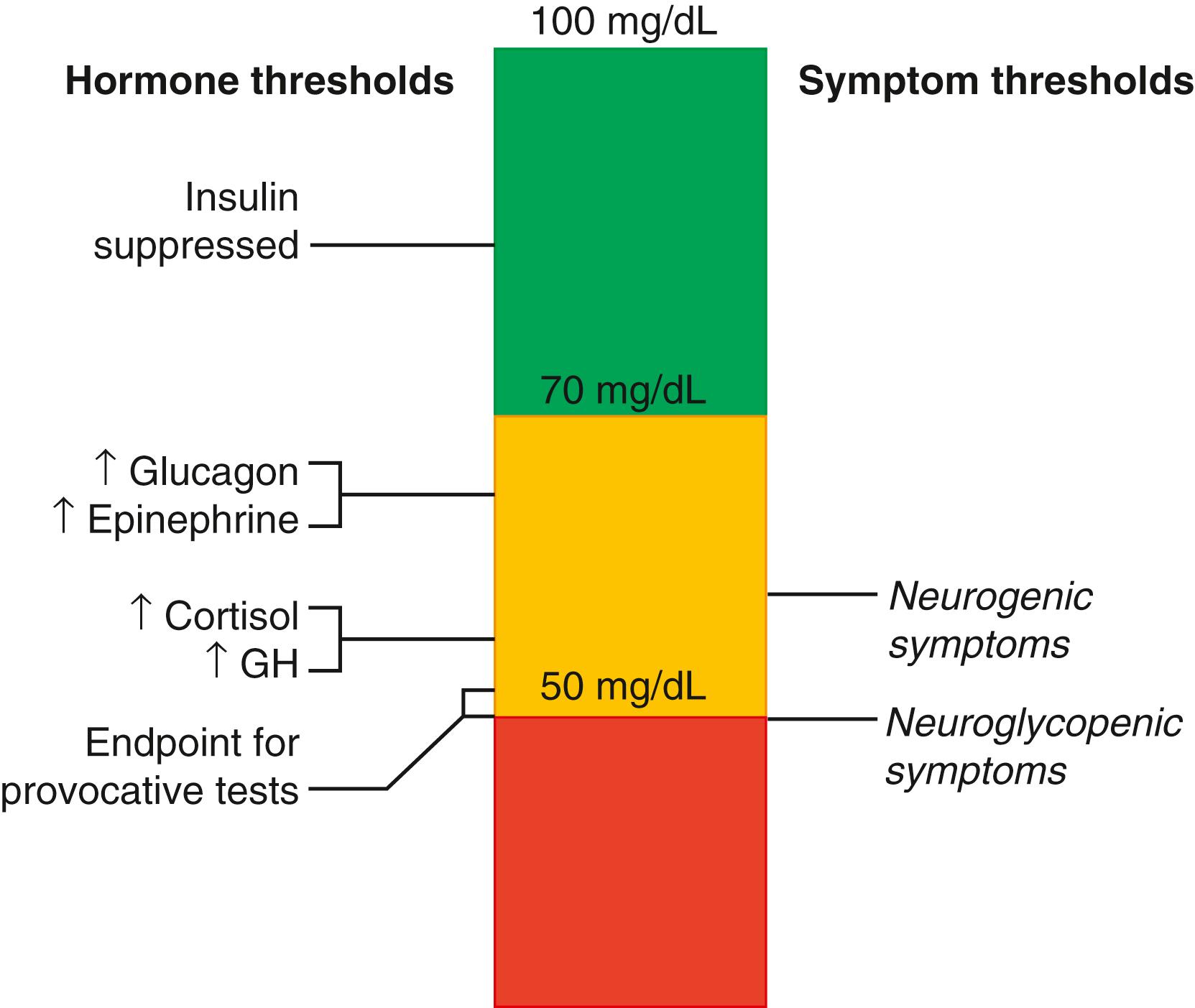
The set-point for glucose reflects the glucose concentration required for optimal rates of energy production in the brain and other tissues. Newborns in the first few days of life have a transitional period of hypoglycemia, where the blood glucose is not maintained at the normal set-point level, for reasons that have not been clearly defined, but, as discussed in the section “Transitional Neonatal Hypoglycemia in Normal Newborns,” are most likely due to persistence of the fetal lower β cell threshold for insulin release.
