Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Jaundice is a common transitional phenomenon in newborn infants and may have been first described in the Chinese textbook On the Origins and Symptoms of Diseases written in CE 610. It is not clear when the association between neonatal jaundice and yellow staining of the brain was first noticed, but the staining phenomenon later known as kernicterus was first described in 1875 by Johannes Orth, who had performed an autopsy on an infant that died at 2 days of age with very intense jaundice. The term kernicterus , the German word for jaundice of the nuclei (or basal ganglia), was coined by Christian Georg Schmorl, in a landmark article in 1904. Of the 120 jaundiced infants he had autopsied, 114 had jaundiced brains, but only 6 of the 120 exhibited a pattern of more intense yellow color of the basal ganglia and medulla oblongata, which gave rise to the term. More than 100 years have passed since then, but the explanation for this localization phenomenon still eludes medical researchers.
In addition to severe jaundice and lethargy progressing to stupor and coma, the clinical picture in the infants who died included hypotonia, increasing tone in extensor groups, retrocollis/opisthotonos, seizures, ophthalmoplegia (paresis of upward gaze), fever, high-pitched cry, and poor sucking. During the first half of the 20th century it became clear that the pathoanatomic finding of jaundiced brain nuclei had a clinical correlation in infants who survived extreme jaundice. Clinical manifestations that appeared to evolve during the first days/weeks to 2 to 3 months of life included hypertonicity, opisthotonos, absent Moro reflex, high-pitched cry, and poor feeding. From then on until approximately 2 years of age, marked delay of motor development became obvious, with decreased muscle tone, hyperreflexia, and persistence of immature postural patterns. Athetosis could begin towards the end of the second year of life but might not become apparent until 8 to 9 years of age. The degree of athetosis was quite variable, from hardly detectable (except to the trained observer) to completely disabling. Some degree of hearing loss was present in most patients. Paresis of upward gaze appears to be fairly obligate in kernicterus but is rare in other types of cerebral palsy. Intellectual deficits were not necessarily present, and only a minority of the patients had developmental delay. The sequelae of bilirubin encephalopathy may present a many-faceted picture, and a proposal for updated nomenclature that incorporates these aspects is discussed below.
There appears to be general agreement among present-day bilirubin researchers that the toxic influence of bilirubin on brain cells is the primary and causative factor in kernicterus. However, as yet no agreement exists on which mechanism(s) might mediate brain bilirubin toxicity. This chapter provides an overview of research on the effects and interactions of bilirubin with the brain, and discusses the relative merits and weaknesses of several theories on the “basic mechanism of bilirubin neurotoxicity.”
Kernicterus, strictly speaking, refers to the pathoanatomic finding of intense yellow coloring of the basal ganglia and associated structures superimposed on a paler yellow background as first described by Orth and Schmorl. When Georg Schmorl coined the term kernicterus , the term kern (i.e., basal ganglia) probably included most of the subcortical structures of forebrain grey matter. Present-day neuroanatomists use the term basal ganglia more to describe a set of functionally related cell groups rather than in a strictly topographic sense, a concept illustrated in Figs. 163.1 and 163.2 . This evolving terminology should be kept in mind when considering the study of Zuelzer and Mudgett, who compared the frequency of staining of brain areas/structures in kernicterus cases. In descending order of frequency, they found that stained areas included the hippocampus, thalamus, hypothalamus, corpus striatum, medulla, olives, pons, and dentate nucleus. In these areas ultrastructural findings have included membrane alterations, dense cytoplasmic bodies thought to represent degenerated mitochondria, and calcium granules. Changes of this nature are likely to be irreversible and to represent the pathoanatomic correlate of the chronic, clinical after effects of bilirubin brain toxicity, until recently called kernicterus. Thus, a discussion of the mechanisms of bilirubin-induced brain injury must necessarily include events that cause or are associated with cell death.
