Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Regulated cell death mainly occurs via extrinsic apoptosis, intrinsic apoptosis, necroptosis, and mitochondrial permeability transition (MPT)–driven regulated necrosis. Autophagy operates as a bona fide cell death mechanism in a few, mostly developmental, settings.
Oncogenesis results from multiple molecular alterations, one of which frequently impairs the ability of cancer cells to die in response to exogenous or endogenous signals.
Several oncoproteins and oncosuppressor proteins regulate, either directly or in an indirect fashion, the molecular machinery for apoptotic or necrotic cell death.
Targeting deregulated cell death signaling pathways in cancer constitutes a clinical reality and underlies promising approaches for the development of novel anticancer regimens.
Perhaps biased by their focus on the cell's vital functions, biologists have disregarded the existence of programmed cell death (PCD; see later for a definition) for a long time. Sporadic observations of PCD were made throughout the 19th century by scientists such as Carl Vogt, August Weismann, Ludwig Stieda, Élie Metchnikoff, Walther Flemming, Sigmund Mayer, and John Beard. The concept of PCD was first theorized in the 1960s, thanks to the work of Sir Richard Lockshin. In 1972 John Kerr, Alastair Currie, and Andrew Wyllie introduced the term apoptosis (from Greek apo, “from, off, without,” and ptosis, “falling”) to describe one type of cell death that manifests with peculiar morphologic traits. At that time and for the subsequent 30 years, apoptosis was thought to be the only form of PCD, an oversimplistic notion that has been invalidated only in this century. In the middle of the first decade of the century, it indeed became clear that other subroutines of cell death, notably necrosis, can occur in a regulated fashion and account for some instances of PCD.
Regulated cell death (RCD) constitutes a conserved mechanism whose usefulness trespasses evolutionistic barriers. For instance, in unicellular organisms that grow in colonies (such as yeast), the controlled demise of old and damaged cells increases the probability that fit individuals will survive adverse environmental conditions, and hence will perpetuate their genes. Conversely, in metazoans (including humans), PCD is critical for embryonic and postembryonic development, as well as for the maintenance of adult tissue homeostasis. In line with this notion, defects in the molecular mechanisms that mediate cell death contribute to the development of a wide array of human diseases. On one hand, the excessive demise of postmitotic cells decisively contributes to the pathogenesis of diseases encompassing ischemia (of the heart, brain, and kidney) and neurodegeneration. On the other hand, insufficient rates of cell death have been associated with autoimmune disorders and cancer.
The first hint that the molecular machinery for cell death is involved in oncogenesis came in the mid-1980s, when it was found that a translocation between chromosomes 14 and 18 (t14;18), which is common in lymphoma patients, leads to the overexpression of the protein BCL2. Subsequent work clarified that BCL2 promotes lymphomagenesis by inhibiting the programmed demise of excessive B cells rather than by stimulating their proliferation. This was the first demonstration that disabled cell death constitutes one of the hallmarks of cancer, irrespective of the histologic origin of malignant cells. During the subsequent two decades, it rapidly became clear that defects in the signaling pathways leading to cell death not only contribute to oncogenesis and tumor progression, but also determine, at least in some instances, the resistance of neoplastic cells to chemotherapy and radiotherapy.
Our understanding of cell death is constantly advancing, and this knowledge has already been translated into multiple therapeutic successes. Nevertheless, future investigations will have to provide deeper insights into the cancer-associated defects of cell death, in all its forms.
In cell death research, the attribute “programmed” is used to highlight the implication of one particular instance of cell death in developmental programs (and hence its physiologic relevance), whereas the adjective “regulated” is employed to stress the notion that one particular instance of cell death can be inhibited by specific pharmacologic or genetic interventions (and hence is mediated by genetically encoded molecular mechanisms). Thus all instances of PCD are regulated by definition, but not vice versa. Additional recommendations on how to define specific cell death subroutines based on morphologic or biochemical parameters have been formulated in the past few years, and will be respected throughout this chapter. The Nomenclature Committee on Cell Death (NCCD) has expressed doubts regarding the causal implication of specific biochemical processes in RCD. Indeed, inhibiting the mechanisms that until now were considered to be the cause of RCD (in all its instances) generally delays, but does not prevent, cell death (at least in mammalian organisms). This has far-reaching conceptual and therapeutic implications, especially for the development of cytoprotective clinical interventions.
According to currently accepted models, there are two main types of cell death: apoptosis and necrosis. Additional cell death subroutines with very specific biochemical traits have been described, although they often constitute particular cases of apoptosis and necrosis. Macroautophagy (hereafter referred to as autophagy) has also been implicated as a potential mechanism of cell death, a notion that remains highly debated (see later).
