Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Parasites are an important cause of human morbidity and mortality worldwide. They include a diverse array of single-celled eukaryotic organisms (protozoans), multicellular worms (helminths), and arthropods that live in or on the human body and can cause mild to severe life-threatening disease. While many parasites primarily infect impoverished individuals in tropical and subtropical regions of the world, others cause infection in the world’s temperate regions including those living in resource-rich, affluent countries. The study of parasitology has gained a renewed importance in recent decades due to the ease in which humans, animals, and food can move rapidly and widely across the globe and the increased number of individuals who are immunocompromised and at risk for severe disease.
This chapter provides an overview of human parasitology and the general laboratory approaches for identifying important parasites in various specimens. Both conventional and state-of-the-art diagnostic methods are covered, including light microscopy, serology, antigen detection, and nucleic acid amplification. Particular attention is paid to testing in blood and fecal specimens, as these are the most common specimens received in the clinical laboratory for detection of parasites. The most important human parasites are discussed individually, with an emphasis on the fundamental clinical and biologic information needed for accurate diagnosis and management of infection. The categories of parasites covered include the blood and tissue protozoa, intestinal protozoa, intestinal helminths, tissue helminths, and medically important arthropods.
Parasitology is one of the oldest medical fields. Because many parasites can be seen with the unaided eye, they have been appreciated and studied for longer than most bacteria and viruses, and descriptions of what are clearly parasites are found in the written records of nearly every culture, including the famous Ebers papyrus of ancient Egypt. Despite medical advances, parasites continue to cause a significant global burden of disease today. Malaria alone caused an estimated 405,000 deaths in 2018, while millions of others died from amebiasis, schistosomiasis, strongyloidiasis, leishmaniasis, ascariasis, African trypanosomiasis, and Chagas disease ( Table 88.1 ). Many parasitic infections are considered to be neglected tropical diseases (NTDs), since they are found in the tropics and subtropics and disproportionately affect impoverished individuals. However, the US Centers for Disease Control and Prevention (CDC) has also identified five neglected parasitic infections (NPIs): toxoplasmosis, Chagas disease, toxocariasis, trichomoniasis, and cysticercosis; these pose a significant public health concern to people living in the United States, thus emphasizing that parasites are not restricted to tropical settings. The rapid movement of humans, animals, and food throughout the world has also served to enhance the spread of parasitic disease. Thus clinicians and laboratorians must be equipped to recognize and treat parasitic infections in the patients they serve.
| Disease (Parasite) | Global Health Impact | Estimated Number of Annual Deaths |
|---|---|---|
| Protozoal Infections | ||
| African trypanosomiasis a ( Trypanosoma brucei ) | <5000 currently infected | Unknown |
| Amebiasis ( Entamoeba histolytica ) | 28 million illnesses/yr | 1470 |
| Ascariasis ( Ascaris lumbricoides ) | 1.3 million DALYs | 6248 |
| Chagas disease a ( Trypanosoma cruzi ) | 8 million infected worldwide; 300,000 in the United States | 10,000 |
| Giardiasis ( Giardia duodenalis ) b | 28 million cases/yr | Unknown |
| Leishmaniasis, visceral a | 300,000 currently infected worldwide | 65,000 |
| Leishmaniasis, cutaneous a | 1 million currently infected | 26,000 |
| Malaria ( Plasmodium spp.) | 228 million new cases/yr | 405,000 |
| Helminth Infections | ||
| Cestodiases | 340,864 illness/yr | 36,500 |
| Food-borne trematodiasis (primarily Clonorchis, Opisthorchis, Fasciola, and Paragonimus ) a | 56 million currently infected; 218,000 new infections | 7500 |
| Lymphatic filariasis a ( Wuchereria bancrofti, Brugia spp.) | 120 million currently infected | Unknown |
| Schistosomiasis a ( Schistosoma spp.) | 240 million currently infected; 2,543,000 DALYs | >200,000 in sub-Saharan Africa |
| Soil-transmitted helminthiases a (primarily Ascaris lumbricoides, Trichuris trichiura, and hookworms; also Strongyloides stercoralis ) | 1.5 billion currently infected | >1000 |
| Arthropod Infestations | ||
| Scabies a (Sarcoptes scabiei) | >200 million currently infected | Unknown |
a Classified by the World Health Organization as a Neglected Tropical Disease (NTD).
b Previously known as Giardia intestinalis and Giardia lamblia .
Nearly 400 species of helminths (worms), protozoa, and arthropods have been reported to parasitize humans, including some very rare and “accidental” infections. The most common parasites that infect humans are listed in Tables 88.2–88.4 . Not all parasites are considered to be pathogens, and some exist in the host solely or primarily as commensals. The clinical severity of infection is often dependent on the immune status of the host ( Table 88.5 ), and individuals at increased risk for acquiring many parasitic infections and suffering complications of infection are the immunocompromised, neonates, the elderly, and asplenic individuals. Humans protect themselves against parasitic infections through a variety of nonspecific innate and adaptive immune mechanisms, including mucosal barriers and cellular and humoral-mediated defenses. In general, helminths elicit production of eosinophilia, while protozoa do not. ,
| Classification | Specific Parasites |
|---|---|
| Supergroup Amoebozoa | |
| Intestinal amebae | Endolimax nana, Entamoeba spp., Iodamoeba buetschlii |
| Free-living amebae (Discosea) | Acanthamoeba spp., Balamuthia mandrillaris |
| Supergroup Excavata | |
| Intestinal flagellates | Chilomastix mesnili, Enteromonas hominis, Giardia duodenalis, b Pentatrichomonas hominis, Retortamonas intestinalis |
| Genitourinary flagellate | Trichomonas vaginalis |
| Free-living ameboflagellate (Heterolobosea) | Naegleria fowleri |
| Hemoflagellates, kinetoplastids | Leishmania spp., Trypanosoma brucei, Trypanosoma cruzi |
| Supergroup Sar (Stramenopiles-Alveolata-Rhizaria) | |
| Stramenopiles | Blastocystis spp. |
| Apicomplexa | |
| Haemosporidia | Plasmodium spp. |
| Piroplasmida | Babesia spp. |
| Coccidia | Toxoplasma gondii, Cyclospora cayetanensis, Cystoisospora ( Isospora ) belli, Sarcocystis spp. |
| Gregarines | Cryptosporidium spp. |
| Ciliate (Ciliophora) | Balantioides coli |
| Supergroup Opisthokonta | |
| Helminths | |
| Nematodes (roundworms) | Intestinal, filarial, and zoonotic nematodes; see Table 88.3 . |
| Cestodes (tapeworms) | Intestinal and tissue cestodes; see Table 88.3 . |
| Trematodes (flukes) | Intestinal, liver, lung, and blood flukes; see Table 88.3 . |
| Acanthocephala (thorny-headed worms) | See Table 88.3 . |
| Arthropods | See Table 88.4 . |
a Parasites are grouped according to the “Revised classification of eukaryotes,” , with commonly used conventional or descriptive groupings below each supergroup.
b Previously known as Giardia intestinalis and Giardia lamblia .
| Classification | Common Examples |
|---|---|
| Nematodes (roundworms) | |
| Intestinal nematodes | Ancylostoma duodenale, Ascaris lumbricoides, Capillaria philippinensis, Enterobius vermicularis, hookworms, Strongyloides stercoralis, Trichuris trichiura |
| Filarial nematodes | Brugia spp., Dirofilaria spp., Loa loa, Mansonella spp., Onchocerca volvulus, Wuchereria bancrofti |
| Zoonotic nematodes | Angiostrongylus spp., Anisakis spp. Baylisascaris procyonis, Capillaria hepatica , Gnathostoma spp., Pseudoterranova spp. , Toxocara spp., Trichinella spp. |
| Cestodes (tapeworms) | |
| Intestinal cestodes | Dibothriocephalus/Diphyllobothrium/Adenocephalus spp., Dipylidium caninum, Hymenolepis nana, Hymenolepis diminuta, Taenia spp. |
| Tissue cestodes | Echinococcus spp., Taenia solium (cysticercosis), other Taenia spp. (coenurus), Spirometra spp. (sparganosis) |
| Trematodes (flukes) | |
| Intestinal flukes | Fasciolopsis buski, Heterophyes heterophyes, Metagonimus yokogawai |
| Liver flukes | Clonorchis sinensis, Fasciola hepatica, Opisthorchis spp. |
| Lung flukes | Paragonimus spp. |
| Blood flukes | Schistosoma spp. |
| Acanthocephala (thorny-headed worms) | Moniliformis moniliformis, Macracanthorhynchus spp. |
| Classification | Common Examples |
|---|---|
| Hexapoda (insects) | Lice, fleas, bed bugs, kissing bugs, beetles, stinging bees, wasps, ants, sand flies, mosquitoes, biting midges, black flies |
| Arachnida (arachnids) | Hard ticks, soft ticks, mites, spiders, scorpions |
| Diplopoda (millipedes) | Several poisonous species |
| Chilopoda (centipedes) | Several venomous species |
| Crustacea (crustaceans) | Copepods, pentastomes (tongue worms), crabs/crayfish |
| CLINICAL MANIFESTATIONS | ||
| Parasite | Immunocompetent Host | Immunocompromised Host |
| Acanthamoeba spp., Balamuthia mandrillaris | Amebic keratitis, usually in contact lens wearers. CNS involvement is rare. Responsive to treatment, but corneal infection may require transplant; infection may recur if incompletely excised. | Same risk for amebic keratitis. Possible involvement of skin, lungs, and central nervous system (granulomatous amebic encephalitis, GAE). GAE is a subacute to chronic infection, which is nearly always fatal. |
| Babesia spp. | Asymptomatic or self-limited febrile illness. Responsive to treatment. | Extensive hemolysis, high parasite load, death (particularly in asplenic individuals). Prolonged therapy (>3 months) and exchange transfusion may be required. |
| Cryptosporidium spp. | Self-limited watery diarrhea. Responsive to treatment. | Protracted severe watery diarrhea (up to 17 L/day fluid loss). Occasional dissemination to biliary and respiratory tracts. Refractory to treatment while patient is immunocompromised. |
| Cyclospora cayetanensis | Self-limited watery diarrhea. Responsive to treatment. | Protracted severe diarrhea. Responsive to treatment. |
| Cystoisospora belli | Self-limited watery diarrhea. Responsive to treatment. | Protracted severe watery diarrhea. Occasional dissemination to regional lymph nodes. Responsive to treatment. |
| Entamoeba histolytica | Most infections are asymptomatic. Manifestations may include colitis, dysentery, ameboma, disseminated infection (i.e., amebic liver abscess). Responsive to treatment. | Increased risk for severe manifestations. Responsive to treatment. |
| Giardia duodenalis | Most infections are asymptomatic. Manifestations may include self-limited watery diarrhea with malabsorption, chronic symptoms with fatigue, reactive arthritis, allergies. Responsive to treatment, but chronic symptoms may persist. | Increased risk for severe manifestations, including protracted watery diarrhea with malabsorption. Responsive to treatment. |
| Leishmania spp. | Many infections are asymptomatic. Cutaneous, mucocutaneous, and visceral forms of infection. Cutaneous and visceral forms are responsive to treatment, but mucocutaneous disease may be less responsive. | Increased risk for severe manifestations including progression to visceral disease and mucocutaneous leishmaniasis. Cutaneous form is generally responsive to treatment, but visceral and mucocutaneous disease may be less responsive. High risk for relapse after treatment. |
| Plasmodium spp. | Infection ranges from asymptomatic to severe and life-threatening, depending on the infecting species. Responsive to treatment. | Increased risk for severe manifestations. Responsive to treatment. |
| Sarcoptes scabiei | Many infections are asymptomatic. Mild to moderate itching with focal anatomic involvement. Responsive to topical therapy. | Crusted (Norwegian) scabies with widespread anatomic involvement, formation of crusts, high mite burden; highly infectious. Secondary bacterial infections common. Often refractory to treatment; topical and oral agents recommended. |
| Strongyloides stercoralis | Many infections are asymptomatic. Manifestations include Loeffler syndrome (acute infection), abdominal pain, diarrhea. Auto-infection allows for infection to persist for decades. Responsive to treatment. | Hyperinfection syndrome, severe colitis, peritonitis, pneumonia, respiratory failure, disseminated disease, Gram-negative sepsis or meningitis. Fatal if untreated. Responsive to treatment. |
| Toxoplasma gondii | Many infections are asymptomatic or self-limited mononucleosis-like illness with lymphadenopathy. Risk for congenital transmission if infection occurs during pregnancy. Responsive to treatment. | Acute or reactivated active infection, commonly involving the central nervous system. Can also cause pneumonitis, chorioretinitis, hepatitis. Fatal if untreated. Responsive to treatment. |
| Trypanosoma cruzi | Acute, indeterminate, and chronic stages possible. Chronic form may cause cardiomyopathy, megacolon, and megaesophagus years after initial infection. Responsive to treatment in acute and, sometimes, indeterminate stages. Supportive care necessary for irreversible manifestations of chronic disease. | Faster progression to chronic stage. Similar outcome as seen in immunocompetent patients. |
Some parasites have complex life cycles involving multiple hosts. The host in which sexual reproduction occurs is the definitive host, whereas the host in which asexual reproduction occurs is the intermediate host. A paratenic host is one that can sustain the parasite, without the parasite undergoing further development. Knowledge of the parasite life cycles is important for understanding the etiology of the clinical symptoms, determining which specimens or assays are useful for diagnosing infection, and for selecting methods for treatment and prevention of infection.
This chapter provides an overview of human parasitology and the general laboratory approaches used to identify clinically important parasites in human specimens. The reader is referred to a number of excellent texts and atlases for more extensive coverage of specific parasites. The CDC’s Division of Parasitic Diseases and Malaria maintains a website with an extensive image library of common human parasites, and also provides free diagnostic assistance.
Traditional classification schemata place parasites into two Kingdoms: the Animalia (worms and arthropods) and Protozoa. They are further classified into phylum, class, order, and family groupings. Although this classification scheme is familiar to most, it is based on somewhat subjective morphologic, ecologic, and physiologic criteria and does not reliably account for phylogenetic relatedness among organisms in each grouping. More recently, biochemical and molecular analyses, including whole-genome sequencing, have supported the creation of a new classification scheme based on clades rather than traditional hierarchical ranks (first order, second order, and so on). , In this system, human parasites are classified within five clusters called supergroups: the Amoebozoa, Archaeplastida, Excavata, SAR, and Opisthokonta. Although this new system is seemingly complex, many familiar groupings have been retained (e.g., Endamoebidae, Apicomplexa) to facilitate communication and the older hierarchal system is still commonplace for the true animals (e.g., helminths and arthropods). This updated classification system along with familiar groupings is reflected in Tables 88.2–88.4 . For simplicity, we have retained the use of the terms protozoa/protozoans to refer to the diverse array of unicellular eukaryotic parasites that infect humans.
Multiple laboratory methods are available for diagnosis of parasitic infections (see At a Glance ). , , , Microscopy remains the gold standard for detection and identification of many parasites, and allows for direct visualization of many different organisms in clinical specimens. Tests that detect parasite antigens or nucleic acid also exist for specific parasites, and often provide increased sensitivity and specificity over traditional microscopy. Antibody detection is most useful for detection of tissue parasites that are not otherwise easily sampled and is less useful for parasites that are limited to the gastrointestinal tract. Finally, culture methods are available for select parasites, but are usually used mostly in specialized referral centers and research facilities. Blood and feces comprise the bulk of the specimens that are submitted for parasitologic evaluation, but parasites can involve virtually any organ. Therefore the clinical laboratory should be prepared to receive and examine a variety of other specimens for parasites, including urine, sputum, skin scrapings, aspirates, and tissue biopsies.
Microscopy (gold standard method of identification of many parasites)
Macroscopic (gross) identification of helminths and arthropods
Microscopic identification of protozoans and helminth eggs and larvae in stool and other specimen types
Examination of thick and thin blood films for blood parasites
Histopathologic identification of parasites in biopsy and autopsy specimens
Culture techniques
Free-living amebic infections
Naegleria fowleri
Acanthamoeba spp.
Strongyloides stercoralis
Trichomonas vaginalis
Serologic (antibody) testing (primarily used to detect disseminated or tissue-based infections)
Toxoplasma gondii
Entamoeba histolytica extra-intestinal disease
Toxocara spp. and Baylisascaris procyonis (visceral larva migrans)
Taenia solium (Cysticercosis)
S. stercoralis
Schistosoma spp.
Trypanosoma cruzi
Antigen detection methods
Giardia duodenalis
Cryptosporidium spp.
E. histolytica
T. vaginalis
Plasmodium spp.
Wuchereria bancrofti and Brugia spp. (lymphatic filariasis)
Molecular amplification methods
G. duodenalis
Cryptosporidium spp.
Cyclospora cayetanensis
E. histolytica
T. gondii
Plasmodium spp.
Babesia spp.
T. vaginalis
Both protozoan and helminthic parasites can be found in blood. The primary protozoan pathogens are the agents of malaria ( Plasmodium spp.), babesiosis ( Babesia spp.), Chagas disease ( Trypanosoma cruzi ), and African trypanosomiasis ( Trypanosoma brucei ) , while the helminthic parasites are the filarial worms, Wuchereria bancrofti, Mansonella spp., Brugia spp., and Loa loa . , All of these parasites can be detected using conventional thin and thick blood films, while concentration techniques provide increased sensitivity for the extracellular parasites. Unfixed (wet) blood preparations can also be examined for the presence of motile trypanosomes and microfilariae. , All specimens should be considered potentially infectious and handled using standard precautions. ,
Thick and thin blood films are the most commonly used preparations for detection of blood parasites. , , Blood can be obtained via venipuncture, or by finger-stick or earlobe puncture, and then spread onto clean, grease-free glass microscope slides. If the latter collection methods are used, then blood must be allowed to flow freely from the puncture site before collection is attempted to prevent contamination of the specimen with tissue fluid or the alcohol disinfectant. Ideally, the slides are prepared at the bedside immediately after obtaining the blood. However, if the blood must be transported to the laboratory prior to slide preparation, then it should be collected using ethylenediaminetetraacetic acid (EDTA) as the anticoagulant. It is important to prepare the slides as soon as possible after collection, since prolonged exposure to EDTA can result in distortion of the parasite morphology, particularly with Plasmodium spp.