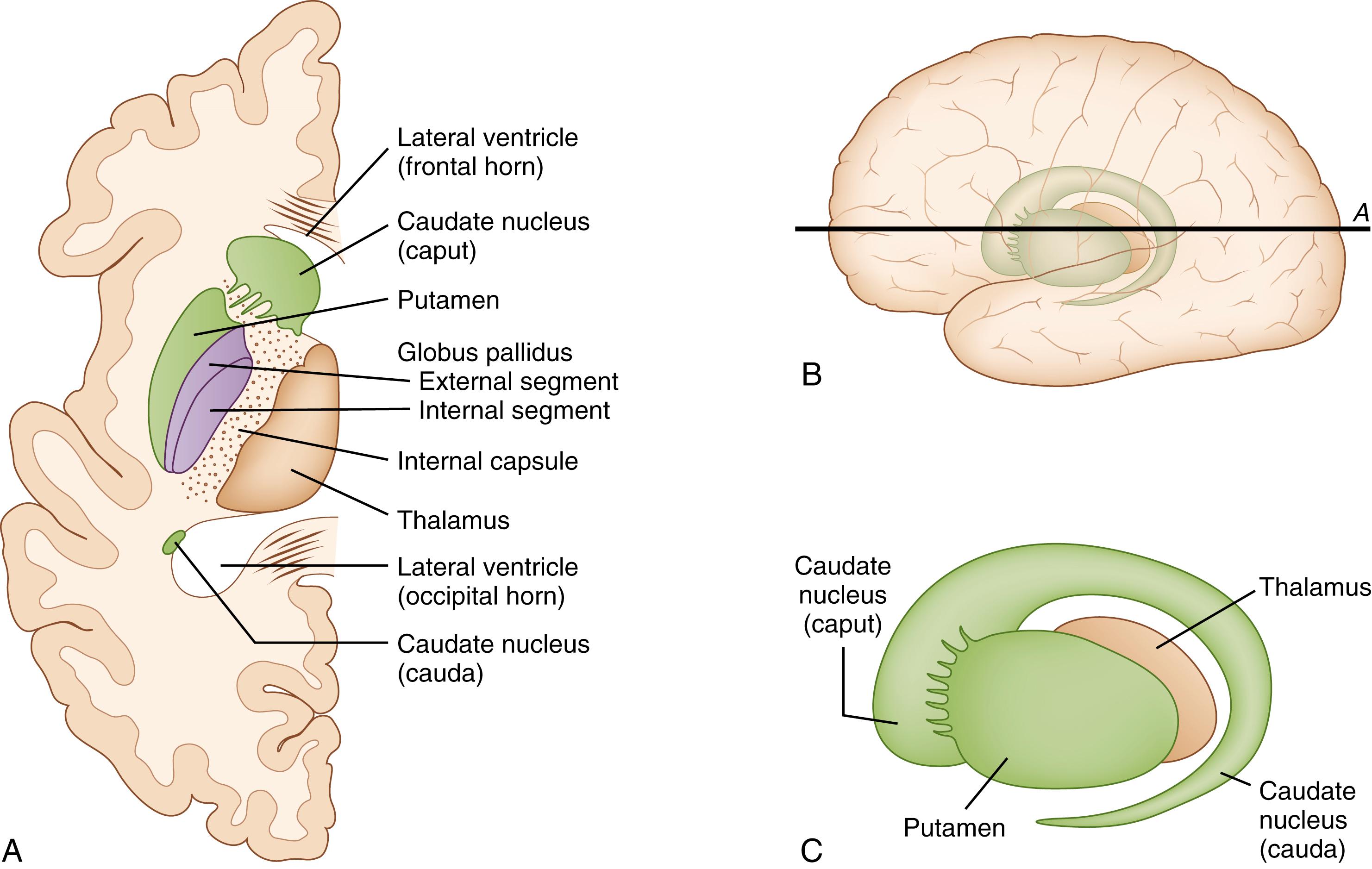
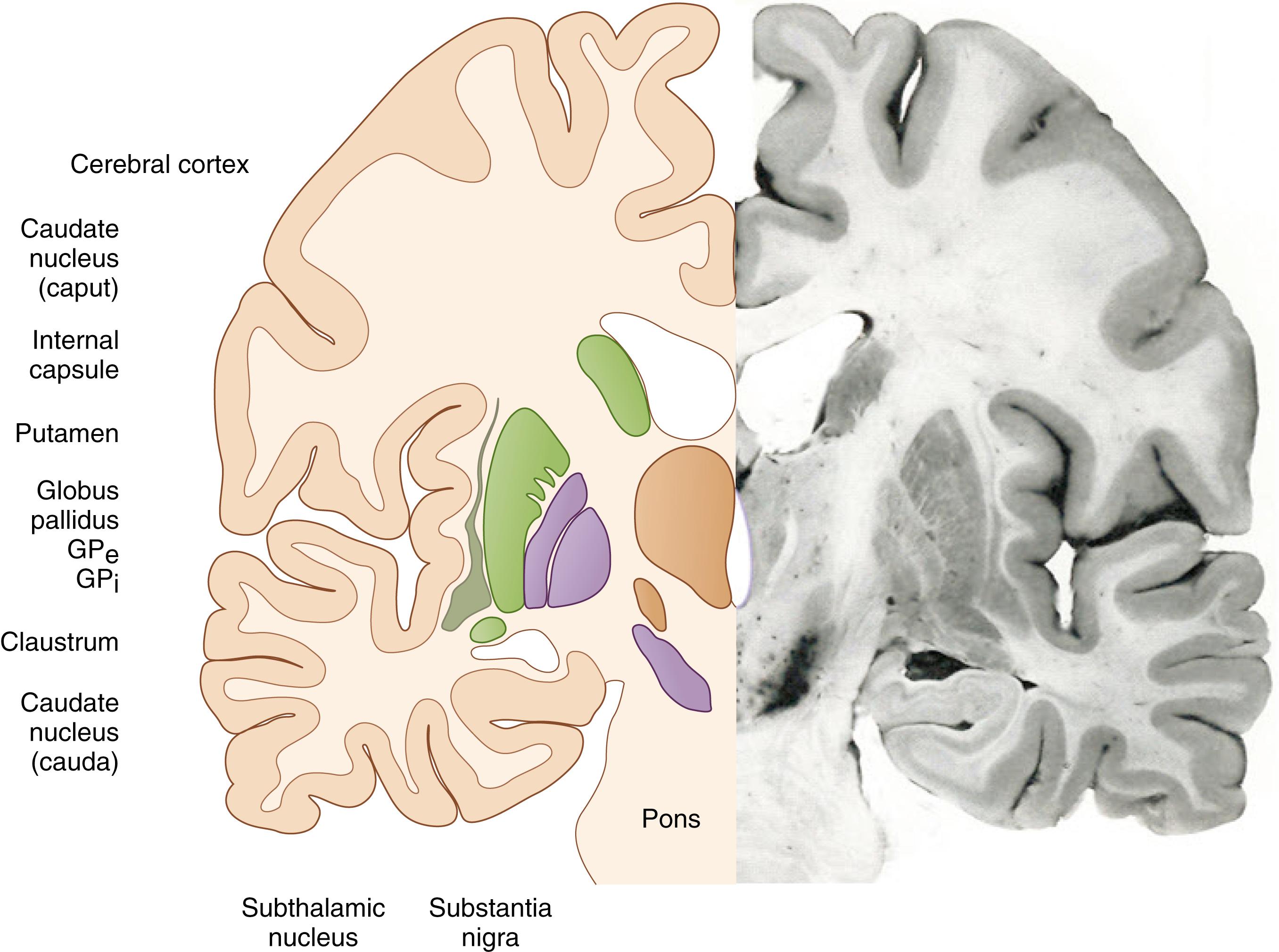
With modern imaging techniques, such as magnetic resonance imaging, lesions may be seen in both the globus pallidus and the subthalamic nucleus. Lesions in the globus pallidus tend to be more intense than those in the subthalamic nucleus, whereas lesions in the auditory brain stem nuclei and cerebellum are often not noted on routine magnetic resonance imaging scans. Magnetic resonance imaging scans done within a few weeks of the neurotoxic jaundice episode in babies who develop chronic sequelae tend to show bilateral hyperintensities in the globus pallidus on T1 weighting, without change on T2 weighting. Subsequently, the magnetic resonance imaging scan may become apparently normal, but later T2 and fluid-attenuated inversion recovery images become hyperintense.
However, bilirubin effects on the brain often appear to be transitory. Thus, neonates with significant jaundice frequently exhibit lethargy or drowsiness, hypotonia, and feeding problems. Evoked response studies, both in human infants and experimental animals, have given more objective evidence of such reversible toxicity, although permanent changes have been found in some subjects. , The increased incidence of apnea in jaundiced premature infants, as compared with less jaundiced controls, appears to abate after the first week of life and is associated with progression of changes in the auditory brain stem response. This type of reversible toxicity is not kernicterus, yet there is little doubt that it reflects bilirubin effects on the brain.
Recently a group of bilirubin experts proposed a revision of the terminology used to describe and classify bilirubin effects on the brain. They introduced kernicterus spectrum disorder (KSD) as an overarching term to reflect the range of bilirubin effects, which includes both acute and chronic signs