Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
antibody-mediated rejection
acute tubular necrosis
chronic allograft nephropathy
cytomegalovirus
calcineurin inhibitor
calcineurin inhibitor toxicity
delayed graft function
donor-specific antibody
electron microscopy
focal segmental glomerulosclerosis
glomerular basement membrane
human leukocyte antigen
interstitial fibrosis and tubular atrophy
membranous glomerulonephritis
membranoproliferative glomerulonephritis
periodic acid-Schiff
peritubular capillary
posttransplant lymphoproliferative disease
polyomavirus tubulointerstitial nephritis
T cell-mediated rejection
transplant glomerulopathy
tubular basement membrane
thrombotic microangiopathy
Many thanks to a coauthor of a prior version, Shamila Mauiyyedi, MD, and to Dr. Paul J. Kurtin, for his useful suggestions on the manuscript.
Renal biopsy remains the “gold standard” for the diagnosis of episodes of graft dysfunction that occur commonly in patients after transplantation. Studies have indicated that the results of a renal allograft biopsy change the clinical diagnosis in 30% to 42% and therapy in 38% to 83% of patients, even after the first year. Most important, unnecessary immunosuppression was avoided in 19% of patients. The biopsy is also a gold mine of information on pathogenetic mechanisms, a generator of hypotheses that can be tested in experimental animal studies and in clinical trials. Finally, the biopsy serves, in turn, to validate the hypothesis tested in such trials. Renal biopsy interpretation currently relies primarily on histopathology complemented by immunologic molecular probes. Quantitative gene expression analysis methods may be implemented more in the future as those techniques are further validated and approved for clinical use.
This chapter describes the relevant light, immunofluorescence, and electron microscopy (EM) findings of the most common lesions affecting the renal allograft and their differential diagnosis, citing references largely limited to human pathologic studies after 1990. The discussion is broadly divided into allograft rejection and nonrejection pathology, with an emphasis on differential diagnosis of acute and chronic allograft dysfunction. Grading systems of acute and chronic rejection are discussed further in those sections. Additional references and details are available in a comprehensive review.
At least seven nonsclerotic glomeruli and two arteries (bigger than arterioles) must be present in a renal allograft biopsy for adequate evaluation. Using these criteria, the sensitivity of a single core is approximately 90%, and the predicted sensitivity of two cores is about 99%. However, adequacy depends entirely on the lesions seen in the biopsy: one artery with endarteritis is sufficient for the diagnosis of acute cellular rejection (TCMR), even if no glomerulus is present; similarly, immunofluorescence or EM of one glomerulus is adequate to diagnose membranous glomerulonephritis (MGN). In contrast, a large portion of cortex with a minimal infiltrate does not exclude rejection. Subcapsular cortex often shows inflammation and fibrosis and is not representative. Diagnosis of certain diseases is even possible with only medulla (acute humoral rejection [acute AMR], polyomavirus tubulointerstitial nephritis [PTN]). However, a normal medulla does not rule out rejection. Frozen sections for light microscopy are of limited value, because frozen artifacts preclude accurate evaluation. The diagnostic accuracy of frozen sections was 89% compared with paraffin sections. Rapid (2-hour) formalin/paraffin processing is used at Massachusetts General Hospital for urgent and weekend biopsies.
The biopsy is examined for glomerular, tubular, vascular, and interstitial pathology including: (1) transplant glomerulitis, glomerulopathy, and de novo or recurrent glomerulonephritis; (2) tubular injury, isometric vacuolization, tubulitis, atrophy, or intranuclear viral inclusions; (3) endarteritis, fibrinoid necrosis, thrombi, myocyte necrosis, nodular medial hyalinosis, or chronic allograft arteriopathy; (4) interstitial infiltrates of activated mononuclear cells, edema, or neutrophils, fibrosis, and scarring. Arteries and arterioles are particularly scrutinized, because the diagnostic lesion often lies there.
A typical immunofluorescence panel (used at Massachusetts General Hospital) detects IgG, IgA, IgM, C3, kappa and lambda light chains, C4d, albumin, and fibrin in cryostat sections. C4d, a complement fragment, is used to identify AMR; the other stains are primarily for recurrent or de novo glomerulonephritis. Immunohistochemistry (IHC) in paraffin sections is indicated in the differential diagnosis of lymphoproliferative or viral diseases and may be used for C4d. EM is valuable when de novo or recurrent glomerular disease is suspected and to evaluate peritubular capillary (PTC) basement membranes.
The ideal diagnostic classification of renal allograft pathology should be based on pathogenesis, have therapeutic relevance, and be reproducible. The current classification based on Banff and other systems ( Table 25.1 ), meets these criteria.
|
Biopsy of the cadaveric donor kidney is sometimes used to determine the suitability of the kidney for transplantation. Objective pathologic criteria based on outcome that could be applied to the renal biopsy as a screening test have not been fully established, as donor biopsies are not always performed and controlled trials have not been done. One of the major problems in assessing the donor kidney is that this is usually done with cryostat sections, often by local pathologists in the middle of the night. Using arbitrary criteria risks that kidneys will be discarded needlessly. In several large studies, the outcome at 1 to 5 years has not measurably correlated with pathologic lesions. As rejection and patient death from complications diminish, the influence of the quality of the graft is likely to increase. Both donor biopsies and reperfusion biopsies can be quite helpful in assessing the baseline status of the graft, although reperfusion biopsies do not provide aid in donor selection.
Glomerulosclerosis is one feature that is readily assessed in frozen section, by the most casual observation. Glomerulosclerosis >20% correlates with poor graft outcome In several studies, donor serum creatinine did not distinguish the different degrees of glomerulosclerosis found on biopsy, although that has been demonstrated by other studies. In one study, the odds ratio for poor outcome remained significant after adjustment for donor age, rejection episodes, or panel reactive antibody. Five-year graft survival was strikingly diminished in recipients of grafts with >20% glomerulosclerosis compared with those 0% sclerosis (35% vs. 80%). However, other large studies have failed to detect a major effect of glomerulosclerosis >20%, if adjusted for the age of the donor or renal function. At least 25 glomeruli are needed to correlate with outcome. A wedge biopsy may not be representative, because it includes mostly outer cortex, the zone where glomerulosclerosis and fibrosis due to vascular disease is most severe, therefore a needle biopsy is recommended. Even though many other studies try to correlate fibrosis or vascular disease, reproducibility of scoring these lesions, even on permanent sections in broad daylight, is notoriously poor. At this time histologic evaluation is recommended in donors with any evidence of renal dysfunction, a family history of renal disease, or whose age is >60 years. Histologic selection of optimal kidneys from donors over age 60 years can result in a graft survival rate similar to that of grafts from younger patients.