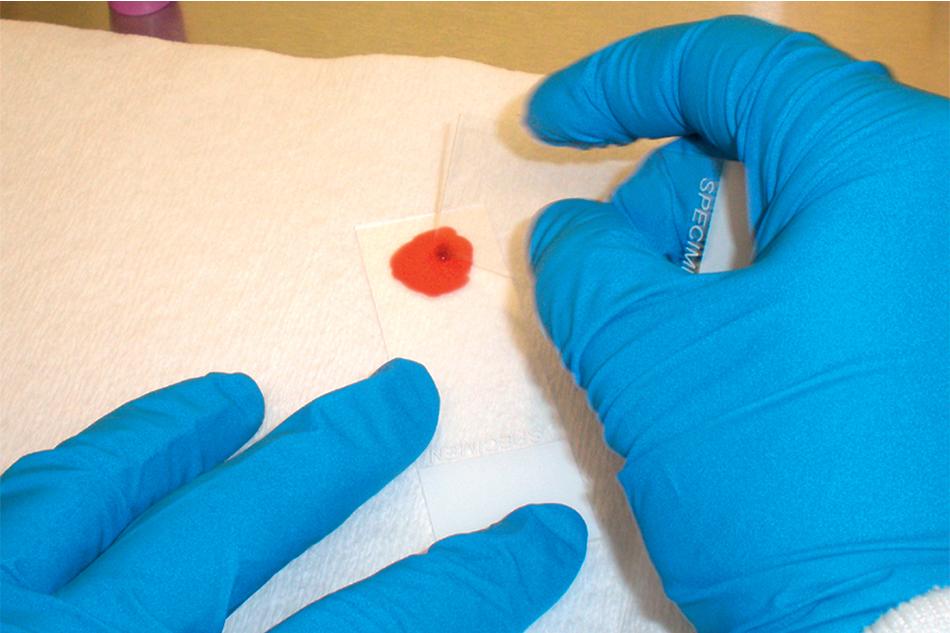
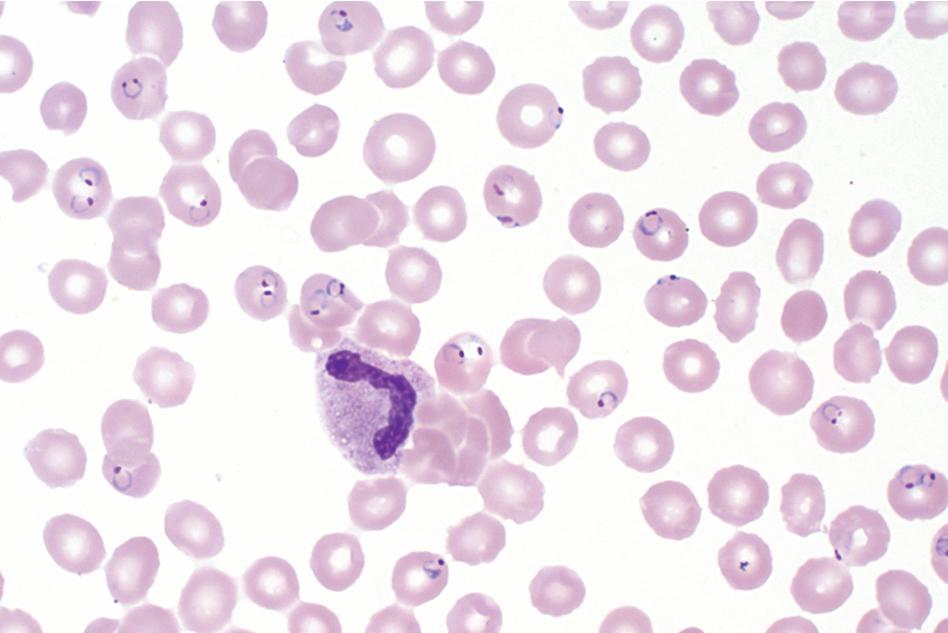
Thin blood films are prepared in the same manner as for hematology evaluation, with creation of a thin layer of intact, mostly nonoverlapping blood cells. To make the thin film, one drop of blood (approximately 0.05 mL) is placed at one end of the slide, and a second slide is used to sweep the drop along the length of the slide. The well-prepared thin film contains a “feathered” edge of approximately 1.5 to 2 cm in length in which the erythrocyte and parasite morphology ( Plasmodium and Trypanosoma spp.) are ideal for microscopic examination ( Fig. 88.1 ). , The slide is fixed in methanol prior to staining, allowing the erythrocytes (and intraerythrocytic parasites, if present) to remain intact during staining. Therefore the thin film provides the ideal morphology for determining the species and percent parasitemia of intraerythrocytic parasites.
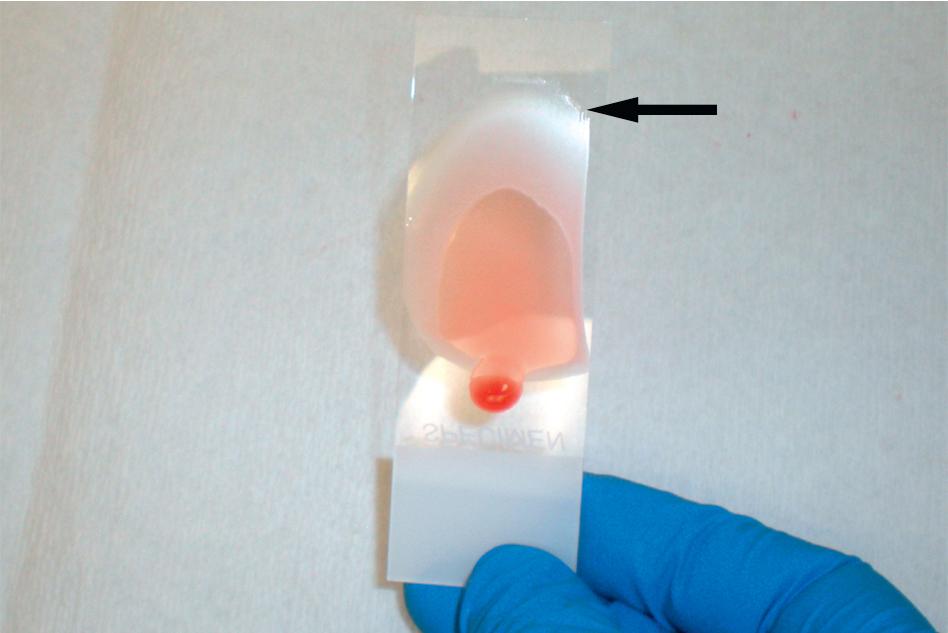
As the name implies, the thick film provides a greater concentration of blood than the thin film (16 to 30 times more blood per microscopic field than with the thin film) and therefore provides a more sensitive preparation for parasite screening. The reason that it is possible to examine this thicker film of blood is that the erythrocytes are lysed during the staining process so that only leukocyte nuclei, platelets, and parasites (if present) are visible. , , The thick film is made by placing one to two drops of blood on the slide, and then using a second slide (or wooden stick) to spread the blood into a circular film. The ideal thick film should be 1.5 to 2 cm in diameter and of a thickness through which small typed print (e.g., newsprint) can just be read. The blood film must then be allowed to thoroughly dry so that it does not detach during the staining process. While older methods recommended thick film drying times of 8 to 12 hours, this prolonged period can be avoided by gently pushing down with the corner of the spreader slide while creating the film, thereby creating minute scratches on the carrier slide ( Fig. 88.2 ). This greatly improves adherence of the thick film to the carrier slide and allows for staining to take place as soon as the film is visibly dry (within 30 to 60 minutes). Regardless of the method used to distribute the blood on the slide, it is important not to fix the film in methanol since the erythrocytes will not effectively lyse during staining.
Giemsa is the preferred stain for both the thick and thin films since it provides the best contrast between the intraerythrocytic parasites and hemoglobin. It must be made fresh daily and is usually prepared by diluting Giemsa stock solution with phosphate-buffered distilled or deionized water. A small quantity of Triton X-100 (5 to 10%) can be added to the buffer to improve the contrast and staining of the organisms. The buffer pH should be at 7.0 to 7.2 to allow for optimal visualization of parasite-associated intracytoplasmic inclusions (i.e., Schüffner stippling and Maurer clefts). Wright-Giemsa and Wright stains may also be used, but the thick films must first be lysed in water since these stains commonly incorporate alcohol as a fixative. These stains generally have a pH of less than 6.8, and therefore stippling will usually not be seen. The Field stain may also be used as a rapid method for thick and thin blood film staining and is well suited for field use.
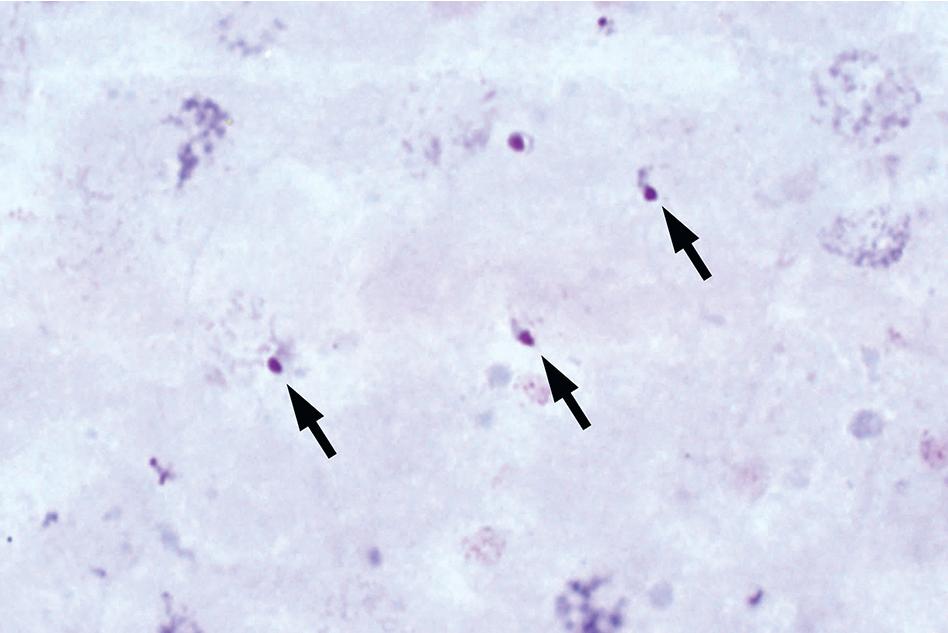
The entire thick and thin films should be examined using low magnification (i.e., 10× or 20× objective) to allow for detection of larger blood parasites such as microfilariae. At least 100 thick film fields or 200 thin film fields should also be examined using high magnification (100× oil immersion objective) before issuing a negative report ( Figs. 88.3 and 88.4 ). Malaria and babesiosis are potentially life-threatening conditions, and therefore testing should be performed on a STAT basis. , Some laboratories have opted to use rapid immunochromatographic tests for initial malaria testing due to their ease of use and interpretation (see the “Immunodiagnostic Methods” section later in this chapter). It is important to note that these methods are less sensitive than thick and thin blood film microscopy, particularly for non– Plasmodium falciparum infections and low levels of parasitemia. Therefore confirmatory testing using conventional blood films should still be performed, particularly in nonendemic settings. ,
Concentration techniques are recommended for more sensitive detection of the larger blood parasites (trypanosomes and microfilariae) and organisms that infect white blood cells (Leishmania). , , , One relatively simple technique is the creation and examination of a buffy coat smear. In this method, anticoagulated blood is centrifuged to separate the denser erythrocytes from plasma and allow for formation of an intervening buffy coat layer. This layer, which primarily contains leukocytes and platelets, can be drawn off and used to prepare a wet mount (for identifying motile organisms) or Giemsa-stained blood films. The Knott’s concentration and membrane filtration techniques are also useful for detection of microfilariae, particularly in low-density infections. Like the buffy coat preparation, the Knott’s concentration method uses centrifugation to separate the larger microfilariae. However, the blood is first lysed with 2% formalin, allowing the microfilariae to concentrate in the sediment. The sediment can be examined as a wet or stained preparation. With the membrane filtration technique, approximately 1 mL of blood is lysed and passed through a filter with 3-to 5-μm pores to trap the microfilariae. The filter is then removed and placed on a microscope slide for examination.
Most intestinal protozoa and helminths can be identified through microscopic examination of fecal specimens. While conventional microscopy (i.e., the “ova-and-parasite” or “O&P” examination) is commonly performed, newer tests for detection of parasite antigen or nucleic acid offer commonly used, sensitive methods for detection of specific parasites such as Giardia duodenalis, Cryptosporidium spp., and Entamoeba histolytica . , The stages of intestinal protozoal parasites that can be identified microscopically are the active, motile trophozoite (“troph”) forms and the inactive, environmentally resistant cyst or oocyst forms. , , Eggs (ova) are the most common form of intestinal helminths seen in stool specimens, although larvae are the primary form seen in Strongyloides stercoralis infection. Less commonly, adult forms of various worms may be submitted for identification. Examination of both a concentration and a permanently stained preparation is recommended for optimal sensitivity. , , ,
Proper collection and handling are essential for optimal recovery and identification of parasites in fecal specimens. Examination of poorly preserved specimens or those containing interfering substances (e.g., antibiotics, barium, bismuth, oily laxatives, and nonabsorbable antidiarrheal medications) provide little to no clinical value and should be avoided. , , Ideally, specimens should be collected at least 7 days after completion of antibiotic therapy or product use. Fecal specimens should be collected in a clean container (not contaminated with urine, water, or soil) and may be submitted to the laboratory in a fresh or preserved state. Table 88.6 lists the most common preservatives and the types of preparations that can be made with each. The conventional two-vial preservative consists of 5 to 10% buffered formalin and polyvinyl alcohol (PVA). More recently, formalin- and mercury-free commercial single-vial proprietary fixatives have become increasingly popular due to their relative lack of toxicity and ease of use. , , It is important to note that not all preservatives are amenable for use with enzyme immunoassay (EIA) and polymerase chain reaction (PCR) tests. , Fresh specimens must be examined as soon as possible (within 30 minutes for soft or liquid specimens or within 1 hour for semi-formed specimens) to avoid degradation of fragile trophozoites. If it is not possible to examine fresh specimens within this time frame, they should be placed immediately into an appropriate preservative. Vials of preservative should be given to patients for collection so that multiple specimens may be collected over a period of time. All specimens should be considered potentially infectious and handled using standard precautions. ,
| EXAMINATION TECHNIQUE | ||
|---|---|---|
| Fixative | Concentration Wet Mount | Permanently Stained Smear |
| None (fresh stool) a | Yes | Yes |
| 5 or 10% formalin (including buffered formalin) | Yes | No |
| Merthiolate-iodine-formalin (MIF) | Yes | Yes, polychrome intravenous stain |
| Sodium acetate–formalin (SAF) | Yes | Yes, iron hematoxylin stain |
| Schaudinn’s fixative b | Yes (rarely used) | Yes, trichrome or iron hematoxylin stains |
| Polyvinyl alcohol (PVA) b | Yes (rarely used) c | Yes |
| Modified PVA | Yes (rarely used) c | Yes |
| Single-vial fixatives d | Yes | Yes, use of proprietary stain may be recommended. |
a Fresh stool must be examined as soon as possible (ideally within 1 hour) after being passed to avoid degradation of parasite forms.
b Contains mercury, which is poisonous and requires special disposal.
c Concentration techniques have been described using PVA but are suboptimal for detection of some parasites.
d Many single-vial fixatives are now commercially available (e.g., ECOFIX, TOTAL-FIX, PROTO-FIX, ParaPak SVT, Parasafe, Alcorfix) and can be used for all of the examination techniques listed above.
Ideally, three or more specimens (collected on consecutive days or every other day over a 10-day period) should be examined for optimal detection of most parasites; up to seven specimens must be examined for optimal detection of S. stercoralis.
Fecal specimens should be examined macroscopically for consistency (e.g., watery, soft, formed) and the presence of blood, mucus, and whole worms and proglottids. The consistency of the specimen can provide important clues to the types of organisms that might be present; trophozoites are more frequently found in watery to soft specimens, while cysts are generally the only stages found in formed specimens.
A portion of the specimen can then be selected for microscopic examination, taking care to sample bloody or mucoid areas that may contain higher numbers of trophozoites. Three types of microscopic preparations may be used for parasite detection: the direct wet mount of fresh unpreserved stool, the wet mount of preserved concentrated stool, and the permanently stained smear.
Each preparation has specific limitations and advantages. The direct wet mount is made by placing a small amount of stool onto a slide and mixing it with a drop of 0.85% saline. The specimen is then coverslipped, and the entire specimen is examined using the 10× objective (i.e., 100× magnification). At least 1/3 of the coverslip should also be examined using the 40× objective (i.e., 400× magnification). A second (optional) preparation may be made in a similar fashion by using a drop of 1:5 dilution of Lugol iodine instead of saline in order to increase the contrast of protozoal trophozoites and cysts. Direct examination of the fresh, unpreserved specimen is used primarily for detection of motile parasites (larvae and trophozoites), and it is therefore essential to examine the specimen shortly after passage, before motile parasites die and begin to degrade. Since it is generally more important to preserve parasite morphology than motility, laboratories that cannot examine specimens within the narrow recommended time frame should forego the direct preparation in favor of placing the specimen immediately into a preservative.
Concentrated preparations can be made using either fresh or preserved feces (see Table 88.6 ). The two methods for specimen concentration are sedimentation and flotation. , Of these, sedimentation methods are most commonly used in human clinical parasitology laboratories. In these methods, parasites settle to the bottom of the specimen via centrifugation, whereas with flotation methods, parasites float to the top of a solution with a high specific gravity. , , Most concentration methods also incorporate filtration/straining and defatting steps to remove extraneous fecal material. The classical method for concentrating human fecal specimens in the diagnostic laboratory is the formalin-ether sedimentation method, which uses formalin as a preservative and fixative and ether as a defatting agent. , This procedure is still commonly used worldwide, although ethyl acetate is often used in place of ether, given the high flammability of the latter. Prior to concentration, a filtration step is generally performed to remove large particulate matter from the stool. Filtration may occur using a simple mesh screen or employing a specially designed commercial filtration and concentration device. The concentrated wet mount is examined in the same manner as the direct wet mount, using low and high magnifications, with or without a second preparation using iodine. Examination of the concentrate allows for identification of protozoa and helminth eggs and larvae but is suboptimal for detection of small protozoa such as Dientamoeba fragilis . Fecal leukocytes, erythrocytes, and Charcot-Leyden crystals (slender crystals formed during eosinophil breakdown) can also be identified ( Fig. 88.5 ). The presence of fecal leukocytes is suggestive of an inflammatory cause of the patient’s symptoms such as invasive bacterial or parasitic infections, or inflammatory bowel disease, while the presence of eosinophils/Charcot-Leyden crystals may suggest a parasitic etiology or other cause of eosinophilia.
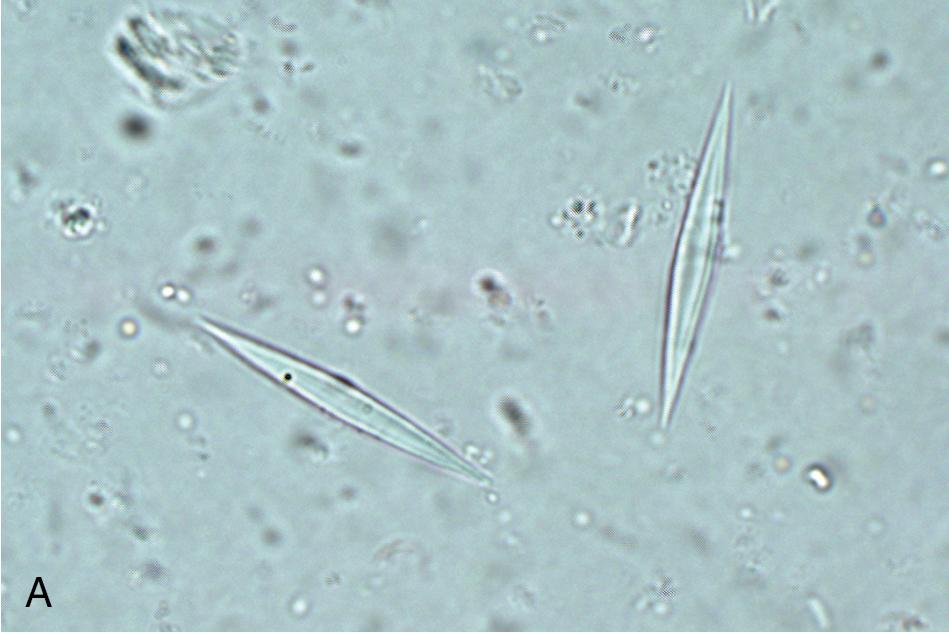
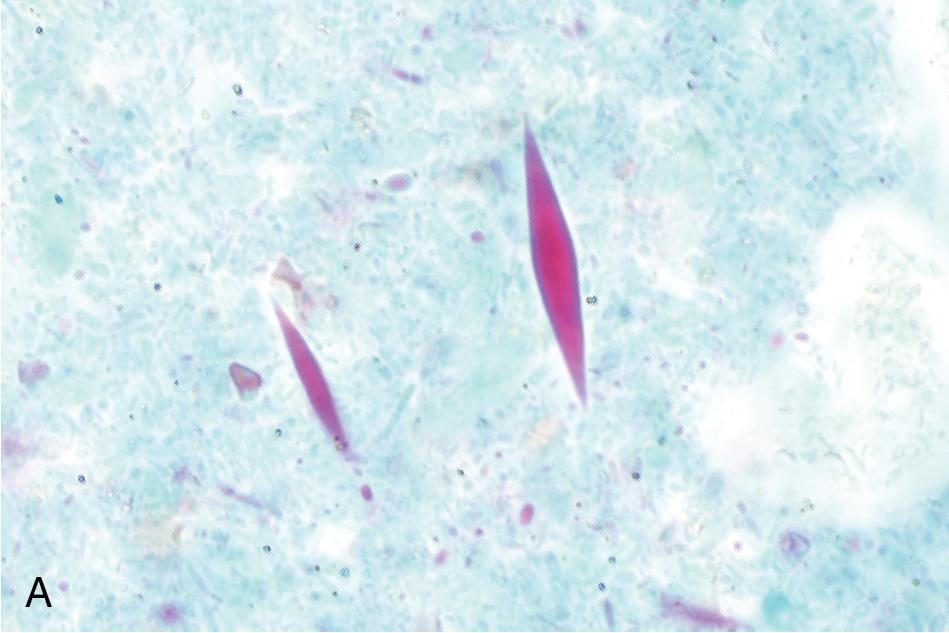
The third component of a complete O&P examination is the permanently stained smear. This preparation is used primarily to identify protozoa since it provides greater sensitivity for these parasites than concentrate and direct preparations alone. , However, it is not recommended for identification of helminth eggs and larvae, since the stain may obscure the features of these larger objects. The stained smear is useful for providing a permanent record of the patient’s specimen and allows for sharing of slides among laboratories. , , It is made by spreading feces onto a slide in such a way that thick and thin regions are created, and then stained with a method that allows for good protozoal nuclear and cytoplasmic differentiation. While historically the permanent stained smear was made from unconcentrated preserved stool, it is becoming more commonplace to make the smear of a concentrated specimen, especially when using single-vial spin-column technology. The iron-hematoxylin stain was traditionally used due to its superior definition of key cytoplasmic and nuclear characteristics, but it is technically challenging and has largely been replaced with the Wheatley modification of the trichrome stain in the United States ( Fig. 88.6 ). Proprietary versions of the trichrome stain may be recommended for specimens preserved in commercial single-vial preservatives, so it is important to check with the manufacturer when selecting a stain for routine use.