as well as type and severity of damage. Proposed modifier terms include mild, moderate, and severe. Subtypes may then be described in terms of auditory (auditory-predominant), motor (motor-predominant), and classical (involving both auditory and motor dysfunction). Further, the authors suggest retaining the term acute bilirubin encephalopathy (ABE), as it correctly describes the neurologic effects of ongoing bilirubin exposure. However, the term chronic bilirubin encephalopathy (CBE) should be discarded in favor of KSD, because CBE may be misinterpreted to suggest that bilirubin exposure is still happening, although the signs and symptoms we observe are in fact the long-term sequelae of an acute, time-limited bilirubin exposure that happened in the past. Similarly the term bilirubin-induced neurologic dysfunction (BIND) has been used both as a scoring system and as a descriptive term for a range of bilirubin effects on brain function and should be abandoned in favor of KSD. The authors also proposed a set of guidelines to define levels of severity for motor and auditory sequelae.
It is not clear whether ABE and KSD represent the extreme ends of a continuum of toxicity, or whether separate and distinct mechanisms are involved in cell death versus a transitory disturbance in neuronal signaling. Even moderate degrees of hyperbilirubinemia may have discernible long-term effects on behavior, and questions relating to the sensitivity and specificity of the tools used to study the long-term effects of neonatal jaundice will have to be resolved before we can determine what effects are reversible. This chapter therefore discusses bilirubin-induced brain injury in a wide sense, including bilirubin effects on processes that may not result in cell death and residual damage. Whether such effects may permanently alter cell functions without causing cell death is at present not known.
