Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
This chapter focuses on the molecular and cellular changes that underlie heart failure with a reduced ejection fraction (HFrEF), with an emphasis on the role of neurohormonal activation and left ventricular (LV) remodeling as the primary determinants for disease progression in HF. The hemodynamic, contractile, and wall motion disorders in HF are discussed in the chapters on cardiac contraction and relaxation ( Chapter 46 ), echocardiography ( Chapter 16 ), cardiac catheterization ( Chapter 22 ), and radionuclide imaging ( Chapter 18 ). The clinical assessment of patients with HF is discussed in Chapter 48, Chapter 51 discusses the pathogenesis of HF with preserved ejection fraction.
As shown in Figure 47.1A , HFrEF is initiated after an index event either damages the heart muscle, with a resultant loss of functioning cardiac myocytes or, alternatively, disrupts the ability of the myocardium to generate force, thereby preventing the heart from contracting normally. This index event may have an abrupt onset, as in the case of a myocardial infarction (MI); it may have a gradual or insidious onset, as in the case of hemodynamic pressure or volume overloading; or it may be hereditary, as in the case of many of the genetic cardiomyopathies. Regardless of the nature of the inciting event, the feature that is common to each of these index events is that they all, in some manner, produce a decline in pumping capacity of the heart. The circulatory changes that arise from impaired myocardial pump function are sensed by peripheral arterial baroreceptors as “underfilling” of the circulation. These sensory and other peripheral receptors (e.g., metaboreceptors and ergoreceptors) activate a series of compensatory mechanisms discussed below that lead to changes in heart rate and cardiac contractility, salt and water retention, and constriction of the peripheral blood vessels.
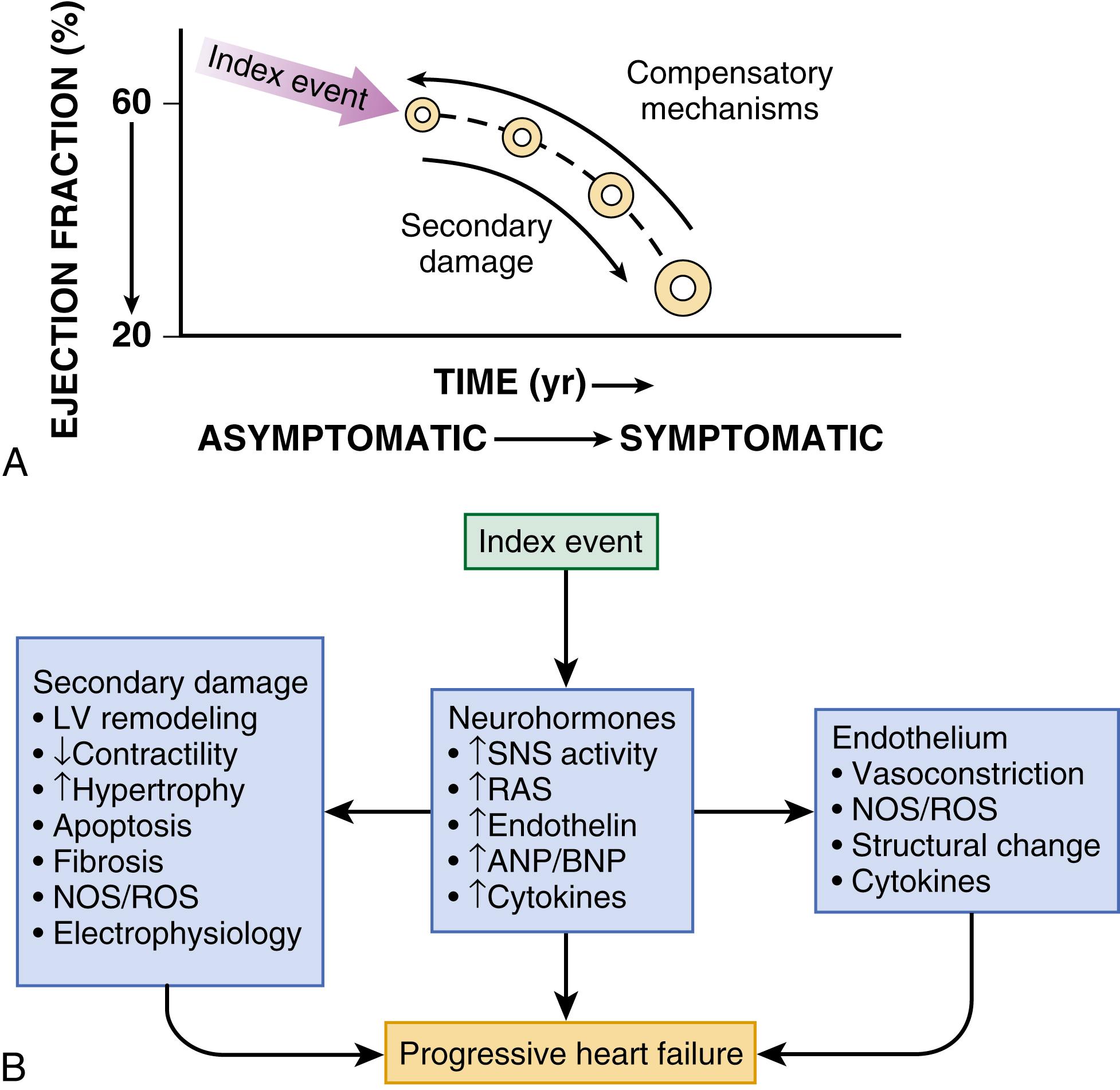
In some patients LV function will recover spontaneously after resolution or removal of the inciting stress that compromised myocardial function; however, in a significant proportion of patients LV function will remain depressed. Some patients with LV dysfunction will remain asymptomatic or minimally symptomatic after the initial decline in pumping capacity of the heart, or symptoms develop only after the dysfunction has been present for some time. The precise reasons why patients with LV dysfunction remain asymptomatic are not known. One potential explanation is that a number of compensatory mechanisms that become activated in the setting of cardiac injury or depressed cardiac output are sufficient to modulate LV function within a physiologic/homeostatic range, such that the patient’s functional capacity is preserved or is depressed only minimally. However, sustained activation of neurohormonal systems leads to peripheral vasoconstriction, salt and water retention by the kidney, as well as a series of end-organ changes within the myocardium that contribute to worsening LV dilation (referred to as LV remodeling) and LV dysfunction ( Fig. 47.1B ). As HF progresses patients undergo the transition from asymptomatic to symptomatic HF.
A growing body of experimental and clinical evidence suggests that HF progresses as a result of the overexpression of biologically active molecules that are capable of exerting deleterious effects on the heart and circulation (see Fig. 47.1B ). The portfolio of compensatory mechanisms that have been described thus far includes activation of the sympathetic nervous system (SNS) and the renin-angiotensin aldosterone system (RAAS), which are responsible for maintaining cardiac output through increased retention of salt and water; peripheral arterial vasoconstriction and increased contractility; and inflammatory mediators that are responsible for mediating cardiac repair and remodeling. It bears emphasis that neurohormone is largely a historical term, reflecting the original observation that many of the molecules that were elaborated in HF were produced by the neuroendocrine system and thus acted on the heart in an endocrine manner. It has since become apparent, however, that a great many of the so-called classic neurohormones such as norepinephrine (NE) and angiotensin II are synthesized directly within the myocardium by myocytes and thus act in an autocrine and paracrine manner. Nonetheless, the important unifying concept that arises from the neurohormonal model is that the overexpression of portfolios of biologically active molecules contributes to disease progression by virtue of the deleterious effects these molecules exert on the heart and circulation.
The decrease in cardiac output in HF activates a series of compensatory adaptations that are intended to maintain cardiovascular homeostasis. One of the most important adaptations is activation of the sympathetic (adrenergic) nervous system, which occurs early in the course of HF. Activation of the SNS in HF is accompanied by a concomitant withdrawal of parasympathetic tone ( eFig. 47.1 ). Although these disturbances in autonomic control initially were attributed to loss of the inhibitory input from arterial or cardiopulmonary baroreceptor reflexes, increasing evidence indicates that excitatory reflexes also may participate in the autonomic imbalance that occurs in HF. In healthy persons, “high-pressure” carotid sinus and aortic arch baroreceptors and “low-pressure” cardiopulmonary mechanoreceptors provide inhibitory signals to the central nervous system (CNS) that repress the sympathetic outflow to the heart and peripheral circulation. Under normal conditions, inhibitory inputs from high-pressure carotid sinus and aortic arch baroreceptors and the low-pressure cardiopulmonary mechanoreceptors are the principal inhibitors of sympathetic outflow, whereas discharge from the nonbaroreflex peripheral chemoreceptors and from muscle metaboreceptors are the major excitatory inputs to sympathetic outflow. The vagal limb of the baroreceptor heart rate reflex also is responsive to arterial baroreceptor afferent inhibitory input. Healthy persons display low sympathetic discharge at rest and have a high heart rate variability. In patients with HF, however, inhibitory input from baroreceptors and mechanoreceptors decreases and excitatory input increases, with the net result that there is a generalized increase in sympathetic nerve traffic and blunted parasympathetic nerve traffic, leading to loss of heart rate variability and increased peripheral vascular resistance.
