Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Note: The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Extensive cutaneous thermal injury invariably results in the severe derangements of cardiovascular function and end-organ perfusion known as burn shock . Shock is an abnormal physiologic state in which tissue perfusion is insufficient for oxygen and nutrient delivery and cellular waste removal. Before the 19th century, investigators demonstrated that, after a burn, fluid is lost from the blood and the blood becomes thicker; in 1897, saline infusions for severe burns were first advocated. Frank Underhill derived a more complete understanding of burn pathophysiology when he demonstrated that unresuscitated burn shock was associated with increased hematocrit values, which are secondary to fluid and electrolyte loss after burn injury. Thus increased hematocrit values after severe burn injury are a consequence of a plasma volume deficit. Cope and Moore furthered these finding, demonstrating that the hypovolemia of burn injury resulted from fluid and protein translocation into both burned and unburned tissues.
Animal and clinical studies have established the importance of fluid resuscitation for burn shock. Investigations have focused on correcting the rapid and massive fluid sequestration in the burn wound and the resultant hypovolemia. The literature contains a large experimental and clinical database on the circulatory and microcirculatory alterations associated with burn shock and edema generation in both the burn wound and unburned tissues. Substantial research has focused on identifying and defining the mechanisms and effects of the many inflammatory mediators produced and released after burn injury.
Burn shock occurs from the coalescence of three cardinal causes: (1) hypovolemia resulting from intravascular fluid leaking into the interstitial space causing burn edema, (2) cardiac depression due to humoral factors and loss of preload, and (3) increased systemic vascular resistance. Later in the resuscitation process vasoplegia can replace the increase in system vascular resistance. Burn shock is a complex process of circulatory and microcirculatory dysfunction that is not easily or fully repaired by fluid resuscitation. Severe burn injury results in significant distributive shock and substantial tissue trauma, both of which cause the formation and release of many local and systemic mediators. Burn shock results from the interplay of direct tissue injury, hypovolemia, and the release of multiple mediators of inflammation, with effects on both the microcirculation and the function of the heart and lungs. Subsequently burn shock continues as a significant pathophysiologic state even if hypovolemia is corrected. Increases in pulmonary and systemic vascular resistance (PVR, SVR) and myocardial depression occur despite adequate preload and volume support. Such cardiovascular dysfunctions can further exacerbate the whole-body inflammatory response into a vicious cycle of accelerating organ dysfunction.
This chapter examines our current understanding of the pathophysiology of the early events in burn shock, focusing on the many facets of the microcirculatory, organ, and systemic effects resulting directly from burns and circulating mediators. Intracellular pathways are not presented.
Inflammatory shock mediators, both local and systemic, that are implicated in the pathogenesis of burn shock include histamine, serotonin, bradykinin, nitric oxide, oxygen radicals, tumor necrosis factor (TNF), interleukins, and products of the eicosanoid acid cascade including prostaglandins and thromboxanes. Additionally certain hormones and mediators of cardiovascular function are elevated several fold after burn injury: these include epinephrine, norepinephrine, vasopressin, angiotensin II, and neuropeptide-Y. Other mediators and unknown factors yet to be defined are also involved.
Burn injury causes extravasation of plasma into the burn wound. Extensive burn injuries are hypovolemic in nature and are characterized by hemodynamic changes similar to those that occur after hemorrhage, including decreased plasma volume, cardiac output, and urine output and an increased SVR with resultant reduced peripheral blood flow. However, whereas in hemorrhage there is a fall in hematocrit with blood loss due to autotransfusion of interstitial fluid into the vasculature, in burn shock hematocrit may rise due to plasma extravasation. This is particularly common when fluid therapy is inadequate.
As in the treatment of other forms of hypovolemic shock, the primary initial therapeutic goal is to promptly restore intravascular volume and to preserve tissue perfusion and minimize tissue ischemia. However burn resuscitation is complicated not only by severe burn wound edema, but also by extravasated and sequestered fluid and protein in unburned soft tissue. Large volumes of resuscitation solutions are required to maintain intravascular volume during the first several hours after an extensive burn.
