Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In recent years, there has been an exponential expansion of our understanding of the epidemiology and adverse outcomes associated with neonatal acute kidney injury (AKI). From a multitude of single-center studies and in the recent multicenter Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study, AKI is now known to be associated with several adverse outcomes (increased length of stay, length of mechanical ventilation, mortality) in a variety of neonatal populations. Furthermore, neonatal AKI may increase the risk of chronic kidney disease (CKD) later in life. AKI can no longer be viewed as an incidental finding, but rather an independent risk factor for poor outcomes.
Neonates are vulnerable to AKI secondary to maternal, neonatal, and perinatal risk factors associated with the transition from intrauterine to extrauterine life (e.g., perinatal asphyxia, sepsis, nephrotoxic medications). Subsequently, factors associated with critical illness will predominate the risk for AKI. Finally, neonatal AKI may differ and have differing long-term implications, depending on the neonate’s gestational age, as nephrogenesis continues until 32 to 36 weeks of gestation. AKI in premature infants, in particular, may therefore have significant implications for long-term renal health and the development of CKD.
Future strategies to prevent and treat AKI will require a better understanding of the pathophysiology of neonatal AKI, risk factors associated with AKI, and more sensitive and specific methods for the detection of AKI. The purpose of this chapter is to describe the pathophysiology of neonatal AKI, identify associated risk factors, highlight recent epidemiology studies, and discuss the gaps in knowledge that need to be filled to improve outcomes.
The unique and rapidly changing physiology surrounding birth and the first few months of life creates challenges in the diagnosis of neonatal AKI. Many aspects of physiology unique to term or premature neonates pertinent to the study of AKI predispose them to AKI or create challenges for the rapid identification of an episode of neonatal AKI by clinicians.
Nephrogenesis begins at the fifth week of gestation and continues until 34 to 36 weeks gestation, yielding the adult complement of 200,000 to 2.7 million nephrons at the time of term delivery. , Autopsy data of full-term, generally healthy neonates provide evidence that the wide variation in nephron endowment is established early in life. , With nearly two-thirds of nephron formation occurring during the third trimester, nephrogenesis may be limited and disrupted by preterm birth. Rodriguez and colleagues found that premature infants who survived to 40 days of age had fewer layers of glomeruli than their term counterparts. When stratified for AKI status, those infants exposed to AKI had fewer glomerular layers than those without AKI. Sutherland and colleagues noted that premature infants seemed to have accelerated maturation of the glomeruli, with a smaller nephrogenic zone, suggesting early cessation of nephrogenesis, as well as signs of glomerular hypertrophy, dilation of Bowman space, and shrunken glomerular tufts. The concept of nephron loss during nephron development was evaluated in a recent rat study where unilateral nephrectomy during nephrogenesis led to a greater degree of glomerular and tubular damage in adulthood, as compared to the group that underwent nephrectomy after the epoch of nephrogenesis. Given their decreased number of filtration units, premature and very low-birth-weight (VLBW) infants have less renal reserve and are particularly susceptible to various renal insults encountered in the neonatal intensive care unit (NICU). ,
The newborn kidney undergoes important hemodynamic changes in the perinatal period. In contrast to adult kidneys, which receive 20% to 30% of cardiac output, the developing fetal kidney receives only 2.5% to 4% of cardiac output. Over time, the proportion of renal cardiac output seen by the kidney increases from 6% at 24 hours to 10% at 1 week and 15% to 18% at 6 weeks of age. Changes in renal perfusion pressure after birth occur because of increasing peripheral vascular resistance, coupled with decreasing renal vascular resistance. The decreased renal vascular resistance is driven by two main factors: a redistribution of renal blood flow within the kidney from the medulla and inner cortex during the fetal period to the outer cortex after birth, and a change in the balance of vasoconstrictive and vasodilatory factors affecting glomerular arteriolar resistance ( Fig. 164.1 ).