Depending on the laboratory, the available resources, and the primary purpose of the stool parasite examination, any or all three of these preparations may be used. Many parts of the world, including those in which the primary concern is detection of helminth ova, will only examine a wet mount preparation of unconcentrated or concentrated stool. However, many laboratories in the United States, Australia, Canada, and parts of Europe will examine a permanent smear in addition to the concentrated wet preparation to provide additional sensitivity for detection of protozoa. Of note, both a concentrated wet preparation and permanent smear must be examined by laboratories accredited through the College of American Pathologists, including those outside of the United States.
Finally, additional special stains may be performed for sensitive detection of the coccidia and the microsporidia. , Modified acid-fast stains (modified Kinyoun method, modified acid-fast dimethyl sulfoxide, auramine-O) or modified safranin stains are recommended for detection of coccidian oocysts in stool preparations. , , Instructions for performing staining procedures are available elsewhere. , ,
A number of other techniques exist for the morphologic detection of parasites in fecal specimens. , , A method that is uncommonly used in the United States and Europe, but may be used in endemic settings, is egg enumeration for estimation of intestinal worm burden. This may be requested to determine the need for treatment and to monitor therapeutic efficacy. Methods for enumerating eggs include the Stoll dilution egg count, direct smear method of Beaver, and the Kato-Katz technique. , ,
Finally, a number of concentration and culture techniques are used to increase the sensitivity of S. stercoralis detection, including the Baermann concentration, Harada-Mori filter paper test-tube culture, and stool agar culture methods. , , These methods also allow for recovery of other nematodes including those shed as eggs in stool. With the Baermann technique, a portion of feces is placed on gauze that is suspended on a wire or nylon sieve over the top of a funnel. The funnel is held upright in a ring stand and attached to a section of rubber hose, which is clamped shut. The funnel is then filled with water up to the level of the gauze, so that the feces just barely touches the water. This allows nematode larvae that are present in the fecal specimen to migrate through the gauze into the water, from where they settle to the bottom of the funnel via gravity. Water can be sampled from the hose after 2 or more hours and centrifuged so that the sediment can be examined for the presence of larvae. ,
The Harada-Mori filter paper test-tube method is similar to the Baermann concentration technique in that fecal material is placed on permeable substance (filter paper) and placed in contact with nonchlorinated water. , This method can also be used to detect nematodes in soil samples. With this method, the filter paper strip is placed into a test tube and the tube is allowed to stand upright at room temperature (25 to 28 °C) for 10 days. Larvae that are present in the sample will migrate into the water and drift to the bottom of the tube. A small amount of water is withdrawn from the bottom of the tube daily for 10 days and examined for the presence of live larvae. Hookworm and Trichostrongylus eggs in the specimen may also hatch during this time frame and release detectable larvae. Although the Baermann and filter paper test-tube culture methods provide greater sensitivity for detection of S. stercoralis larvae than conventional O&P testing, they are time-consuming and potentially hazardous (due to the infectivity of the larvae) and therefore rarely used in the clinical laboratory.
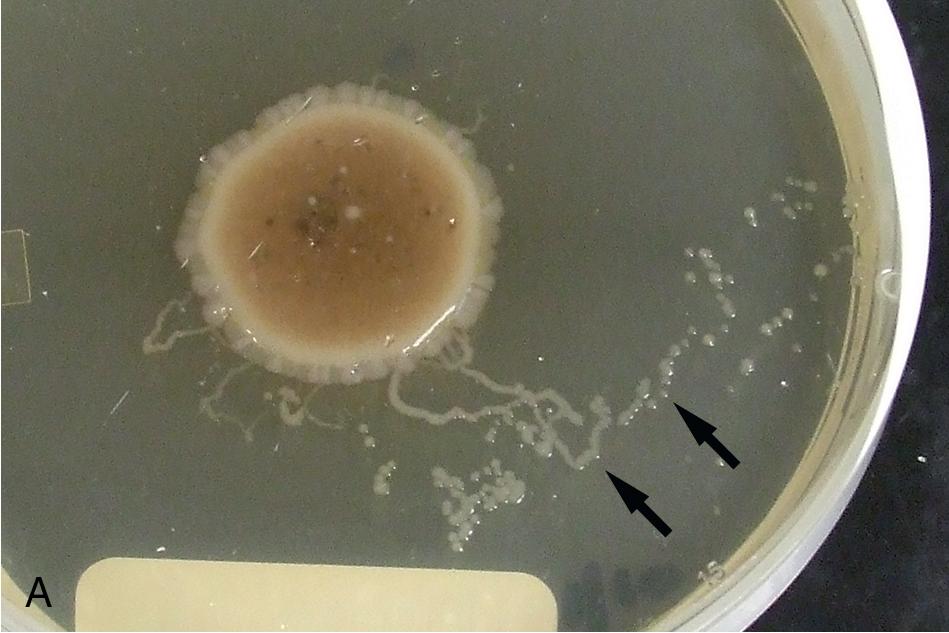
The stool agar culture method provides a safer, simpler, and more sensitive alternative to the Baermann technique and is therefore better suited for routine clinical use. , , , Using this method, approximately 2 g of feces is placed in the center of an agar plate and incubated at room temperature. Larvae that are present in the specimen will migrate from the feces into the agar and carry fecal bacteria with them. The bacteria grow in the tracks of the migrating larvae and produce visual evidence of their presence ( Fig. 88.7 ), thus allowing for easier detection. The plates should be incubated at room temperature for 2 or more days (agar side up) and examined daily for evidence of larval tracks leading away from the stool. Migrating larvae from positive agar culture plates should be examined microscopically to confirm the identity of the infecting nematode. Any type of nutrient agar that can sustain bacterial growth is suitable for the stool agar culture, but clear agars are easier to macroscopically examine for larval migration tracks. Despite the increased sensitivity provided by the stool agar culture, testing multiple specimens may still be required for detection of latent S. stercoralis infection. , As noted earlier, the filariform larvae of S. stercoralis and hookworms are infectious, and therefore testing should be conducted wearing gloves. For additional protection of laboratory staff, stool agar culture plates should be sealed with cellulose tape and placed in a clear plastic bag for daily visual inspection. Feces should not be refrigerated since this will kill the larvae and cause false-negative results. ,
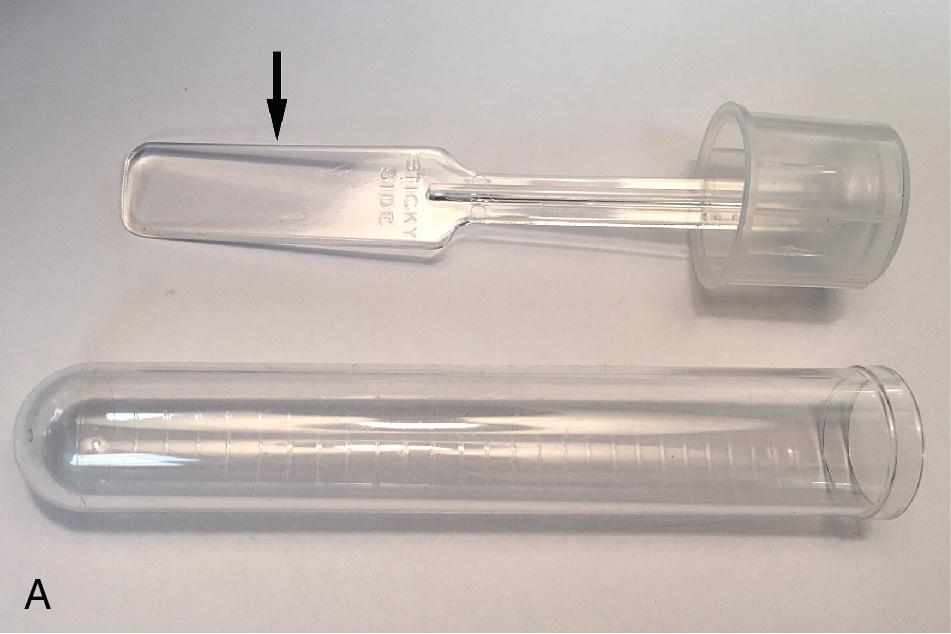
Unlike other intestinal nematodes, stool is not the preferred specimen for detection of pinworm ( Enterobius vermicularis ) since eggs are deposited directly onto the perianal skin folds rather than in feces. Therefore sampling of the perianal skin using the cellulose tape test is preferred for detecting this helminth. Taenia spp. eggs may also be detected since proglottids containing eggs may migrate out of the anus. With this method, clear (non-frosted) adhesive cellulose tape (e.g., Scotch tape) is pressed onto the perianal skin, and then placed onto a glass slide for microscopic examination for detection of pinworm eggs, and occasionally adult females. Commercial adhesive paddle devices such as the Swube (Becton Dickinson, Franklin Lakes, New Jersey) and the Pinworm Collector (Scientific Device Laboratory, Des Plaines, Illinois) provide an alternative sampling device that is easier to use by parents and caregivers ( Fig. 88.8 ). Sampling should be performed early in the morning before the patient bathes or defecates. Gloves should be worn during specimen collection and examination, since E. vermicularis and Taenia solium eggs may be infectious. A single negative exam does not rule our pinworm infection, and up to six exams may be required for optimal sensitivity. Although pinworm eggs and/or worms may occasionally be detected in fecal specimens, the O&P is considered a suboptimal method for diagnosing pinworm infections.
Direct duodenal sampling may allow for enhanced detection of S. stercoralis, G. duodenalis, Cryptosporidium spp., and Cystoisospora belli, and may, less commonly, recover Clonorchis sinensis, Opisthorchis spp., and Fasciola hepatica . , , Specimens may be collected by upper endoscopy or through use of the “string test” (Entero-test, gelatin-capsule method). , With the string test, the patient swallows a weighted gelatin capsule that contains a spool of nylon string. The string also protrudes from one end of the capsule and is taped to the patient’s cheek or neck. Once swallowed, the gelatin capsule dissolves, and the string unwinds and is carried to the duodenum by peristalsis. Organisms that are present in the duodenum will adhere to the string and will then be removed when the string is pulled out of the patient approximately 4 hours later. Forceps are used to remove any adherent material (e.g., intestinal mucus) from the string for subsequent microscopic examination. With both duodenal aspirates and the string test, the material is examined by wet or stained preparation for the presence of parasites, in a similar manner to routine stool examinations. ,
Urine, urethral and vaginal discharge, and prostatic secretions may be examined for Trichomonas vaginalis, a sexually transmitted protozoal parasite. , , This was traditionally done in the physician’s office using a wet mount of the specimen in saline, and could also be used to identify yeasts and pseudohyphae (suggestive of candidiasis) and clue cells (bacterial vaginosis) in vaginal secretions. T. vaginalis trophozoites are identified by their characteristic morphology and “jerky” motility. Unfortunately, this is a relatively insensitive method for diagnosis of trichomoniasis and is less commonly used in the United States due to regulatory/proficiency requirements for physician-provided microscopy. Instead, nucleic acid amplification tests (NAATs) are recommended for detection of T. vaginalis infection in men and women. , If imidazole drug resistance is suspected, culture is required for antimicrobial susceptibility testing (see the “Parasite Culture Techniques” section later in this chapter). ,
Urine may also be examined for Schistosoma haematobium eggs and, less commonly, microfilariae. , , , For detection of S. haematobium, several urine specimens should be examined over a period of several days to maximize the likelihood of detection. The optimal time for collection is between 10:00 am and 2:00 pm, since this is when peak egg shedding is thought to occur. Use of membrane filtration (as described earlier for microfilariae) ( Fig. 88.9 ) or collection of a 24-hour urine specimen may increase detection.

A variety of helminthic and protozoal parasites may be detected in respiratory specimens such as sputa, bronchial aspirates, and bronchoalveolar lavage specimens. The parasites most commonly seen in these specimens include S. stercoralis larvae, Paragonimus spp. eggs, Echinococcus spp. protoscoleces and free hooklets, and E. histolytica trophozoites. Specimens are usually examined as a wet mount, but a permanently stained slide is also appropriate for detection of protozoans. The type of stain used depends on the suspected organisms. Cryptosporidium spp. oocysts may rarely be seen and are best detected using a modified acid-fast stain.
Cerebrospinal fluid (CSF) can be examined as a wet mount or stained preparation for trypanosomes ( T. brucei ) , free-living amebae (FLA) ( Naegleria fowleri ), and rarely T. solium (cysticercosis larval form), S. stercoralis, Echinococcus granulosus, Angiostrongylus cantonensis (L4 larvae), and various microfilariae. , Specimens should be centrifuged, and the pellet used for examination. It is important not to refrigerate the specimen if it will also be used for culture of N. fowleri.
A number of parasites may be found in tissue aspirates, abscesses, cysts, and biopsies, and the type of preparation and stains should be selected based on the clinical presentation and suspected parasite. , , In addition to the wet mount preparation, Giemsa-stained aspirate smears and tissue “touch preps” (impression smears) are particularly useful for identification of the protozoa, including the hemoflagellates, T. brucei and Leishmania spp., and FLA. Leishmania spp. amastigotes are most commonly seen within macrophages in bone marrow, liver, lymph node, skin biopsies, and splenic aspirates. Culture of the aspirated or biopsied material should be performed to increase the sensitivity of detection and to determine the infecting species if required for treatment purposes; , , , see the “Parasite Culture Techniques” section later in this chapter. T. brucei trypomastigotes can also be seen in lymph node aspirates, whereas E. histolytica causes abscesses in the liver, and less commonly, the lung, brain, and other organs, and the FLA ( N. fowleri, Acanthamoeba spp., Balamuthia mandrillaris ) are found primarily in brain tissue. All can be detected using wet mounts and Giemsa-stained preparations, although the trichrome stain is preferred for detection of E. histolytica. , , It is important to note that E. histolytica trophozoites are often few in number and may be difficult to identify among necrotic host cells; therefore culture, antigen-detection methods, or PCR of aspirated or biopsied material may be useful. Culture or PCR is also recommended for enhanced detection of the FLA, N. fowleri , and Acanthamoeba spp.; see “Parasite Culture Techniques” later in this chapter. , ,
The helminths most commonly found in tissue aspirates, cysts, and biopsies are Echinococcus spp., T. solium (cysticercosis larval form), and S. stercoralis filariform larvae (in hyperinfection syndrome). These can usually be identified using wet mounts, but can also be highlighted with a variety of stains including Giemsa and trichrome.
Skin biopsies (e.g., bloodless skin snips) submitted for detection of Onchocerca volvulus or Mansonella streptocerca should be placed in 1 mL saline for incubation at room temperature for up to 4 hours. , The skin snip and the saline are then examined for detection of motile microfilariae.
Lastly, human muscle biopsies (or animal meat) sent for suspected trichinosis are generally examined by compressing the fresh specimen between two glass slides (“squash prep”) or using a trichinoscope that compresses the muscle in a similar manner. , Bladder and rectal biopsies may be examined in a similar fashion for detection of Schistosoma spp. eggs. Specimens can also be submitted for routine histologic identification of Schistosoma eggs or Trichinella larvae.
Skin scrapings are the preferred specimen for detection of Sarcoptes scabiei mites, eggs, and fecal pellets (scybala). Specimens should be obtained from the infected area using a scalpel blade. A drop of mineral oil should be applied to the blade and allowed to flow onto the region of skin being sampled since this will facilitate adherence of the mites to the blade. Because the mites live within the epidermis, the area should be vigorously scraped, such that flecks of blood are seen in the oil. The material is then transferred to a glass slide using a wooden applicator stick. Another drop of oil is added, and the material is mixed together. The slide can then either be coverslipped for immediate examination, or covered with another slide and rubber-banded together for transportation to the laboratory. , This method can also be used for the detection of Demodex mites. Gloves should be worn during collection and examination of skin scrapings due to the highly infectious nature of the S. scabiei mites.
Corneal scrapings are used for detecting Acanthamoeba spp. cysts and trophozoites. , , Giemsa is sufficient for detection of both Acanthamoeba cysts and trophozoites, but Calcofluor white will detect the cysts only. Smears are generally insensitive for detection of amebic keratitis, and therefore culture and/or PCR should also be performed on corneal specimens. Swabs are suboptimal for collecting ocular material for testing.
The use of artificial intelligence (AI) is becoming increasingly popular in diagnostic microbiology laboratories, everything from automated bacterial plate readers to the use of machine learning using a deep convolutional neural network (CNN) to detect bacteria on a Gram stain. The use of AI in parasitology is still in its infancy, but there has been proof-of-concept work for Plasmodium and Babesia in blood and helminth eggs and protozoan cysts in wet mounts of stool. Recently, a large reference lab in the United States clinically validated an assay that uses a CNN to detect protozoan trophozoites in stool stained with trichrome.
Culture methods have been described for a wide variety of parasites including most protozoa and some helminths. However, culture is rarely performed in the clinical laboratory due to the infrequent nature of requests and the complexity of the methods and associated quality control and is therefore usually limited to specialized public health laboratories (e.g., CDC) and the research setting. The parasites that are most commonly cultured for clinical reasons are T. vaginalis, Leishmania spp., Trypanosoma spp., Acanthamoeba spp., N. fowleri, and E. histolytica. Culture techniques for certain nematodes may also be performed, such as the Harada-Mori filter paper strip culture described earlier in this chapter. These techniques are described in detail elsewhere , and are not explained in depth here.
Cultures may be described as xenic or axenic, based on the presence or absence, respectively, of other organisms. Examples of axenic cultures that may be encountered in the clinical setting include those for T. vaginalis and Leishmania spp. T. vaginalis can be grown in a variety of nutrient cultures, and a commercially available culture system (INPOUCH TV, Biomed Diagnostics, Inc., White City, Oregon) exists. Culture provides greater sensitivity than wet mount for detection of T. vaginalis , but still has inferior sensitivity to NAATs. , Leishmania and T. cruzi can be grown effectively using Novy-MacNeal-Nicolle (NNN) medium. The CDC provides a collection kit to providers in the United States that can then be returned for culture and species identification. While the CDC will receive kits from other countries, kits are only provided to US-based clinicians unless special arrangements are made.