For a long time, instances of cell death have been catalogued as apoptotic based on purely morphologic manifestations, including cytoplasmic and nuclear shrinkage (pyknosis), nuclear breakdown (karyorrhexis), and plasma membrane blebbing. This morphologic definition, reflecting the original observations by Kerr, Currie, and Wyllie, has been abandoned in favor of a biochemical one. Thus, the term apoptosis is now used to define an instance of RCD that is precipitated by the activation of caspase 3 (CASP3), implying that it can be delayed by chemical CASP3 inhibitors as well as by specific genetic interventions that inactivate CASP3. Apoptosis can be triggered by the activation of either of two distinct but not mutually exclusive signaling pathways. Thus, whereas extrinsic apoptosis is initiated by an extracellular stimulus acting on plasma membrane receptors, intrinsic apoptosis follows the permeabilization of mitochondrial membranes driven by an intracellular stimulus. Of note, the enzymatic activity of CASP3, caspase 6 (CASP6), and caspase 7 (CASP7)—which are cumulatively known as executioner caspases—is responsible for multiple (but not all) classic morphologic and biochemical manifestations of apoptosis, including karyorrhexis, internucleosomal DNA degradation, and the exposure of the phospholipid phosphatidylserine on the outer surface of the plasma membrane.
Extrinsic apoptosis is frequently elicited by the ligand-induced activation of plasma membrane proteins of the death receptor family, such as Fas cell surface death receptor (FAS) and the tumor necrosis factor (TNF) superfamily member 1A (TNFRSF1A, best known as TNFR1). Alternatively, apoptotic signals can be transduced by the so-called dependence receptors (such as Patched 1, PTCH1) when the extracellular concentration of trophic factors, such as Sonic Hedgehog (SHH), falls below a critical threshold level. This means that, at odds with death receptors, dependence receptors deliver proapoptotic signals in the absence rather than in the presence of their ligands. Death receptors undergo spontaneous trimerization owing to the so-called preligand assembly domain (PLAD). Ligand binding stabilizes this configuration and hence allows for the recruitment of several proteins at the cytoplasmic tails of death receptors, including (but in some instances not limited to) pro-caspase 8 (pro-CASP8), receptor interacting serine/threonine kinase 1 (RIPK1, also known as RIP1), FAS-associated protein with a death domain (FADD), and cellular inhibitor of apoptosis proteins (cIAP1 and cIAP2, which are E3 ubiquitin ligases that also inhibit apoptosis owing to their ability to interfere with caspase activation). This multiprotein platform is known as death-inducing signaling complex (DISC) and stimulates the conversion of pro-CASP8 into caspase 8 (CASP8), which proteolitically activates CASP3 and hence initiates the degradative cascade that precipitates extrinsic apoptosis. Of note, the components of the signaling pathways that emanate from specific death receptors exhibit some degree of variation, yet all converge (in apoptosis-permissive conditions; see later) to the activation of CASP8. The molecular mechanisms that link dependence receptors to CASP3 activation are not precisely understood, yet appear to involve the adaptor protein DRAL and CASP9 ( Fig. 5.1 ).
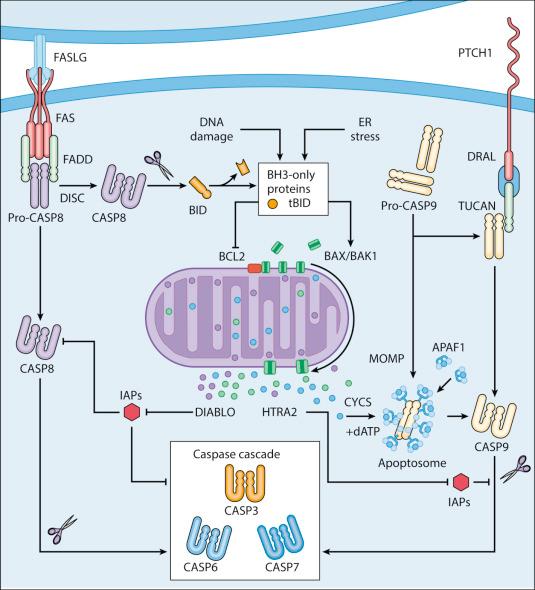
Intrinsic apoptosis (also known as mitochondrial apoptosis) can be activated by a plethora of stimuli, including intracellular damage (to virtually any of the subcellular compartments) and oncogenic stress (see later). Cells are equipped with a heterogeneous set of intracellular sensors that respond to microenvironmental perturbations by activating signaling pathways for the reestablishment of homeostasis and the repair of damage, and then, if damage is irreparable, by igniting intrinsic apoptosis. The central step in this cascade is regulated by the integrity of mitochondrial structure and function. Indeed, if proapoptotic signals are predominant, mitochondrial membranes get permeabilized, resulting in the abrupt cessation of adenosine triphosphate (ATP) synthesis and other metabolic functions, as well as in the spillage of several proteins that are normally confined in the mitochondrial intermembrane space. These proteins include cytochrome c, somatic (CYCS, a semisoluble component of the mitochondrial respiratory chain), diablo IAP-binding mitochondrial protein (DIABLO), and HtrA serine peptidase 2 (HTRA2). Once in the cytosol, CYCS—together with dATP and the adaptor protein apoptotic peptidase activating factor 1 (APAF1)—drives the assembly of the apoptosome, a supramolecular platform for the activation of CASP9. DIABLO and HTRA2 also stimulate caspase activation, although indirectly, by sequestering and/or degrading cytosolic caspase inhibitors of the IAP family. Other mitochondrial proteins with cytotoxic potential are released in the cytosol during the course of intrinsic apoptosis, including apoptosis inducing factor, mitochondria associated 1 (AIFM1, which normally contributes to the stability and function of respiratory complex I) and endonuclease G (ENDOG). Although both AIFM1 and ENDOG can translocate to the nucleus and mediate large-scale DNA degradation independently of caspases, their implication in intrinsic apoptosis has been dismissed.