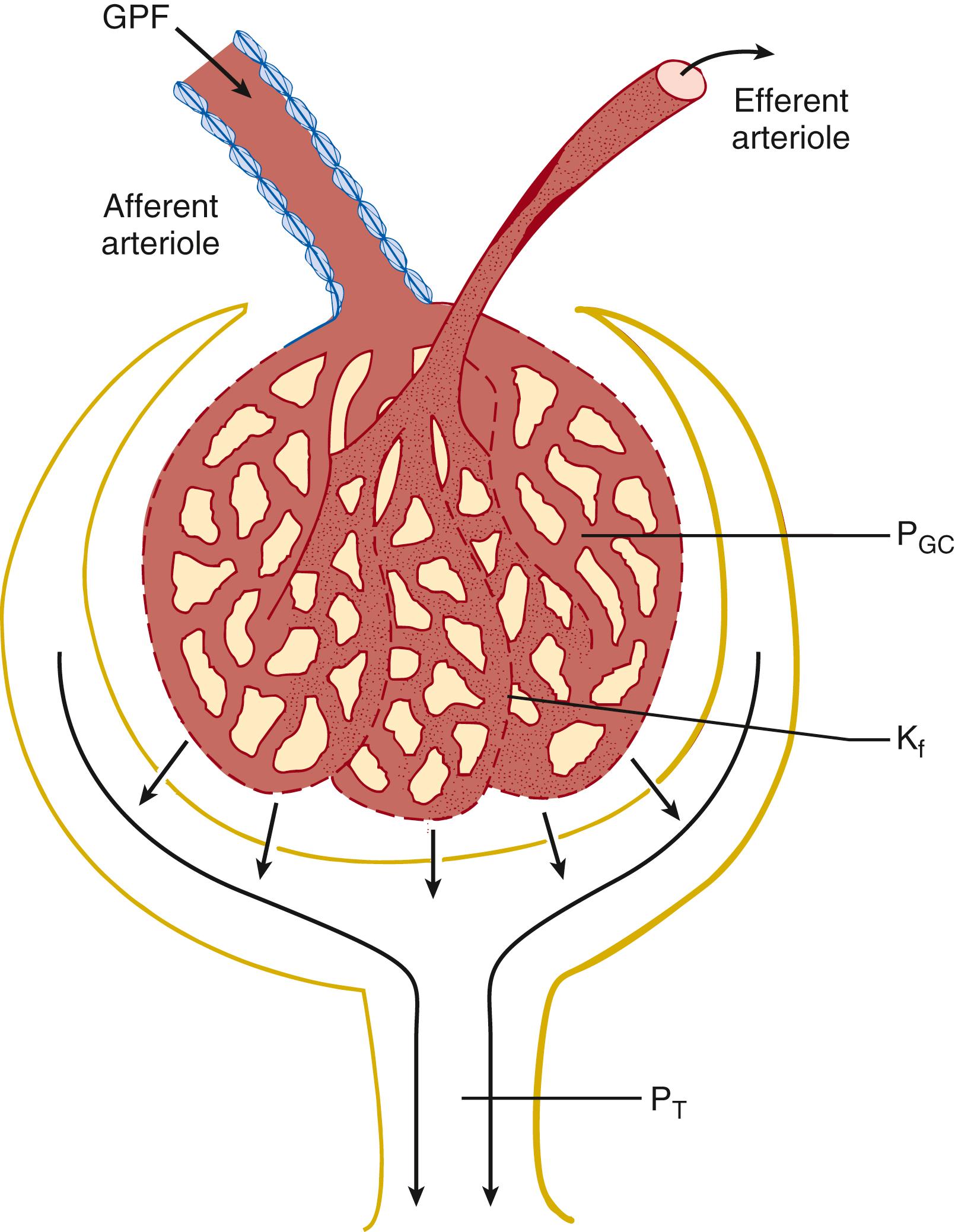
The renin-angiotensin-aldosterone system is a primary determinant of renal vasoconstriction and a key mechanism for maintaining the glomerular filtration rate (GFR) in the newborn. This pathway is active throughout fetal development and in the newborn period. Angiotensin II acts via the AT 1 receptor, causing vasoconstriction at the afferent and efferent arterioles. Angiotensin II increases GFR by a causing greater degree of constriction of and increase in resistance at the efferent arteriole relative to the afferent arteriole, increasing GFR. Other important renal vasoconstrictors active in the newborn period include endothelin, adenosine, and sympathetic innervation. ,
Vasodilators, including prostaglandins and atrial natriuretic peptide, increase renal blood flow by causing vasodilatation of the afferent arteriole. They counterbalance the effects of angiotensin II, catecholamines, and other vasoconstrictive substances in the neonatal kidney. , It follows then that nonsteroidal antiinflammatory drugs such as indomethacin, a potent prostaglandin inhibitor used to treat patent ductus arteriosus in premature infants, can cause severe, though usually reversible, acute kidney injury (AKI). Maternal exposure to nonsteroidal antiinflammatory drugs also predisposes neonates to decreased renal perfusion and AKI.