Certain aspects of bilirubin chemistry and solubility are inextricably linked to bilirubin neurotoxicity and therefore are briefly discussed here. The main bilirubin isomer in humans is bilirubin IXα ( Z , Z isomer), which exists either as a charged dianion or as bilirubin acid. The eight hydrophilic groups of the dianion impart some water solubility at neutral pH, whereas the presence of intramolecular hydrogen bonds in the bilirubin acid results in near-insolubility in water. , The hydrophobic bilirubin IXα isomer appears to be responsible for the toxic effects, whereas the water-soluble isomers are believed to be nontoxic. Bilirubin becomes water soluble when it is conjugated to glucuronic acid in the hepatocytes, and when bound to albumin for transport in serum. Isomerization of the bilirubin molecule during phototherapy also results in molecules that are more polar (photoisomers).
Bilirubin experts disagree on the issue of bilirubin solubility and its impact on studies of bilirubin brain toxicity, hence the claims that many experiments on bilirubin toxicity have been done at nonphysiologic concentrations, and thus the results may not be relevant. Others argue that concentrations of bilirubin for in vitro studies must mimic those found in the brains of infants with kernicterus, as well as those found in experimental animals with clinically relevant levels of serum bilirubin.
Bilirubin IXα ( Z , Z ) is an amphipathic molecule but, with respect to membranes, behaves as lipophilic; thus it binds to and crosses phospholipid membranes. It is presumably this characteristic that enables bilirubin IXα ( Z , Z ) to cross the intact blood-brain barrier and enter the brain. A minute amount of bilirubin (in the nanomolar range) is always present in plasma as “free” or unbound bilirubin. According to the “free bilirubin theory,” it is this unbound bilirubin moiety that enters the brain to produce neuronal injury. Increased concentrations of unbound bilirubin in the blood will, by the laws of equilibrium, shift more bilirubin into tissues, including the brain. Bilirubin is bound to albumin at primary and secondary binding sites, with higher affinity at the primary site, such that the concentrations of unbound bilirubin in serum are normally very low. , , Factors that increase the concentrations of unbound bilirubin, and thus augment bilirubin entry into brain, include altered albumin characteristics, changes in pH, and the presence of exogenous or endogenous binding competitors. An epidemic of kernicterus occurred in the 1950s and was shown to be related to the use of sulfisoxazole. In subsequent years it was shown that many drugs compete with bilirubin for binding to serum albumin. Bilirubin “displacers” are now well recognized to increase the risk of bilirubin neurotoxicity in jaundiced infants, and this has significantly influenced the use and choice of drugs in neonatal medicine. Numerous animal studies show that the blood-brain equilibrium for bilirubin is shifted towards the brain when bilirubin-displacing substances are administered. ,
The water solubility of bilirubin photoisomers raises questions regarding their neurotoxicity. McDonagh and Lightner proposed decades ago that bilirubin photoisomers ought to be less toxic than the predominant IXα ( Z , Z ) isomer. Also, by virtue of their polarity the photoisomers should not easily cross the blood-brain barrier, lacking a specific transporter. Thus, the theoretical arguments are chemically and physiologically coherent. The reports of apparent reversibility of acute intermediate-to-advanced stage bilirubin encephalopathy with timely aggressive therapy may also be interpreted to support this hypothesis. , Unfortunately, experimental models to test this hypothesis have been hard to devise. Thus many of the in vitro studies of bilirubin photoisomers in cultured cells have methodologic issues that limit the conclusions that can be drawn. However, a recent well-controlled study exposed a human neuroblastoma cell line (SH-SY5Y) to bilirubin-IXα (Z,Z), or lumirubin, or a mixture of bilirubin-IXα (Z,E/E,Z) isomers. These had been carefully prepared and purified. While the cells exposed to the IXα (Z,Z) isomer exhibited significant loss of viability that increased with time, viability was not affected in cells exposed to photoisomers.
Entry of bilirubin into brain is a sine qua non for neurotoxicity. An unstable state of equilibrium appears to exist between bilirubin in the blood and bilirubin in the brain, an equilibrium influenced by a number of factors ( Fig. 163.3 ). The blood-brain gradient of bilirubin is high; in experimental animals with an intact blood-brain barrier the brain bilirubin concentrations are only 1% to 2% of the serum bilirubin concentrations. Molecules that can cross the blood-brain barrier appear to share certain characteristics, including (1) low molecular weight (<400 daltons), (2) increased lipid solubility, and (3) not being a substrate for an efflux transporter. Bilirubin has a molecular weight of 585; its lipid solubility depends on the isomeric form, , and it is a substrate for an efflux transporter (P-gp). Thus, native bilirubin really does not satisfy any of these criteria. This may explain why bilirubin entry into brain is limited. The blood-brain barrier may be more permeable to bilirubin in neonates than in mature subjects, and in studies with experimental animals the brain-to-blood ratios appear to be higher in subcortical regions in immature organisms, suggesting the possibility that the subcortical areas may be more accessible to bilirubin entry. On the other hand, albumin permeability is equally restricted in both young and old subjects. Studies involving P-gp (a bilirubin transporter) indicate that expression of this efflux pump for bilirubin in endothelial cells increases with maturation and may contribute to the perceived immaturity of the blood-brain barrier in the newborn.