Other lesions may cause the transplant surgeon or pathologist to argue against use of the graft. Arterial intimal fibrosis increases the risk of delayed graft function (DGF) and has a slight effect on 2-year graft survival (6% decrease). Thrombotic microangiopathy (TMA) with widespread but less than 50% glomerular thrombi increases the likelihood of DGF and primary nonfunction, but unaltered 2-year graft survival can still be observed. Likewise, deceased donor kidneys with fibrin thrombi in up to essentially 100% of glomeruli due to presumed disseminated intravascular coagulation have been transplanted successfully with initial DGF but eventual stable allograft function. Despite initial DGF, It has been shown that donor-derived glomerular fibrin thrombi can resolve after donor kidney transplantation, sometimes quite rapidly.
Reversal of diabetic glomerulosclerosis and IgA nephropathy have been reported, as well as membranous glomerulonephritis, lupus nephritis, membranoproliferative glomerulonephritis (MPGN), and endotheliosis due to preeclampsia (personal observation).
Hyperacute rejection refers to immediate rejection (typically within minutes to hours) of the kidney upon perfusion with recipient blood, where the recipient is presensitized to alloantigens on the surface of the graft endothelium. During surgery, the graft kidney becomes soft and flabby; and livid, mottled, purple, or cyanotic in color; and urine output ceases. The kidney subsequently swells, and widespread hemorrhagic cortical necrosis and medullary congestion appears. The large vessels are sometimes thrombosed.
Early lesions show marked accumulation of platelets in glomerular capillaries’ lumina that appear as amorphous, pale pink, finely granular masses in hematoxylin and eosin (H&E) stained slides (negative on periodic acid Schiff [PAS] stains). Neutrophil and platelet margination then occur over the next hour or so along damaged endothelium of small arteries, arterioles, glomeruli, and PTCs, and the capillaries fill with a sludge of compacted red cells and fibrin. The larger arteries are usually spared. The neutrophils do not infiltrate initially but form “chain-like” figures in the PTCs without obvious thrombi. The endothelium is stripped off the underlying basal lamina, and the interstitium becomes edematous and hemorrhagic. Intravascular coagulation occurs and cortical necrosis ensues over 12 to 24 hours. The medulla is relatively spared, but is ultimately affected as the whole kidney becomes necrotic. Widespread microthrombi are usually found in the arterioles and glomeruli and can be detected even in totally necrotic samples. The small arteries may show fibrinoid necrosis. Mononuclear infiltrates are typically sparse. One case showed CD3+ cells in the adventitia of small arteries and in the surrounding interstitium. By EM, neutrophils attach to injured glomerular endothelial cells. The endothelium is swollen—separated from the glomerular basement membrane (GBM) by a lucent space. Capillary loops and PTCs are often bare of endothelium. Platelet, fibrin thrombi, and trapped erythrocytes occlude capillaries.
The site of antibody and complement deposition is determined by the site of the target endothelial alloantigens. Hyperacute rejection due to preexisting anti-HLA class I antibodies may show C3, C4d, and fibrin throughout the microvasculature. ABO antibodies (primarily IgM) also deposit in all vascular endothelium. Cases with anticlass II antibodies may have IgG/IgM primarily in glomerular capillaries and peritubular capillaries, where class II is normally conspicuous. In antiendothelial-monocyte antigen cases, IgG is primarily in PTCs, rather than glomeruli or arteries. Often antibodies cannot be detected in the vessels, even though they can be eluted from the kidney. In these cases C4d should be positive in PTCs and more useful than immunoglobulin stains. Occasional cases, particularly intraoperative biopsies, may be negative for C4d (A. H. Cohen, Cedar Sinai Hospital, Los Angeles, personal communication), perhaps related to focally decreased perfusion or insufficient time to generate substantial C4d amounts.
The differential diagnosis of hyperacute rejection includes ischemia and major vascular thrombosis. The major diagnostic feature of hyperacute rejection is C4d deposition in PTCs and the prominence of neutrophils in capillaries. Although the finding of antibody and C4d deposition in PTCs is diagnostic when present, negative immunofluorescence stains do not exclude hyperacute rejection. Exogenous antibody (rabbit or horse antilymphocyte serum) can cause severe endothelial injury sometimes with C4d deposition mimicking hyperacute rejection. Hyperacute rejection typically has more hemorrhage, necrosis, and neutrophil accumulation in glomeruli and PTCs than acute tubular necrosis (ATN), although glomerular neutrophils alone are associated with ischemia. Major arterial thrombosis has predominant necrosis with little hemorrhage or microthrombi and PTC neutrophils are not prominent. Renal vein thrombosis shows marked congestion and relatively little neutrophil response.
Acute rejection typically develops in the first 2 to 6 weeks after transplantation, but can arise in a normally functioning kidney from 3 days to 10 years or more, or in a graft affected by other conditions, such as ATN, calcineurin inhibitor toxicity, or chronic rejection. Acute rejection may be cell mediated, humoral, or both (see Table 25.1 ). TCMR is mediated primarily by T cells reacting to donor histocompatibility antigens in the kidney and is much more common than acute humoral rejection, due to donor-specific antibodies (i.e., acute antibody-mediated rejection), although the latter is now recognized with greater frequency and has a worse prognosis. Only since 1999 has the distinction between the two been clearly made in the literature.