A delicate balance of homeostatic mechanisms in the neonate is required to maintain renal perfusion and GFR in the postnatal period. Although it rises quickly after birth, the GFR of the newborn infant is still low compared with that of older children and will not reach the level associated with normal renal function in adults until 18 to 24 months of age. A number of maternal exposures and perinatal events can disrupt the homeostatic balance and lead to decreased renal blood flow, impaired renal perfusion, and AKI. Common causes of renal hypoperfusion in the NICU include low intravascular volume from fluid losses such as insensible losses, chest tubes, blood loss, nasogastric suction, abdominal drains, and chronic diuretic use. Poor cardiac preload can occur from high mean airway pressures or congenital heart disease; congestive heart failure can also lead to poor perfusion. Low oncotic pressure can lead to low intravascular volume. Correction of the underlying problem with restoration of adequate renal blood flow is critical for the normalization of renal function.
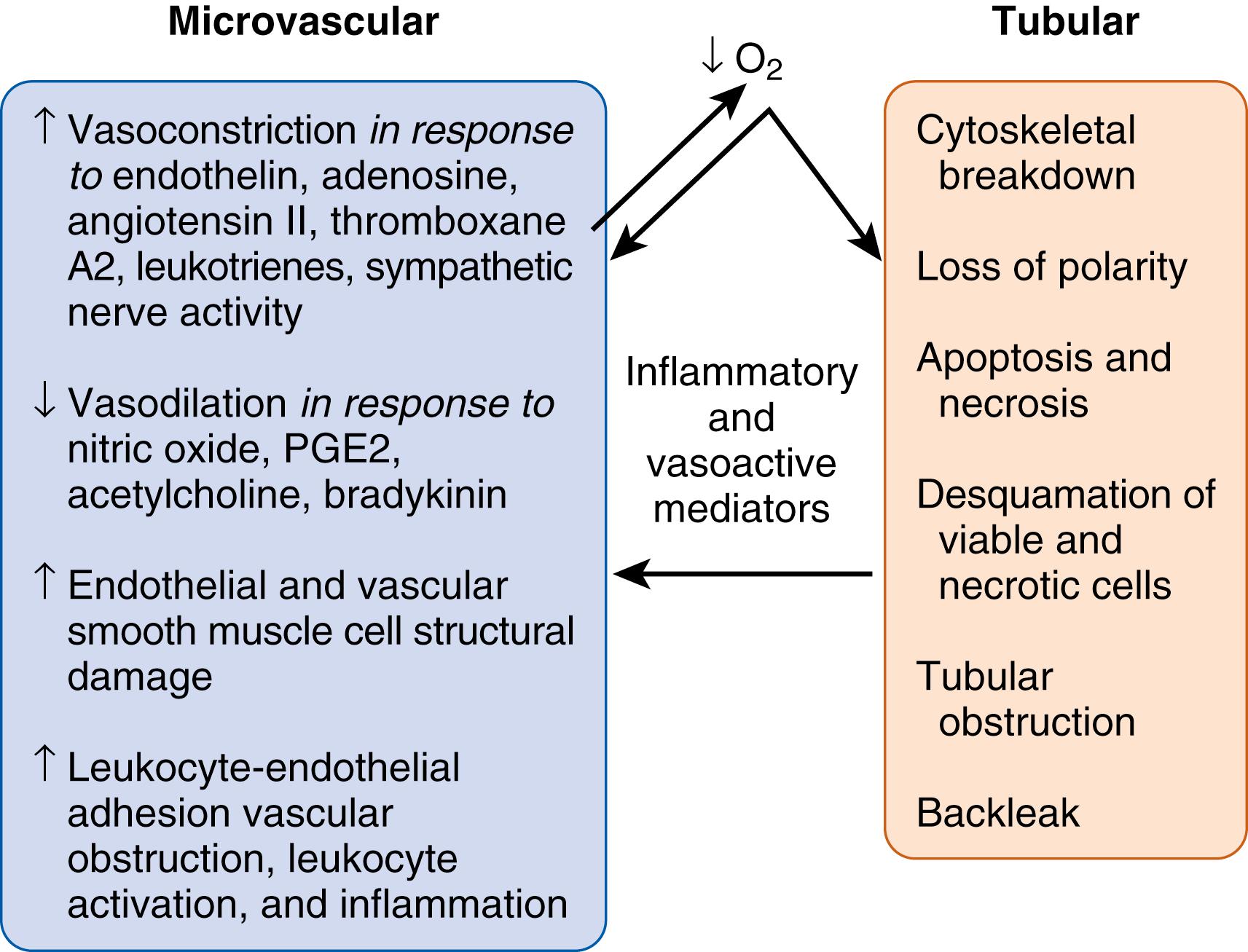
Prolonged renal hypoperfusion or severe acute hypoperfusion events from perinatal asphyxia, poor cardiac function, hypoalbuminemia, or hypotension can cause inadequate oxygen delivery resulting in cellular injury and more pronounced organ dysfunction, such as acute tubular necrosis. A series of important events have been well described in the course of ischemic AKI. These include epithelial cell injury with subsequent structural changes and in some cases cell death, endothelial injury and dysfunction, inflammation, and repair ( Fig. 164.2 ). These events correspond with the phases of ischemic AKI classically described as the initiation, extension, maintenance, and recovery. Decreased renal blood flow leads to inadequate intracellular ATP levels and subsequent cellular injury or death depending on the severity of the insult (initiation phase). The areas that have the highest metabolic demand and decreased ability to convert to anaerobic metabolism (including the proximal tubule and the medullary thick ascending limb) are particularly susceptible to hypoxic-ischemic injury. In the proximal tubule, the apical brush border is lost through the detachment of the microvilli that subsequently form membrane-bound “blebs,” which are released into the tubular lumen.