![Fig. 163.3, Factors that modulate the bilirubin-brain equilibrium. A number of factors can modulate bilirubin uptake into and clearance from brain. These include the avidity of bilirubin binding to serum albumin, the presence of competitors for the albumin binding site, immaturity or other factors that may affect blood-brain permeability, the presence and activity or expression of membrane pumps in the blood-brain barrier, and brain blood flow. A, Albumin; A-B, albumin-bound bilirubin; B, (unbound) bilirubin; BV , blood vessel; CSF, cerebrospinal fluid; MRP1, multidrug resistance–associated protein 1 (also referred to as ATP binding cassette subfamily C member 1 [ABCC1); P-gp , phosphoglycoprotein (also referred to as multidrug resistance protein 1 [MDR1] or ATP-binding cassette subfamily B member 1 [ABCB1]); ST, stroma; TJ, tight junction. Fig. 163.3, Factors that modulate the bilirubin-brain equilibrium. A number of factors can modulate bilirubin uptake into and clearance from brain. These include the avidity of bilirubin binding to serum albumin, the presence of competitors for the albumin binding site, immaturity or other factors that may affect blood-brain permeability, the presence and activity or expression of membrane pumps in the blood-brain barrier, and brain blood flow. A, Albumin; A-B, albumin-bound bilirubin; B, (unbound) bilirubin; BV , blood vessel; CSF, cerebrospinal fluid; MRP1, multidrug resistance–associated protein 1 (also referred to as ATP binding cassette subfamily C member 1 [ABCC1); P-gp , phosphoglycoprotein (also referred to as multidrug resistance protein 1 [MDR1] or ATP-binding cassette subfamily B member 1 [ABCB1]); ST, stroma; TJ, tight junction.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PathophysiologyofKernicterus/2_3s20B9780323712842001634.jpg)
Bilirubin may interact with biologic membranes in a way that affects their function, thus it seems reasonable to ask whether bilirubin could also affect the blood-brain barrier. In that regard, perturbation of blood-brain barrier function by bilirubin might be secondary to the toxic effects of bilirubin on glial cells. , Studies addressing the question of direct bilirubin effects on the blood-brain barrier found that pre-exposure to bilirubin increased the permeability of the blood-brain barrier both to a dye and to bilirubin itself. ,
P-gp is a member of the adenosine triphosphate (ATP)-binding cassette superfamily of membrane transporters, which are expressed both in normal and in diseased tissues and appear to limit the entry of xenobiotics into cells. P-gp (encoded by ABCB1 ) is localized on the luminal (blood) side of the blood-brain barrier. Bilirubin is a substrate for P-gp and may also inhibit P-gp function. , This has several interesting implications for the interaction between bilirubin and brain. Brain bilirubin content in P-gp-deficient mice after an intravenous bilirubin bolus was almost twofold higher than in wild-type mice. Drugs known to inhibit P-gp significantly enhanced bilirubin entry into rat brain after an intravenous bilirubin bolus. P-gp function appears to be regulated by phosphorylation, and phosphorylation has been linked to enhanced activity of P-gp. , This provides a possible link to understanding how bilirubin might increase blood-brain barrier permeability, , as bilirubin has been shown to inhibit phosphorylation of a wide range of protein-peptide substrates. ,
In astrocytes, which are important for blood-brain barrier function, bilirubin appears to mediate up-regulation of multidrug resistance–associated protein 1 (MRP1) (a member of the ATP-binding cassette transporter superfamily), resulting in decreased sensitivity to bilirubin toxicity. However, other findings suggest that astrocytes are sensitive to toxicity, and particularly that immature cells are more vulnerable than mature cells. , In mouse embryo fibroblasts, intracellular accumulation of [ 3 H]bilirubin, as well as cytotoxicity, were significantly greater in cells from MRP1-deficient mice than in cells from wild-type controls. The implications of these findings remain to be elucidated and clearly need further study.
Opening of the blood-brain barrier by radiation, inflammation, asphyxia/hypoxia, hyperosmolality, and hypercarbia has been shown to increase bilirubin entry into the brain in a number of studies. , , , , The latter three conditions may occur quite frequently in sick neonates and appear to be relevant for clinical management of jaundice in neonatal intensive care unit (NICU) infants. In hypercarbia, most, but not all, of the bilirubin enters brain as the unbound molecule; however, with hyperosmolality considerable entry of albumin into the brain also occurs, and in the jaundiced individual a large proportion of this albumin will carry bilirubin. In hypercarbia the rapid entry of bilirubin into the brain is increased compared to control conditions, but clearance is also rapid. In contrast, with hyperosmolality it appears that bilirubin remains in the brain significantly longer than under control conditions. Whether such prolonged exposure is associated with an increased risk of toxicity is speculation, but some clinical data suggest that not only the level of serum bilirubin but also the duration of exposure is associated with risk of neurologic sequelae.