T cells react to donor histocompatibility antigens expressed in the tubules, interstitium, vessels, and glomeruli, separately or in combination ( Table 25.2 ). The donor ureter is also affected but rarely sampled.
| Suspicious/borderline | Tubulitis + infiltrate Tubulitis (t1, t2, or t3) with minor interstitial infiltrate (i0 or i1) or infiltrate (i2, i3) with mild tubulitis (t1) |
| Type I | Tubulitis >4 cells/tubule + infiltrate >25% A: with 5–10 cells/tubule (t2), orB: with >10 cells/tubule (t3) |
| Type II | Mononuclear cells under arterial endothelium A: <25% luminal area, orB: ≥25% luminal area |
| Type III | Transmural arterial inflammation, or fibrinoid arterial necrosis with accompanying lymphocytic inflammation |
The prominent microscopic feature of TCMR is a pleomorphic interstitial infiltrate of mononuclear cells, accompanied by interstitial edema and sometimes hemorrhage ( Fig. 25.1 ). The infiltrate is typically patchy, both in the cortex and medulla. The infiltrating cells are primarily T cells and macrophages. Activated T cells (lymphoblasts) with increased basophilic cytoplasm, nucleoli, and occasional mitotic figures indicate increased synthetic and proliferative activity. Granulocytes are not uncommonly present but rarely prominent. When neutrophils are conspicuous, the possibility of AMR or pyelonephritis should be considered. Eosinophils are present in about 30% of biopsies with rejection and can be abundant, but are rarely more than 2% to 3% of the infiltrate. Abundant eosinophils (10% of infiltrate) are associated with endarteritis (Banff type II). Mast cells increase, as judged by tryptase content, and correlate with edema. Acute rejection with abundant plasma cells has been described as early as the first month associated with poor graft survival. Infiltrating T cells express cytotoxic molecules, namely perforin, FasL, granzyme A and B, and TIA-1/GMP-17, and tumor necrosis factor-β (lymphotoxin). Apoptosis of the infiltrating T cells can be demonstrated with the TdT-uridine-nick end label (TUNEL) technique, probably as a result of activation-induced cell death, and would thereby serve to limit the immune reaction.
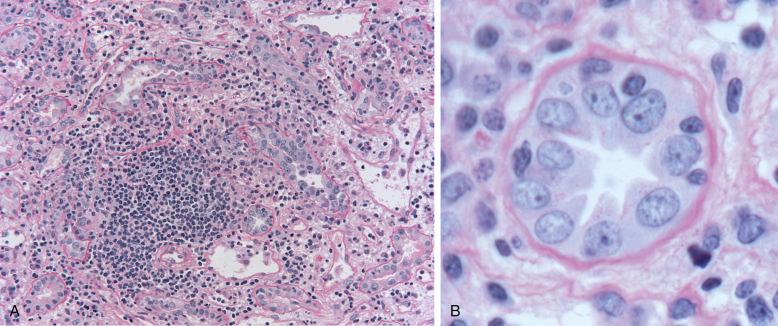
Mononuclear cells invade tubules and insinuate between tubular epithelial cells, a process termed “tubulitis” (see Fig. 25.1 inset), which is best appreciated in sections stained with PAS or a silver stain to delineate the tubular basement membrane (TBM). All cortical tubules (proximal and distal) as well as the medullary tubules and the collecting ducts may be affected. Tubular cell apoptosis occurs, which correlates with the number of cytotoxic cells and macrophages in the infiltrate. Tubular epithelial cells express human leukocyte antigen–DR (HLA-DR), intercellular adhesion molecule 1 (ICAM 1), and vascular cell adhesion molecule 1 (VCAM-1) in increased amounts in TCMR and express the costimulatory molecules CD80 and CD86. Tubules also synthesize tumor necrosis factor-α, transforming growth factor-β1, IL-15, osteopontin, and vascular endothelial growth factor (VEGF). Increased expression of S100A4 may signal the process of epithelial to mesenchymal transition (EMT), which may actually consist of an in situ epithelial response rather than a true emigration of tubular epithelial cells into the interstitium. Thus, as suggested by proceedings at a Banff conference on allograft pathology, EMT may be better thought of as an epithelial to mesenchymal phenotype (EMP). Some tubular cell-derived molecules have the potential to inhibit acute rejection, such as protease inhibitor-9 (PI-9), the only known inhibitor of granzyme B and IL-15, which inhibits expression of perforin.
CD8+ and CD4+ cells invade tubules. Intratubular T cells with cytotoxic granules, and CD4+FOXP3+ cells accumulate selectively in the tubules, compared with the interstitial infiltrate. T cells proliferate once inside the tubule, as judged by the marker Ki67 (MIB-1), which contributes to their concentration within tubules, in addition to selective invasion. Increased tubular HLA-DR, tumor necrosis factor (TNF)α, IFNγ receptor, IL-2 receptor, and IL-8 are detectable by immunoperoxidase study in TCMR. Several adhesion molecules are increased on tubular cells during rejection, including ICAM-1 (CD54) and VCAM-1, and correlate with the degree of T cell infiltration.
Signs of tubular cell injury can be detected by TUNEL apoptosis assay. Increased numbers of TUNEL+ tubular cells are present in acute rejection, compared with normal kidneys. The frequency was significantly lower in cyclosporin A (CsA) toxicity or ATN. The degree of apoptosis correlates with the cytotoxic cells in the infiltrate, consistent with a pathogenetic relationship. Prominent apoptosis of the infiltrating T cells has also been detected at a frequency comparable to that in the normal thymus (1.8% of cells). Others have described occasional TUNEL+ lymphocytes. Apoptosis probably occurs in infiltrating T cells as a result of activation-induced cell death and would thereby serve to limit the immune reaction. Little, if any, immunoglobulin deposition is found by immunofluorescence in TCMR, which is characterized primarily by extravascular fibrin accumulation in the interstitium and not uncommonly increased C3 along the TBM. The C3 is largely derived from tubular cells. C3 may have a role in the pathogenesis of acute rejection, because C3-deficient mouse kidneys have prolonged survival. C4d deposition in PTCs indicates an antibody-mediated component.