Xenic culture methods are commonly used for intestinal amebae and some FLA ( Acanthamoeba spp., N. fowleri ). When fecal samples are cultured for intestinal amebae, both pathogenic and nonpathogenic organisms may be amplified, as well as fecal bacteria. The bacteria serve as a food source and allow for proliferation of the amebae. For culture of Acanthamoeba and N. fowleri, there are usually insufficient bacteria present in the specimen to serve as a food source, and therefore bacteria (e.g., Escherichia coli ) are added to the culture medium prior to specimen inoculation. When only a single organism is added to the culture, it is referred to as monoxenic . A commonly used method is the non-nutrient agar culture, in which the agar is plated with a lawn of bacteria prior to adding the specimen (e.g., corneal scrapings, contact lenses, CSF, brain biopsy). N. fowleri trophozoites are cold-sensitive, and therefore specimens should not be refrigerated prior to culturing. It is important to note that B. mandrillaris, an FLA that causes central nervous system infection, will not grow with this method.
Immunodiagnostic methods are important tools for supporting the diagnosis or screening for the presence of parasitic infections. They are also used commonly for epidemiologic studies. In areas of the developing world where infection with parasites is common, the utility of immunodiagnostic assay is limited by its low positive predictive value for acute infections. Several assays have been developed for the detection of either antigens or antibodies of common parasites, including EIAs, lateral-flow immunochromatographic assays (LFICAs), indirect immunofluorescence assays (IFAs), direct fluorescence antibody assays (DFAs), immunoblots, Western blots, radioimmunoassays, and immunodiffusion assays. Some assays for common parasites are commercially available. For more uncommon infections (e.g., paragonimiasis), serologic testing is often performed at specialized reference laboratories including the CDC. The following section will review the principle of commercially available assays for the detection of antigens and antibodies of the most common parasites.
Most commercially available assays are designed to detect antigens of enteric protozoa such as E. histolytica, G. duodenalis, and Cryptosporidium spp. The two most commonly used commercial methods are EIAs and LFICAs ( Table 88.7 ). , Commercial assays are also available for detection of D. fragilis, Plasmodium spp., W. bancrofti, and T. vaginalis , but depending on the organism may not be US Food and Drug Administration (FDA) approved for routine clinical diagnosis in the United States .
| Targeted Analyte | Manufacturer | Test | Methodology |
|---|---|---|---|
| Cryptosporidium spp. | Alere (Abbott) | Cryptosporidium II | EIA |
| Trinity Biotech | Uni-Gold Cryptosporidium | LFICA | |
| Remel (Thermo Scientific) | Xpect Cryptosporidium | LFICA | |
| Remel (Thermo Scientific) | ProSpecT Cryptosporidium | EIA | |
| IVD Research, Inc. | Cryptosporidium stool antigen detection | EIA | |
| Entamoeba histolytica | Alere (Abbott) | E. histolytica II | EIA |
| Alere (Abbott) | E. histolytica QUIK CHEK | LFICA | |
| Remel (Thermo Scientific) | ProSpecT E. histolytica | EIA | |
| Cellabs | Entamoeba CEIA Path | EIA | |
| Giardia duodenalis a | Alere (Abbott) | Giardia II | EIA |
| Trinity Biotech | Uni-Gold Giardia | LFICA | |
| Remel (Thermo Scientific) | Xpect Giardia | LFICA | |
| Remel (Thermo Scientific) | ProSpecT Giardia Microplate | EIA | |
| Remel (Thermo Scientific) | ProSpecT Giardia EZ Microplate | EIA | |
| IVD Research, Inc. | Giardia Stool Antigen Microwell | EIA | |
| Giardia/Cryptosporidium | Alere (Abbott) | Giardia/Cryptosporidium QUIK CHEK | LFICA |
| Alere (Abbott) | Giardia/Cryptosporidium CHEK | EIA | |
| Remel (Thermo Scientific) | Xpect Giardia/Cryptosporidium | LFICA | |
| Remel (Thermo Scientific) | ProSpecT Giardia/Cryptosporidium Microplate | EIA | |
| IVD Research, Inc. | Giardia/Cryptosporidium Combo Stool Antigen Detection Microwell | EIA | |
| Meridian Bioscience, Inc. | ImmunoCard STAT! Crypto/Giardia | LFICA | |
| Meridian Bioscience, Inc. | MERIFLUOR Cryptosporidium/Giardia | DFA | |
| Trichomonas vaginalis | Genzyme Corp. | OSOM Trichomonas Rapid Test | LFICA |
| Xenotope Diagnostics Inc. | XenoStrip-Tv Trichomonas | LFICA | |
| Plasmodium spp. b | Alere (Abbott) | BinaxNOW Malaria | LFICA |
| BIO-RAD | OptiMAL | LFICA | |
| Premier Medical Corp. | First Response Malaria P.F/P.v Antigen Strips | LFICA | |
| Apacor | CareStart Malaria (Pan) | LFICA | |
| Alere (Abbott) | SD BIOLINE Malaria Ag | LFICA | |
| Wuchereria bancrofti | Alere (Abbott) | BinaxNOW Filariasis | LFICA |
a Previously known as Giardia intestinalis and Giardia lamblia .
b The BinaxNOW Malaria test is the only commercially available assay cleared by the Food and Drug Administration (FDA) for diagnosis of malaria in the United States. Many other assays are available outside of the United States, and some have superior performance. Listed here are several that scored highly for detection of P. falciparum by World Health Organization (WHO) and Foundation for Innovative New Diagnostics (FIND) product testing.
EIAs are commonly used in the parasitology laboratory and generally have the benefit of established performance and utility for many other tests in the laboratories. They also usually demonstrate good sensitivity and specificity for detection of fecal protozoa when compared to the O&P examination. Most EIA methods require 2 to 3 hours to perform and can be read manually or using a spectrophotometer. EIAs commonly use microtiter plates containing immobilized monoclonal antibodies (capture antibodies) to the parasite antigens of interest. The test sample is mixed with conjugate antibodies (e.g., polyclonal antibodies against the parasite antigens) and added to the microtiter plate containing the monoclonal antibodies. If the antigen is present, an immune complex will form and remain bound when washed to remove unbound antibodies. A substrate is then added and, if antigen/antibody complexes are present, a color will develop as a result of an enzymatic reaction (e.g., peroxidase).
LFICAs are available for the detection of parasite antigens in stool, blood, and vaginal/cervical samples (see Table 88.7 ). In general, LFICAs are designed to be used as point-of-care tests and are relatively simple to perform. Results are usually available within a short time frame (15 to 30 minutes). It is important to note, however, that analytical sensitivity and specificity may be lower than reported for EIAs. The typical assay design consists of a nitrocellulose membrane test strip that has been coated with mouse or rabbit “capture” antibodies to the parasite antigen of interest on the test line portion of the strip. Another portion of the strip is coated with goat anti-mouse or anti-rabbit Ig to serve as a control for the test mechanics. To perform the test, straight or diluted samples are added to a loading chamber, where the specimen migrates to a conjugate pad containing immobilized antibodies. These antibodies have been conjugated with colloidal gold or colored latex particles and are specific to the targeted parasite antigen. If parasite antigen is present in the specimen, then antigen/antibody complexes are formed, and these migrate along the nitrocellulose test strip via capillary action until reaching the control and test lines. An immune complex will form if the sample is positive for the targeted antigen and is visible as a colored line or dot ( Fig. 88.10 ). A positive control line reaction should always be detected to confirm that the test was performed correctly.
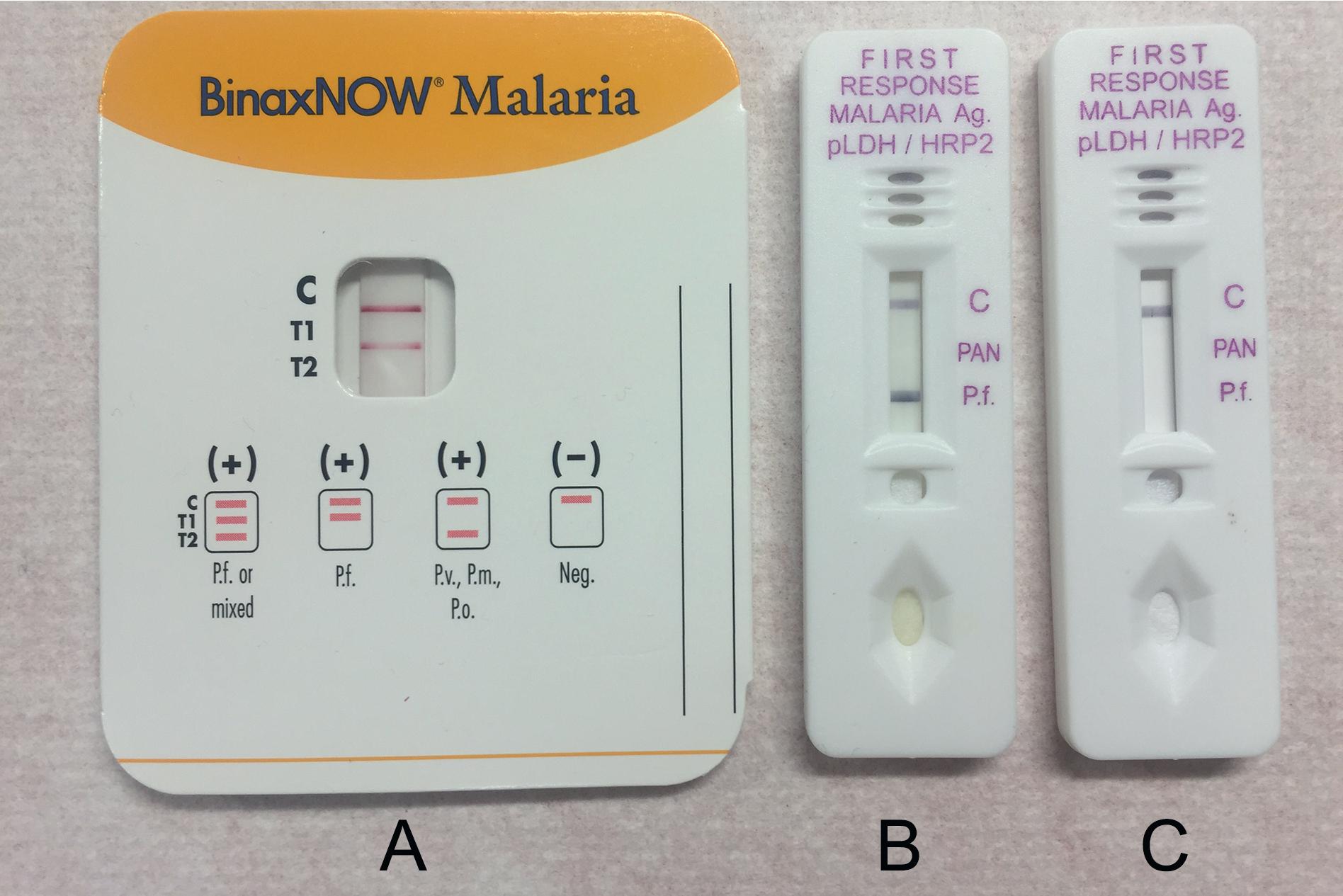
LFICAs are available for Cryptosporidium spp., E. histolytica, E. histolytica/Entamoeba dispar, Giardia , Plasmodium spp., and W. bancrofti (see Table 88.7 ). The sensitivity and specificity of these assays vary and are highest in regions with a higher prevalence of the disease. Also, not all assays are capable of species-specific identification; for example, some Entamoeba spp. antigen tests detect both E. histolytica and E. dispar, and do not differentiate between them. Due to the limited sensitivity and specificity of some tests, additional laboratory testing may be recommended for confirmation of negative results. It should be noted that some of these (e.g., those for W. bancrofti ) are not FDA approved for routine clinical diagnosis in the United States.
Detection of parasite-specific IgM- and/or IgG-class antibodies is useful for estimating the geographic distribution and prevalence of an infection, determining previous exposure status, during traceback investigations of parasitic infections acquired by blood transfusion, and to support the clinical diagnosis. In the latter case, measurement of IgM or a fourfold increase in the serum IgG titer between baseline and convalescent specimens is necessary for diagnosing a recent infection. Detection of antibodies is particularly indicated for some systemic parasitic infections (e.g., toxoplasmosis, Chagas disease, cysticercosis) in which an invasive sample cannot be easily collected and when traditional microscopic examinations are negative. In general, antibody detection methods are less useful for parasites that are limited to the gastrointestinal tract, since a detectable immune response is not often formed.
Methods used to detect antibodies include a variety of capture sandwich immunoassays, such as chemiluminescent magnetic microparticle immunoassay (CMIA), electrochemiluminescence immunoassay analyzer (ECLIA), and microparticle enzyme immunoassay (MEIA), as well as indirect immunofluorescent assays, and simple EIAs. The principle of the immunoassay sandwich test relies on the capture of the antibody of interest by a monoclonal anti–human antibody that is coupled to particles (e.g., magnetic beads) and bound to a solid surface. A conjugate antibody that is bound with a label or enzyme is complexed with the parasite antigen and added to the reaction plate containing the monoclonal antibody. If the antibody to the parasite is present, it will be detected by an enzymatic, fluorescent, or chemiluminescent reaction.
Commercial methods are available for the detection of antibodies to Toxoplasma gondii, T. cruzi, and E. granulosus . A well-known limitation of antibody detection methods for parasitic infection is the potential for false-positive results due to cross-reactivity with other closely related parasites. For example, significant serologic cross-reactivity may occur between Echinococcus spp. and T. solium.
Molecular diagnostic methods for parasitic infections have traditionally been performed using laboratory-developed assays in specialized reference or research laboratories. , This is still true for most parasitic infections. However, in recent years, several assays have become widely available for the molecular diagnosis of enteric pathogens and T. vaginalis ( Table 88.8 ). , Most commercially available enteric pathogen assays also detect other common bacterial and viral causes of gastroenteritis using pathogen-specific primers and/or probes. The sensitivity and specificity of molecular methods is high, ranging from 90 to 100% when compared to microscopic or antigen-based methods. Methodologies used vary, but most assays use the PCR method to amplify parasite DNA. Detection of amplified products is performed using specific fluorescent probes (real-time PCR), DNA intercalating dyes, or bead suspension arrays. Other amplification methods include loop-mediated isothermal amplification (LAMP), nucleic acid sequence-based amplification (NASBA), strand-displacement amplification (SDA), and helicase-dependent amplification (HAD).
| Manufacturer | Test | Parasite Target(s) Included | Acceptable Specimen Types | Methodology | Fda-ivd/Ce-ivd |
|---|---|---|---|---|---|
| Intestinal protozoa | |||||
| Beckton, Dickinson | BD MAX Enteric Parasite Panel |
|
|
|
Yes/Yes |
| BioFire Diagnostics (bioMérieux) | FilmArray Gastrointestinal Panel |
|
Preserved stool |
|
Yes/Yes |
| Luminex | xTAG Gastrointestinal Pathogen Panel |
|
Unpreserved stool |
|
Yes/Yes |
| R-Biopharm | RIDAGENE Parasitic Stool Panel |
|
Stool, unspecified |
|
No/Yes |
| Savyon Diagnostics | Gastrointestinal Panel |
|
Unpreserved stool |
|
No/Yes |
| AusDiagnostics | Faecal Pathogens A |
|
Unpreserved stool |
|
No/Yes |
| Trichomonas vaginalis | |||||
| Cepheid | Xpert Trichomonas vaginalis | T. vaginalis |
|
|
Yes/Yes |
| Quidel | AmpliVue Trichomonas Assay | T. vaginalis | Vaginal swabs | Helicase-dependent amplification (HDA) | Yes/Yes |
| Gen-Probe | APTIMA Trichomonas vaginalis Assay | T. vaginalis |
|
Transcription-mediated amplification (TMA) hybridization protection assay (HPA) | Yes/Yes |
| Becton Dickinson | BD ProbeTec Trichomonas vaginalis (TV) Qx Amplified DNA Assay | T. vaginalis |
|
Strand displacement amplification | Yes/Yes |
| Becton Dickinson | BD Affirm VPIII | T. vaginalis | Vaginal swabs | DNA hybridization probe | Yes/Yes |
| Roche Diagnostics | cobas TV/MG | T. vaginalis |
|
PCR | Yes/Yes |
Molecular methods have been developed and validated for many parasites including enteric protozoa, intestinal helminths, Plasmodium spp., Babesia spp., and T. gondii. The most common nucleic acid targets used for parasite detection include the 5.8S rDNA gene, 18S rDNA gene, internal spacer regions 1 and 2, and the small subunit rDNA. Molecular methods are particularly useful in the diagnosis of acute infections and in the species determination of organisms that cannot be easily differentiated by microscopic or some immunodiagnostic methods (e.g., E. histolytica and E. dispar ). Limitations of many molecular methods include the need for a highly specialized laboratory setting, expensive reagents and instrumentation, and highly skilled personnel for performing and interpreting the assays. Some of the newer molecular commercial assays have simpler, sample-to-answer designs that allow implementation in a variety of settings. However, the current cost of most of these assays is still high, thus limiting their potential impact in resource-poor countries, where many parasites are endemic.
As with any laboratory test, appropriate quality assurance and quality control measures must be incorporated into all parasite testing procedures. , , A complete and well-written procedure manual should be readily available and reviewed every 1 to 2 years. Reference texts and atlases, as well as positive slides and specimens should also be easily accessible. All laboratory staff performing testing for parasites must undergo regular competency assessment and participate in proficiency testing for the tests that they perform. This is especially important for parasites since they may be infrequently encountered in nonendemic settings but are important to accurately identify. ,
Although laboratory-acquired parasitic infections are rare, collection and manipulation of specimens submitted to the laboratory for the detection of parasites should be handled with care as many specimen types, including fecal samples, may contain infective parasite forms. Furthermore, other bacterial, viral, and fungal pathogens may be present in the specimens. Formalin or other fixatives are useful for reducing, but not eliminating, the infectious risk. , Gloves and, if indicated, protective eyewear, are recommended when handling fresh (unpreserved) specimens, worms/worm sections, and arthropods. Additional protective measures may be indicated for patients in whom certain conditions (e.g., Ebola virus disease) are suspected.
Finally, hazardous chemicals such as mercury-containing fixatives and ether should be eliminated wherever possible. Several mercury-free reagents now exist, and ethyl acetate can be used in place of ether. , The performance of heavy-metal alternatives may vary compared to standard reagents, and therefore any changes in procedure or reagents need to be fully validated by the laboratory prior to reporting results for patient specimens.
Microscopy remains the gold standard for detection and identification of many parasites.
Blood and feces are the most common specimens that are submitted for parasitologic evaluation, but other important specimen types include urine, sputum, duodenal aspirates, tissue biopsies, cyst and abscess material, and skin scrapings.
Culture methods are available for select parasites but are infrequently utilized except in specialized referral centers and research facilities.
Antigen detection methods, including enzyme immunoassays and lateral flow immunochromatographic assays are widely used for detection of enteric protozoa and Plasmodium species.
Antibody detection methods are most useful for detecting evidence of systemic infection in which parasites are not otherwise easily sampled.
Nucleic acid amplification methods are becoming increasingly important for detection and identification of parasites, such as the intestinal protozoa, Leishmania species, Toxoplasma gondii, and Trichomonas vaginalis.
Formalin and other preservatives lessen, but do not completely eliminate, the infectious disease risk associated with specimens submitted for laboratory analysis.
The blood and tissue protozoa comprise a number of genetically diverse organisms, including members of the Apicomplexa ( Plasmodium spp. , Babesia, T. gondii ), the hemoflagellates (also known as kinetoplastids: T. brucei, T. cruzi, Leishmania spp.), and several FLA ( N. fowleri, Acanthamoeba spp., and B. mandrillaris; see Table 88.2 ). The microfilariae (first instar larvae) of the filarial worms can also be found in blood, and they are discussed in the “Blood and Tissue Helminths” section later in this chapter.
Malaria is a hematogenous, mosquito-transmitted infection caused by apicomplexan parasites (Haemosporidia) in the genus Plasmodium . , , The four species that cause human malaria are P. falciparum, P. malariae, P. vivax, and P. ovale ( P. ovale curtisi and P. ovale wallikeri ). Other zoonotic species may also occasionally cause human disease, with P. knowlesi being recently recognized as a significant source of human infection in parts of Southeast Asia.