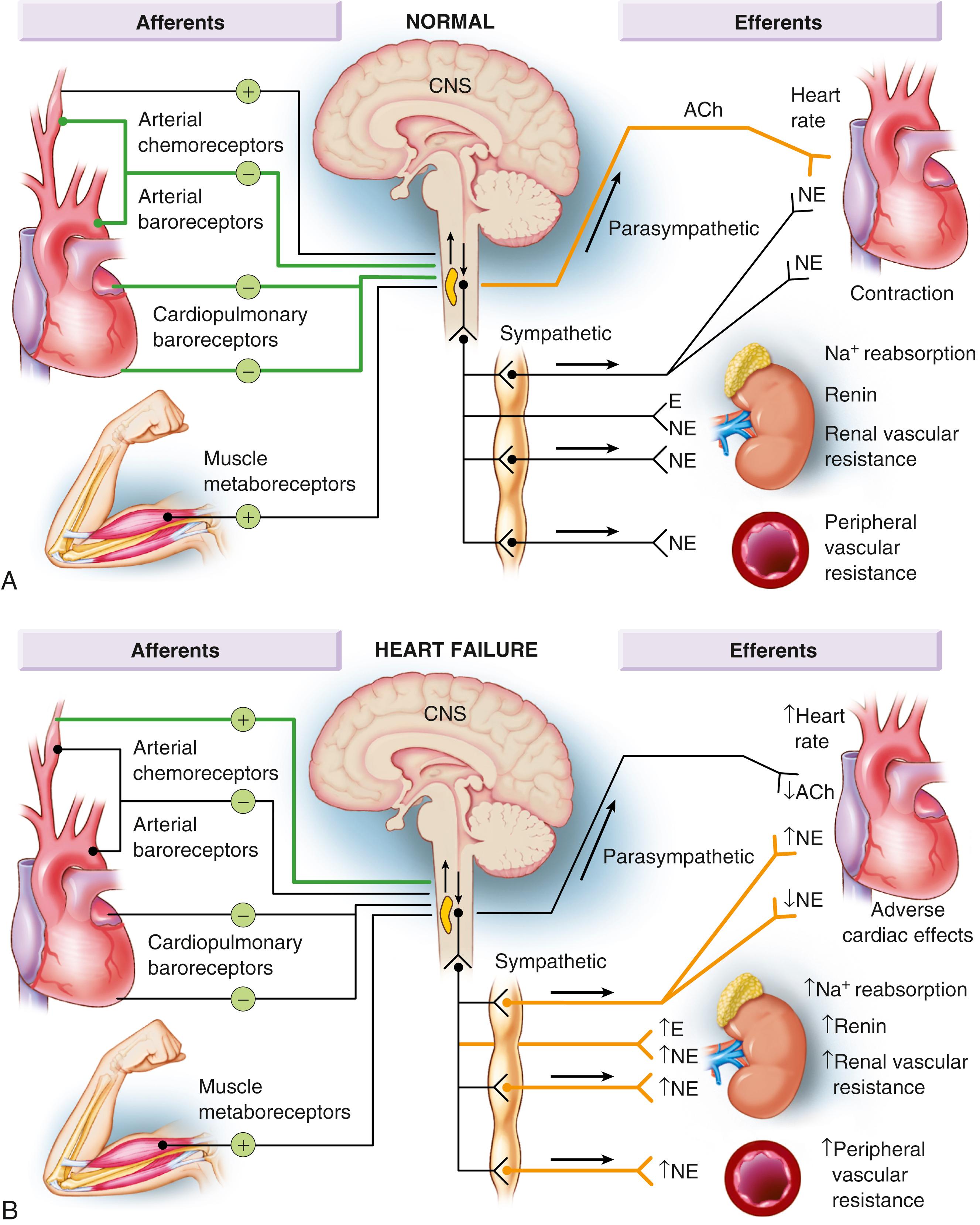
As a result of the increase in sympathetic tone, there is an increase in circulating levels of NE, a potent adrenergic neurotransmitter. The elevated levels of circulating NE result from a combination of increased release of NE from adrenergic nerve endings and its consequent “spillover” into the plasma, as well as reduced uptake of NE by adrenergic nerve endings. In patients with advanced HF, the circulating levels of NE in resting patients are two to three times those found in normal persons. Indeed, plasma levels of NE predict mortality in patients with HF. Whereas the normal heart usually extracts NE from the arterial blood, in patients with moderate HF the coronary sinus NE concentration exceeds the arterial concentration, indicating increased adrenergic stimulation of the heart. However, as HF progresses there is a significant decrease in the myocardial concentration of NE. The mechanism responsible for cardiac NE depletion in severe HF is not clear and may relate to an “exhaustion” phenomenon resulting from the prolonged adrenergic activation of the cardiac adrenergic nerves in HF. In addition, there is decreased activity of myocardial tyrosine hydroxylase, which is the rate-limiting enzyme in the synthesis of NE. In patients with cardiomyopathy, iodine 131 ( 131 I)–labeled metaiodobenzylguanidine (MIBG), a radiopharmaceutical that is taken up by adrenergic nerve endings, is not taken up normally, suggesting that NE reuptake is also depressed.
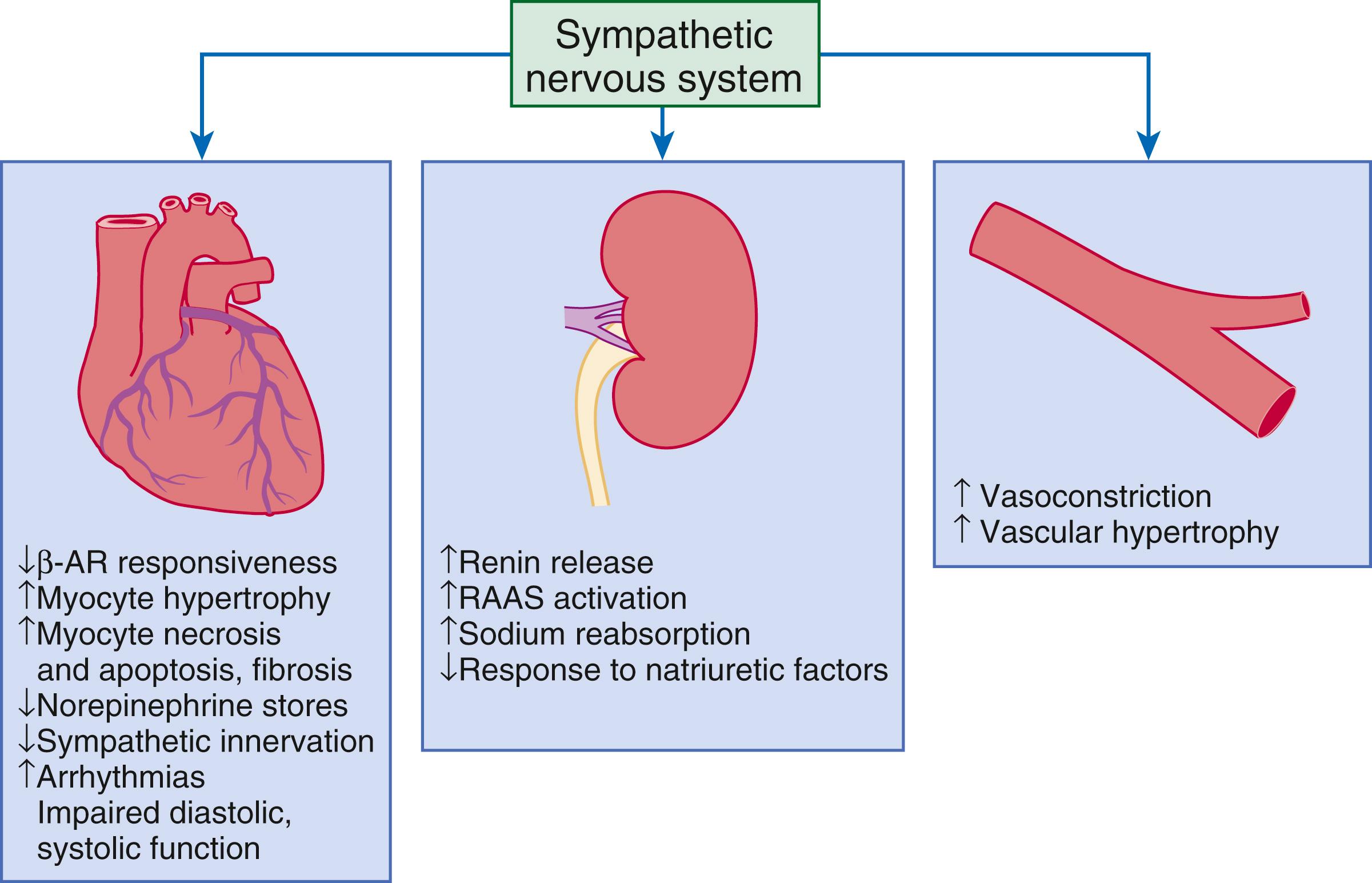
Increased sympathetic activation of the beta 1 -adrenergic receptor results in increased heart rate and force of myocardial contraction, with a resultant increase in cardiac output (see Chapter 46 ). In addition, the heightened activity of the adrenergic nervous system leads to stimulation of myocardial alpha 1 -adrenergic receptors, which elicits a modest positive inotropic effect, as well as peripheral arterial vasoconstriction ( Fig. 47.2 ). Although NE enhances both contraction and relaxation and maintains blood pressure, myocardial energy requirements are augmented, which can intensify ischemia when myocardial oxygen (O 2 ) delivery is restricted. The augmented adrenergic outflow from the CNS also may trigger ventricular tachycardia or even sudden cardiac death, particularly in the presence of myocardial ischemia. Thus, activation of the SNS provides short-term support that has the potential to become maladaptive over the long term. Moreover, increasing evidence suggests that apart from the deleterious effects of sympathetic activation, parasympathetic withdrawal also may contribute to the pathogenesis of HF. Withdrawal of parasympathetic nerve stimulation has been associated with decreased nitric oxide (NO) levels, increased inflammation, increased sympathetic activity, and worsening LV remodeling. Several clinical trials with direct vagal nerve stimulation did not meet their primary endpoint but additional studies are ongoing.