Gene expression studies of graft tissue have revealed that transcripts for proteins of cytotoxic T lymphocytes (CTLs), such as granzyme B, perforin, and Fas ligand and the master transcription factor for CTLs, T-bet, are characteristic of TCMR. Graft CTL-associated transcripts (CATs) precede tubulitis in mouse kidney grafts. Treatment of rejection is followed by a measurable decrease of CATs. However, knockout of either granzyme or perforin does not prevent acute rejection, suggesting they are not essential. IFNγ mRNA is detectable in fine needle aspirates 1 week before the clinical onset of rejection. Other genes associated with acute rejection are IFNγ, TNFβ, TNFα, RANTES (regulated on activation, normal T cell expressed and secreted, also known as also Chemokine (C-C motif) ligand 5 [CCL5]), and macrophage inflammatory protein 1-alpha (MIP-1-alpha, also known as Chemokine [C-C motif] ligand 3 [CCL3]); no elevation of TGFβ or IL-10 is detected.
Infiltration of mononuclear cells under arterial and arteriolar endothelium is the pathognomic lesion of TCMR ( Fig. 25.2 ). Many terms have been used for this process, including “endothelialitis,” “endothelitis,” “endovasculitis,” “intimal arteritis,” or “endarteritis.” We prefer the last term, which emphasizes the type of vessel (artery vs. vein) involved and the site of inflammation. Mononuclear cells that are sometimes attached to the endothelial surface are insufficient for the diagnosis of endarteritis; however, they probably represent the early phase of this lesion. Endarteritis in TCMR must not be confused with fibrinoid necrosis of arteries. The latter is characteristic of acute AMR and can also be seen in thrombotic vasculopathy. Regrettably, some still do not separate these lesions, regarding all “vascular rejection” as predominately humoral.
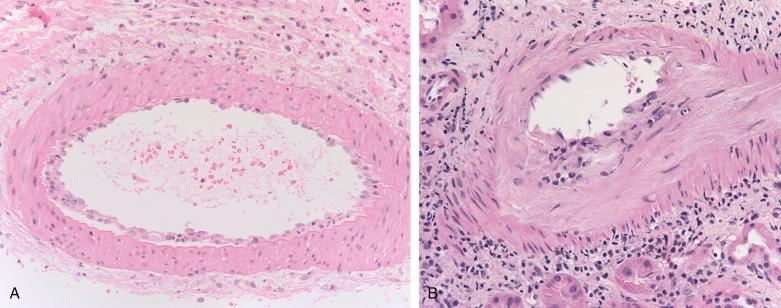
Endarteritis has been reported in 35% to 56% of renal biopsies with TCMR. Many do not find the lesion as often, which may possibly be ascribed to inadequate sampling, overdiagnosis of rejection (increasing the denominator), patient population with respect to medication adherence (severity of rejection), or the timing of the biopsy with respect to antirejection therapy. Endarteritis lesions affect arteries of all sizes including the arteriole, although the lesions affect larger vessels preferentially. For example, in a detailed analysis, 27% of the artery cross sections were affected, vs. 13% of the arterioles. A sample of four arteries would have an estimated sensitivity of about 75% in the detection of type II rejection. Thus a sample may not be considered adequate to rule out endarteritis unless several arteries are included. “Arteriolitis” has the same significance as endarteritis. Endarteritis can occur in cases with little or no interstitial infiltrate or tubulitis, arguing that it has a distinct pathogenetic mechanism, and even in cases with “isolated endarteritis,” that finding is an independent risk factor for kidney transplant failure. In severe cases, a transmural mononuclear infiltrate affects the media, with focal necrosis of the myocytes, features that constitute type III rejection (transmural inflammation or fibrinoid necrosis). Although this occasionally occurs in the absence of demonstrable antibodies, it is more typical of AMR.
Endothelial cells are typically reactive with increased cytoplasmic volume and basophilia. The endothelium shows disruption and lifting from supporting stroma by infiltrating inflammatory cells. Occasionally endothelial cells are necrotic or absent, however, thrombosis is rare. Endothelial apoptosis occurs and increased numbers of endothelial cells appear in the circulation. The media usually shows little change. In severe cases a transmural mononuclear infiltrate may be seen (termed “type III rejection”). The cells infiltrating the endothelium and intima are T cells and monocytes, but not B cells. Both CD8+ and CD4+ cells invade the intima in early grafts, but later CD8+ cells predominate, suggesting that class I antigens are the primary target. Vascular endothelial cell apoptosis can be detected in sites of endarteritis.
Normal arterial endothelial cells express class I antigens, weak ICAM-1, and little or no class II antigens, or VCAM-1. During acute rejection the endothelium of arteries expresses increased HLA-DR and ICAM-1 and VCAM-1. This adhesion molecule upregulation occurs in association with CD3+ 82 and CD25+ 80 infiltrating mononuclear cells. Endothelial cells also have decreased endothelin expression in rejection with endarteritis, but not in tubulointerstitial rejection.
In most TCMR cases, glomeruli are spared or show minor changes, typically a few scattered mononuclear cells (T cells and monocytes) and occasionally segmental endothelial damage ( Fig. 25.3 ). A severe form of this glomerular injury, termed “transplant glomerulitis” or “acute allograft glomerulopathy,” develops in a minority of cases (<5%), manifested by hypercellularity, injury, and enlargement of endothelial cells; infiltration of glomeruli by mononuclear cells; and by webs of PAS-positive material. Crescents and thrombi are rare. Endarteritis often accompanies the transplant glomerulitis. The glomeruli contain numerous CD3+ and CD8+ T cells and monocytes. Fibrin and scant immunoglobulin and complement deposits are found in glomeruli. This variant of cellular rejection has been associated with certain viral infections, such as cytomegalovirus (CMV) infection and hepatitis C virus, although viral antigens are not in the glomerular lesions.
Unique patterns of rejection have been observed under novel immunosuppression regimens. For example, following pronounced lymphocyte depletion from alemtuzumab (CAMPATH-1H), TCMR with a prominent monocyte population (i.e., an acute monocytic rejection) has been described. In these cases, much of the interstitial rejection infiltrate stains for CD68, correlating with renal dysfunction and tubular stress, shown by HLA-DR staining of the tubules. Under these conditions, T cells did not correlate with renal dysfunction or HLA-DR staining.