According to current models, mitochondrial outer membrane permeabilization (MOMP) is initiated at the mitochondrial outer membrane (OM) by the pore-forming activity of proapoptotic multidomain proteins of the Bcl-2 family. These proteins, such as BCL2 associated X, apoptosis regulator (BAX), and BCL2 antagonist/killer 1 (BAK1), contain several Bcl-2 homology (BH) domains as well as a transmembrane domain that allow for their constitutive or inducible insertion into the OM. MOMP execution by BAK1 and BAX is regulated by other members of the same protein family. In particular, antiapoptotic proteins such as BCL2, apoptosis regulator (BCL2), BCL2-like 1 (BCL2L1, best known as BCL-xL), and MCL1, BCL2 family apoptosis regulator (MCL1) inhibit MOMP by binding to BAX and BAK1 and hence by maintaining them in an inactive conformation. Conversely, the so-called BH3-only proteins (small members of the Bcl-2 family that often contain only the BH3 domain) can promote the pore-forming activity of BAK1 and BAX by two different mechanisms. Thus, BH3-only proteins can either stimulate the conformational activation of BAX and BAK1 in a direct fashion or competitively displace BAX, BAK1 or other BH3-only proteins from inhibitory interactions with BCL2, BCL-xL and MCL1. BH3-only proteins can be regulated at the transcriptional level as well as by rapid posttranslational modifications (e.g., phosphorylation, proteolytic processing), de facto constituting sensors of intracellular stress that directly impinge on the regulation of intrinsic apoptosis.
Of note, the signaling pathways leading to extrinsic and intrinsic apoptosis exhibit some degree of cross talk. Thus, whereas in some cells including lymphocytes (type I cells) the activation of death receptors leads to pro-CASP3 processing and apoptosis without any mitochondrial involvement, in other cells such as hepatocytes (type II cells), CASP8 not only activates CASP3 but also mediates the proteolytic cleavage of the BH3-only protein BH3 interacting domain death agonist (BID), generating a MOMP-inducing fragment. Thus, in type II cells, MOMP functions as an amplifier of apoptotic signaling by eliciting the CASP9-mediated activation of CASP3. Interesting to note, whether cells respond to death receptor ligation in a type I- or type II-like manner depends on the expression levels of the cIAP-like protein X-linked inhibitor of apoptosis (XIAP). The cross talk between extrinsic and intrinsic apoptosis is pathophysiologically relevant in vivo, as demonstrated by the fact that the hepatocytes of Bid −/− mice are partially protected from FAS-induced apoptosis (see Fig. 5.1 ).
Classically, necrosis was defined as an instance of cell death lacking the peculiar morphological manifestations of apoptosis as well as the accumulation of cytoplasmic vacuoles that characterize autophagic cells. Somehow, this was in line with the belief that necrosis would always proceed in an unregulated fashion and would only terminate accidental instances of cell death. In the 1990s, Vandenabeele's and Schulze-Osthoff's groups demonstrated that engagement of FAS does not always lead to cell death via extrinsic apoptosis. This observation instilled in some researchers the suspicion that, similar to apoptosis, necrosis also might be orchestrated by a refined molecular machinery; this ignited an intense wave of research that has not yet come to an end. Important to note, regulated necrosis occurs during mammalian development, in particular at the bone growth plate (i.e., the zone of the bone that controls its length), as well as during adult tissue homeostasis, for instance in the lower regions of intestinal crypts. Moreover, multiple instances of necrotic RCD have been involved in the pathophysiology of diseases including viral infection, neurodegeneration, ischemia, and others.