In contrast with the SNS, the components of the renin-angiotensin system (RAS) are activated comparatively later in HF. The presumptive mechanisms for RAS activation in HF include renal hypoperfusion, decreased filtered sodium reaching the macula densa in the distal tubule, and increased sympathetic stimulation of the kidney, leading to increased renin release from juxtaglomerular apparatus. As shown in Figure 47.3 , renin cleaves four amino acids from circulating angiotensinogen, which is synthesized in the liver, to form the biologically inactive decapeptide angiotensin I. Angiotensin-converting enzyme (ACE) cleaves two amino acids from angiotensin I to form the biologically active octapeptide (1 to 8) angiotensin II. Most ACE activity (approaching 90%) in the body is found in tissues; the remaining 10% is found in a soluble (non–membrane-bound) form in the interstitium of the heart and vessel wall. The importance of tissue ACE activity in HF is suggested by the observation that ACE messenger RNA (mRNA) and ACE-binding sites and ACE activity are increased in explanted human hearts. Angiotensin II also can be synthesized using renin-independent pathways through the enzymatic conversion of angiotensinogen to angiotensin I by kallikrein and cathepsin G ( Fig. 47.3A ). The tissue production of angiotensin II also may occur along ACE-independent pathways, through the activation of chymase. This latter pathway may be of major importance in the myocardium, particularly when the levels of renin and angiotensin I are increased by the use of ACE inhibitors. Angiotensin II itself can undergo further proteolysis to generate three biologically active fragments: angiotensin III (2 to 8), angiotensin IV (3 to 8), and angiotensin 1 to 7 ( Fig. 47.3B ). The latter results mainly from angiotensin II cleavage by angiotensin converting enzyme 2 (ACE2), which is a type I transmembrane carboxypeptidase with 40% homology to ACE. ACE2 has also been identified as the cellular receptor of SARS-CoV-2 (see also Chapter 94 ).
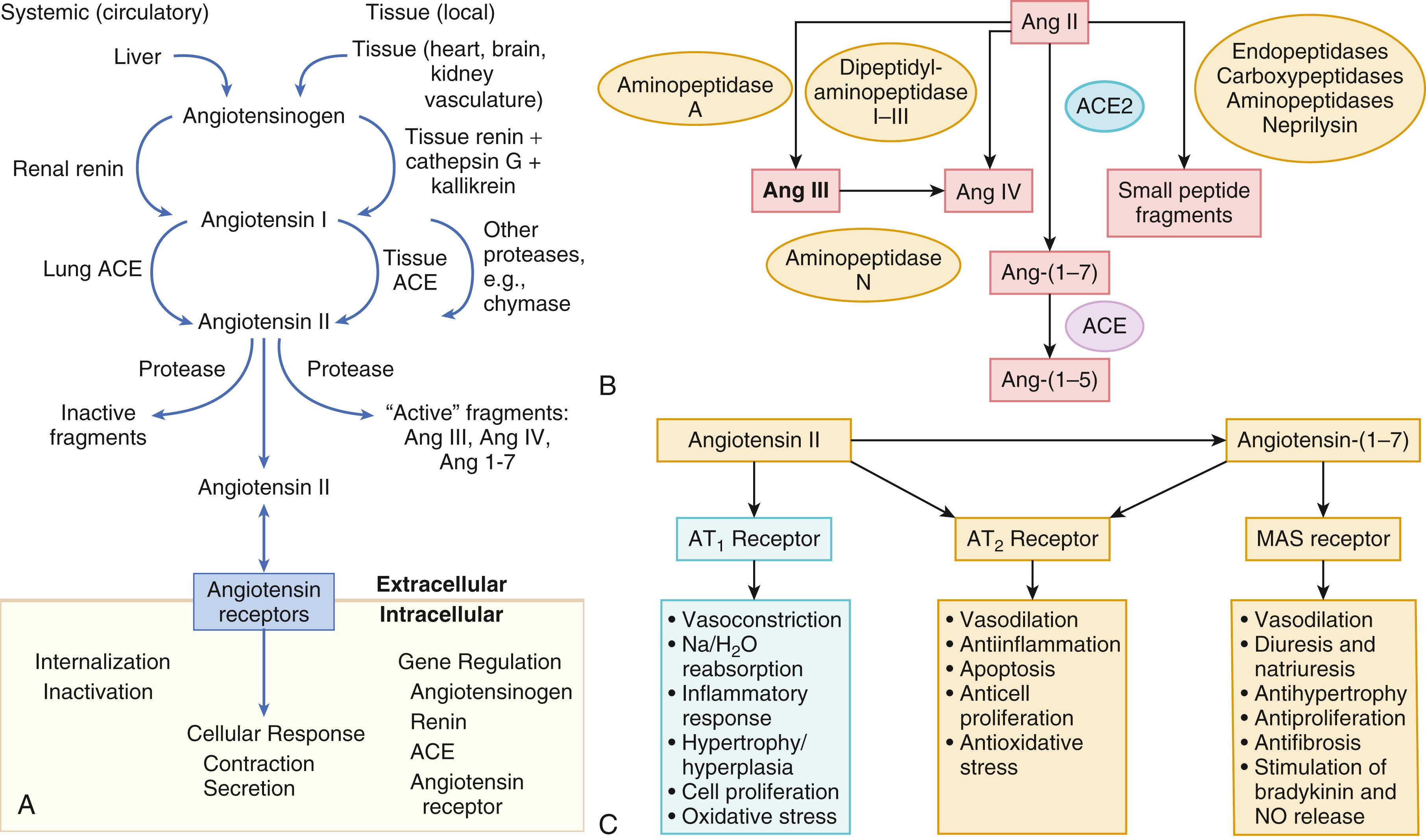
Angiotensin II exerts its effects by binding to two G protein–coupled receptors (GPCRs), the angiotensin type 1 (AT 1 ) and angiotensin type 2 (AT 2 ) receptors. The predominant angiotensin receptor in the vasculature is the AT 1 receptor. Although both AT 1 and AT 2 receptor subtypes are present in human myocardium, the AT 2 receptor predominates in a 2:1 molar ratio. Cellular localization of the AT 1 receptor in the heart is most abundant in nerves distributed in the myocardium, whereas the AT 2 receptor is localized more specifically in fibroblasts and the interstitium. Activation of the AT 1 receptor leads to vasoconstriction, cell growth, aldosterone secretion, and catecholamine release, whereas activation of the AT 2 receptor leads to vasodilation, inhibition of cell growth, natriuresis, and bradykinin release ( Fig. 47.3C ). Studies have shown that the AT 1 receptor and mRNA levels are downregulated in failing human hearts, whereas AT 2 receptor density is increased or unchanged, so that the ratio of AT 1 to AT 2 receptors decreases. The MAS receptor is a GPCR that is expressed primarily in the brain and testes but also in the heart (see Fig. 47.3C ).
Angiotensin II has several important actions that are critical to maintaining short-term circulatory homeostasis. The sustained expression of angiotensin II is maladaptive, however, leading to fibrosis of the heart, kidneys, and other organs. Angiotensin II can also lead to worsening neurohormonal activation by enhancing the release of NE from sympathetic nerve endings, as well as stimulating the zona glomerulosa of the adrenal cortex to produce aldosterone. Analogous to angiotensin II, aldosterone provides short-term support to the circulation by promoting the reabsorption of sodium in exchange for potassium in the distal segments of the nephron. However, the sustained expression of aldosterone may exert harmful effects by provoking hypertrophy and fibrosis within the vasculature and the myocardium, contributing to reduced vascular compliance and increased ventricular stiffness. In addition, aldosterone provokes endothelial cell dysfunction, baroreceptor dysfunction, and inhibition of NE uptake, any of which may lead to worsening HF. The mechanism of action of aldosterone in the cardiovascular system appears to involve oxidative stress, with resultant inflammation in target tissue. Although the exact role of angiotensin III (2 to 8), angiotensin IV (3 to 8), and angiotensin 1 to 7 in HF are not known, experimental studies suggest that angiotensin 1 to 7 counteracts the effects of angiotensin II, and attenuates LV remodeling. In contrast, angiotensin III directly stimulates the zona glomerulosa of the adrenal glands to produce aldosterone, which promotes sodium resorption in the distal collecting duct of the kidney. Angiotensin III also has an important role in vasopressin release in the brain, which controls water retention in the distal collecting duct of the kidney. Angiotensin III in the brain can also modulate cardiac nervous sympathetic hyperactivity, as well as LV remodeling after MI.