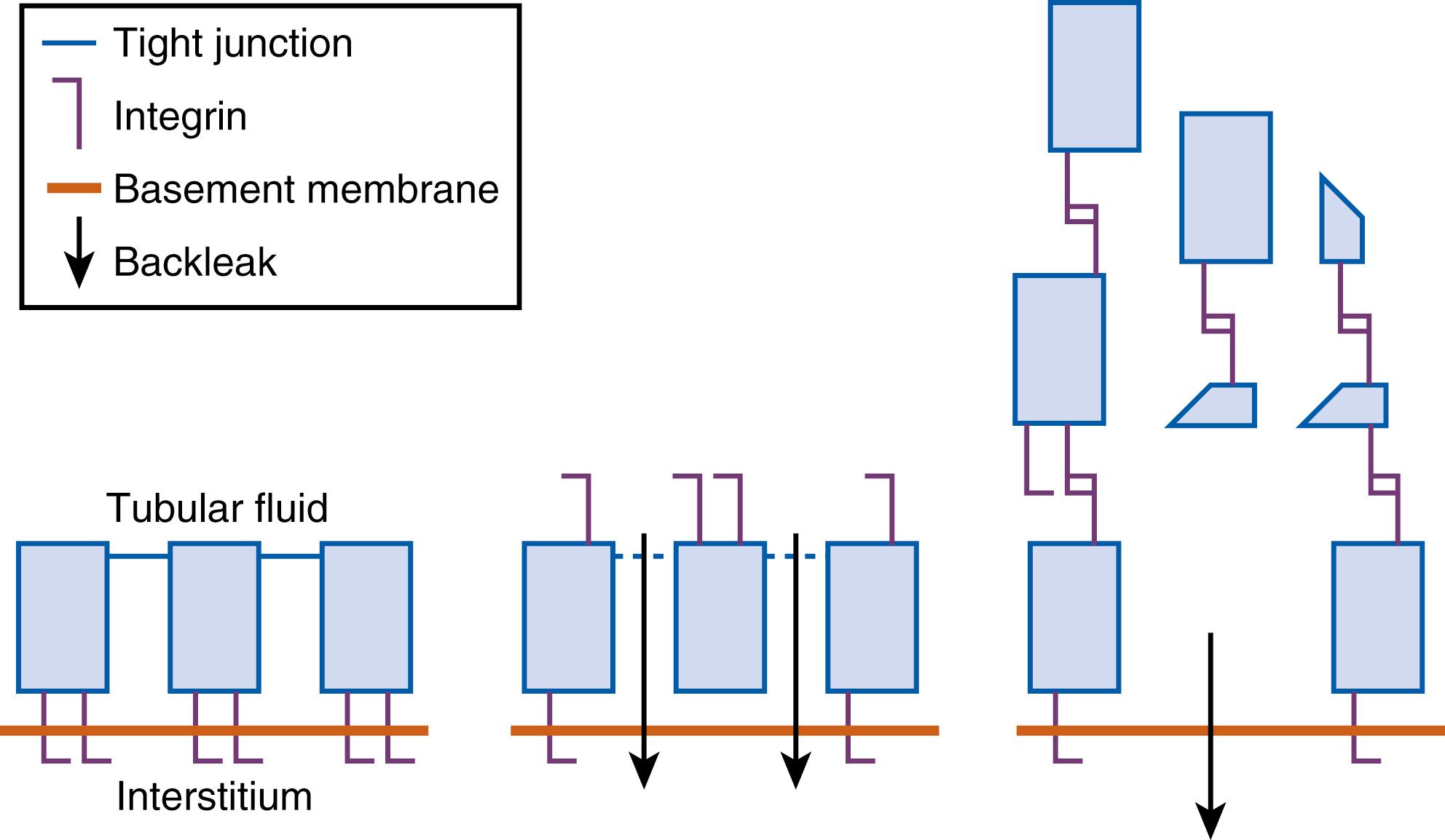
The actin cytoskeleton, a key element for maintaining cell structure and function, also undergoes important structural changes after the loss of intercellular adhesion junctions that anchor the epithelial cells to the basement membranes. These structural elements normally serve important roles in paracellular transport, cell polarity, and cell morphology. Loss of their integrity allows back-leak of the glomerular filtrate into the interstitium, as well as sloughing of the epithelial cells into the tubular lumen. Detached cells, cellular debris, and microvilli blebs may form granular casts that obstruct the tubular lumen and lead to loss of function.