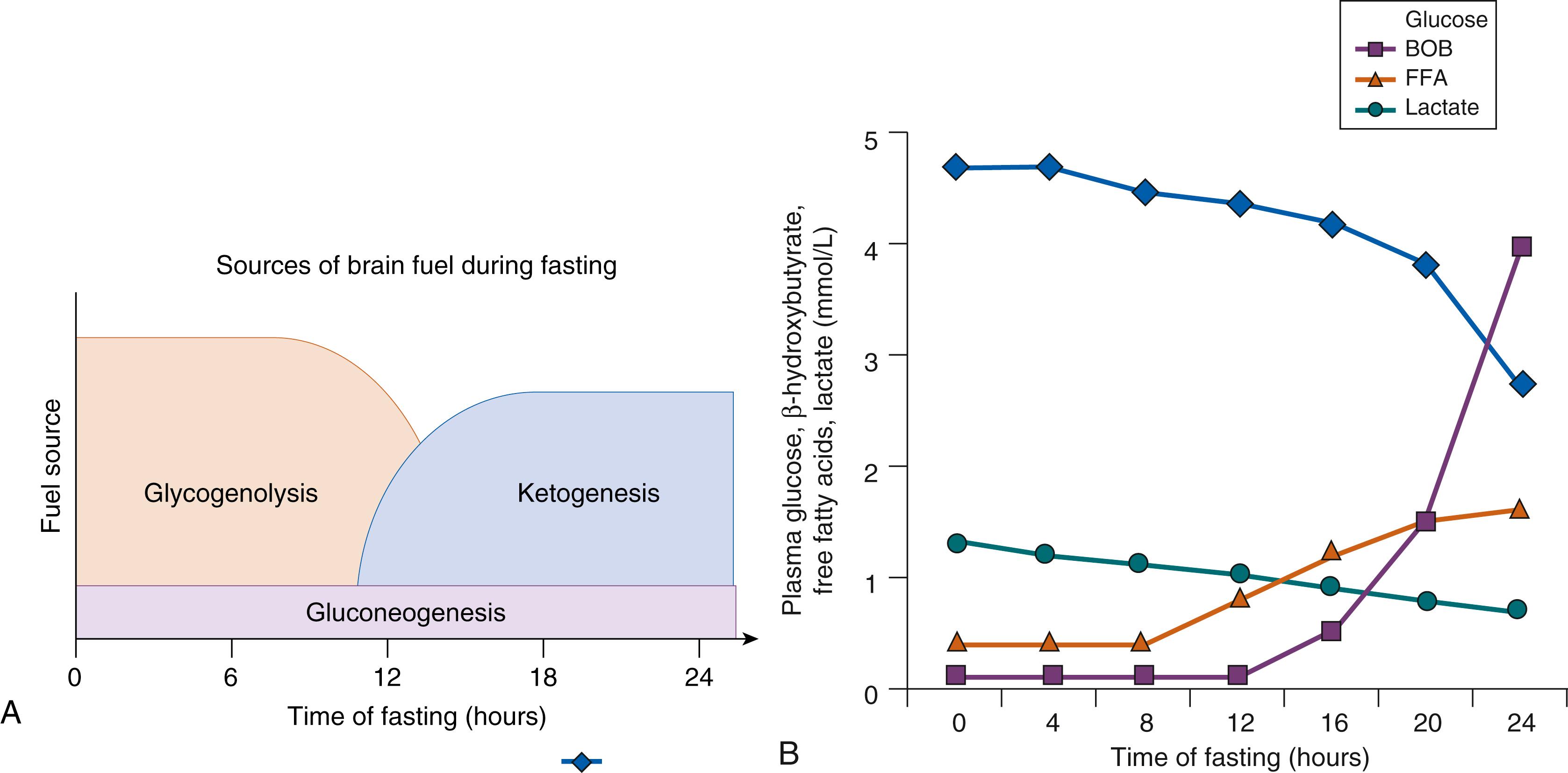
The changes in source of substrates for brain metabolism during increasing periods of fasting are shown in Fig. 152.2 A and B. Following a meal, brain metabolism initially depends on glucose released from hepatic glycogen stores ( glycogenolysis ) and then on hepatic glucose synthesis ( gluconeogenesis ) using substrates such as amino acids generated by protein turnover in peripheral tissues, especially muscle. As fasting is extended and hepatic glycogen reserves become depleted, glucose production declines and free fatty acids, derived from the release of triglycerides stored in adipose tissue ( lipolysis ), become the major fuel for peripheral tissues, such as muscle. Although the brain cannot oxidize fatty acids directly, it can use fat stores indirectly after conversion of free fatty acids to ketones (β-hydroxybutyrate and acetoacetate) by hepatic ketogenesis ; these ketones serve as a major fuel for the brain when their plasma concentrations become elevated, and can partially replace the need for glucose.