Malaria, from the Italian “mal aria,” meaning “bad air,” is one of the most important infections worldwide. It is a disease of antiquity, and several mutations have evolved over the course of human evolution that provide some protective benefit against severe malaria. Among the most well known are sickle cell trait, hemoglobin C, hemoglobin E, hereditary spherocytosis, <alpha>-and <beta>-thalassemia, and glucose-6-phosphate dehydrogenase (G6PD) deficiency. See Chapter 78 for further information.
According to the World Health Organization (WHO) World Malaria Report 2015, 3.2 billion individuals are at risk for malaria ( Fig. 88.11 ), and millions do not have access to reliable services and medications to prevent and treat the disease. There were an estimated 228 million cases of malaria in 2018 (95% confidence interval: 206 to 258 million) and 405,000 deaths. Of the malaria-related deaths, 94% occurred in the WHO African Region, with Nigeria, the Democratic Republic of the Congo, the United Republic of Tanzania, Angola, Mozambique, and Niger accounting for the most deaths. An estimated 67% of all deaths worldwide (272,000) were in children younger than 5 years of age. Malaria is found primarily in tropical and subtropical regions of the world today, although P. vivax is also found in some temperate regions, and used to be widespread in the United States. P. falciparum and P. vivax are the two most widely encountered species worldwide, followed by P. malariae and P. ovale . A list of countries in which malaria is endemic can be found in the CDC Yellow Book, which is an important source of information for travelers and physicians that care for international travelers, as well as the CDC Red Pages. , In nonendemic countries, malaria is seen primarily in immigrants and travelers. The majority of imported malaria is detected in patients who have traveled to an endemic region to visit friends and relatives (VFRs), as these individuals do not usually seek medical advice prior to traveling, tend to visit more rural settings where there is an increased exposure to mosquito bites, and tend to stay for longer periods of time. In 2016, there were 2088 cases of imported malaria reported to the CDC, of whom 69.4% were VFRs.
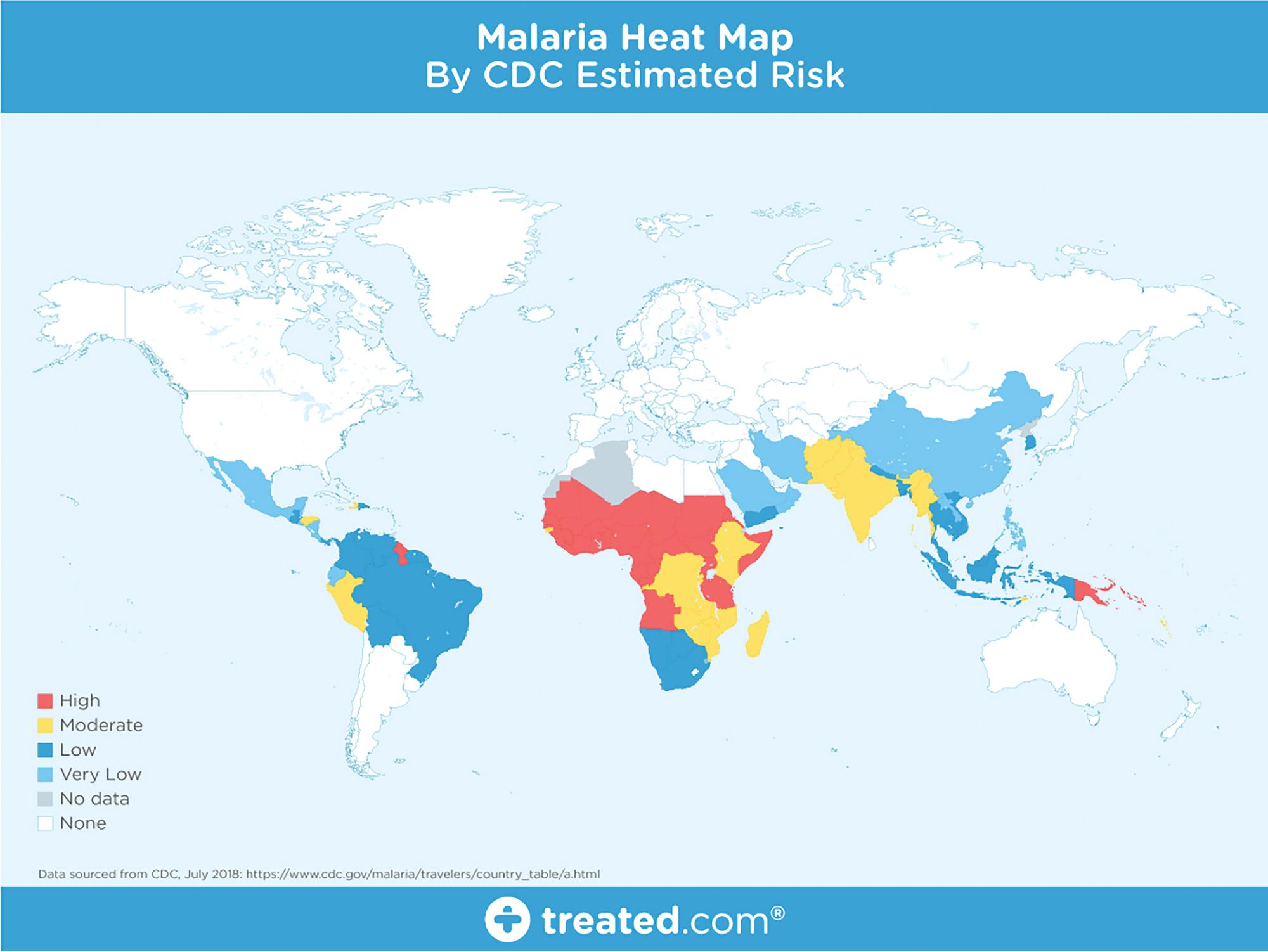
Despite these sobering figures, there have been significant reductions in malaria incidence and mortality rates due to extensive global malaria campaigns. Between 2010 and 2018, incidence rates fell from 71 to 57 cases per 1000 population at risk. Furthermore, malaria has fallen from number 9 in all causes of cause-specific disability-adjusted life year (DALYs) in 2000 to number 12 in 2012, and is no longer in the top 20 causes as of 2016. Malaria was estimated to cause 37,369,000 DALYs in 2016. These successes have been due to extensive efforts in controlling the Anopheles mosquito vector, providing highly effective artemisinin-based combination therapies (ACTs), and providing intermittent preventive treatment during pregnancy.
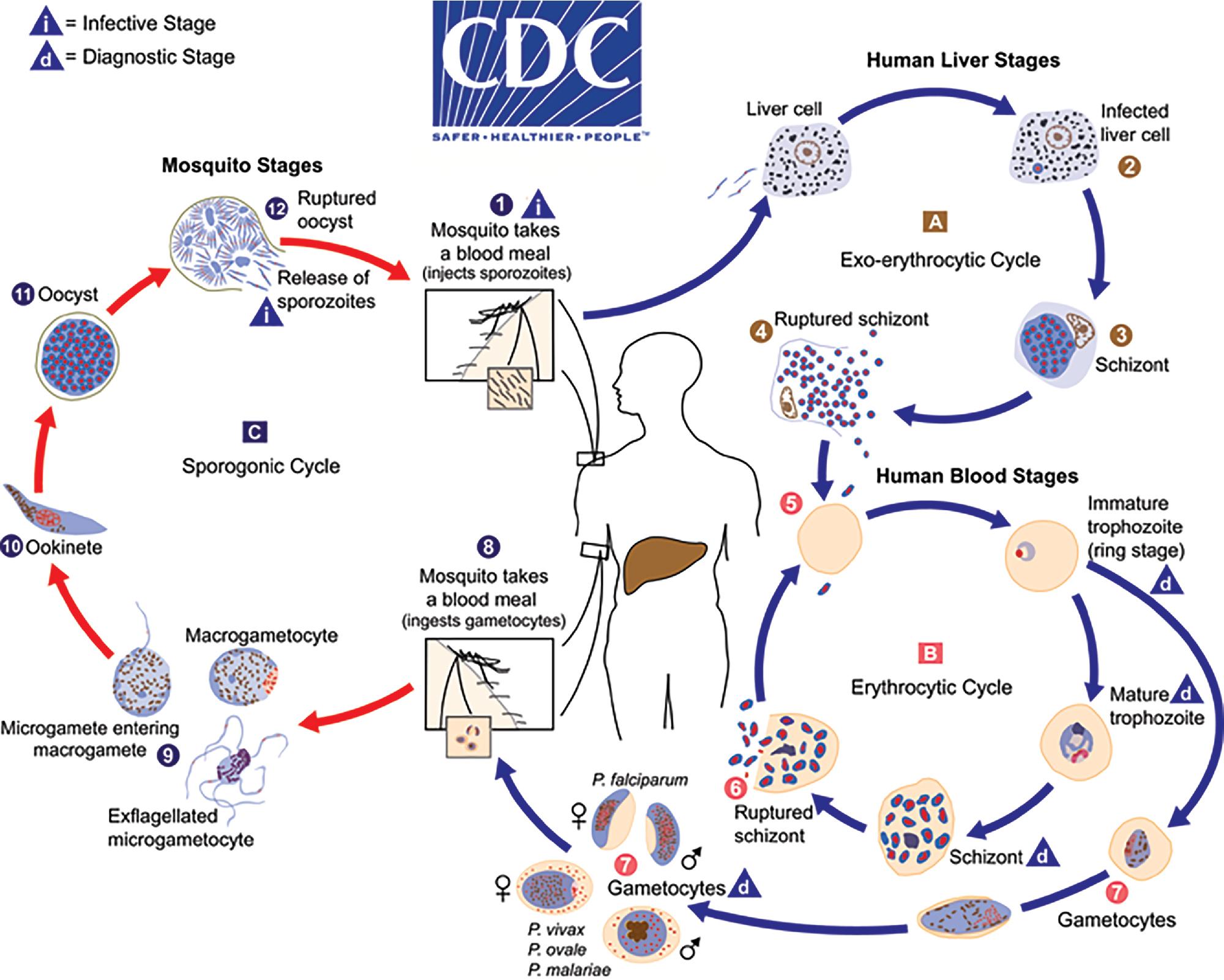
Human Plasmodium spp. have a complex life cycle involving sexual reproduction in an Anopheles mosquito vector and asexual reproduction in the human host ( Fig. 88.12 ). It is important to understand that an initial asymptomatic liver stage precedes erythrocytic infection by 8 to 40 days (depending on the species) and that dormant hypnozoite stages remain in the liver with P. vivax and P. ovale infection, which may reactivate weeks, months, or years later. Other, less common ways that humans acquire infection are transplacental spread (congenital infection) and through blood transfusion. , The asexual forms of infection are seen in humans, but rarely exflagellated microgametes and ookinetes (sexual life cycle stages) may be seen in human blood only if the blood specimen is allowed to sit at room temperature for a prolonged period of time prior to examination. ,
Malaria can range from asymptomatic to severe, life-threatening disease ( Table 88.9 ). The classic symptom is the malaria fever paroxysm, which begins abruptly when parasite destruction of erythrocytes and subsequent release of parasite antigens into the peripheral blood results in host cytokine production. With long-standing infection, erythrocytes may begin to rupture in synchrony, resulting in the classic (but rarely seen) fever cycles (see Table 88.9 ). Signs and symptoms of severe disease include generalized convulsions, hypoglycemia, respiratory distress, renal failure, circulatory collapse, and unarousable coma. Nonimmune individuals, pregnant women (and their fetuses), and young children are at greatest risk for severe disease. P. falciparum is associated with the highest morbidity and mortality due in part to its ability to infect erythrocytes of all ages, leading to high levels of parasitemia, anemia, jaundice, and splenomegaly. , In comparison, other human Plasmodium spp. preferentially infect old ( P. malariae ) or young ( P. vivax and P. ovale ) erythrocytes, and cause significantly less destruction. Erythrocytes infected with P. falciparum also sequester in small blood vessels due to receptor-mediated cytoadherence to endothelial cells and other erythrocytes, resulting in accumulation of infected cells in the microvasculature. This phenomenon, called sequestration, is associated with local tissue hypoxia, cytokine release, and metabolic acidosis, particularly in the brain, kidney, and lung. , Sequestration also occurs in the placenta due to cytoadhesion of infected erythrocytes to syncytiotrophoblasts, resulting in poor fetal outcomes. Other Plasmodium spp. do not sequester, and cause significantly lower mortality, although rare fatal infections have been reported due to P. vivax and P. knowlesi infection. , , Given the broad range of clinical symptoms associated with malaria, and the potential for life-threatening infections, malaria should be considered in all patients who travel to endemic areas and have unexplained fever.
| Characteristic | Plasmodium falciparum | Plasmodium malariae | Plasmodium vivax | Plasmodium ovale |
|---|---|---|---|---|
| Fever cycle period | 36–48 h | 72 h | 44–48 h | 48 h |
| Erythrocyte stage infected | All stages | Old cells | Young cells | Young cells |
| Sequestration of infected erythrocytes | Yes | No | No | No |
| Degree of disease severity a | Mild to severe, life-threatening | Mild to moderate | Mild to severe, rarely life-threatening | Mild |
| Central nervous system involvement | Common | Rare | Infrequent | Rare |
| Nephrotic syndrome | Rare | Common | Infrequent | Rare |
| Degree of host inflammatory response | High | Low | Very high | Low |
| Ability to cause relapsing disease | No | No, but possible recrudescence | Yes (due to activation of hypnozoite stage) | Yes (due to activation of hypnozoite stage) |
| Endemic regions | Large regions of the tropics and subtropics, especially Africa and Asia | Narrow range; tropics | Large regions of the tropics, subtropics, and temperate areas; mostly absent in West Africa | Select regions of the tropics in sub-Saharan Africa and Southeast Asia |
a Mild disease may be seen in patients with preexisting immunity.
A number of drugs are available for preventing and treating malaria, including ACTs. At this time, the only ACT that is readily available in the United States is artemether-lumefantrine (Coartem, Novartis, East Hanover, NJ), although intravenous (IV) artesunate is available through the CDC malaria hotline under an expanded-access investigational new drug (IND) protocol for patients in the United States with severe malaria and those who are unable to tolerate oral medications. Selection of therapy is complex due to the widespread resistance of P. falciparum, and to a lesser extent, P. vivax to chloroquine and other antimalarials. It is therefore important to know the causative species, travel history, degree of parasitemia, clinical status of the patient, and patient medications and drug allergies, since this will help direct treatment. Patients with P. vivax or P. ovale infection must be given primaquine, in addition to a primary therapeutic antimalarial, in order to eradicate the hepatic hypnozoite stage. , Primaquine can cause hemolytic anemia in patients with G6PD deficiency, and therefore screening for this disorder is recommended before initiating therapy (for further details, see Chapter 78 for a discussion on enzymes of the red blood cell).
In addition to antimalarial therapy, supportive care may be necessary in patients with respiratory or renal failure, lactic acidosis, and hypoxia. Exchange transfusion may be used for cases in which the parasite load exceeds 10% of circulating red blood cells. While this method is no longer recommended by the CDC, the American Society for Apheresis guidelines support its use for patients with severe malaria and high parasitemia.
Malaria is a potentially life-threatening disease, and testing for malaria should be performed immediately and be available 7 days a week on a 24-hour basis. , , Examination of thick and thin blood films is the gold standard for diagnosing malaria but requires considerable expertise for accurate screening and interpretation. If this expertise or resources are not available, then laboratories should consider using a rapid malaria LFICA or transport the specimen immediately to a nearby laboratory. , In the past decade, malaria molecular tests (primarily PCR) have become available at reference and public health laboratories. While these methods usually offer equal or greater sensitivity and specificity than conventional blood films, they are rarely performed rapidly enough to be used for primary diagnosis. Finally, malaria serologic tests may be useful in some settings, primarily for epidemiologic studies, but play little to no role for detection of acute disease.
Testing a single blood specimen may be insufficient for ruling out a diagnosis of malaria. The Clinical and Laboratory Standards Institute (CLSI) recommends examining repeat blood films every 6 to 8 hours for up to 3 days before excluding the diagnosis of malaria. There are no similar guidelines for repeat testing with LFICAs or PCR. Of note, DNA and/or antigen may remain detectable for days to weeks after successful treatment, and therefore NAATs and LFICAs cannot be used as a “test of cure.”
As mentioned earlier in the section on microscopic examination of blood specimens, microscopic examination of Giemsa-stained thick and thin blood films remains the gold standard for diagnosis of malaria. At least two thin and two thick blood films should be prepared as described earlier in this chapter. The thick film is up to 20 times more sensitive than the thin film for detection of Plasmodium parasites and is therefore the preferred method for screening (see Fig. 88.3 ). The reported detection threshold is approximately 10 to 50 parasites/μL blood, although this may be different in field conditions (e.g., 500 parasites/μL blood). The thin film, in contrast, is ideal for determining the species of the infecting Plasmodium spp., since the erythrocytes remain intact with this method (see Fig. 88.4 ). Species determination is made based on a number of morphologic features as shown in Figs. 88.3 , 88.4 , and 88.13–88.20 . Among the most important features are the size of the infected erythrocyte, presence or absence of cytoplasmic inclusions (when stain pH is 7.0 to 7.2), and the stages of parasites present ( Table 88.10 , Figs. 88.13–88.20 ). Importantly, all stages (early and late trophozoites, schizonts, and gametocytes) are generally seen in peripheral blood smears for all species except P. falciparum, in which only early trophozoites (ring forms) and gametocytes are usually seen. This is because late-stage asexual forms (late-stage trophozoites and schizonts) of P. falciparum are contained in erythrocytes that are sequestered in the microvasculature. While rare, the presence of P. falciparum late-stage asexual forms in the peripheral blood is indicative of severe, overwhelming infection. When examining blood films for malaria, it is important to also look for other blood parasites (trypanosomes, microfilariae), bacteria (intraleukocytic morulae of Ehrlichia spp./ Anaplasma phagocytophilum and relapsing fever Borrelia spirochetes), and yeasts (e.g., Histoplasma capsulatum ). The presence of extracellular bacteria and yeast could be indicators of infection or contaminants, the latter of which might require investigative actions. Mixed infections with multiple Plasmodium spp. may also be observed, with rates of co-infection varying widely by region and methods used for Plasmodium species detection. In malaria cases reported to the CDC with onset of illness in 2016, co-infection was noted in 1.1% of cases. Finally, it is essential to differentiate Plasmodium spp. from the similar-appearing Babesia spp. parasites ( Table 88.11 ).