Opening of the blood-brain barrier may contribute to or exacerbate bilirubin neurotoxicity. , , In vitro studies suggest that albumin blocks the toxic effects of bilirubin when present in equimolar concentrations. , , Opening of the blood-brain barrier (e.g., with hyperosmolality) permits the entry of albumin-bound, as well as unbound, bilirubin, and, because the equilibrium balance in serum is heavily tilted towards albumin-bound bilirubin, the observation of increased toxicity in vivo is somewhat surprising. Earlier studies had suggested that signs of toxicity might best be predicted by total brain bilirubin content, and enhanced binding to albumin appeared to be protective. More recently, Daood and Watchko demonstrated an association between calculated free bilirubin levels in brain and signs of toxicity in a Gunn rat pup model.
The role of brain blood flow may be best illustrated by studies of hypercarbia, which results in increased brain blood flow. , , , These studies show that increased brain blood flow in experimental animals is associated with increased entry of bilirubin into brain. One may speculate that with increased brain blood flow each circulating bilirubin molecule passes the blood-brain barrier more often and thus has more opportunity to equilibrate with bilirubin in the brain.
If we accept the likelihood that bilirubin IXα ( Z , Z isomer), in the presence of an intact blood-brain barrier, enters the brain primarily in the unbound form, it is reasonable to ask in what form bilirubin is found in the brain. The majority of cerebrospinal fluid (CSF) proteins originate from blood, and albumin constitutes 35% to 80% of CSF proteins , ; thus we cannot rule out the possibility that a small fraction of the bilirubin that enters the brain in a jaundiced newborn may be albumin-bound. The experimental studies that have measured brain bilirubin concentrations have mostly been done using methods that do not distinguish between bound and unbound bilirubin. ,
Several groups have studied the relationship between bilirubin in serum and bilirubin in CSF, as well as bilirubin and protein (or albumin) in CSF. CSF-unconjugated-bilirubin was found to correlate with total serum bilirubin (TSB), , and also to CSF total protein or albumin, although one study failed to verify the CSF bilirubin-to-protein relationship. In one study, CSF-bilirubin was also found to correlate with serum free bilirubin measurements. Meisel and colleagues measured CSF bilirubin in jaundiced newborns and found a mean value of 4.5 ± 3.1 μmol/L in the presence of a mean total serum bilirubin value of 221 ± 130 μmol/L. Thus, the CSF bilirubin value was approximately 2% of the serum bilirubin value. Several other groups also found CSF bilirubin concentrations in the low micromolar range. , The bilirubin:albumin (B:A) ratio in CSF was 0.32 ± 0.22, and Meisel and colleagues suggested that the concentration of free bilirubin in CSF must be low. However, given the brain-CSF barrier and the hypothesized role of CSF as a “sink” for brain bilirubin, it is not clear that these findings can be directly related to the question of bilirubin in brain tissue. Interpretation of these data is further complicated by the fact that within the CSF space there is a gradient of blood-derived proteins from the ventricles to the lumbar space, which for albumin is 2.5-fold. Applied to the data from Meisel and colleagues, the albumin concentration in the ventricles would be only 40% of that in the lumbar CSF, bringing the B:A ratio in the ventricular space to approximately 0.8, assuming that CSF bilirubin content is uniformly distributed within the CSF space. Whether this latter assumption holds is unknown. However, this question seems worthy of further investigation, as a higher B:A ratio in the ventricular CSF would theoretically be accompanied by a higher concentration of free bilirubin.
Daood and Watchko have calculated unbound bilirubin levels in the central nervous system (CNS) of homozygous Gunn rat pups after administration of sulfadimethoxine, using published in vivo albumin-bilirubin binding constants, and found a value that appears to be approximately two-thirds of the measured bilirubin content in whole brain. Their calculations assumed that in situ flushing of the brain vasculature completely clears these blood vessels of blood, thus also removing intravascular bilirubin as well as albumin. However, an earlier study had examined this assumption, using approximately the same volume-for-weight to flush the rat brain vasculature in situ, and using both 51 Cr-labeled erythrocytes and 125 I-IgM to estimate brain blood volume. Approximately 30% to 40% of pre-flushing brain blood volume was still present after flushing. Applying this correction to Daood’s and Watchko’s data would probably change their estimate. Thus, the question of binding of bilirubin in brain warrants further study.
Organic anion transporters, such as MRPs, may play a role in limiting bilirubin accumulation in the CSF and in keeping bilirubin out of brain. Preliminary data appear compatible with a role for MRP1 (encoded by ABCC1 ), which appears to be localized on the basolateral face of the choroid plexus epithelium of the blood-CSF barrier. ,
The question of how bilirubin localizes to the basal ganglia will be addressed here, although whether the localization phenomenon is related to bilirubin entry into or clearance from brain is not clear. The predilection of bilirubin for certain brain regions was described early and was primarily based on visual inspection of brain slices. , However, more objective, albeit limited, data are available from human subjects. Claireaux and colleagues extracted bilirubin from the brains of four infants who died with severe jaundice. They found approximately 35 nmol/g in the nuclear regions and 8 nmol/g in the remainder of the brains, speculating that bilirubin concentrations may have been several-fold higher in the most intensely stained areas of the nuclei.