Studies have included simultaneous bone marrow and kidney transplantation protocols in attempt to induce tolerance to the transplanted organ. In these studies, human leukocyte antigen (HLA)-mismatched renal transplants have been performed; withdrawal of maintenance immunosuppression has been accomplished in some of the patients with relatively preserved renal function. In several of these patients, a capillary leak or engraftment syndrome has been observed around 10 days after a simultaneous kidney/bone marrow transplant preceded by a nonmyeloablative conditioning regimen. In this “engraftment syndrome,” acute tubular injury is accompanied by congested PTCs containing mononuclear cells and red blood cells. IHC shows that the cells are primarily CD68+MPO+ mononuclear cells and CD3+CD8+ T cells, the latter with a high proliferation index (Ki67+). XY chromosome fluorescence in situ hybridization (FISH) has been used to demonstrate that the PTC cells are recipient derived, correlating with chimerism studies showing a simultaneous decline in circulating donor cells and recovery of recipient circulating cells. PTC endothelial injury can also be seen on EM in these cases. The etiology of the syndrome remains undefined, and others have performed combined kidney and bone marrow transplants without observing this phenomenon. With modifications in the combined kidney and bone marrow transplantation protocol, it is possible that the “engraftment syndrome” can be eliminated or at least attenuated; this suggests that “engraftment syndrome” may not be an accurate term for what may actually just be a form of transient acute kidney injury.
TCMR typically has a diffuse, interstitial mononuclear cell infiltrate, whereas patients with CNI toxicity (CNIT) and those with stable function have only focal mononuclear cell infiltrates ( Table 25.3 ). Endarteritis or C4d+ is found extremely rarely, if ever, in CNIT and if either is present, is the most discriminating feature for acute rejection. Prominent tubulitis favors acute rejection, because it is less prominent in acute tubular necrosis, particularly in the proximal tubules. However, tubulitis has been documented in renal transplants with dysfunction due to lymphoceles (obstruction) and in urine leaks, possibilities that need to be considered and excluded by other techniques. Acute obstruction typically has some dilation of the collecting tubules, especially in the outer cortex. Edema and a mild mononuclear infiltrate are also common.
| (A) Classification Categories | |
| Category 1: Nonspecific changes or normal biopsy | |
| Category 2: Antibody-mediated changes | |
| Acute/active ABMR | If all three features are present, they are considered diagnostic. If 1 and 2 or 1 and 3 below are present, a “suspicious” designation can be made. a |
|
|
| Chronic active ABMR | If all three features are present, they are considered diagnostic. If 1 and 2 or 1 and 3 below are present, a “suspicious” designation can be made. a |
|
|
| C4d staining without evidence of rejection | Only if three features are present:
|
| Category 3: Borderline changes suspicious for acute TCMR | |
|
|
| Category 4: TCMR | |
| Acute TCMR (grade/type) |
|
| Chronic active TCMR (grade) |
|
| Interstitial fibrosis and tubular atrophy (IFTA, grade) |
|
| Category 6: Changes not considered to be caused by chronic or acute rejection (see “Nonrejection Injury” in Table 25.1 ) | |
a Designate if C4d positive or C4d negative.
b C4d2 or C4d3 by immunofluorescence on frozen sections or C4d0 >0 by immunohistochemistry on paraffin sections.
c At least moderate (≥ moderate ) microvascular inflammation ([g + ptc] ≥2) can be sufficient for this requirement; however, in the presence of acute TCMR, borderline infiltrate, or infection, ptc ≥2 alone is not sufficient, and g must be ≥1.
d Increased gene transcript/classifier expression is considered sufficient for this requirement “if thoroughly validated” in biopsy tissue.
e DSAs can be substituted by C4d staining or expression of validated transcripts/classifiers in criteria 2; however, extensive DSA testing (including non-HLA antibodies if HLA antibody testing is negative) is still advised if criteria 1 and 2 are not met.
1 These arterial lesions may be indicative of ABMR, TCMR, or mixed ABMR/TCMR.
2 ≥ seven layers in one cortical peritubular capillary and five or more in two additional capillaries, avoiding portions cut tangentially.
3 Severely atrophic tubules have three features: (1) diameter <25% of unaffected or minimally affected tubules; (2) undifferentiated-appearing, flattened, or cuboidal epithelium; and (3) pronounced wrinkling and/or thickening of the tubular basement membrane. Other known causes of i-IFTA should be excluded.