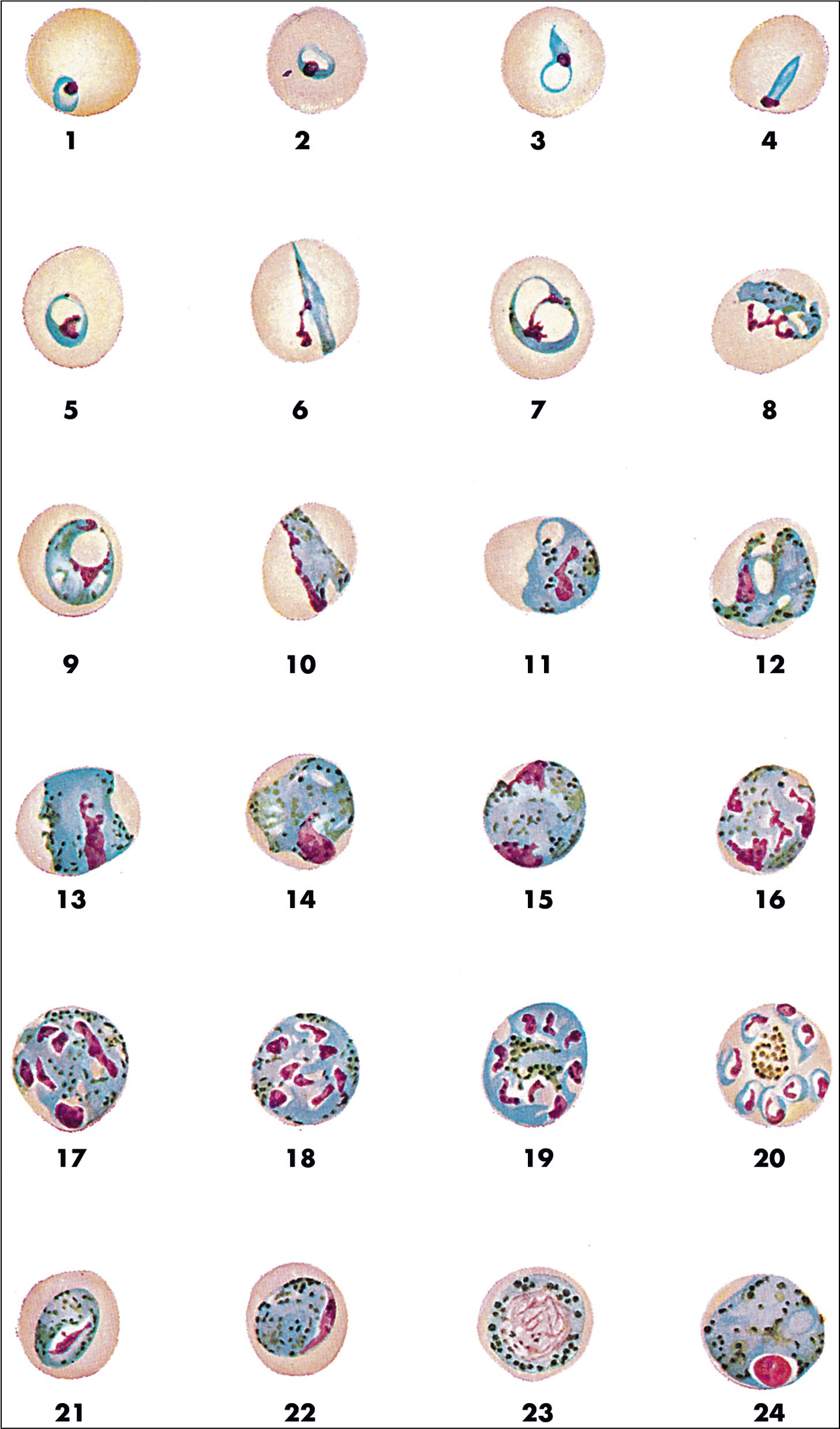
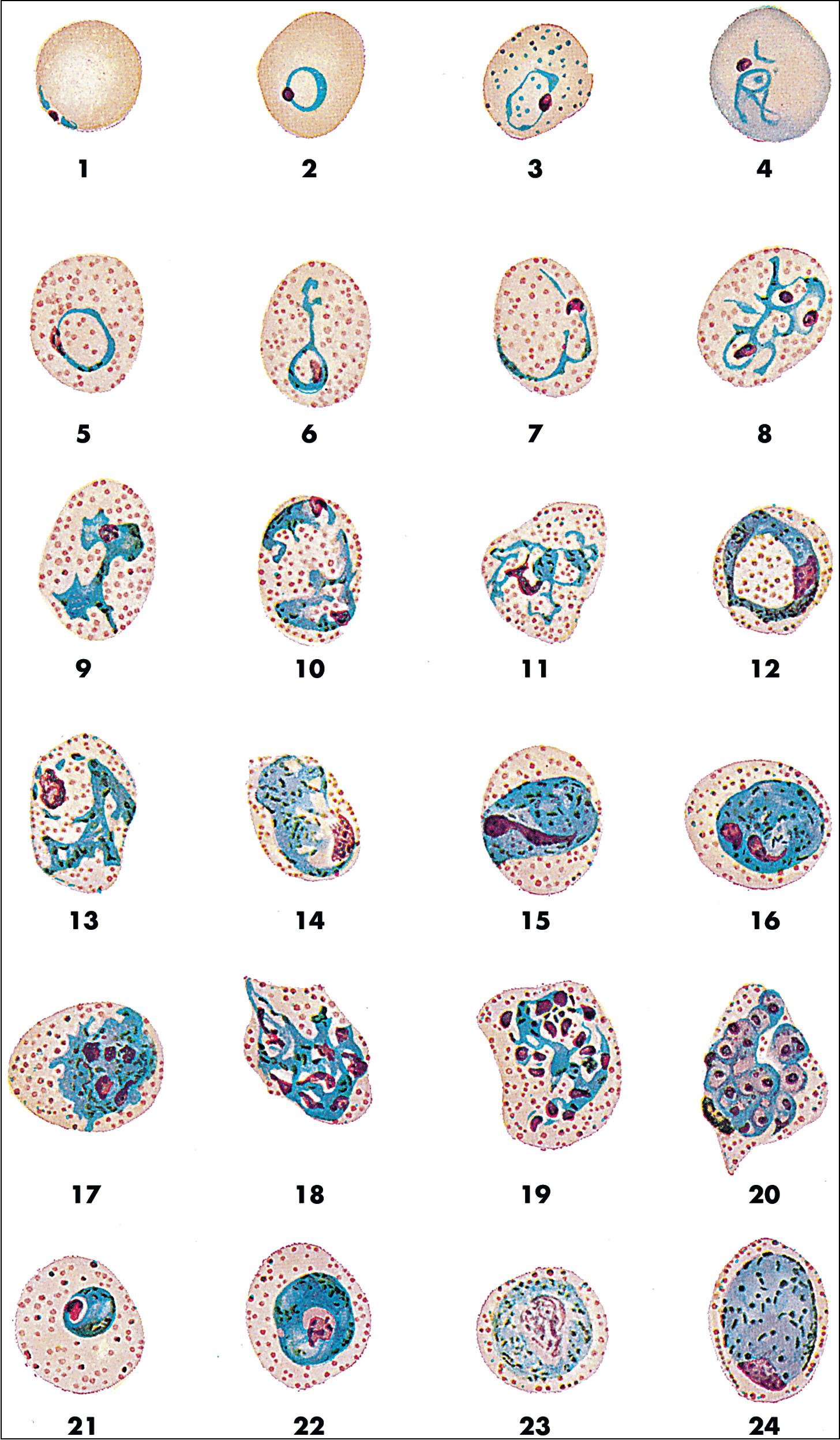
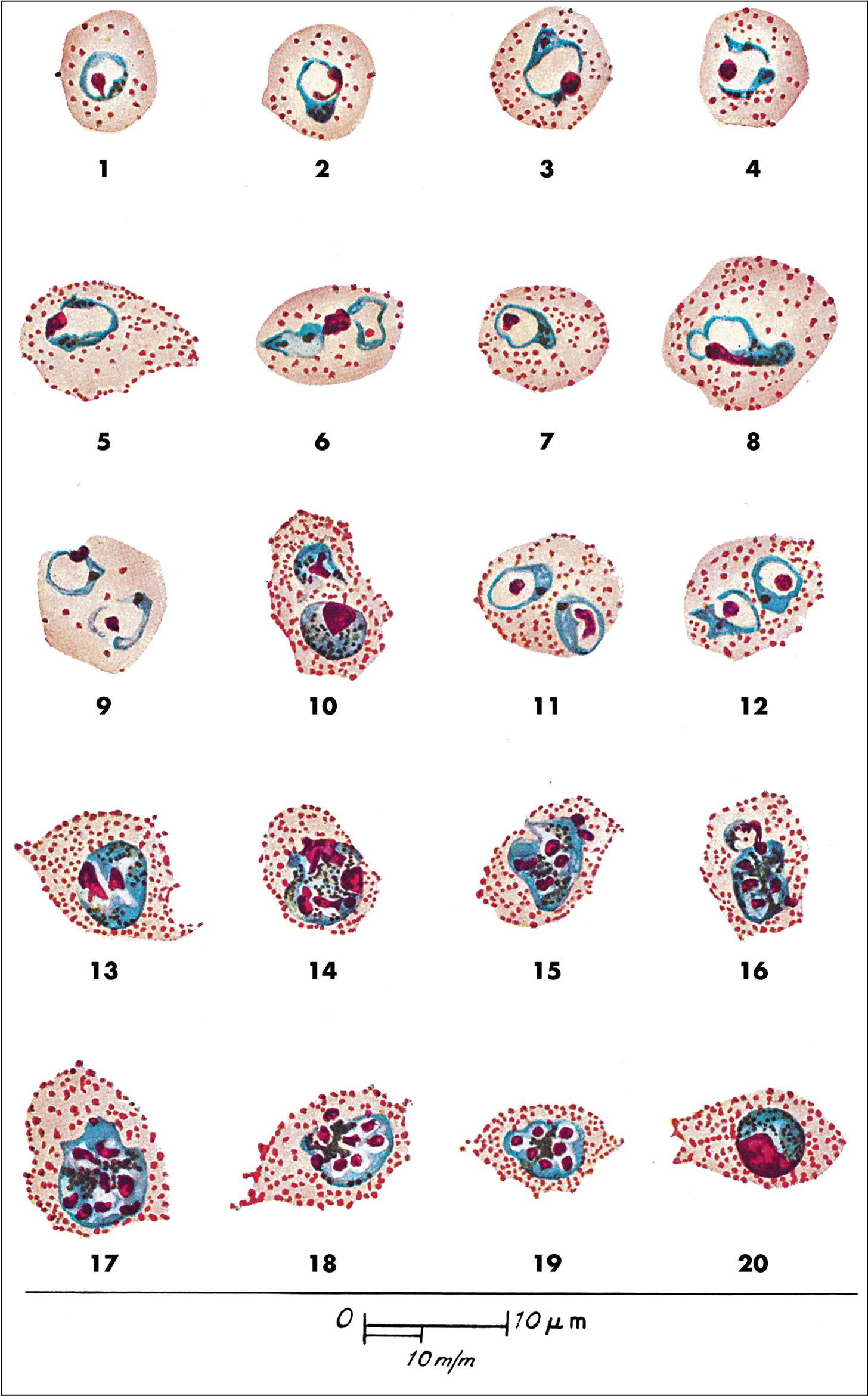

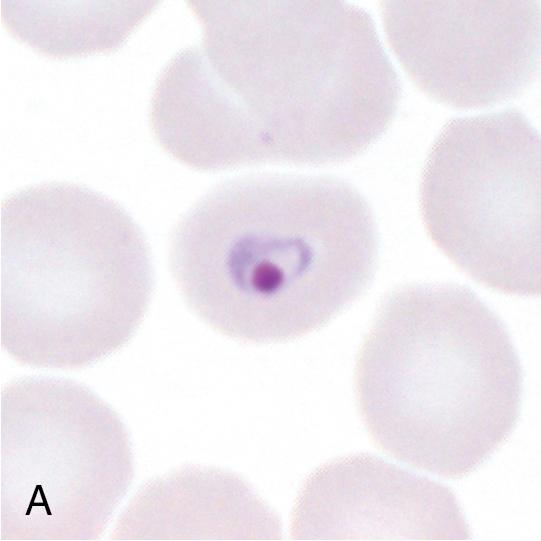
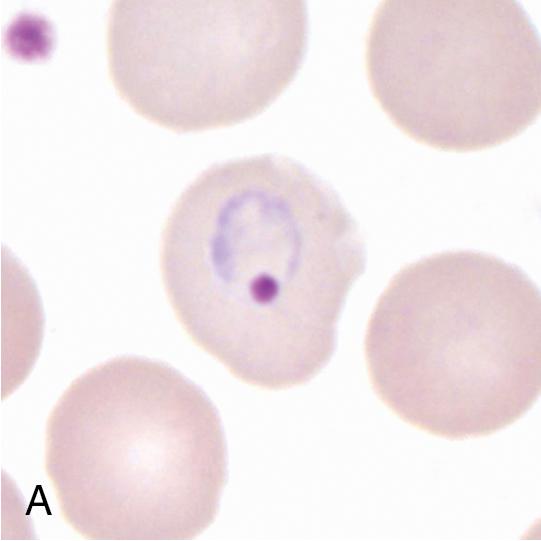
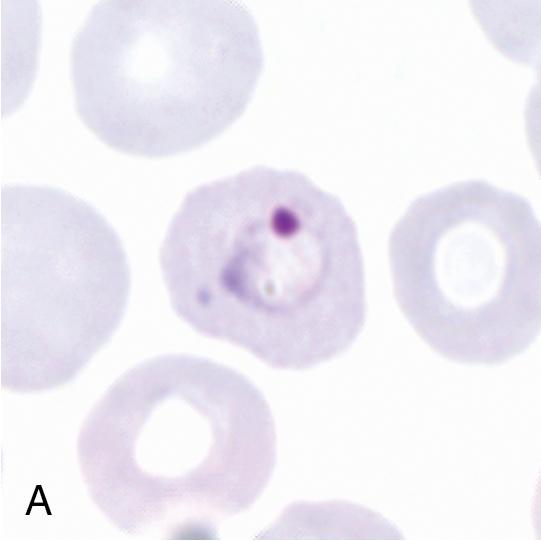
| Species a | Appearance of the Infected RBC | Intracellular Inclusions b | Degree of RBC Infectivity | Characteristic Features |
|---|---|---|---|---|
| Plasmodium falciparum | Normal size, multiple infections of RBCs common | Rarely Maurer clefts | High | Usually only rings and gametocytes seen. Delicate rings, <1/3 of RBC diameter, appliqué and “headphone” forms; “banana-shaped” gametocytes; delicate brown-black pigment, often in one mass. Mature schizonts (rarely seen, except in very heavy infections) have 8–24 merozoites. Percent parasitemia may exceed 2%. |
| Plasmodium malariae | Small to normal size | Rarely Ziemann stippling | Low | All stages seen; small coarse rings, ≤1/3 of RBC diameter, growing rings form basket, band and bird’s eye forms. Schizont with 6–12 merozoites, occasionally in a “rosette” configuration; round-oval gametocytes. Coarse brown pigment. Percent parasitemia <2%. |
| Plasmodium vivax | Enlarged size, multiple infections of RBCs not uncommon | Schüffner dots (stippling) | Low | All stages seen; ring forms >1/3 of RBC diameter, ameboid forms; schizonts with 12–24 merozoites; round-oval gametocytes. Delicate yellow-brown pigment. Percent parasitemia <2%. |
| Plasmodium ovale | Enlarged size, round-to-oval, occasionally fimbriated | Schüffner dots (stippling) | Low | All stages seen; ring forms >1/3 of RBC diameter, more compact than P. vivax; schizonts with 6–14 merozoites, round-oval gametocytes. Coarse brown pigment. Percent parasitemia <2%. |
a Zoonotic species, such as Plasmodium knowlesi, are not included in this table. P. knowlesi is geographically restricted to parts of Southeast Asia.
b Inclusions are best visualized using a Giemsa stain with a pH of 7.0 to 7.2.
| Morphologic Feature | Plasmodium falciparum | Babesia spp. |
|---|---|---|
| Size of infected RBC | Normal | Normal |
| Parasite stages in peripheral blood | Ring forms; less commonly “banana-shaped” gametocytes. High degree of RBC infectivity possible. | Ring forms only. High degree of RBC infectivity possible. |
| Ring form characteristics | Small size, <1/3 diameter of RBC, appliqué and “headphone” forms. Multiply infected RBCs common. | Small size, 1/3–1/6 diameter of RBC; irregular, spindle, and ameboid forms; single or multiple chromatin dots. Rarely, “Maltese cross” formation. Multiply infected RBCs common. |
| Presence of intracellular inclusions | Rarely Maurer clefts | None |
| Extracellular forms | Rare | Common |
| Pigment | Brown-black | None |
Determining the percentage of infected erythrocytes can be performed using the thick or thin blood film, and is useful for predicting prognosis, determining treatment approaches, and monitoring response to therapy. Formulas are widely available for this purpose. , ,
A number of other microscopic methods have also been described for malaria detection, including stains for hemozoin and nucleic acid. One of the commonly used methods is the QBC Malaria Test (Drucker Diagnostics, Port Matilda, Pennsylvania), which uses the fluorescent acridine orange stain to highlight parasites in centrifuged blood. This and other fluorescent stain methods may decrease screening time and provide increased sensitivity but requires fluorescence microscopy and may be difficult to interpret due to nonspecific staining patterns.
A large number of LFICA antigen detection methods are commercially available, and are increasingly used for screening, and in some cases, primary diagnosis. Commonly used targets include histidine-rich protein II (HRP-II), parasite lactate dehydrogenase, and parasite aldolase. The WHO, in collaboration with several other national and international organizations, recently reviewed the performance of 164 assays using patient-derived P. falciparum and P. vivax, and reported wide variation in sensitivity and specificity for detection of these parasites. Significant variation in heat stability and ease of use was also observed. At this time, only a single LFICA (BinaxNOW Malaria Test, Abbott., Abbott Park, IL) is cleared by the US FDA for diagnosis of malaria in the United States (see Fig. 88.10 ). According to the WHO product testing of malaria RDTs this assay has 100% sensitivity for detection of P. falciparum at a concentration of 2000 to 5000 parasites/μL, but only 91.1% sensitivity for detection of P. falciparum at 200 parasites/μL. Sensitivity for detection of non- falciparum species is significantly lower, with sensitivities for P. vivax detection reported at 85 and 10% at concentrations of 2000 to 5000 and 200 parasites/μL, respectively. In a prospective study in a clinical setting, investigators reported an overall BinaxNOW sensitivity and specificity of 84.2 and 99.8%, respectively. This study also detected one case of P. falciparum infection that was misclassified as a non- falciparum species. Given these results, all negative BinaxNOW Malaria Test results must be confirmed using conventional blood film microscopy. Microscopy is also important for detecting mixed infections and determining the degree of parasitemia, and thus blood microscopy is also indicated for positive BinaxNOW Malaria Test results.
Serologic assays use EIA and IFA formats, with antigens prepared from the different human Plasmodium spp. As previously mentioned, serologic testing is primarily used for epidemiologic studies and screening blood donors. , It may also be useful for determining previous exposure history, particularly in cases of recrudescent, relapsing, and untreated infection in patients from nonendemic settings. ,
A number of molecular diagnostic assays have been described for detection of Plasmodium nucleic acid, including PCR, DNA/RNA hybridization, and LAMP methods. , , There are currently no FDA-cleared tests, but multiple CE-marked kits are available, and laboratory-developed methods are widely used in research, public health, and reference laboratories. , Depending on the assay design, these assays may provide increased sensitivity and superior detection of mixed infections when compared to conventional microscopy. However, they require expensive reagents and instruments, a high level of technical expertise, and laboratory facilities suitable for high-complexity testing; they are therefore not generally suited for use in endemic settings. , , The primary uses for molecular testing are confirmation of diagnosis, detection of subclinical levels of infection in endemic settings, recognition of mixed Plasmodium infections, and species identification in cases in which morphology is suboptimal. Molecular methods are also useful for detecting single-nucleotide polymorphisms associated with antimalarial drug resistance.
Other laboratory methods are rarely used for diagnosis. Histopathology is not usually useful for detection of acute disease but may be important in autopsy studies. In tissues, evidence of malaria is seen primarily as hemozoin pigment (parasite-derived hemoglobin breakdown product) within erythrocytes in small blood vessels. “Ring hemorrhages” are commonly seen in cerebral malaria ( Fig. 88.21 ).

Some newer, promising laboratory techniques include detection of hemozoin-generated nanobubbles across intact skin and flow cytometry for malaria screening and diagnosis. ,
Finally, the use of digital image analysis and AI continues to be investigated for detection of malaria parasites and hemozoin using whole blood and stained blood films. While these approaches currently lack sensitivity compared with conventional light microscopic analysis, they hold promise for simplifying malaria detection and may even be adaptable for use with a cell phone.