Edema develops when the rate at which fluid is filtered out of the capillaries exceeds the flow in the lymph vessels. Edema formation often follows a biphasic pattern. An immediate and rapid increase in the water content of burned tissue is seen in the first hour after burn injury. A second and more gradual increase in fluid flux of both the burned skin and unburned soft tissue occurs during the first 12–24 hours after burn injury. The amount of edema formation in burned skin depends on the type and extent of injury, on whether fluid resuscitation is provided, and on the type and volume of fluid administered. Fluid resuscitation elevates blood flow and capillary pressure thereby contributing to further fluid extravasation. Without sustained IV replacement of intravascular fluid losses edema formation is somewhat self-limited, as tissue blood flow and capillary pressure decrease.
Edema formation in thermally injured skin is characterized by an extremely rapid onset. Tissue water content can double within the first hour after burn. Leape found a 70–80% increase in water content in a full-thickness burn wound 30 minutes after burn injury, with 90% of this change occurring in the first 5 minutes. There was only a modest increase in burn wound water content after the first hour in nonresuscitated animals. In resuscitated animals or animals with small wounds, adequate tissue perfusion continues to “feed” the edema for several hours. Demling et al. used dichromatic absorptiometry to measure edema development during the first week after an experimental partial-thickness burn injury on one hind limb in sheep. Although edema formation was rapid, with more than 50% occurring in the first hour, maximum water content was not present until 12–24 hours after burn injury. The mass of the burned tissue is significantly less than that of the remainder of the body. As such most fluid shifts likely occur from the blood into the unburned tissue due to the humoral actions of inflammatory mediators causing endothelial activation and glycocalyx injury.
An understanding of the physiologic mechanisms of the rapid formation of burn edema requires an understanding of the mechanisms of microvascular fluid balance. Under physiologic steady-state conditions blood pressure in capillaries causes filtration of fluid into the interstitial space. The bulk of the filtrate is removed from the interstitial space by lymphatic drainage. Fluid transport across the microcirculatory wall in normal and pathological states has been described by the original “classic” Starling equation that did not include the glycocalyx:
Starling sought to explain the interaction of physical forces that govern fluid transfer between intravascular and extravascular compartments. J v is the flux (flow rate) of fluid that crosses the microvasculature barrier. K f is the capillary filtration coefficient, which is the product of the surface area and hydraulic conductivity (water permeability) of the capillary wall; P c is the capillary hydrostatic pressure; P if is the interstitial fluid hydrostatic pressure; π p is the colloid osmotic pressure of plasma; π if is the colloid osmotic pressure of interstitial fluid; σ is the osmotic reflection coefficient. Edema occurs when the lymphatic drainage rate (J L ) does not keep pace with the increased J v ( Fig. 8.1 ). Fig. 8.1 shows the key structures and microvascular forces of the classic Starling equation.
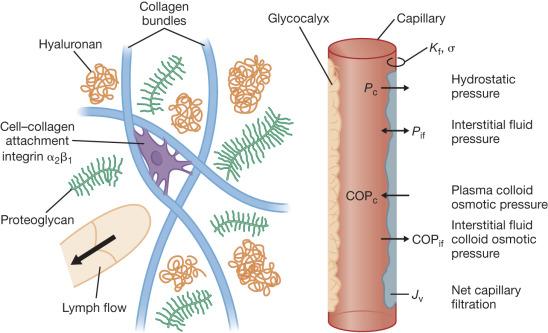
There is now evidence that suggests the plasma colloid osmotic pressure does not exert its full effect at the capillary wall because of a protective barrier that excludes protein: the glycocalyx on the luminal endothelium. The effective colloid osmotic absorptive force is generated by the gradient across the glycocalyx. However the role of the glycocalyx in burns is largely unexplored. It remains useful to review each term of the classic Starling equation and its role in burn edema before addressing the possible the implications of a modified Starling equation.