Attempts to recreate this staining pattern in animal models have met with variable success. Burgess and colleagues , found regional differences in bilirubin concentrations in piglets that had received a [ 3 H]bilirubin infusion and were exposed either to hypercarbia or hyperosmolality. However, the question remains whether these differences were due to variations in bilirubin entry into or disappearance from brain, or whether they might be related to redistribution or binding to specific tissue or cell elements. Cannon and colleagues examined regional bilirubin concentrations in 15- to 19-day-old Gunn rat pups pretreated with sulfadimethoxine and found significant differences in bilirubin localization. The cerebellar bilirubin concentration was 18.9 μg/g, whereas the brain stem and cortex contained 10.7 and 4.7 μg/g, respectively. These numbers are overall surprisingly similar to those previously found in human brains. The brain bilirubin concentrations were higher in the male pups, suggesting that sex may modulate brain bilirubin uptake or clearance. However, no clear explanation was offered for how these regional differences arose, nor could they be said to mimic a typical kernicteric pattern. Attempts to recreate a kernicteric staining pattern in Sprague Dawley rats through infusion of bilirubin and manipulations of bilirubin binding, blood-brain barrier opening, and brain blood flow have not been succesful. These studies also failed to show interregional differences between bilirubin uptake into or clearance from brain. Other studies have examined bilirubin metabolism in brain, and, although differences were found, the findings did not conform to or explain the staining pattern of kernicterus. Although, as previously discussed, membrane transporters may play a role in extruding bilirubin from brain and in modulating toxicity, there is no evidence for region-specific differences in the expression of either ABCB1 or ABCC1 .
Hemolysis is believed to be implicated in the mechanisms underlying bilirubin neurotoxicity, and this is reflected in most therapeutic guidelines, which call for more aggressive management in the presence of hemolysis. , However, the mechanisms underlying this increased toxicity are not known. In rats with a chemically induced hemolytic anemia neither entry into nor clearance of bilirubin from the brain were different from that in control rats after an intravenous bolus of bilirubin. This obviously does not model the immunologic aspects of anemia in infants with blood group incompatibility and thus cannot discount the possibility that blood-brain barrier permeability may be affected by such mechanisms. However, immunology is not involved in the hemolysis of glucose 6-phosphate dehydrogenase deficiency, where increased risk of kernicterus is well described. Rhesus incompatibility with a hematocrit less than 35% had an odds ratio of 48.6 (95% confidence interval, 14 to 168) for ABE and/or death, or a pathologic neurologic examination at discharge in infants admitted to Cairo University Children’s Hospital. However, ABO incompatibility with a similarly low hematocrit was not associated with ABE or neurologic abnormalities at discharge in this population. In other studies both ABO and Rh incompatibility were risk factors for KSD. , Whether other products of hemolysis than bilirubin could increase bilirubin neurotoxicity has apparently not been studied.
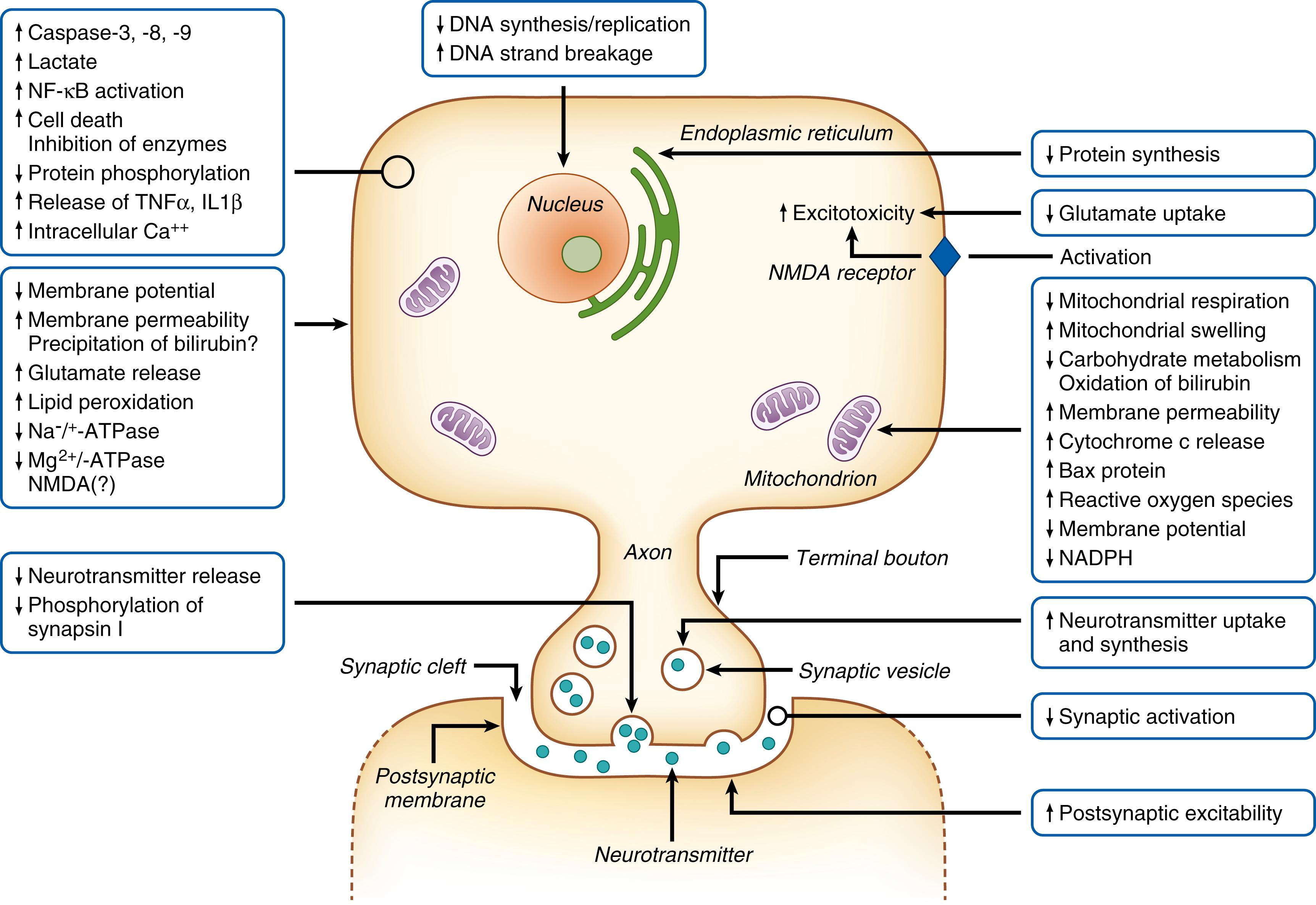