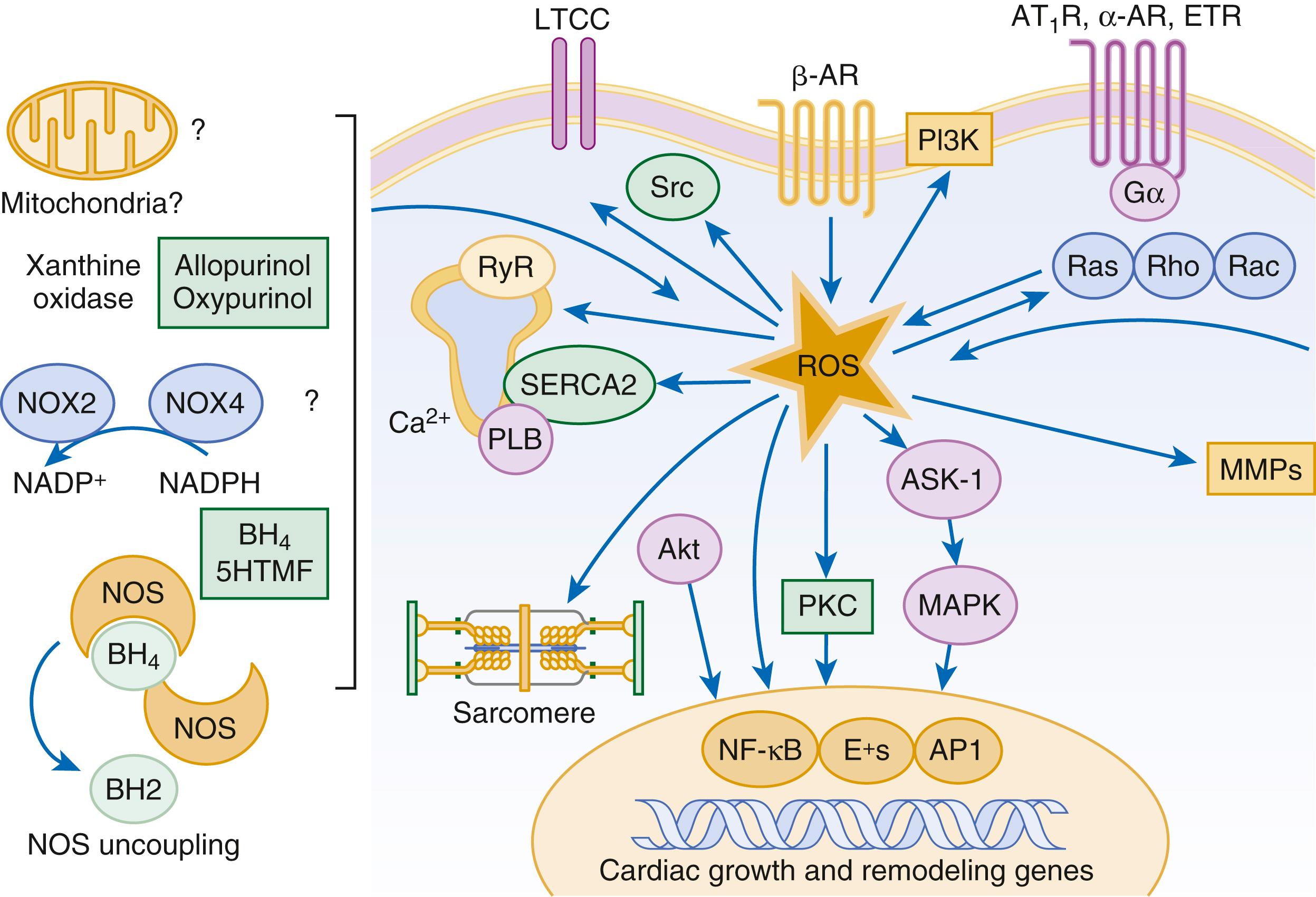
Reactive oxygen species (ROS) are a normal byproduct of aerobic metabolism. In the heart, the potential sources for ROS include the mitochondria, xanthine oxidase, and nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase ( Fig. 47.4 ). ROS can modulate the activity of a variety of intracellular proteins and signaling pathways, including essential proteins involved in myocardial excitation-contraction coupling, such as ion channels, sarcoplasmic reticulum (SR) calcium release channels, and myofilament proteins, as well as signaling pathways that are coupled to myocyte growth. “Oxidative stress” occurs when the production of ROS exceeds the buffering capacity of antioxidant defense systems, leading to an excess of ROS within the cell. Substantial evidence indicates that the level of oxidative stress is increased both systemically and in the myocardium of patients with HF. Oxidative stress in the heart may be caused by reduced antioxidant capacity and increased production of ROS, which may arise secondary to mechanical strain of the myocardium, neurohormonal stimulation (angiotensin II, alpha-adrenergic agonists, endothelin-1 [ET-1]), or inflammatory cytokines (tumor necrosis factor [TNF], interleukin [IL]-1). Excessive mitochondria-derived ROS in cardiac myocytes have been demonstrated in experimental models of HF and may contribute to contractile dysfunction in advanced HF. Increased xanthine oxidase expression and activity have been reported in canine rapid pacing–induced HF and patients with end-stage HF. Moreover, increased expression and activity of myocardial NADPH oxidases have been demonstrated in both experimental and human HF in cultured cardiac myocytes, ROS stimulate myocyte hypertrophy, reexpression of fetal gene programs, and apoptosis. ROS also can modulate fibroblast proliferation and collagen synthesis and trigger increased matrix metalloproteinase (MMP) abundance and activation. ROS also can affect the peripheral vasculature in HF by decreasing the bioavailability of NO. These and other observations have led to the suggestion that strategies to reduce ROS may be of therapeutic value in patients with HF. However, xanthine oxidase inhibition with allopurinol to reduce oxidative stress in hyperuremic patients with HF did not improve clinical status or cardiac function in a clinical trial.
The importance of aldosterone, independent of angiotensin II, has been demonstrated by clinical trials (see Chapter 50 ) showing that low-dose spironolactone increased the survival of patients with New York Heart Association (NYHA) class II to IV systolic HF, as well as improved survival after MI, independent of changes in volume or electrolyte status.
One of the signatures of advancing HF is increased salt and water retention by the kidneys. Traditional theories have ascribed this increase to either “forward” failure, which attributes sodium retention to inadequate renal perfusion as a consequence of impaired cardiac output, or “backward” failure, which emphasizes the importance of increased venous pressure in favoring transudation of salt and water from the intravascular to the extracellular compartment. These mechanisms have largely been supplanted by the concept of decreased effective arterial blood volume , which postulates that despite blood volume expansion in HF, inadequate cardiac output sensed by baroreceptors in the vascular tree leads to a series of compensatory neurohormonal adaptations that resemble the homeostatic response to acute blood loss. As illustrated in Figure 47.5 , a falling cardiac output or redistribution of the circulating blood volume is sensed by baroreceptors in the left ventricle, aortic arch, carotid sinus, and renal afferent arterioles. The loss of inhibitory input from arterial or cardiopulmonary baroreceptor reflexes leads to sustained activation of the SNS and the RAS. An implantable barostimulation device that activates the carotid baroreceptors to decrease sympathetic activation and increase vagal tone improved quality of life, exercise capacity, and NT-proBNP in patients with symptomatic HF in the BeAT-HF (Barostimulation for Heart Failure).
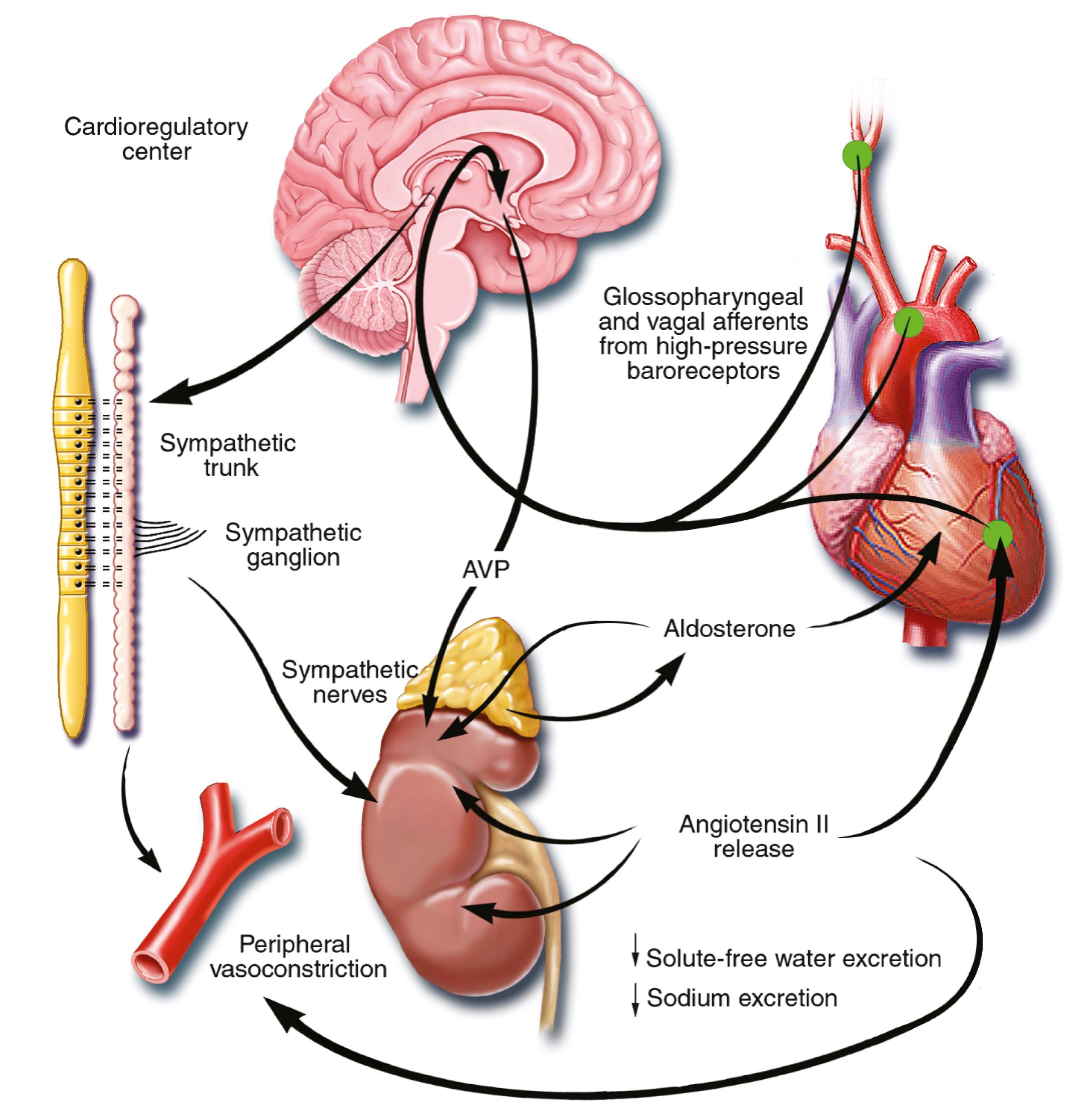
There is little evidence to suggest that a primary renal abnormality is responsible for the initial sodium retention in the heart; however, there is mounting evidence that secondary changes in the kidney contribute importantly to volume overload as HF progresses. Volume overload in HF is multifactorial and is secondary, at least in part, to several factors that have the potential to cause increased sodium reabsorption, including activation of the SNS, activation of RAS, reduced renal perfusion pressures, and blunting of renal responsiveness to natriuretic peptides. Increased renal sympathetic nerve–mediated vasoconstriction leads to decreased renal blood flow, as well as increased renal tubular sodium and water reabsorption throughout the nephron. Renal sympathetic stimulation also can lead to the nonosmotic release of arginine vasopressin (AVP) from the posterior pituitary, which reduces the excretion of free water and contributes to worsening peripheral vasoconstriction, as well as increased endothelin (ET) production. Increased renal venous pressure can also lead to renal interstitial hypertension, with the development of tubular injury and renal fibrosis.