The disruption of the actin cytoskeleton results in changes in cell polarity and function. Na + -K + -ATPase pumps normally found on the basolateral membrane redistribute to the apical membrane and allow bidirectional transport of sodium and water. As a result, cellular sodium may be transported back into the tubular lumen, resulting in a high fractional excretion of sodium characteristic of acute tubular necrosis. Further decline in GFR may then result from the activation of tubuloglomerular feedback by the delivery of high sodium concentrations to the distal tubule ( Fig. 164.3 ).
The impact of the ischemic injury on tubular epithelial cells depends on the severity and duration of the event. If the underlying condition is corrected in a timely fashion and adequate perfusion is restored, cells may demonstrate structural and functional recovery. With more severe injury, cells may undergo either apoptosis or necrosis.
In addition to tubular injury, ischemia results in endothelial cell dysfunction that in turn perpetuates ongoing ischemia and further renal injury (extension phase). As noted previously, certain segments of the nephron are more profoundly affected by poor perfusion than others. Ischemia may persist in those areas even after renal blood flow has been restored, highlighting the important concept that ischemic AKI is driven by changes in the microcirculation and not solely by systemic pressure or global renal blood flow. The endothelium undergoes loss of structural integrity that results in increased microvascular permeability. Vascular permeability allows the passage of chemokines and cytokines between the blood compartment and adjacent renal tubules, promoting inflammation in both regions. In addition, ischemia induces a prothrombotic environment, leading to microvascular congestion and dysfunction.
Endothelial dysfunction and damage have implications for long-term kidney outcomes, as data suggest that the vasculature does not regenerate or recover in the way that the tubular epithelial cells can. This lack of recovery, or “vascular dropout,” and subsequent scar formation may be key risk factors for the development of CKD after AKI , (see the section entitled “Long-Term Outcomes and Progression to Chronic Kidney Disease” later in this chapter).
Inflammation plays an important role in the perpetuation of tubular and endothelial injury, resulting in apoptosis and necrosis (maintenance phase). A number of inflammatory cells and chemical mediators influence this process. Toll-like receptors 2 and 4, components of the innate immune system, have been identified as important components in the development of AKI. These proteins are expressed on the renal epithelium and are upregulated during ischemic AKI, further amplifying the inflammatory process. Moreover, the deletion of TLR2 or TLR4 protects against renal injury in animal models. ,
The kidney has many mechanisms responsible for protection, repair, and recovery (recovery phase). Restoration of blood flow is followed by repair of cells with mild injury; cells with more severe injury go through a process of dedifferentiation during which they appear flattened with an ill-defined brush border. Cells that have not undergone necrosis or apoptosis are able to proliferate across the basement membrane and undergo restoration of the cytoskeleton and proper cell polarity. Cell recovery is also aided by the heat shock proteins, a system that assists recovery in the proper folding of both denatured and newly synthesized proteins. , An association between genetic polymorphisms that result in low HSP72 protein expression and increased risk of AKI in VLBW neonates was demonstrated in a Hungarian neonatal cohort. Heme oxygenase 1, an enzyme with antiinflammatory effects, conferred resistance to apoptosis in an in vitro study. Kidney injury molecule 1 and transmembrane glycoprotein nonmetastatic melanoma B (NMB) have been identified in animal models as phagocytic proteins that promote cell repair and remodeling through the removal of apoptotic cellular debris. ,
The tubular and endothelial cell damage that occurs during ischemic AKI leads not only to dysfunction within the kidney but also to a systemic inflammatory response that may impact distant organs. This inflammatory dysregulation is partly due to dysfunctional immune, inflammatory, and soluble mediator metabolism. , Thus AKI can lead to pulmonary inflammation, brain injury, and cardiac dysfunction.