| (B) Quantitative Criteria | ||||
| Quantitative Criteria (Lesion) | 0 | 1 | 2 | 3 |
| i (interstitial Inflammation) |
i0: none or trivial (<10% of unscarred cortex) | i1: 10%–25% of unscarred cortex inflamed | i2: 26%–50% of unscarred cortex inflamed | i3: >50% of unscarred cortex inflamed |
| ti (total interstitial inflammation) |
ti0: none or trivial (<10% of cortex) | ti1: 10%–25% of scarred and unscarred cortex | ti2: 26%–50% of scarred and unscarred cortex | ti3: >50% of scarred and unscarred cortex |
| i-IFTA (inflammation in interstitial fibrosis and tubular atrophy) |
i-IFTA0: no inflammation or <10% of scarred cortical parenchyma | i-IFTA1: inflammation in 10%–25% of scarred cortical parenchyma | i-IFTA2: inflammation in 26%–50% of scarred cortical parenchyma | i-IFTA3: inflammation in >50% of scarred cortical parenchyma |
| t (tubulitis) |
t0: no tubular mononuclear cells | t1: 1–4 cells/tubular cross section 1 | t2: 5–10 cells/tubular cross section 1 | > 10 cells/tubular cross section 2 |
| V (arteritis) |
v0: no arteritis | v1: mild to moderate arteritis in ≥1 arterial cross section | v2: severe arteritis with ≥25% luminal area lost in ≥1 arterial cross section | v3: transmural arteritis and/or fibrinoid change and medial smooth muscle necrosis with vascular lymphocytic infiltrate |
| g (glomerulitis) 3 |
g0: none | g1: <25% of glomeruli | g2: segmental or global in 25%–75% of glomeruli | g3: mostly global in >75% of glomeruli |
| ptc 4 (peritubular capillaritis) |
ptc0: Absent or < 10% of cortical PTCs | ptc1: 3–4 luminal inflammatory cells 3 | ptc2: 5–10 luminal inflammatory cells 3 | ptc3: >10 luminal inflammatory cells |
| ci (interstitial fibrosis) |
ci0: ≤5% of cortical area | ci1: 6%–25% of cortical area | ci2: 26%–50% of cortical area | ci3: >50% of cortical area |
| ct (tubular atrophy) |
ct0: none | ct1: ≤ tubular atrophy | ct2: 26%–50% tubular atrophy | ct3: >50% tubular atrophy |
| cg (transplant glomerulopathy) |
cg0: no GBM double contours | cg1: GBM double contours in ≤25% of capillary loops 5 | cg2: Double contours in 26%–50% of capillary loops | cg3: Double contours in >50% of capillary loops |
| mm (mesangial matrix increase) |
mm0: none | mm1: ≤25% of nonsclerotic glomeruli | mm2: 26%–50% of nonsclerotic glomeruli | mm3: 50% of nonsclerotic glomeruli |
| cv (arterial fibrous intimal thickening) 6 |
cv0: arterial fibrous intimal thickening | cv1: arterial fibrous intimal thickening with 1%–25% luminal narrowing | cv2: arterial fibrous intimal thickening with 26%–50% luminal narrowing | cv3: arterial fibrous intimal thickening with >50% luminal narrowing |
| ah (arteriolar hyalinosis) |
ah0: none | ah1: mild-moderate in ≤1 arteriole | ah2: moderate to severe in >1 arteriole | ah3: Severe in many arterioles |
| aah (arteriolar hyaline thickening) 7 |
aah0: no lesions typical of CNI arteriolopathy | aah1: 1 arteriole, not circumferential | aah2: >1 arteriole, not circumferential | aah3: Any number of arterioles, circumferential |
| C4d IF by immunofluorescence | C4d0: 0% of biopsy area, considered negative | C4d1: 1 to <10% of biopsy area, considered minimal/negative | C4d2: 10%–50% of biopsy area, considered focal unknown | C4d3: >50% of biopsy area, considered diffuse positive |
| C4d IHC by immunohistochemistry | C4d0: 0% of biopsy area, considered negative | C4d1: 1% to <10% of biopsy area, considered minimal/unknown | C4d2: 10%–50% of biopsy area, considered focal positive | C4d3: >50% of biopsy area, considered diffuse positive |
1 Tubulitis can be considered per tubular cross section or per 10 tubular cells.
2 t3 can also be diagnosed if or ≥ two areas of tubular basement membrane destruction accompanied by i2/i3 inflammation and t2 tubulitis elsewhere in the biopsy.
3 Complete or partial occlusion of ≥1 glomerular capillary by leukocyte infiltration and endothelial cell enlargement.
4 Comment on extent (focal ≤ 50%; diffuse >50%) and composition (neutrophils and mononuclear cells).
5 In the severely affected glomerulus; also note number and percent sclerotic. Furthermore, cg1a denotes no GBM double contours by light microscopy but GBM double contours by electron microcopy (EM) with endothelial swelling and/or subendothelial electron lucent widening, and cg1b can denote ≥1 double contours in ≥1 non-sclerotic glomerulus, confirmed by EM if available.
6 Characterized by features of chronic rejection (fibrointimal thickening/neointima formation ± breach of internal elastic lamina or presence of occasional mononuclear or foam cells, ± breaks in elastic lamina).
7 Alternate scoring for hyaline arteriolar thickening (not always used diagnostically) due to calcineurin inhibitors (CNI).
| Acute Rejection | CNI Toxicity | |
|---|---|---|
| Interstitium | ||
| Infiltrate | Moderate–marked | Absent–mild |
| Edema | Usual | Can be present |
| Tubules | ||
| Tubular injury | Usual | Usual |
| Vacuoles | Occasional | Common |
| Tubulitis | Prominent | Minimal–absent |
| Arterioles | ||
| Endothelialitis | Can be present | Absent |
| Smooth muscle degeneration | Absent | Sometimes present |
| Mucoid intimal thickening with red cells | Absent | Sometimes present (TMA) |
| Arteries | ||
| Endothelialitis | Common | Absent (rare mononuclear TMA) |
| Peritubular capillaries | ||
| C4d | May be positive | Negative |
| Glomeruli | ||
| Mononuclear cells | Often | Rare |
| Thrombi | Occasional | Occasionally prominent (TMA) |
Interstitial mononuclear inflammation and tubulitis occur in a variety of diseases other than acute rejection, such as drug-induced (allergic) or infectious tubulointerstitial nephritis. When eosinophils are more abundant than usual for rejection and eosinophils invading tubules are identified, then drug allergy may be favored over rejection. The presence of endarteritis permits a definitive diagnosis of active rejection. Lymphocytes commonly surround vessels (without medial involvement), a nonspecific feature, and must not be confused with endarteritis. Tubulitis is often present in atrophic tubules and does not indicate acute rejection. The diagnosis of acute pyelonephritis should be raised when active inflammation and abundant intratubular neutrophils are present. A note of caution though because in acute AMR, neutrophilic tubulitis with neutrophil casts can be seen; a C4d stain will help in distinguishing between these. A positive urine culture will also separate infection from rejection.
Polyoma virus interstitial nephritis (BK virus) is often diagnosed by the presence of the enlarged, hyperchromatic tubular nuclei with lavender viral nuclear inclusions, often in collecting ducts. However, these may be inconspicuous, and diligent study of multiple sections may be required. Other clues are prominent apoptosis of tubular cells and abundant plasma cells, which invade tubules. IHC for the polyoma SV40 large T antigen or in situ hybridization for BK polyomavirus and EM (even of paraffin) will confirm the diagnosis. Sometimes BK virus infection, with its exuberant plasmacytic infiltration and activated immunoblasts may be confused with the plasmacytic hyperplasia form of posttransplant lymphoproliferative disease, which also should be considered in the differential diagnosis of acute cellular rejection. Rarer infections, including microsporidia, should also be considered in biopsies with interstitial inflammation.