AVP is a pituitary hormone that plays a central role in the regulation of free water clearance and plasma osmolality (see Fig. 47.5 ). Under normal circumstances, AVP is released in response to an increase in plasma osmolality, leading to increased retention of water from the collecting duct. Of note, circulating AVP is elevated in many patients with HF, even after correction for plasma osmolality (i.e., nonosmotic release), and may contribute to the hyponatremia that occurs in HF. The cellular effects of AVP are mediated mainly by interactions with three types of receptors, termed V 1 , V 2 , and V 3 (previously V 1b ). The V 1 receptor, the most widespread subtype, is found primarily in vascular smooth muscle cells. The V 3 receptor has a more limited distribution and is located mainly in the CNS. The V 2 receptors are found primarily in the epithelial cells in the renal collecting duct and the thick ascending limb. AVP receptors are members of the GPCRs. The V 1 receptors mediate vasoconstriction, platelet aggregation, and stimulation of myocardial growth factors, whereas V 3 modulates adrenocorticotropic hormone (ACTH) secretion from the anterior pituitary. The V 2 receptor mediates antidiuretic effects by stimulating adenyl cyclase to increase the rate of insertion of water channel−containing vesicles into the apical membrane. Because the vesicles contain preformed functional water channels, termed aquaporins, their localization in the apical membranes in response to V 2 stimulation increases the water permeability of the apical membrane, leading to water retention. The “vaptans,” vasopressin receptor antagonists with V 1 (relcovaptan) or V 2 (tolvaptan, lixivaptan) selectivity or nonselective V 1 /V 2 activity (conivaptan), have been shown to reduce body weight and reduce hyponatremia in clinical trials (see Chapter 49, Chapter 50 ).
Increased renal sympathetic activity leads to increased renin production by the kidneys, with a resultant sustained activation of RAAS, despite an expanded extracellular volume. Angiotensin II facilitates retention of sodium and water by multiple renal mechanisms, including a direct proximal tubular effect, as well as through activation of aldosterone, which leads to increased sodium resorption in the distal tubule. Angiotensin II also stimulates the thirst center of the brain and provokes the release of AVP and aldosterone, both of which can lead to further dysregulation of salt and water homeostasis.
| Vasoconstrictors |
| Catecholamine |
|
| Peptide |
|
| Lipid |
|
| Vasodilators |
| Catecholamine |
|
| Peptide |
|
| Gas |
|
| Lipid |
|
A number of counterregulatory neurohormonal systems become activated in HF to offset the deleterious effects of the vasoconstricting neurohormones ( eTable 47.1 ). Metabolites of vasodilatory prostaglandins, including prostaglandin E 2 (PGE 2 ) and prostacyclin (PGI 2 ), are elevated in patients with HF. In addition to being a vasodilator, PGE 2 enhances renal sodium excretion and modulates the antidiuretic action of AVP. One class of the most important counterregulatory neurohormonal systems that become activated in HF are the natriuretic peptides, including ANP and brain (B-type) natriuretic peptide (BNP). Under physiologic conditions, ANP and BNP function as natriuretic hormones that are released in response to increases in atrial and myocardial stretch, often secondary to excessive sodium intake. Once released, these cardiac peptides act on the kidney and peripheral circulation to unload the heart, through increased excretion of sodium and water, while inhibiting the release of renin and aldosterone ( Fig. 47.6 ). In the setting of RAAS activation, the release of ANP and BNP may serve as an important counterregulatory mechanism that maintains sodium and water homeostasis. However, for reasons that are not entirely clear, the renal effects of the natriuretic peptides appear to become blunted with advancing HF, leaving the effects of RAAS unopposed. Potential reasons for this blunting include low renal perfusion pressure, relative deficiency or altered molecular forms of the natriuretic peptides, and decreased levels of natriuretic peptide receptors.
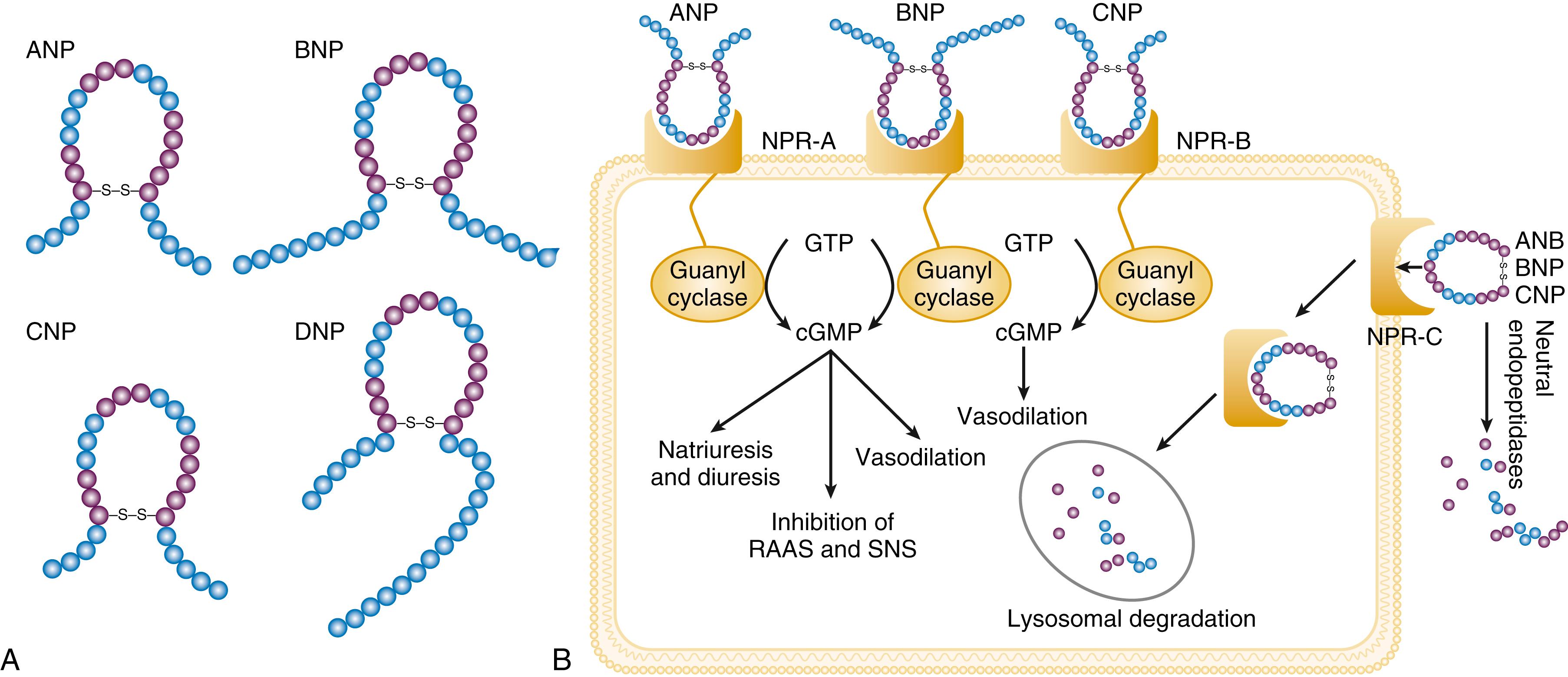
The natriuretic peptide system consists of five structurally similar peptides: ANP, urodilatin (an isoform of ANP), BNP, C-type natriuretic peptide (CNP), and dendroaspis natriuretic peptide (DNP) ( Fig. 47.6A ). ANP, a 28–amino acid peptide hormone, is produced principally in the cardiac atria, whereas BNP, a 32–amino acid peptide originally isolated from porcine brain, was later identified as a hormone that was primarily produced in the cardiac ventricles. Both ANP and BNP are secreted in response to increasing cardiac wall tension; however, other factors such as neurohormones (e.g., angiotensin II, ET-1, catecholamines) or physiologic factors (e.g., age, gender, renal function) may also play a role in their regulation. The biosynthesis, secretion, and clearance of BNP differs from ANP, suggesting that these two natriuretic peptides have discrete physiologic and pathophysiologic roles. Whereas ANP is secreted in short bursts in response to acute changes in atrial pressure, the activation of BNP is regulated transcriptionally in response to chronic increases in atrial/ventricular pressure. ANP and BNP initially are synthesized as prohormones that are subsequently proteolytically cleaved, respectively, by corin and furin, to yield large, biologically inactive N-terminal fragments (NT-ANP and NT-BNP) and smaller, biologically active peptides (i.e., ANP and BNP). ANP has a relatively short half-life of approximately 3 minutes, whereas BNP has a plasma half-life of approximately 20 minutes. CNP, which is located primarily in the vasculature, also is released as a prohormone that is cleaved into biologically inactive form (NT-CNP) and a 22–amino acid, biologically active form (i.e., CNP).