Babesiosis is a hematogenous, tick-transmitted infection caused by apicomplexan parasites ( Piroplasmida ) in the genus Babesia . It is primarily a zoonotic disease, but humans can become infected with several Babesia spp. through the bite of an Ixodes species tick. , ,
Unlike malaria, babesiosis is primarily a disease of temperate climates. It is zoonotic and in nature cycles between rodents and Ixodes ticks. Highly endemic regions in the United States are the northeastern and upper Midwestern states, as well as areas of the South-Central and Atlantic states, where B. microti is the predominant pathogen and ticks in the I. scapularis -complex are the tick vectors. Babesia duncani is responsible for a smaller number of cases in the Pacific Northwest and is transmitted by Ixodes pacificus, while B. divergens –like organisms (sometimes referred to as Babesia MO-1) have been reported from patients in the states of Missouri, Kentucky, and Washington. From 2011 to 2015, there were 7612 cases of babesiosis reported to the CDC from 27 states, with most cases coming from 7 states (Connecticut, Massachusetts, Minnesota, New Jersey, New York, Rhode Island, and Wisconsin). The number of annual cases mostly increased each year over this time period, with 1126 cases in 2011 and 2074 in 2015. Most patients were 60 years of age or older.
Substantially fewer cases of babesiosis occur in Europe, with approximately 39 published cases to date. , , Of these, Babesia divergens is responsible for the majority of cases, while Babesia microti and B. venatorum cause a smaller number of cases. , Ixodes ricinus is the primary tick vector in Europe. Elsewhere, rare cases of human babesiosis due to unnamed species have been reported from Taiwan, Japan, South Korea, China, India, South Africa, Egypt, Mozambique, Mexico, and Colombia.
As with malaria, babesiosis is acquired primarily through the bite of an infected vector ( Fig. 88.22 ). Unlike malaria, there is no liver stage, and parasites immediately invade and multiply in erythrocytes. Also, the trophozoite, merozoite, and gamete stages are morphologically similar, appearing as round, oval, and pleomorphic ring stages. In the United States, infections occur primarily during the spring, summer, and fall when the tick vectors are actively seeking blood meals. Less commonly, humans acquire infection transplacentally and through infected blood transfusion (whole blood or blood-derived platelets). , The FDA considers transfusion-transmitted babesiosis to be a relevant threat and now requires blood collection centers in specific high-risk states to test each donation using a licensed B. microti NAAT. There are two FDA-approved NAATs for blood-donor babesiosis screening, as well as an arrayed fluorescent immunoassay for detection of B. microti antibodies (AFIA). The FDA has also approved pathogen reduction devices, capable of reducing B. microti in plasma and platelet components.
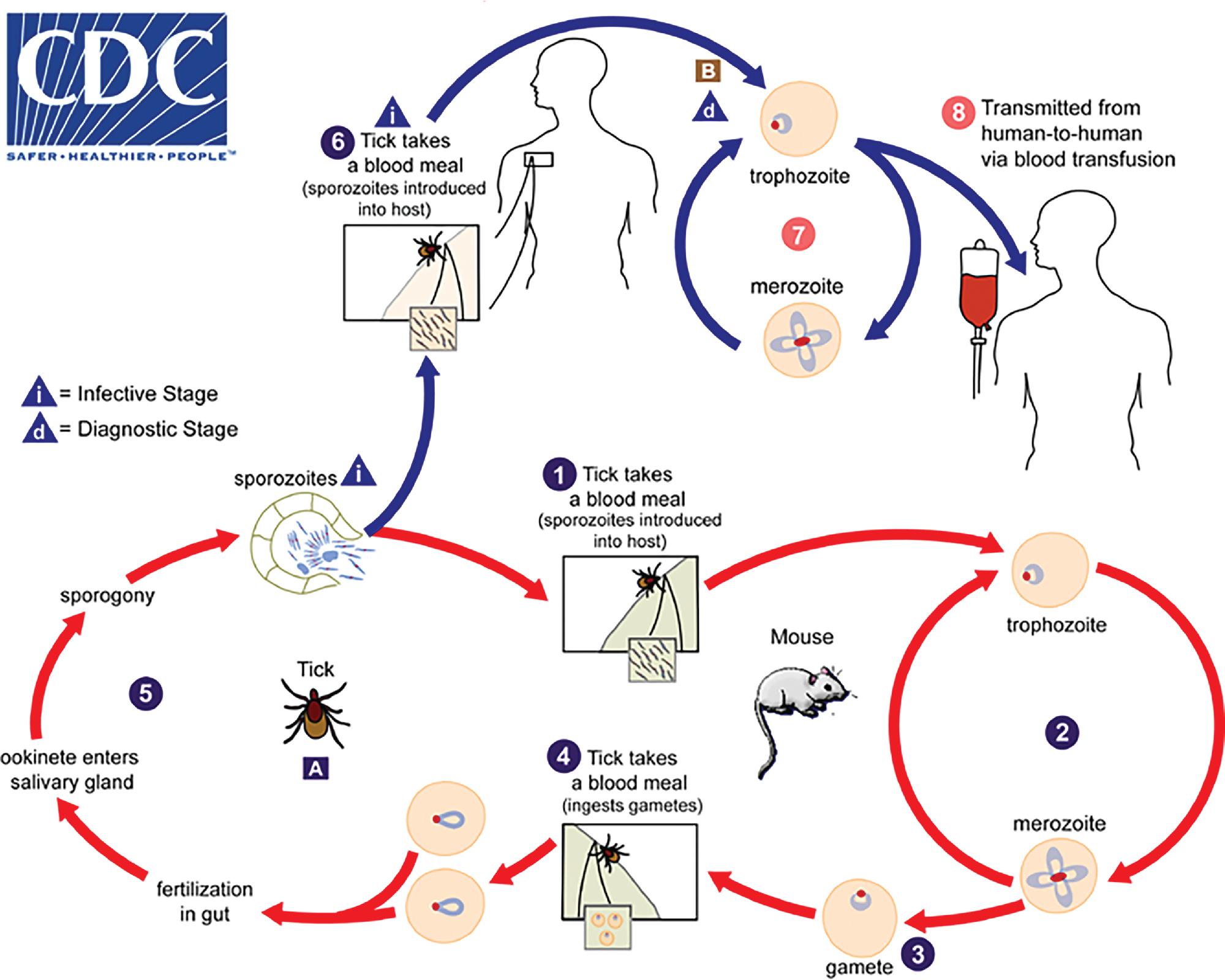
Most patients with babesiosis due to B. microti are asymptomatic. When present, symptoms commonly include fever, headache, and myalgias. , Elderly, immunocompromised, and asplenic individuals are at risk for severe life-threatening disease; complications may include hemolytic anemia, hepatosplenomegaly, hematuria, jaundice, disseminated intravascular coagulation (DIC), respiratory failure, acute renal failure, congestive heart failure, coma, and death (see Table 88.5 ). , Most patients with B. divergens infection have been immunocompromised and presented with severe disease, and most patients with B. divergens –like infections in the United States have similar characteristics. Only a few cases of B. venatorum infection have been described to date, but disease appears to be mild with this agent. ,
Babesiosis is generally treated with combination therapy using atovaquone and azithromycin or clindamycin and quinine. , , Supportive care including vasopressors, mechanical ventilation, dialysis, and blood transfusions may be necessary. As with malaria, high levels of parasitemia (≥10%) should prompt consideration of exchange transfusion therapy. , ,
Testing for acute babesiosis is performed in a similar manner as for malaria, with collection and examination of thick and thin blood films. Due to the potentially life-threatening nature of babesiosis, particularly in immunocompromised and asplenic patients, testing should be performed on an emergent basis. Serology plays little to no role in the diagnosis of acute disease but may be useful for detecting cases of chronic infection, for screening blood donor units, and for epidemiologic studies. There are currently no FDA-approved antigen or molecular detection methods for clinical diagnosis, although PCR is offered by select reference and public health facilities. In vivo culture may also be performed in rare instances at public health laboratories such as the CDC. It is important to note that other infectious agents (e.g., Borrelia burgdorferi and B. mayonii, A. phagocytophilum, Borrelia miyamotoi, Powassan virus) may also be transmitted by Ixodes spp. ticks, and therefore testing for these agents may also be indicated.
Blood films are examined in the same manner as described earlier for malaria, including calculation of percent parasitemia, although clinically relevant breakpoints may be different. , An important consideration is the differentiation of Babesia spp. parasites from the morphologically similar-appearing Plasmodium spp. (see Table 88.11 ), especially when a patient’s travel history is not available. In particular, P. falciparum shares several morphologic features with Babesia spp.: they are both present primarily as thin intraerythrocytic ring forms, may cause high parasitemia levels, and can produce multiply infected cells. However, Babesia parasites usually demonstrate greater pleomorphism in size and shape than P. falciparum , are more likely to have extracellular forms, and can exhibit the characteristic (although uncommon) tetrad (“Maltese cross”) form ( Fig. 88.23 ). Babesia spp. also do not produce hemozoin pigment, and they do not have morphologically distinct gametocytes like Plasmodium spp. , , , Of note, it is not possible to differentiate the human-infecting Babesia spp. by morphology alone, but the causative agent can usually be inferred by the patient’s travel history.
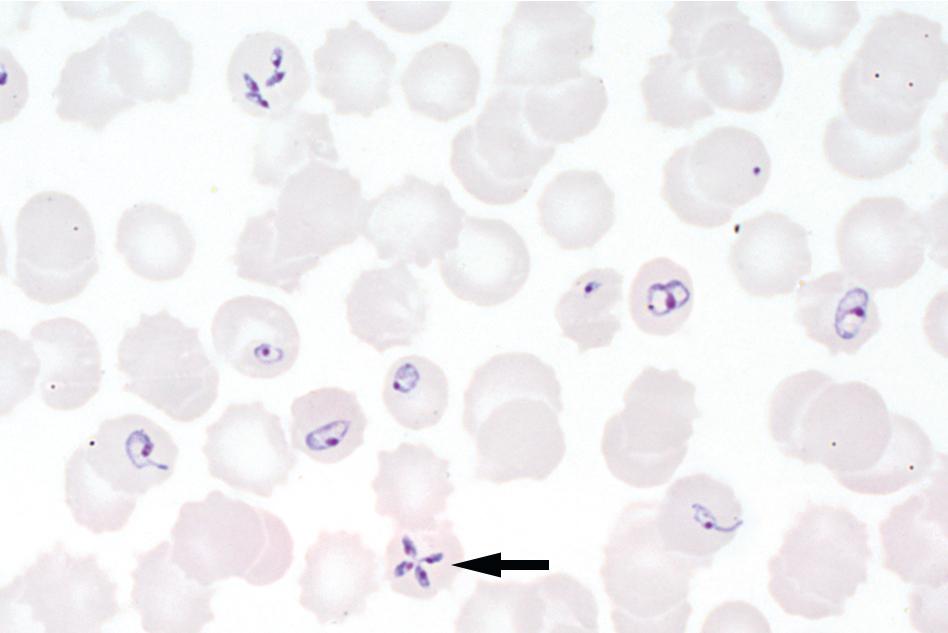
Antibodies to Babesia are usually detectable 2 weeks after illness onset and may remain detectable for several years after infection. , The preferred method for detection of serum antibodies against B. microti is IFA, and commercial kits for B. microti are available in the United States and Europe. Reported sensitivities and specificities of B. microti IFA are 88 to 96% and 90 to 100%, respectively. , False-positive results may be seen in patients with rheumatoid arthritis and other connective tissue disorders, and false-negative results have been reported in immunocompromised or asplenic patients. There does not appear to be significant cross-reactivity with antibodies to other Babesia spp., so specific IFA assays are recommended for each species. ,
Laboratory-developed PCR assays have been described for B. divergens and B. microti and may be used for initial testing if they can be performed rapidly . These assays generally offer increased detection sensitivity compared to examination of thick blood films, with reported detection limits of less than 0.0001% parasitemia. Commercially available tests such as the Meridian Bioscience Illumigene Babesia LAMP assay (Cincinnati, OH) are also available for clinical diagnosis, as well as commercially available NAATs for blood donor screening.
The hemoflagellates are protozoa characterized by the presence of a large mitochondrion called a kinetoplast . This structure can be seen using light microscopy and is an important identifying morphologic feature for both motile and nonmotile forms of the parasites ( Fig. 88.24 ). The two genera of hemoflagellates that infect humans are Trypanosoma and Leishmania . The two main human pathogens are T. brucei and T. cruzi. Trypanosoma rangeli has also been reported to cause asymptomatic human infection in the Americas and must be distinguished from the known pathogens. There are multiple Leishmania spp. that infect humans, and the severity and type of infection vary by the infecting species, prior exposure, and host’s immune status. Both Trypanosoma and Leishmania parasites are transmitted to humans through the bite of an infected arthropod vector.
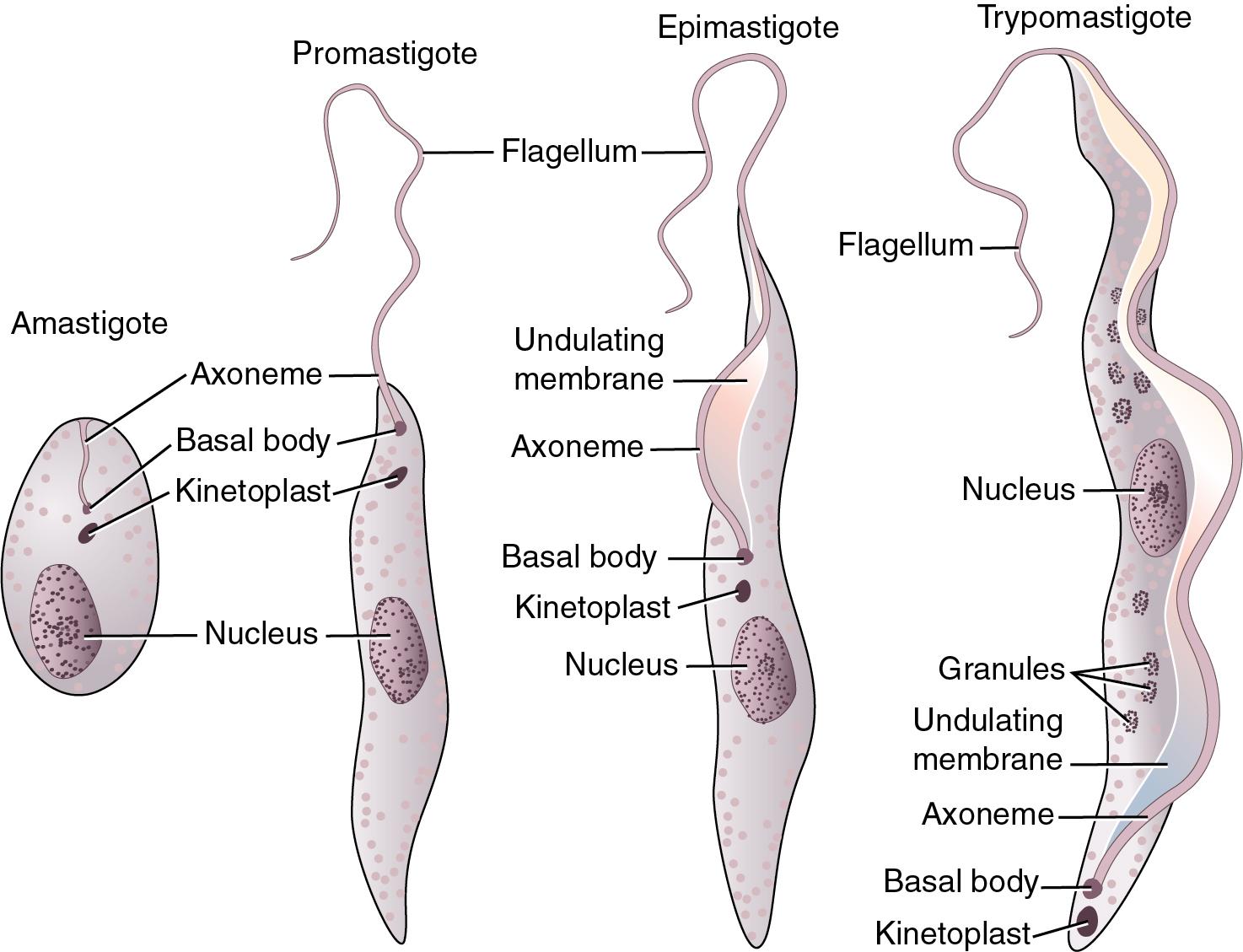
Human African trypanosomiasis (HAT), also known as African sleeping sickness, is one of the 20 NTDs currently recognized by the WHO. There are two morphologically indistinguishable subspecies of T. brucei that cause distinct forms of human disease: T. brucei rhodesiense causes East African sleeping sickness (acute African trypanosomiasis) and T. brucei gambiense causes West African sleeping sickness (chronic African trypanosomiasis). Both are transmitted through the bite of an infected tse tse fly (genus Glossina ).
T. brucei is found primarily in sub-Saharan Africa. T. brucei gambiense is endemic in 24 countries of west and central Africa, and is responsible for the majority (>97%) of human cases. In contrast, T. brucei rhodesiense is endemic in 13 countries in eastern and southeastern Africa and is considered to be primarily a zoonosis, causing only ~3% of reported human cases. Numbers of reported cases have dropped significantly due to the success of multiple control efforts; between 1999 and 2014, new cases of West African trypanosomiasis fell from 27,862 to 3679 (77%), and new cases of East African trypanosomiasis fell from 619 to 117 (71%). There were only 977 cases detected in 2018, which is a record low for this historic disease.
The life cycle in humans is relatively simple. Infective (metacyclic) trypomastigotes are injected into skin when an infected tse tse fly takes a blood meal. These enter the circulatory system, transform into bloodstream trypomastigotes (see Fig. 88.24 ), and are carried throughout the body. They eventually reach the lymphatics, and later, the CSF. The motile trypomastigote is the only stage seen in humans (compared to T. cruzi, which also has a nonmotile amastigote stage), and these forms replicate in the blood and other body fluids through binary fission. The tse tse fly becomes infected when it ingests trypomastigotes with its blood meal from an infected host. The parasite then undergoes further replication to form procyclic trypomastigotes, which migrate from the gut and transform into epimastigotes. These multiply in the tse tse fly salivary gland and transform into metacyclic trypomastigotes, which can be injected into the next host.
HAT generally occurs in three stages, beginning with a trypanosomal chancre at the site of inoculation. , This is followed by the hemolymphatic stage during which trypomastigotes may be found in the blood and lymph nodes. During this time period, patients may present with fever, pruritus, and lymphadenopathy. In the third (late) stage of infection (meningoencephalitic stage), trypomastigotes enter the CSF and cause headache, mental status changes, somnolence, loss of consciousness, and ultimately, coma and death. , These three stages are most pronounced in West African trypanosomiasis, which has a more chronic course. Posterior cervical lymphadenopathy, referred to as Winterbottom sign, may be pronounced. In comparison, East African trypanosomiasis is an acute and rapidly progressive febrile illness, in which patients may die before CNS involvement is conspicuous. , The diagnosis is usually suspected based on the clinical presentation and travel/exposure history. Antitrypanosomal therapy is indicated for all cases of infection. The choice of therapy varies with both the infecting subspecies and the stage of disease. Pentamidine and suramin are used for treatment of T. b. gambiense and T. b. rhodesiense early-stage infection, respectively, while eflornithine and melarsoprol (an arsenic derivative) are used for late-stage (meningoencephalitic) infection with T. b. gambiense and T. b. rhodesiense, respectively. , Potential side effects and toxicities occur with all of these medications, but are most severe for melarsoprol, which causes a life-threatening encephalopathy in 5 to 10% of patients who receive this drug. , Death occurs in approximately one-half of the patients with melarsoprol-induced encephalopathy. In 2019, the WHO published new guidelines for the treatment of HAT, which includes the use of the new medication, fexinidazole, for T. b. gambiense infection.