Acute antibody-mediated rejection (also known as acute humoral rejection, acute AMR, or, as referred to in the latest Banff criteria: “active” AMR ) is a form of renal allograft rejection due to damage by circulating antibodies that react to donor alloantigens on endothelium. These antigens include HLA class I and class II antigens, ABO blood group antigens, and other non-major histocompatibility complex (MHC) antigens, even in HLA-identical grafts. The main risk factors for donor-specific antibody (DSA; this term typically refers to anti-HLA antibody) are blood transfusion, pregnancy, and prior transplant. DSA may arise de novo in the posttransplant period, or alloantibody may be present before transplantation in the case of positive crossmatch (+XM) or ABO blood group incompatible transplants with preconditioning regimens to lower the alloantibody level before transplantation. Hyperacute rejection is an immediate rejection that occurs with high levels of preformed alloantibody directed against the graft.
Traditionally, identification of acute AMR in biopsies is difficult because none of the histologic features is diagnostic, and immunoglobulin deposition was usually not detectable in the graft. Techniques for demonstrating C4d in PTCs, pioneered by Feucht, have substantially improved detection of this condition. Acute AMR may occur in the absence of evidence for T-cell-mediated injury, particularly in +XM transplants; however, it is not uncommon for both to be present, particularly in the later posttransplant period (months to years).
Acute AMR typically presents with clinically severe acute rejection 1 to 3 weeks after transplantation, but also can arise months to years later, associated with decreased immunosuppression or noncompliance. With current therapy, approximately 5% to 7% of recipients develop an episode of acute AMR, and about 25% of biopsies taken for acute rejection have pathologic evidence of an acute AMR component. The main risk factor is presensitization by blood transfusion, pregnancy, or prior transplant, however, the majority have a negative crossmatch at the time of transplantation.
Serologic testing for DSA has become more sensitive in the past decade due to the widespread use of solid-phase assays rather than the older cell-based assays. These assays can be used before transplantation and for posttransplant monitoring for DSA. These more sensitive methods of detecting DSA have brought to light the spectrum of alloantibody-mediated damage (e.g., capillaritis) that may not have been recognized in previous studies.
The three diagnostic criteria for acute AMR are (1) histologic evidence of acute injury (neutrophils in capillaries, acute tubular injury, fibrinoid necrosis), (2) evidence of antibody interaction with tissue (typically C4d in PTCs), and (3) serologic evidence of circulating antibodies to antigens expressed by donor endothelium (typically HLA). Criteria for the diagnosis of acute AMR have been refined over the years. Generally speaking, if only two of the three major criteria are established (e.g., when antibody is negative or not done), the diagnosis can be considered suspicious for acute AMR. Biopsies meeting criteria for both acute AMR and TCMR type I or II are considered to have both forms of rejection. Biopsies with C4d and no pathology are likely a manifestation of “accommodation” (see later).
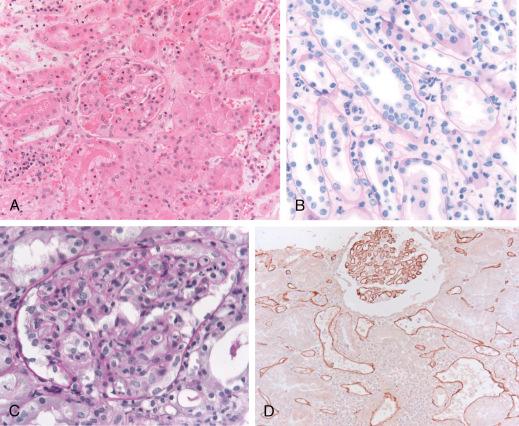
Histologic findings are typically scant to moderate mononuclear interstitial infiltrates, sometimes with prominent neutrophils and increased numbers of macrophages (see Fig. 25.3 ). The extent of mononuclear infiltration often does not meet the criteria for TCMR. PTCs have neutrophils in about 50% of cases and are classically dilated ( Fig. 25.4A ). Interstitial edema and hemorrhage can be prominent. Glomeruli have accumulations of macrophages (∼50% of cases) and neutrophils (∼25% of cases; see Fig. 25.3 ) and occasionally fibrin thrombi or segmental necrosis. Acute tubular injury, sometimes severe, can be identified in many cases and may be the only initial manifestation of acute AMR. Focal necrosis of whole tubular cross sections, similar to cortical necrosis has been reported; 38% to 70% of acute AMR cases may have patchy infarction. Little mononuclear cell tubulitis is found, although a neutrophilic tubulitis with or without neutrophil casts may be prominent, resembling acute pyelonephritis. Plasma cells can be abundant in acute AMR, either early or late after transplantation, sometimes associated with severe edema and increased IFNγ production in the graft. B cells can be also present, but have no apparent diagnostic value.
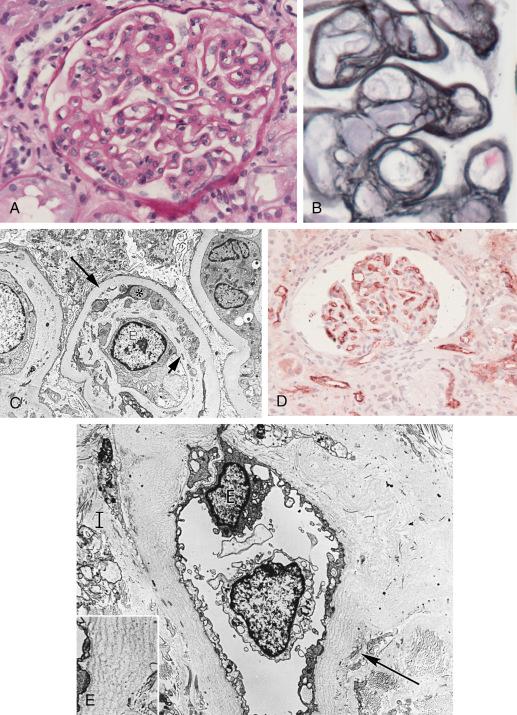
In about 15% of cases small arteries shows fibrinoid necrosis, with little mononuclear infiltrate in the intima or adventitia but with neutrophils and karyorrhectic debris ( Fig. 25.5 ). Arterial thrombosis can be found in 10%, and a pattern resembling TMA has also been reported. Around 75% of cases with fibrinoid necrosis are C4d positive. Presumably the C4d-negative cases had T-cell-mediated rejection or TMA. Antibodies to the angiotensin II type 1 receptor have been detected in a few cases with arterial fibrinoid necrosis, in the absence of capillary C4d deposition. The presence of mononuclear endarteritis in cases of acute AMR strongly suggests a component of T-cell-mediated rejection.