Figure 47.6B illustrates the signaling pathway of the natriuretic peptide system. The natriuretic peptides stimulate the production of the intracellular second-messenger cyclic guanosine monophosphate (cGMP), via binding to the natriuretic peptide A receptor (NPR-A), which preferentially binds ANP and BNP, and the natriuretic peptide B receptor (NPR-B), which preferentially binds CNP. Both NPR-A and NPR-B are coupled to particulate guanylate cyclase. Activation of NPR-A and NPR-B results in natriuresis, vasorelaxation, inhibition of renin and aldosterone, inhibition of fibrosis, and increased lusitropy. The natriuretic peptide C receptor (NPR-C) is not linked to cGMP and serves as a clearance receptor for the natriuretic peptides.
All three natriuretic peptides are degraded by two major mechanisms: NPR-C–mediated internalization, followed by lysosomal degradation and enzymatic degradation by neutral endopeptidase (NEP) 24.11 (neprilysin), which is widely expressed in multiple tissues, where it often is colocalized with ACE. Both ACE and NEP are membrane-bound zinc-containing metallopeptidases involved in the metabolism of a variety of biologic peptides.
Neprilysin, like many other membrane-bound metalloproteases, can be released from the cell surface, producing a non–membrane-associated soluble form that retains catalytic activity. NEP preferentially cleaves small peptides on the N-terminal side of hydrophobic residues ( Fig. 47.6C ). NEP has a wide range of tissue distribution, including vascular endothelium, smooth muscle cells, myocytes, fibroblasts, kidney tubule cells, and nerve cells. NEP degrades multiple peptides, including natriuretic peptides ( Fig. 47.6D ), angiotensin I, angiotensin II, ET-I, adrenomedullin, opioids, bradykinin, chemotactic peptides, enkephalins, and a 14 myloid-β peptide (Aβ). NEP inhibition of degradation of natriuretic peptides results in vasorelaxation, natriuresis, inhibition of hypertrophy, and fibrosis. On the other hand, inhibition of degradation of other vasoactive peptides, such as angiotensin II, angiotensin 1 to 7, and ET, opposes the vasodilatory effects of natriuretic peptides. Accordingly, NEP inhibition has variable effects on blood pressure. NEP inhibition increases urinary kinin levels, which may contribute to its natriuretic effects. NEP plays an important role in clearance of amyloid peptides in the brain. In particular, NEP is of major relevance for degrading the amyloid-beta peptides (Aβ), which play a significant role in neurotoxicity, and formation of amyloid plaques from Aβ aggregates in complex with other proteins is a hallmark of Alzheimer disease. Overexpression of neprilysin ameliorated the development of Alzheimer disease, and disruption of the neprilysin gene induces cognitive dysfunction in a mouse model of Alzheimer disease. Because of the potentially beneficial effects of natriuretic peptides in HF, NEP inhibition was pursued as a rational approach for HF therapy. The early use of omapatrilat, a dual vasopeptidase inhibitor that inhibits both ACE and NEP, was not shown to be more effective than ACE inhibition alone in HF patients. However, the use of a combined AT 1 receptor antagonist and a neprilysin inhibitor (valsartan/sacubitril, LCZ696) was shown to have a favorable impact on HF outcome, including quality of life, exercise capacity, and more importantly, HF hospitalization and total mortality, in the PARADIGM-HF trial (see Chapter 50 ).
The biologic importance of the natriuretic peptides in renal sodium handling has been demonstrated in multiple studies using NPR antagonists, as well as overexpression of ANP or BNP. In experimental HF models, either acute blockade of NPR-A and NPR-B or chronic genetic disruption of NPR-A blunts the renal natriuretic response to acute volume expansion, demonstrating the renal protective action of natriuretic peptide activation. The infusion of a recombinant human ANP and BNP exerts beneficial hemodynamic effects, characterized by decreases in arterial and venous pressures, increase in cardiac output, and suppression of neurohormonal activation in humans, resulting in their clinical development as therapeutic agents for human HF (see Chapter 49 ). In addition to their important biologic role, the natriuretic peptides have provided important diagnostic and prognostic information in HF (see Chapter 48 ).
In patients with HF, the complex interactions between the autonomic nervous system and local autoregulatory mechanisms tend to preserve circulation to the brain and heart while decreasing blood flow to the skin, skeletal muscles, splanchnic organs, and kidneys. This intense visceral vasoconstriction during exercise helps to divert the limited cardiac output to exercising muscle but contributes to hypoperfusion of the gut and kidneys. The most powerful stimulus for peripheral vasoconstriction is sympathetic activation, which releases the potent vasoconstrictor NE. Other vasoconstrictors that contribute to maintaining circulatory homeostasis include angiotensin II, ET, neuropeptide Y, urotensin II, thromboxane A 2 , and AVP (see eTable 47.1 ). The increased sympathetic adrenergic stimulation of the peripheral arteries and the increased concentrations of circulating vasoconstrictors contribute to the arteriolar vasoconstriction and to the maintenance of arterial pressure. The sympathetic stimulation of the veins contributes to an increase in venous tone, which helps to maintain venous return and ventricular filling and to support cardiac performance by Starling’s law of the heart (see Chapter 46 ). For more information see the online supplement “Vasoconstricting Peptides in Heart Failure.” ![]()
As noted, the vasoconstricting neurohormones activate counterregulatory vasodilator responses, including release of natriuretic peptides, NO, bradykinin, adrenomedullin, apelin, and vasodilating PGI 2 and PGE 2 (see eTable 47.1 ). Under normal circumstances, the continuous release of NO (endothelium-derived relaxing factor) from the endothelium counteracts these vasoconstricting factors and allows for appropriate vasodilatory responses during exercise. As HF advances, however, the endothelial cell–mediated vasodilatory responsiveness is lost, which contributes to the excessive peripheral arterial vasoconstriction that is emblematic of advanced HF. Of interest, the vasodilator response can be restored by the administration of l -arginine, a precursor of endothelium-derived NO.
The free radical gas NO is produced by three isoforms of NO synthase (NOS). All three isoforms are present in the heart, including NOS1 (neuronal NOS [nNOS]), NOS2 (inducible NOS [iNOS]) and NOS3 (so-called endothelial-constitutive NOS [eNOS]). NOS1 has been detected in cardiac conduction tissue, in intracardiac neurons, and in the SR of cardiac myocytes. NOS2 is an inducible isoform that is not normally expressed in the myocardium but is synthesized de novo in virtually all cells in the heart in response to inflammatory cytokines. NOS3 is expressed in coronary endothelium and endocardium and in the sarcolemma and transverse (T)-tubule membranes of cardiac myocytes. NOS1 and NOS3 can be activated by calcium or calmodulin, whereas the induction of NOS2 is calcium independent. NO activates soluble guanylate cyclase ( eFig. 47.2A ). Vericiguat, an oral soluble guanylate cyclase stimulator, reduced the composite of death from cardiovascular causes and first HF hospitalization in the VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial (see Chapter 50 ).
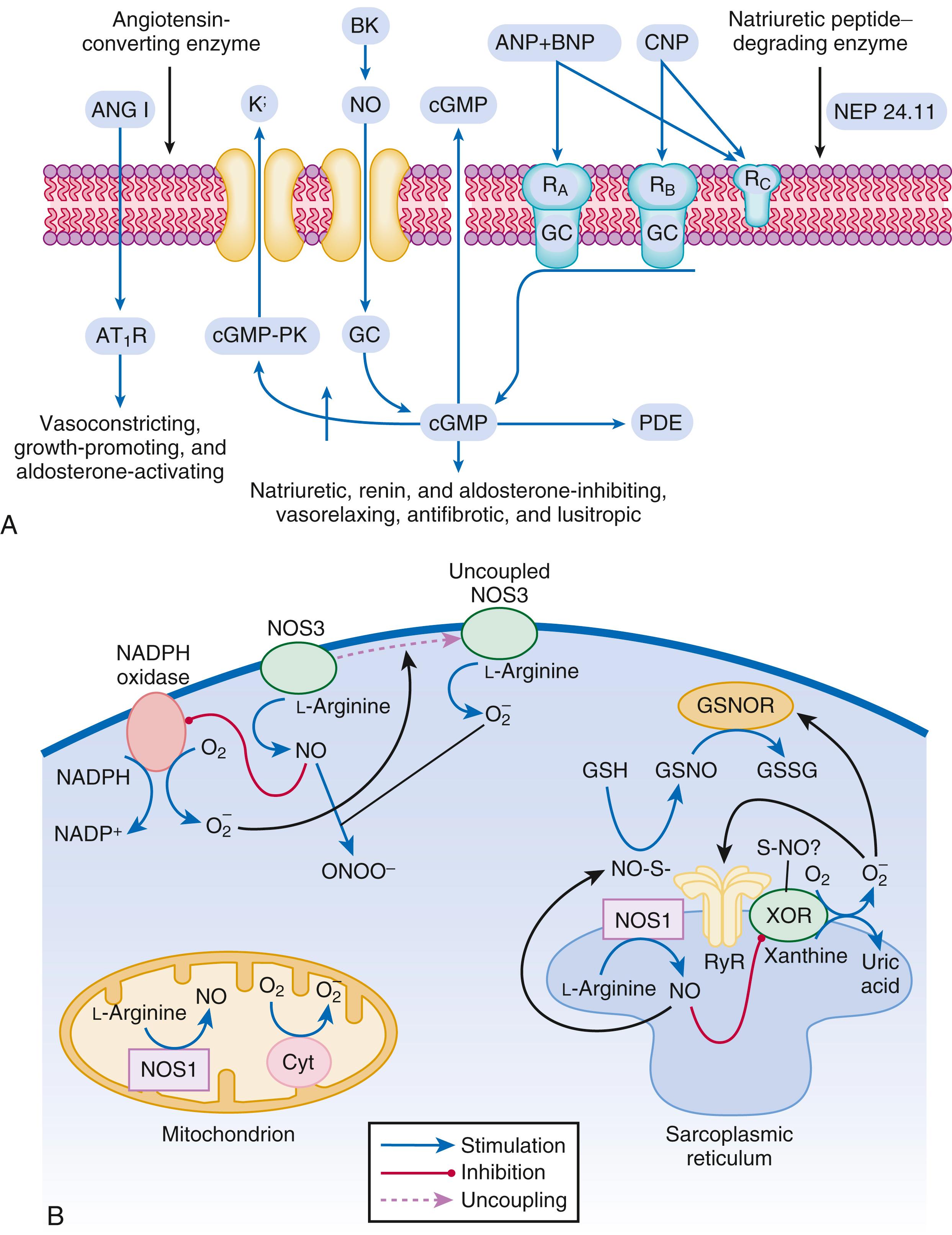
Under normal circumstances, the continuous release of NO (endothelium-derived relaxing factor) from the endothelium counteracts the vasoconstricting factors and allows for appropriate vasodilatory responses during exercise. This activation leads to the production of cGMP, which in turn activates protein kinase G (PKG) and cascade of different signaling events. In normal persons, NO released by endothelial cells mediates vasodilation in the peripheral vasculature through cGMP-mediated relaxation of vascular smooth muscle. In patients with HF, endothelium-dependent NO-mediated dilation of the peripheral vasculature is blunted, which has been attributed to decreased NOS3 expression and activity.