The first in vitro experimental studies of bilirubin toxicity were performed in 1954 and showed that bilirubin inhibited respiration in a rat brain homogenate. In rat liver mitochondria in vitro, bilirubin 300 μmol/L partially inhibited respiration, and phosphorylation was nearly completely inhibited. The interpretation was that bilirubin uncoupled oxidative phosphorylation. This, for many years, was the prevailing theory for the basic mechanism of bilirubin toxicity. A number of in vitro studies also lend some credence to the role of mitochondria in bilirubin toxicity. Thus, bilirubin can affect the mitochondrial membrane, resulting in increased membrane permeability, decreased membrane potential, release of cytochrome c , and triggering of apoptosis. An illustration depicting some of the many different effects and interactions of bilirubin with cells and cellular processes is shown in Fig. 163.4 .
Some in vivo data also support the role of mitochondria. Thus, ultrastructural changes were found in the mitochondria of Gunn rats with bilirubin encephalopathy. , Rats with infusion-induced extreme hyperbilirubinemia exhibited significantly decreased phosphocreatine and ATP levels in the brain, but only after opening the blood-brain barrier with hyperosmolality. In a study of magnetic resonance spectroscopy in infants with severe neonatal jaundice, one of five infants had an abnormally high ratio of lactate to N -acetylaspartate. This was the only infant who also exhibited abnormalities in the basal ganglia on magnetic resonance imaging and one of two who had evidence of KSD during follow-up. The elevated ratio of lactate to N -acetylaspartate may have been the result of changes in mitochondrial function.
However, these data do not unequivocally confirm a role for mitochondria as the primary targets of bilirubin toxicity in vivo. Early electron-microscopic observations in Gunn rats suggested that the mitochondrial changes might result from earlier effects in the cytoplasm. When the effects of bilirubin on L-929 cells in culture were compared with those of known uncouplers of oxidative phosphorylation and inhibitors of reduced nicotinamide adenine dinucleotide oxidase, the results were more indicative of membrane perturbation than of toxic effects on the respiratory chain. Studies in newborn pigs, who received intravenous infusions of bilirubin, failed to show changes in cerebral oxygen, glucose, and lactate metabolism compatible with perturbation of mitochondrial function. Furthermore, bilirubin has been found to affect neurotransmitter metabolism in permeabilized synaptosomes in vitro (synaptic nerve endings that retain their function in vitro) in the presence of high ATP concentrations, so depletion of endogenous ATP could not have contributed to the observed effects. In a study of six infants with extreme jaundice, of whom four were neurologically abnormal at 1 year of age, none demonstrated elevated brain lactate levels. In fact, the findings in that study were more compatible with changes in N -methyl- d -aspartate (NMDA) receptor sensitivity.
For bilirubin to perturb mitochondrial function, it needs to be present in concentrations sufficient to cause toxicity. Whether this is the case has been addressed by only one study, in which [ 3 H]bilirubin was given as an intravenous bolus to rats. The rats were killed after 10 or 30 minutes, and their brains were subjected to subcellular fractionation. Although absolute values for bilirubin could not be computed, the bilirubin content relative to protein in mitochondria was much less than that in the cytoplasm and membrane fractions. In conclusion, whether the mitochondria are the primary targets for bilirubin neurotoxicity remains a theory for which some experimental support exists, but a number of studies point to other possibilities.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here