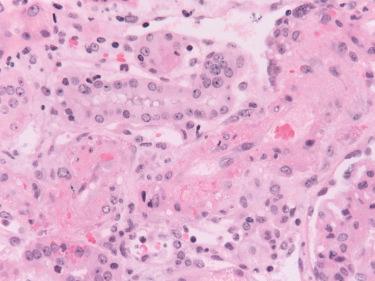
By EM the PTCs are dilated, containing neutrophils. The endothelium is reactive and shows loss of fenestrations. The glomerular endothelium is separated from the GBM by a widened lucent space with endothelial cell swelling and loss of endothelial fenestrations, indicative of injury. Platelets, fibrin, and neutrophils are found in glomerular and PTCs. The small arteries with fibrinoid necrosis show marked endothelial injury and loss, smooth muscle necrosis, and deposition of fibrin.
Feucht and colleagues first drew attention to C4d as a possible marker of an antibody-mediated component of severe rejection. C4d, a fragment of complement component C4, is released during activation of the classical complement pathway by antigen–antibody interaction. Because C4d contains a thioester bond, it binds covalently to tissues at the local site of activation. The covalent linkage explains why C4d remains for several days after alloantibody disappears, because antibody binds to cell surface antigens that can be lost by modulation, shedding, or cell death.
Although immunoglobulin deposition is found in only a minority of cases, C4d is characteristically detected in a widespread, uniform ring-like distribution in the PTCs by immunofluorescence in cryostat sections (see Fig. 25.4B ). Deposition occurs in both the cortex and medulla. Using IHC in formalin-fixed, paraffin embedded tissue, C4d has a similar pattern, although the intensity is variable. “Serum staining” is an artifact of C4d IHC, so PTCs must show clear circumferential staining to be called positive by this technique. Glomerular capillary staining also occurs but is hard to distinguish from C4d normally found in the mesangium in frozen sections stained by immunofluorescence. Formalin fixation eliminates this background staining and demonstrates glomerular C4d in about 30% of acute AMR cases.
Grafts with focal C4d (<50% of PTC) are of uncertain significance and the patient should be monitored closely for donor-reactive antibodies. Two of three studies have failed to show any significant clinical or pathologic difference between cases with focal and diffuse C4d staining. C4d deposition can precede histologic evidence of acute AMR by 5 to 34 days. C4d in 1-week protocol biopsies was followed by clinical acute rejection in 82% of cases and was associated with donor-reactive antibodies.
In the setting of acute rejection, C4d is a specific (96%) and sensitive (95%) marker of circulating antidonor HLA-specific antibodies by the antihuman globulin cytotoxicity test. PTC C4d deposition is associated with concurrent circulating antibodies to donor HLA class I or II antigens in 88% to 95% of recipients with acute rejection. Moreover, C4d deposition and the severity of histologic injury by antibody correlates with the serum DSA level in acute humoral rejection. False negative antibody assays may be due to absorption by the graft as shown by elution from rejected grafts in patients who had no detectable circulating antibody, or it may be due to differences in detection of antibody directed against different HLA alleles and sensitivity of solid-phase methods for particular alloantigens. Alternatively, non-HLA antigens may be the target. C4d-negative acute rejection may show flow cytometry evidence of antidonor reactive antibodies as frequently as 50%, due in part to noncomplement fixing antibodies. Cell based assays have a false positive rate of <10%.
In a comparison of methods for C4d, the triple layer immunofluorescence technique proved the most sensitive, although the difference with IHC in paraffin embedded tissue was small. With fixed tissue, plasma in the capillaries and interstitium may stain for C4d, which interferes with interpretation.
Other components of the complement system have been sought. C3d, a degradation product of C3, was found in PTCs in 39% to 60% of biopsies from HLA-mismatched grafts with diffuse C4d. C3d was usually but not always associated with C4d. C3d correlated with acute AMR in all studies, and was associated with increased risk of graft loss in two series, compared with C3d-negative cases, but C3d provided no convincing additional risk compared with C4d+. The interpretation of C3d stains is complicated by the common presence of C3d along the TBM. Even though C3d should indicate more complete complement activation, it added no diagnostic value to C4d in grafts showing histologic features of acute AMR, except in the setting of ABO-incompatible grafts. Other complement components, such as C1q, C5b-9, and C-reactive protein (CRP) are not conspicuous in PTCs in acute rejection. Lectin pathway components, which activate C4 by binding to microbial carbohydrates, are sometimes detected. Among 18 biopsies with C4d, 16 had diffuse H-ficolin deposition along the PTCs, whereas none of the 42 cases without C4d had H-ficolin. No Mannan-binding lectin serine protease 1 (MASP-1, also known as mannose-associated serine protease 1) or MASP-2 was detectable. The significance of this observation is not clear, because MASP proteins are required to activate C4 via the ficolins or Mannose-binding lectin (MBL).
Natural killer (NK) cells have been the focus of recent research in graft injury, particularly regarding AMR. Microarray analysis has indicated that several DSA-specific gene transcripts show high expression in NK cells, and IHC also shows prominent numbers of peritubular capillary NK cells in these cases. Depletion of NK cells with anti-NK1.1 significantly reduced DSA-induced chronic allograft vasculopathy in a murine cardiac allograft model.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here