The actions of NO on the myocardium are complex and include both short-term alterations in function and energetics and longer-term effects on structure. NO modulates the activity of several key calcium channels involved in excitation-contraction coupling as well as mitochondrial respiratory complexes. This type of regulation is accomplished by spatial localization of different NOS isoforms in distinct cellular microdomains involved in excitation-contraction coupling. Specifically, NOS1 localizes to the SR in proximity to the ryanodine receptor (RyR) and sarcoendoplasmic reticulum calcium–adenosine triphosphatase (SR Ca 2+ -ATPase, SERCA2a), and NOS3 is found in sarcolemmal caveolae compartmentalized with cell surface receptors and the L-type Ca 2+ channel ( e Fig. 47.2B ). NO also participates in mitochondrial respiration, the process that fuels excitation-contraction coupling. The different NOS isoforms also may participate in the process of cardiac remodeling. LV remodeling was ameliorated and survival improved after MI in transgenic mice deficient in NOS2. By contrast, overexpression of NOS3 resulted in improved remodeling after MI. These contrasting effects of NOS2 and NOS3 may reflect the differences in amount of NO produced, which is much higher with NOS2. Emerging evidence indicates an imbalance between increasing free radical production and decreased NO generation in HF, which has been termed the “nitroso-redox imbalance.” NOS uncoupling secondary to a deficiency of tetrahydrobiopterin may further contribute to the nitroso-redox imbalance. The nitroso-redox imbalance probably contributes to disease progression in HF secondary to increased oxidative stress, as well as loss of the peripheral vasodilatory effects of NO.
Kinins are vasodilators that are released from inactive protein precursors (kininogens) through the action of proteolytic enzymes termed kallikreins. The biologic actions of the kinins are mediated by binding to B 1 and B 2 receptors. Most cardiovascular actions are initiated by the B 2 receptor, which is distributed widely in tissues, where it binds bradykinin and kallidin. The B 1 receptor binds the metabolites of bradykinin and kallidin. Stimulation of the B 2 receptor leads to vasodilation, which is mediated by the activation of NOS3, phospholipase A 2 , and adenylyl cyclase. Studies suggest that bradykinin plays an important role in the regulation of vascular tone in HF. The breakdown of bradykinin is catalyzed by ACE and neprilysin, so these enzymes not only lead to the formation of a potent vasoconstrictor (angiotensin II) but also mediate the breakdown of a vasodilator (bradykinin). The augmentation of bradykinin levels likely contributes to the beneficial actions of ACE inhibitors and NEP inhibitors (see Chapter 50 ).
Adrenomedullin is a 52-amino acid vasodilatory peptide that originally was discovered in human pheochromocytoma tissue. Subsequently, high levels of adrenomedullin immunoreactivity were detected in cardiac atrium and adrenal and pituitary glands, with lower levels detected in the ventricle, kidney, and vasculature. Adrenomedullin binds to a number of G-protein coupled receptors (GPRCs) that are present in multiple cell types, including on endothelial and vascular smooth muscle cells. Circulating concentrations of adrenomedullin are elevated in cardiovascular disease and HF in proportion to the severity of cardiac and hemodynamic impairment. Increasing evidence suggests that adrenomedullin may play a compensatory role in HF by offsetting the deleterious effects of excessive peripheral vasoconstriction. For example, the excretion of adrenomedullin that is stimulated by volume overload is thought to be protective by preserving endothelial barrier function. Moreover, adrenomedullin inhibits the renin-angiotensin-aldosterone system. Recently, a new immunoassay that specifically measures the biologically active form of adrenomedullin has been developed and may become a biomarker for tissue congestion.
Apelin is a vasoactive peptide that is an endogenous ligand for the GPCR APJ. The APJ gene encodes a receptor that most closely resembles the angiotensin receptor AT 1 . However, the APJ receptor does not bind angiotensin II. In the cardiovascular system, apelin elicits endothelium-dependent, NO-mediated vasorelaxation and reduces arterial blood pressure. In addition, apelin demonstrates potent inotropic activity without stimulating concomitant cardiac myocyte hypertrophy. Apelin also produces diuresis by inhibition of AVP activity. In experimental animals, apelin concentrations are significantly lower in failing hearts and are increased after treatment with an angiotensin receptor blocking agent. Furthermore, apelin levels are significantly reduced in patients with HF compared with controls and are significantly increased after cardiac resynchronization. The APJ receptor is a bifunctional GPCR that conveys cytoprotective signals after endogenous ligand stimulation and also acts as a mechanosensor to decrease cardiac hypertrophy after hemodynamic pressure overload. Recently, the apelin receptor has been shown to be activated by a novel endogenous peptide ligand referred to as Apela/Elabela/Toddler (ELA). In contrast to apelin, the expression of ELA is mainly enriched in stem cells, the kidney, prostate, and vascular endothelium. ELA exerts similar cardiovascular effects as apelin and may be more potent than apelin. ELA is also downregulated in experimental models and humans with HF. The ELP-AJP axis is currently being evaluated as a therapeutic target in patients with chronic HF.
Although adipose tissue was once considered a simple storage depot for fat, it is now known to synthesize and secrete a family of proteins collectively referred to as adipokines (see eFig. 47.3 ). Adipokines include adiponectin, TNF, plasminogen activator inhibitor type 1 (PAI-1), transforming growth factor-β, and resistin. Leptin is a 16-kDa protein hormone that plays a key role in regulating energy intake and energy expenditure. The product of the ob gene, leptin is predominantly synthesized and secreted by adipocytes, although the heart is also a site of leptin synthesis. The initial role of leptin was thought to decrease appetite through hypothalamic stimulation and thus regulation of food intake. However, elevated circulating levels of leptin and soluble leptin receptor, which act via activating a family of receptor (ob.R) isoforms, are markedly elevated in patients with HF. Leptin may affect myocardial function by promoting activation of both the SNS and the RAS. Moreover, leptin can stimulate the secretion of aldosterone. Several studies suggest that leptin directly induces hypertrophy in both human and rodent cardiac myocytes. Leptin resistance may lead to an accumulation of lipids in non-adipose peripheral tissues, resulting in a variety of “lipotoxic” effects, including cardiac myocyte apoptosis.