AKI in the context of multiorgan dysfunction is of growing interest because AKI portends poor clinical outcomes independently of the severity of illness and comorbid conditions. AKI may play an important role in modulating the inflammatory response in critically ill patients. Proinflammatory mechanisms, including neutrophil migration, cytokine expression, and oxidative stress, increase the risk of injury to other organs in the setting of AKI. Brain and lung development are of particular interest in the neonatal population; thus the impact of AKI on these organ systems is described as follows.
The negative impact of AKI is particularly profound when coupled with comorbid lung injury. Indeed, the kidney plays an important role in the physiologic processes that directly and indirectly influence the ability of the lungs to deliver oxygen. The kidney has an important impact on fluid balance, vascular tone (renin-angiotensin system), acid/base status, and hormone production (erythropoietin). Large observational studies in critically ill adults have shown mortality rates of more than 80% when AKI is complicated by respiratory failure or vice versa. , AKI occurs in up to 82% of critically ill children who require ventilator support, and those with AKI have worse lung function, a higher number of ventilator days, and a poorer oxygenation index than similar children without AKI. , Recent data from the AWAKEN study demonstrate similar findings in neonates. ,
The interaction between lung and kidney injury may be particularly important for premature infants who are at risk of the development of bronchopulmonary dysplasia. Data in preterm neonates, along with near term/term neonates, are becoming available, suggesting a link between fluid balance/AKI and respiratory outcomes. Data from the AWAKEN study show that the peak fluid balance during the first postnatal week and the fluid balance on postnatal day 7 were independently associated with the need for mechanical ventilation on postnatal day 7 in both preterm and near-term neonates. This association persisted even after adjusting for AKI, suggesting an independent impact of fluid on outcomes. In addition, data from the AWAKEN cohort also show that AKI is independently associated with chronic lung disease in premature and near-term/term neonates.
Several animal models have shown that the association between AKI and lung disease is not just about fluid overload. AKI has been shown to impact the lungs by influencing vascular permeability, cytokine profiles, and cellular immunity. , The integrity of the lung epithelium is dependent on tight junctions and channels involved in water and sodium transport, including epithelial sodium channels and aquaporins. Animal models of bilateral nephrectomized mice show a downregulation of these channels in the alveolar epithelium. Animal models of nonoliguric AKI show pulmonary inflammation characterized by an abundance of inflammatory cells and abnormal lung permeability. , Proinflammatory cytokines such as IL-6 and IL-1β triggered by an AKI episode contribute to lung injury. , , AKI has been shown to influence cellular immunity in the lungs, including T-cell handling of antigens and neutrophil migration. , Notably, fluid overload was not a factor in these animal models, suggesting that AKI may lead to abnormal lung function and architecture independently of the effects of volume status. Although the association of AKI with lung injury has been reported in preterm neonates, it is uncertain if the increased risk of bronchopulmonary dysplasia is due to fluid overload or intrinsic lung damage. ,
Both animal and human data, including data from several studies in neonates, have demonstrated an association between AKI and brain injury in the setting of hypoxic-ischemic AKI. In a mouse model of ischemia-reperfusion injury, Liu and colleagues demonstrated a broad range of inflammatory changes and abnormalities in the brain after a severe ischemic AKI event. Specifically, the brains of mice in the study group had higher levels of inflammatory markers, more extensive cellular inflammation, greater injury to susceptible areas of the brain as shown by increased numbers of pyknotic neuronal cells in the hippocampus, and microvascular dysfunction with disruption of the blood-brain barrier. In addition, the mice in the ischemic AKI group demonstrated decreased locomotor activity (behavioral dysfunction) after the AKI event. Several single-center studies have shown an association between AKI during perinatal asphyxia and subsequent neurologic outcomes. , Furthermore, recent data in a single-center study and in the AWAKEN cohort suggest that AKI is independently associated with intraventricular hemorrhage. The authors postulate that this could be due to vasoactive hormone release during AKI, hypertension from fluid overload, or inflammatory mediators that impact the blood-brain barrier.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here