Clinical hypoglycemia is a plasma glucose concentration low enough to cause signs or symptoms of impaired brain function. Recognition of clinical hypoglycemia is most reliably made by Whipple’s Triad (symptoms compatible with hypoglycemia, associated with a low plasma glucose concentration, and relief of symptoms when glucose concentration is restored to normal). Recognition of hypoglycemia may be difficult when the patient cannot communicate symptoms (e.g., young infants and neonates). The glucose set-point (the physiologically normal range of plasma glucose) appears to be the same across all ages from the fetus to adulthood (70 to 100 mg/dL, 3.9 to 5.6 mmol/L, Fig. 152.1 ).
It must be emphasized that clinical hypoglycemia cannot be defined as a specific plasma glucose value. This is because (1) as shown in Fig. 152.1 , the various neural and endocrine responses to hypoglycemia occur across a range of plasma glucose concentrations, (2) brain responses may be altered by availability of alternative fuels (ketones), and (3) brain injury from low glucose concentrations depends on both the degree and duration of hypoglycemia. In addition, there are many potential artifacts in measuring plasma glucose concentrations: (1) venous and capillary blood have lower glucose concentrations than the concentration of arterial plasma glucose to which the brain is exposed, (2) the glucose concentration in plasma is approximately 15% higher than in whole blood due to the volume occupied by red blood cells, (3) glucose concentrations in whole blood specimens decline rapidly with delays in processing due to consumption by red and white blood cells, and (4) glucose measurements by bedside glucometer testing inherently have a 15% imprecision and are also highly susceptible to multiple artifacts of sampling and operator errors.
The symptoms of hypoglycemia reflect brain responses to glucose deprivation. These are not specific to hypoglycemia and fall into two categories. Neurogenic symptoms are caused by the activation of sympathetic nervous system discharge triggered by hypoglycemia. These include both adrenergic symptoms (tachycardia, palpitations, tremor, anxiety) and cholinergic symptoms (sweating, hunger, paresthesias). Awareness of hypoglycemia depends on perception of these neurogenic responses. Neuroglycopenic symptoms reflect brain dysfunction due to deficient glucose supply. The glucose threshold for neurogenic responses to hypoglycemia can be impaired by previous episodes of hypoglycemia for 24 hours or more (hypoglycemia unawareness or hypoglycemia-associated autonomic failure [HAAF]). However, the glucose threshold for neuroglycopenia is not affected by prior exposure to hypoglycemia. In patients with diabetes, HAAF is associated with increased risk of brain damage due to impairment of counter-regulatory responses to hypoglycemia and may also be a risk factor for hypoglycemic injury in neonates with persistent hypoglycemia disorders.
As mentioned, normal newborns in the first 2 days of life routinely have plasma glucose concentrations below the glucose set-point of the body, which is determined by the glucose utilization rates of peripheral tissues. The impact of this transient difference between glucose levels and glucose set-point in normal newborns is not known but is generally assumed to not have permanent adverse consequences if only mild and of brief duration.