Transvascular blood-to-tissue transport of fluid and protein increase with elevation in K f , P c , or π if , and with decreases in P if , or σ. Burn edema is unique in its rapidity compared to other types of edema because it is only in burn edema that all of these variables change significantly in the direction required to increase fluid filtration. Each Starling variable is discussed individually next.
Burn injury causes direct and indirect mediator-modulated changes in the permeability of the blood–tissue barrier of the capillaries and venules. Arturson and Mellander showed that, in the scalded hindlimb of dogs, K f immediately increased two to three times, suggesting that the hydraulic conductivity (water permeability) of the capillary wall increased. K f is a function of both hydraulic conductivity and the capillary surface area. Thus local vasodilation and microvascular recruitment contribute to the increased K f in addition to increased hydraulic conductivity. Measuring K f and the rate of edema formation ( J v ) allowed Arturson and Mellander to determine the changes in transcapillary forces necessary to account for the increased capillary filtration. Their calculations indicated that a transcapillary pressure gradient of 100–250 mm Hg was required to explain the extremely rapid edema formation that occurred in the first 10 minutes after a scald injury. They concluded that only a small fraction of the early formation of burn edema could be attributed to the changes in K f and permeability. They further suggested that osmotically active molecules generating sufficiently large osmotic reabsorption pressures are released from burn-damaged cells. This hypothesis was never confirmed, and subsequent studies described herein show that large increases in filtration forces result from an increased P c , and from a large decrease in P if ( Table 8.1 ).
| Variable | Normal or Baseline | Post-Burn | Δ | References |
|---|---|---|---|---|
| P c | ~25 mm Hg | ~50 mm Hg | ↑ ~25 mm Hg | |
| Π P | 25–30 mm Hg | 15 to 18 mm Hg | ↓ ~10 mm Hg | |
| P if | −2 to 0 mm Hg | ~100 mm Hg non-resuscitated non-perfused skin and −5 mm Hg perfused skin |
↓ ~100 mm Hg ↓ 3–5 mm Hg |
|
| Π if | 10–15 mm Hg | 13–18 mm Hg in burn wound ↓ and with resuscitation hypoproteinemia in unburned skin |
↑ ~3 mm Hg | |
| σ | ~0.9 | ~0.5 | ↓ ~0.4 | |
| K f | ~0.003 mL/min/mm Hg/100 g (leg) | ↑ 2–5× |
In most forms of shock, arteriolar vasoconstriction results in transfer of less arterial pressure to the capillaries; capillary and venous pressures decrease. However in studies using the vascular occlusion technique in the scalded hindlimb of dogs, P c doubled from approximately 25 mm Hg to approximately 50 mm Hg during the first 30 minutes after burn injury and slowly returned to baseline over 3 hours.
Burned tissue has been demonstrated to have a significantly decreased interstitial hydrostatic pressure. Using micropipettes and a tissue oncometer, Lund reported that dermal P if was rapidly reduced from its normal value of −1 mm Hg to less than −100 mm Hg in isolated nonperfused samples of skin. This large negative interstitial hydrostatic pressure constitutes a powerful “suction force” or imbibition pressure promoting microvascular fluid filtration and sustained burn wound edema. In vivo measurements show a temporary reduction of −20 to −30 mmHg; the less negative P if in vivo is due to the continued tissue perfusion and fluid extravasation that relieves the imbibition pressure. Kinsky reported a continued negative pressure providing a partial explanation for the sustained edema during the first 4 hours post injury.