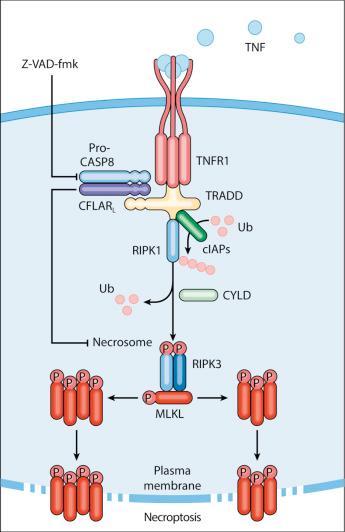
The best-characterized pathway of regulated necrosis, which is known as necroptosis, can be elicited by the ligation of death receptors in conditions in which CASP8 is inhibited (either by pharmacologic or by genetic interventions). In this context, DISC-bound RIPK1 does not get degraded by CASP8 as it occurs during extrinsic apoptosis, but recruits and functionally interacts with its homolog receptor interacting serine/threonine kinase 3 (RIPK3) and mixed-lineage kinase like (MLKL), generating the so-called necrosome. Although PGAM family member 5, mitochondrial serine/threonine protein phosphatase (PGAM5) has been suggested to contribute to necroptosis downstream of RIPK1 and RIPK3 by virtue of its capacity to promote mitochondrial fragmentation, such a possibility has now been discarded. Rather, it is now clear that phosphorylated MLKL can form oligomers that relocalize to the inner leaflet of the plasma membrane and compromise its stability, hence precipitating RCD. Whether phosphorylated MLKL is sufficient to permeabilize the plasma membrane or whether additional factors are required for necroptosis execution remains a matter of debate. RIPK3 activation is tonically inhibited by a heterodimer composed of CASP8 and the long isoform of CASP8 and FADD-like apoptosis regulator (CFLAR), best known as FLIP L , but the underlying molecular mechanisms remain to be elucidated. Moreover, RIPK3 oligomerization and consequent phosphorylation of MLKL is controlled by the RIP homotypic interacting motif (RHIM) of RIPK1, the absence of which results in spontaneous necroptosis. Important to note, stimuli other than death receptor ligands (e.g., alkylating DNA damage, viral infection) trigger bona fide RIPK3- and MLKL-dependent necroptosis ( Fig. 5.2 ).
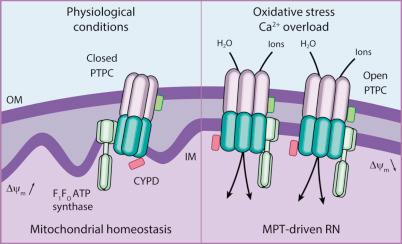
In conditions of intense oxidative stress and/or in the presence of high cytosolic Ca 2+ concentrations, mitochondria experience an abrupt increase in the permeability to ions and small solutes of the mitochondrial inner membrane (IM), a process known as mitochondrial permeability transition (MPT). MPT results in virtually immediate dissipation of the mitochondrial transmembrane potential (Δψ m ), rapid cessation of all Δψ m -dependent mitochondrial activities (including ATP synthesis and protein import), and osmotic breakdown of the organelle. MPT has been ascribed to the activity of a multiprotein protein complex that is assembled at the juxtaposition sites between the OM and the IM, the so-called permeability transition pore complex (PTPC). The main components of the PTPC, including members of the voltage-dependent anion channel (VDAC) family (located in the OM) and the adenine nucleotide translocase (ANT) family (located in the IM), as well as peptidylprolyl isomerase F (PPIF, a protein of the mitochondrial matrix best known as CYPD), normally mediate physiologic functions. For instance, ANT catalyzes the exchange of adenosine diphosphate (ADP) and ATP between the cytosol and the mitochondrial matrix. In response to cytosolic Ca 2+ overload oxidative stress, the PTPC has been proposed to assume a high-conductance conformation leading to rapid mitochondrial breakdown. So far, mouse knockout experiments failed to attribute to specific components of the PTPC a critical role in the regulation of MPT, perhaps because of the existence of multiple and (at least partially) redundant isoforms of these proteins. One notable exception is represented by CYPD, whose absence has been shown to limit pathologic cell death in multiple circumstances, in vitro and in vivo. Thus MPT-driven necrosis can be defined biochemically as a form of RCD that can be retarded by pharmacologic or genetic inhibition of CYPD. Recent findings suggest that the c subunit of the F 1 F O ATPase (which in humans is encoded by ATP5G1, ATP5G2, and ATP5G3 ) may constitute the long-sought pore-forming component of the PTPC, but robust genetic evidence in support of this hypothesis is missing ( Fig. 5.3 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here