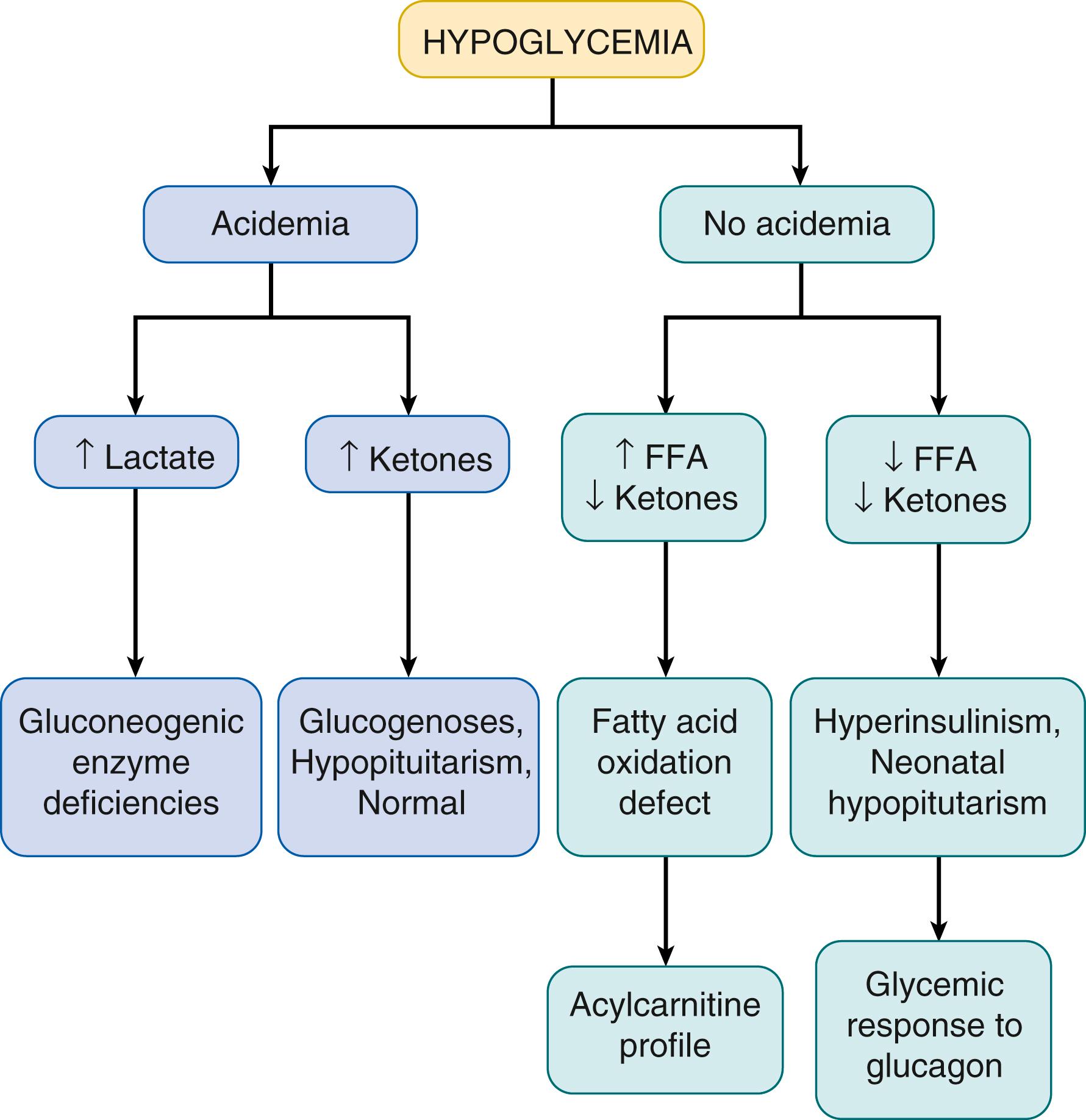
The integrity of the various metabolic and endocrine systems required for glucose homeostasis can be most readily tested by examining changes in the plasma levels of the major fuels at the end of a fasting test when plasma glucose concentrations have fallen toward 50 mg/dL ( Fig. 152.2A ). As shown in Fig. 152.3 , measurement of lactate (a major gluconeogenic substrate), β-hydroxybutyrate (the major ketone), and free fatty acids (released by lipolysis from adipose tissue) provides a robust foundation for the differential diagnosis of hypoglycemia disorders. Normally, at the time plasma glucose has fallen toward 50 mg/dL, plasma lactate remains low (<2.0 mM), but plasma levels of free fatty acids have increased several-fold to greater than 1 to 1.5 mM and levels of β-hydroxybutyrate have risen 10- to 20-fold to greater than 2.0 mM. Because plasma insulin concentrations are frequently not elevated enough to diagnose hyperinsulinism, the diagnosis of hyperinsulinism is most reliably based on evidence of excessive insulin effects, including inappropriate suppression of the levels of free fatty acids and, especially, of β-hydroxybutyrate. Confirmation of hyperinsulinism can be readily done by demonstrating inappropriate conservation of liver glycogen reserves during hypoglycemia as reflected by an inappropriately large (>30 mg/dL) glycemic response to injection of glucagon. It is important to note that plasma cortisol and growth hormone levels are often not high enough to rule out pituitary deficiency and, therefore, specific diagnostic testing for these hormones is frequently necessary. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here