The mechanism for the large decrease in P if is due, at least in part, to the release of cellular tension exerted on the collagen and microfibril networks in the connective tissue via the collagen-binding β 1 -integrins. This tends to expand the interstitial space and induces the imbibition pressure. The integrins are transmembrane adhesion receptors that mediate cell–cell and cell–matrix adhesion, thereby allowing the glycosaminoglycan ground substance, which is normally underhydrated, to expand and take up fluid. McGee et al. confirmed this hydration potential with T2-weighted MRI and noted that it is reversible by application of negative pressure treatment. This supports the mechanism of interfascial rather than colloidal osmotic fluid transfer as a mechanism for burn edema and supports the collagen structural transitions as therapeutic targets.
The osmotic reflection coefficient is an index of the proportion of the full osmotic pressure generated by the concentration gradient of plasma proteins across the microvascular blood-to-tissue barrier. A value of σ = 1.0 represents a barrier impermeable to protein but permeable to water and σ = 0 represents a barrier that is completely permeable to protein and water. The reflection coefficient is traditionally attributed to the endothelial cellular junctions but may well be primarily determined by the glycocalyx. In skin, the normal σ of albumin is reported to be 0.85–0.99. Thermal injury causes an increase in capillary permeability to protein, resulting in a reduced σ, an effective reduction in the absorptive oncotic gradient across the microvascular barrier, and a resulting increase in net fluid filtration. Lymph sampled from burned skin has shown elevated protein concentrations consistent with the large and sustained increases in capillary permeability, whereas a transient and smaller increase in microvascular permeability occurs over 8–12 hours following injury in other soft tissue not directly burned. Pitt et al. estimated the σ for skin from dog hindpaw using a lymph wash-down technique and reported a normal σ of 0.87 for albumin and a reduction to 0.45 after scald injury.
The normal plasma protein concentration of 6–8 g/dL and its associated π p of 25–30 mm Hg, would produce a significant transcapillary absorptive force counterbalancing the other Starling forces that favor filtration. However, the glycocalyx blocks most of the impact of π p on transendothelial fluid movement, as described later. Plasma colloid osmotic pressure decreases in nonresuscitated burn-injured animals as protein-rich fluid extravasates into burn wounds and a significant volume of protein-poor interstitial fluid initially enters the circulation from transvascular reabsorption and lymph of unburned tissue, such as skeletal muscle. Plasma is further diluted and π p is further reduced after crystalloid resuscitation. Increased fluid filtration is less due to the fall in π p and more likely attributed to increased K f , reductions in P if and σ due to a damaged glycocalyx. Initial therapy with colloid solution has always been advocated by some clinicians but is often delayed 8–24 hours after injury based on the reasoning that normalization of microvascular protein permeability in injured tissue must occur before colloid therapy is cost effective. However, the ability of plasma protein and, in particular, albumin to repair the permeability of the glycocalyx suggests a rationale for the earlier use of albumin therapy for burn resuscitation. Evidence for the use of albumin for resuscitation is also covered in Chapter 9 on burn resuscitation. Animal studies have shown that albumin use during burn resuscitation does not reduce the edema in the tissue of burn wounds, but it does reduce total fluid needs and thus reduces edema in unburned tissues. Furthermore, as discussed later, albumin can have a trophic effect on the endovascular glycocalyx, acting to stabilize it.
The π if in skin is normally 10–15 mm Hg or about one-half that of plasma. Experimental studies in animals using lymph as representative of interstitial fluid suggest that the colloid osmotic pressure in lymph from burned skin initially increases 4–8 mm Hg after burn injury. With crystalloid resuscitation, π p and π if decrease because the protein concentration of microvascular filtrate remains less than that of plasma despite an increased permeability. The osmotic reflection coefficient, σ, decreases with burns but never equals zero; thus protein concentration in capillary filtrate is always less than in plasma even in burn-injured skin. Compared to unburned skin, the π i remains significantly higher in the burn wound, supporting the view that sustained increases in protein permeability contribute to the persistence of burn edema. However, compared with the large changes in P c and particularly P if , increased microvascular protein permeability is not the predominant mechanism for the early, rapid rate of edema formation in injured skin.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here