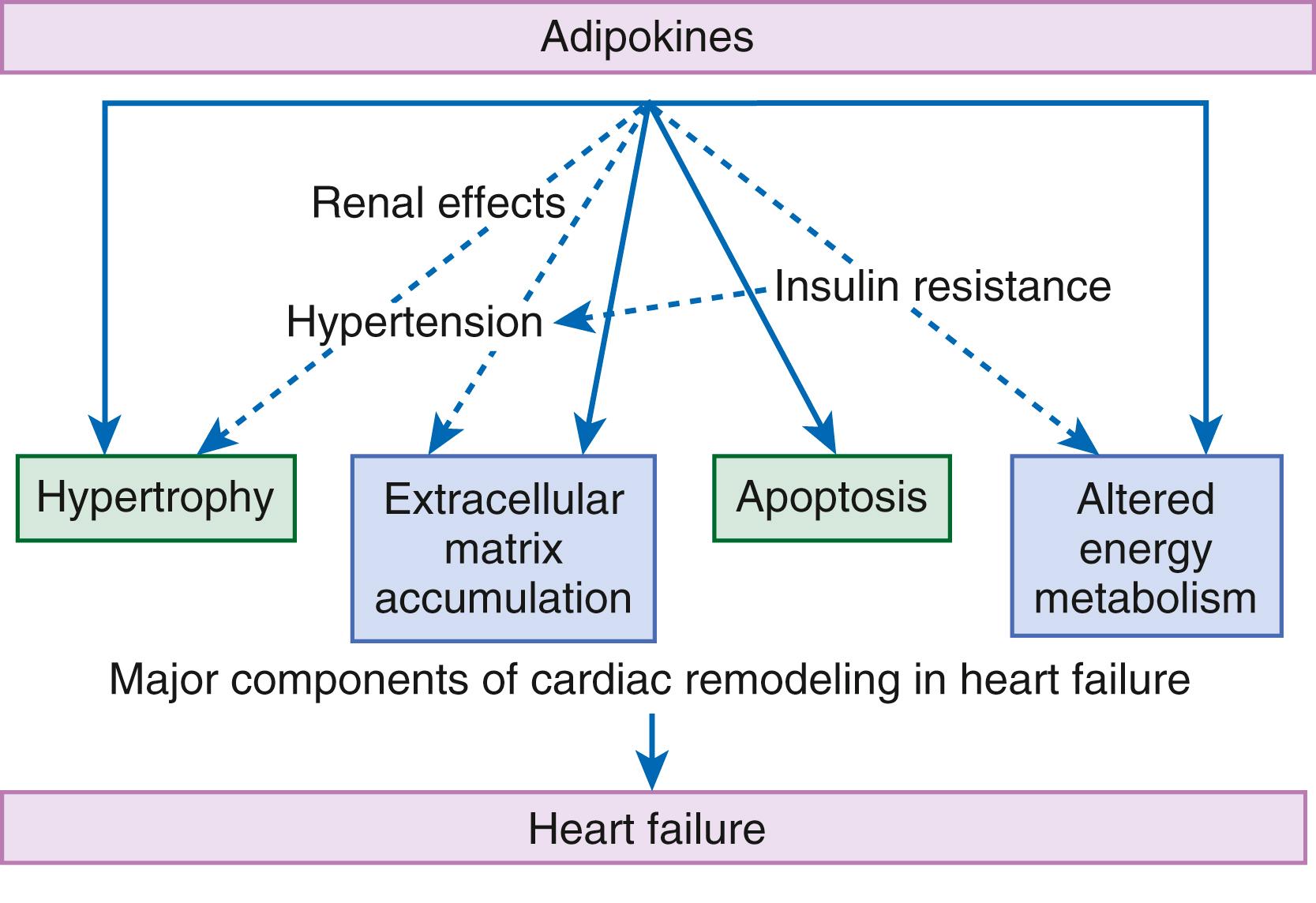
Adiponectin is a 224–amino acid polypeptide that modulates a number of metabolic processes, including glucose regulation and fatty acid oxidation. Although adiponectin initially was thought to be exclusively produced by adipose tissue, recent studies have demonstrated adiponectin expression in the heart. Studies in adiponectin-deficient mice demonstrated progressive cardiac remodeling after hemodynamic pressure overloading, whereas administration of adiponectin diminished infarct size, apoptosis, and TNF production after myocardial ischemia-reperfusion in both wild-type and adiponectin-deficient mice. Adiponectin inhibits cardiac hypertrophy, inflammation, and fibrosis. Moreover, adiponectin suppresses aldosterone secretion. Thus, adiponectin has been proposed as a potential biomarker of HF, as well as a potential therapeutic target for the treatment of HF.
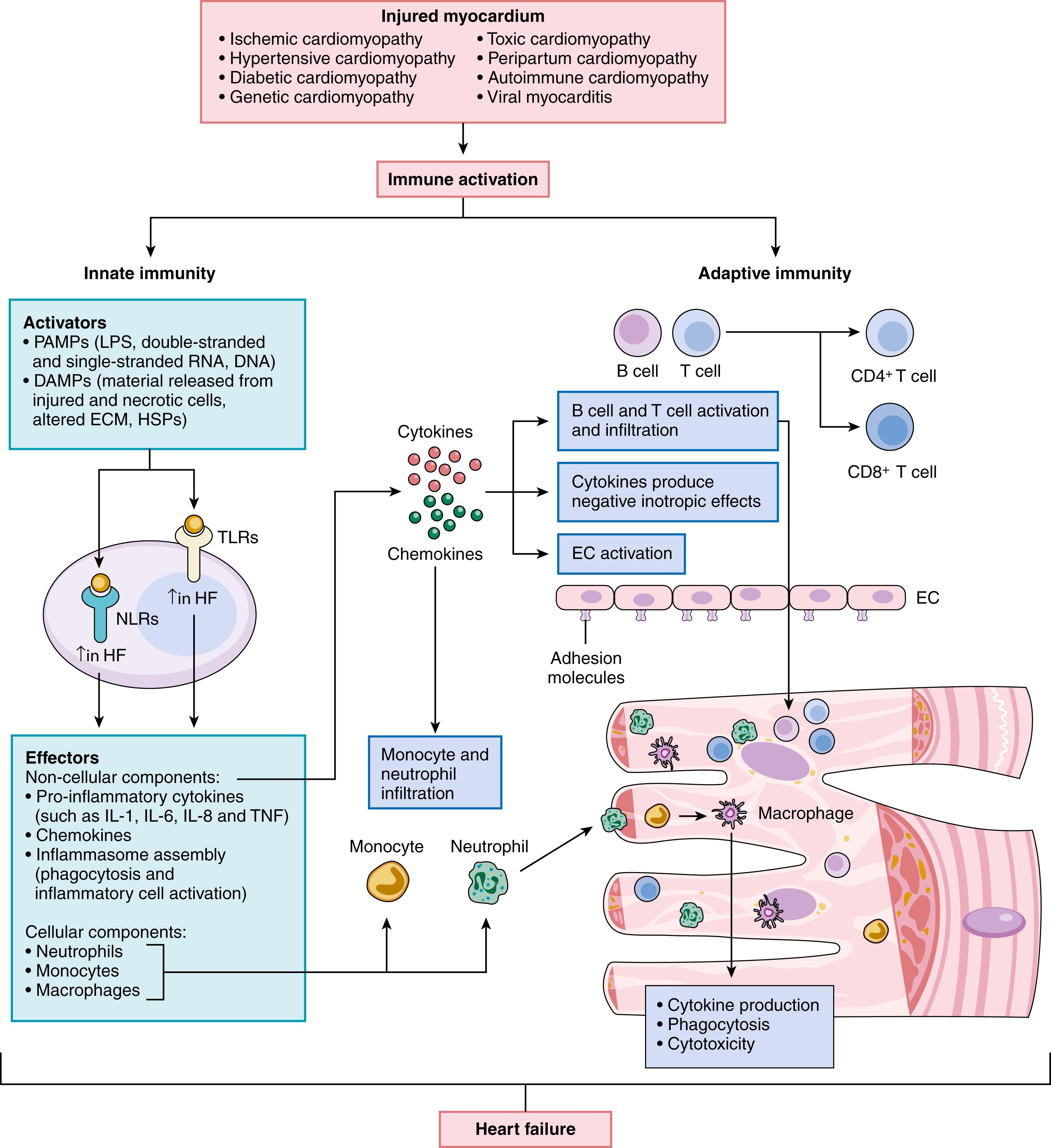
As shown in Figure 47.7 myocardial injury leads to the activation of the innate and adaptive immune systems in the heart. Whereas the innate immune system provides a global, nonspecific defense against tissue injury, the adaptive immune system provides a highly specific response mediated by B cells and T cells. The innate immune system is activated by transmembrane and cytosolic receptors that recognize molecular motifs from endogenous material released by dying or stressed cells (damage-associated molecular patterns [DAMPs]).These receptors, referred to as pattern recognition receptors (PRRs), are expressed by cells residing in the heart, including cardiac myocytes, endothelial cells, and tissue-resident immune cells. PRRs in the heart include Toll-like receptors (TLRs), RIG-I-like receptors (retinoic acid-inducible), NOD-like receptors (NLRs), the NLRP3 inflammasome, pentraxins, and C-type lectin receptors. Transcriptional profiling of human heart samples showed that failing and non-failing hearts have distinct expression profiles of genes related to innate immune responses, and the expression profiles of these genes were different in samples from patients with ischemic and non-ischemic HF. Activation of PRRs by DAMPs initiates downstream signaling cascades that regulate the expression of ensembles of genes encoding pro-inflammatory cytokines, including TNF, IL-1β, and IL-6, as well as chemokines, both of which serve as downstream “effectors” of the innate immune system. Activation of the innate immune system can lead subsequently to the activation of humoral immunity, through recruitment and stimulation of B cells and T cells. Activation of innate and adaptive immune responses provides the heart with a short-term adaptation to increased stress (physiologic inflammation). However, this inflammatory response can become dysregulated and result in chronic inflammation that leads to LV dysfunction and LV remodeling. Circulating levels of proinflammatory cytokines (e.g., CRP, TNF, IL-1β, IL-6) are increased in patients with HF and correlate with adverse patient outcomes. As shown in Table 47.1 , the sustained expression of inflammatory mediators is sufficient to recapitulate virtually all aspects of the HF phenotype, by provoking deleterious changes in cardiac myocytes and nonmyocytes, as well as changes in the myocardial extracellular matrix (ECM). The clinical relevance of these findings is suggested by a prespecified analysis of the CANTOS (Cardiovascular Risk Reduction Study) trial, which showed that targeted anti-cytokine therapy with canakinumab (a monoclonal antibody against IL-1β) reduced HF-related hospitalizations and mortality in patients with previous MI. Importantly, canakinumab-treated patients who achieved on-treatment hsCRP concentrations of less than 2 mg/L had significant reductions in the risk of HF related outcomes, including all-cause death when compared with patients receiving placebo, suggesting that inflammation contributes to the progression of HF.
| Alterations in the Biology of the Myocyte |
|
| Alterations in the Biology of Nonmyocytes |
|
| Alterations in the Extracellular Matrix |
|
| Progressive Myocyte Loss |
|
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here