The primary means of diagnosis is examination of peripheral blood and, in late-stage disease, CSF, for the presence of trypomastigotes. , Trypomastigotes can also be seen in aspirated chancre fluid, lymph nodes, and bone marrow. High total IgM levels are usually detected in the blood and CSF, while pleocytosis (50 to 500 mononuclear cells/μL) is found in the CSF. , In endemic regions, detection of antigen or antibodies is also commonly used, and provides increased sensitivity over microscopic examination. Molecular and culture methods are not widely available and are used primarily in research settings. ,
Extracellular flagellated trypomastigotes can be identified on routine thick and thin blood films, but are often present in low numbers, and therefore concentration techniques such as buffy coat preparations and mini–anion-exchange centrifugation are commonly used to increase diagnostic sensitivity. , ,
The two subspecies of T. brucei are morphologically indistinguishable. They are slender extracellular organisms ranging from 14 to 33 μm in length with a small kinetoplast at the posterior end and a centrally located nucleus ( Figs. 88.24 and 88.25 A). A single flagellum arises from the kinetoplast and runs along the length of the organism as an undulating membrane, leaving the body at the anterior end as a free flagellum. Travel history is usually sufficient for differentiating T. brucei from T. cruzi and T. rangeli since the latter organisms are only found in the Americas.
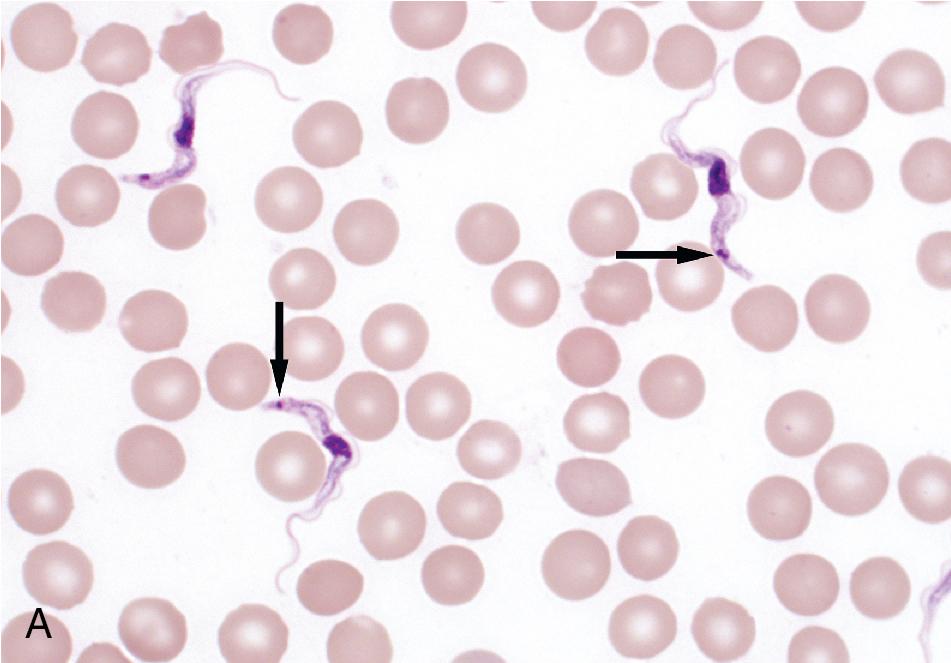
The card agglutination test for trypanosomiasis (CATT) is the primary method used for serologic detection of HAT. The CATT detects antibodies reacting with the variant surface glycoprotein LiTat 1.3 of T. brucei gambiense, and has reported sensitivities of 87 to 98%, although it may miss cases of infection with parasites that lack the gene encoding LiTat 1.3. It is widely used for population screening using blood, serum, or plasma, but is only useful for detection of antibodies to T. brucei gambiense , and false positive results may be observed malaria infections. ,
Several NAATs have been described for detection of T. brucei, but none of these assays are FDA-cleared/approved or CE-marked at the time of preparation of this chapter. , They are primarily used for research purposes, but may also be useful for differentiating between East and West African subspecies when this is not apparent from the clinical presentation and geographic exposure alone.
Histologic features of HAT are nonspecific and may include lymphoplasmacytic and histiocytic infiltrates in the brain and myocardium, with numerous antibody-laden plasma cells (i.e., morulae or Mott) in the Virchow-Robin spaces of the brain.
American trypanosomiasis, also known as Chagas disease, is a vector-borne zoonosis that is endemic to the Americas. It is 1 of 20 NTDs recognized by the WHO, , as well as 1 of the 5 NPIs recognized by the CDC. It is caused by T. cruzi, a hemoflagellate protozoan that exists in humans in both blood and tissue forms, and is transmitted through the bite of a triatomine (reduviid, kissing, assassin, conenose) bug. ,
As many as 8 million individuals are estimated to be infected worldwide, with most infected individuals living in poverty in Latin America. , Common triatomine vector species belong to the genera Rhodnius, Triatoma, and Panstrongylus, and these are found throughout much of the Americas, including parts of the United States. The genera and species of triatomine vector vary from country to country and even among ecologic niches. While some triatomine bugs maintain infection in a sylvatic (i.e., wild) cycle involving animal reservoirs, others infest poorly constructed houses in rural areas and have adapted to a domiciliary life, feeding primarily on humans. ,
Based on the estimated prevalence data in Latin America and immigration rates, the CDC estimates that 300,000 individuals with Chagas disease live in the United States. Most infected individuals in the United States are thought to have acquired infection in Latin America; however, autochthonous (locally acquired) cases have been reported from Texas and Louisiana, and up to 50% of triatomines in these regions were found to be infected with T. cruzi. It should be understood the risk for vector-borne human infection in the United States is low, due to the feeding habits of Nearctic triatomines (with a delay between feeding and defecation) and better housing and infrastructure in the United States.
In comparison to T. brucei, the life cycle of T. cruzi is relatively complex. Infective (metacyclic) trypomastigotes are present in the triatomine bug feces, and these may be inoculated into the bite site or nearby mucosal membranes after the infected triatomine takes a blood meal ( Fig. 88.26 ). , This form of transmission differs from other vector-borne parasitic infections, in which the infective form is usually introduced into the host through the vector’s saliva. Less commonly, humans become infected with T. cruzi through ingestion of food or beverages contaminated with infected triatomine bug feces, as well as through receipt of an infected blood transfusion or organ transplant. Congenital infection is also possible. Once in the body, the parasite alternates between intracellular, nonmotile amastigotes and extracellular, motile trypomastigotes. ,
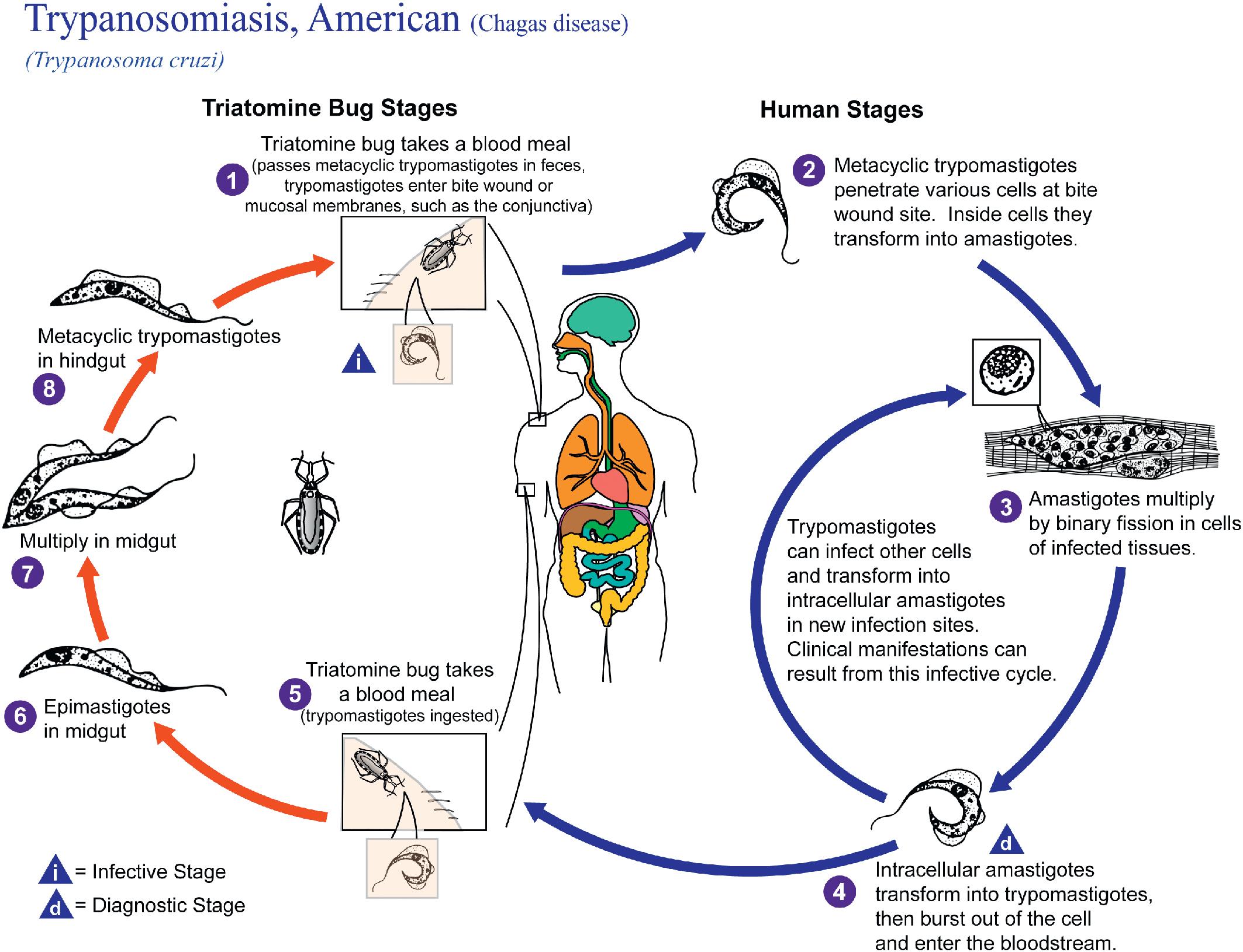
There are two main stages of disease: acute and chronic. , The earliest signs of infection include local swelling and erythema at the infection site. This may include formation of a chagoma (subcutaneous swelling), and unilateral periorbital edema (Romaña sign) when the conjunctiva is the site of inoculation. Localized lymphadenopathy may also occur. , Acute systemic signs and symptoms may be seen 2 to 3 weeks after infection, but only occur in a small subset (~1%) of infected individuals. , Manifestations include high fevers, myalgias, diffuse rash, hepatosplenomegaly, lymphadenopathy, keratitis, subcutaneous edema of the extremities and face, acute myocarditis, and meningoencephalitis. The myocarditis is directly related to replication of amastigotes within the cardiac myocytes and the associated host response, resulting in conduction abnormalities, loss of contractility, and death. , Children younger than 5 years of age are most likely to have severe acute disease. The acute stage lasts for approximately 8 to 12 weeks, after which the parasitemia decreases to undetectable levels and patients enter the chronic stage of infection. , The chronic stage may be asymptomatic, with patients having normal physical examination, electrocardiogram, and radiographic findings. This is sometimes referred to as an indeterminate form of disease, and it can last for years to decades. It is important to note that patients can still transmit infection to biting triatomine bugs during this time, as well as to other humans through donated blood or organs. ,
Approximately 20 to 30% of individuals with indeterminate disease eventually progress to chronic symptomatic Chagas disease and experience one or more of the three “mega” syndromes: megacardia (cardiomyopathy), megaesophagus, and megacolon. , The associated pathology is thought to be due to complex parasite, host, social, and environmental factors and cannot be tied solely to destruction by the replicating amastigotes. Cardiomegaly and cardiac conduction abnormalities are the most common manifestations of chronic Chagas disease. , Nifurtimox and benznidazole are the most commonly used therapies for acute Chagas disease, but they have little benefit in chronic disease. , , Long-term supportive therapies may be necessary for patients with end-stage cardiac or gastrointestinal manifestations of Chagas disease, including dietary modifications and pacemaker use.
The recommended method for laboratory diagnosis is dependent upon the stage of infection. Blood film microscopy is most useful during the acute form of disease since trypomastigotes are present in high numbers and are detectable as early as 10 days after infection. Antigen and molecular-based detection methods may also be performed using peripheral blood. , , The level of parasitemia decreases significantly within the first 90 days of infection, and trypomastigotes are rare or absent during the indeterminate and chronic stages, thus requiring the use of alternate diagnostic modalities. Antibody detection is most commonly used for detection of chronic infections, while NAAT is used for acute cases or cases of reactivation and while monitoring seropositive patients who have undergone recent organ transplantation. ,
Trypomastigotes of T. cruzi are detected in peripheral blood using the same methods described for malaria and T. brucei. , , , Parasites can be seen in thick and thin blood films and buffy coat preparations and must be differentiated from the similar-appearing trypomastigotes of T. brucei and T. rangeli. , , Amastigotes and trypomastigotes may also be seen in lymph node and chagoma aspirates. Amastigotes of T. cruzi are small (1 to 5 μm diameter), round-to-oval, and have a nucleus and rod-shaped kinetoplast (see Fig. 88.24 ). Trypomastigotes of T. cruzi are slender, ranging in length from 12 to 30 μm in length, and have a large kinetoplast at the posterior end and a centrally located nucleus (see Figs. 88.24 and 88.25 B). A single flagellum arises from the kinetoplast and runs along the length of the organism as an undulating membrane, leaving the body at the anterior end as a free flagellum. The larger size of the posterior kinetoplast is the most reliable feature for differentiating T. cruzi from T. brucei and T. rangeli . The patient’s travel history will also usually allow for differentiation of the American and African trypanosomes.
Serologic testing is commonly used for diagnosing chronic Chagas disease, evaluating patients from endemic areas prior to organ transplantation, and is also used for blood donor screening. Several testing formats have been described, including EIA, IFA, complement fixation, and indirect hemagglutination. , , The use of recombinant antigens and synthetic peptides have been reported to provide sensitivities and specificities of greater than 90% when used in endemic settings, but cross-reactivity has been reported in patients with T. rangeli infection and leishmaniasis. Due to the risk of false positive serologic results, confirmatory testing with a second serologic test using a different antigen is recommended.
Several serologic assays are FDA-approved for blood donor screening. The FDA issued guidance for donor screening in 2010 and 2017 for the use of serologic tests to reduce the risk for T. cruzi transmission in blood products, which includes one-time testing of each donor using an FDA-approved test. Donations that are found to be reactive with a screening test must be tested by an additional, more specific, licensed, approved or cleared supplemental test. Donors whose blood tests positive or indeterminate by a supplemental test should be indefinitely deferred.
Blood, aspirates, and tissues can be cultured in NNN media or a suitable animal model. These methods are uncommonly performed but may allow for increased detection of chronic disease or low-grade parasitemias.
NAATs, specifically PCR, may be used for primary detection of acute and congenital disease, reactivation of disease (e.g., post organ transplantation), and monitoring response to therapy. Some assays have very high analytical sensitivity, detecting as few as one parasite in 20 mL of whole blood. However, NAATs performed on blood are not diagnostically useful for identifying individuals in the chronic or indeterminate phase, given the low amounts of circulating DNA.
In endemic areas, xenodiagnosis is sometimes used to detect low-grade and chronic infections. With this method, trypanosome-free triatomine bugs are allowed to feed on patients under evaluation. The feces of the bugs are then examined monthly for 3 months for the presence of epimastigotes.
On occasion, tissue biopsy and histopathologic examination is used to identify amastigotes in infected tissues. Parasites are most commonly seen in the heart, and rarely in other tissues. Tissue sections generally show collections of amastigotes within “pseudocysts” in association with lymphoplasmacytic inflammation and fibrosis ( Fig. 88.27 A). The amastigotes of T. cruzi are morphologically indistinguishable from those of Leishmania spp., and clinical presentation, site of amastigotes, and travel history may aid in distinguishing the two diseases. Amastigotes of T. cruzi may also resemble small yeasts such as H. capsulatum. This can usually be accomplished by the type of infected cell/tissue, size of the collection of organisms, and stain reaction using fungal stains.
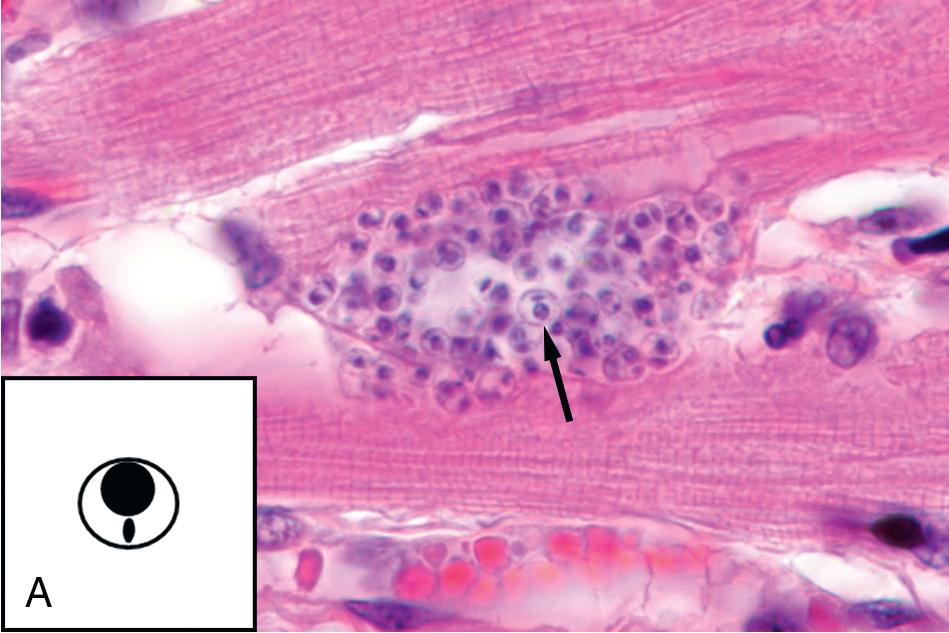
Leishmaniasis is a disease of the reticuloendothelial system caused by hemoflagellate parasites in the genus Leishmania. There are three main forms of disease: visceral leishmaniasis (VL), cutaneous leishmaniasis (CL), and mucocutaneous (a.k.a. mucosal) leishmaniasis (MCL). The type and severity of disease depends on several factors, including the infected parasite species, host immunity, and prior exposure. All species that infect humans are transmitted through the bite of infected phlebotomine sand flies in the genera Phlebotomus (Old World) or Lutzomyia (New World) and have animal reservoirs.
Leishmaniasis is considered an NTD by the WHO, with an estimated 700,000 to 1 million new cases (1 million CL cases, 300,000 VL cases) and 26,000 to 65,000 attributable deaths occurring annually. , There are more than 1 billion people at risk of infection worldwide. Most (95%) cases of CL occur in the Americas, the Middle East, the Mediterranean basin (including parts of Spain and Italy), and Central Asia, with greater than 95% of cases occurring in Afghanistan, Algeria, Brazil, Colombia, Rian, Iraq, and the Syrian Arab Republic. Endemic foci of CL are also found in Texas and Oklahoma. Causative species include the Old World species, Leishmania major, L. tropica, and L. aethiopica, and the New World species, L. mexicana, L. venezuelensis, L. amazonensis, and L. peruviana. Local geographic names for cutaneous disease include Oriental sore, Delhi boil, Baghdad boil, Aleppo evil, and chiclero ulcer. In comparison, most cases of VL occur in Brazil, East Africa, and Southeast Asia, with L. donovani and L. infantum (Old World) and L. chagasi (New World) being the primary etiologic agents. Of note, L. infantum and L. chagasi have many genetic similarities and are considered by many to be the same organism, or subspecies of the same organism. Ongoing control and elimination programs have made significant progress in reducing VL in India, Bangladesh, and Nepal. Finally, MCL is found only in select parts of the world. Classical MCL occurs in the Americas due to L. braziliensis and related species in the Viannia subgenus. Additionally, a mucosal form of disease is seen with L. aethiopica infection in Ethiopia, and rare cases of mucosal involvement with L. donovani have also been reported. Nearly 90% of cases of MCL occur in Brazil, Bolivia, Ethiopia, and Peru.
Humans acquire Leishmania infection primarily through the bite of an infected phlebotomine sand fly ( Fig. 88.28 ). Like T. cruzi, the motile parasites become intracellular amastigotes shortly after inoculation; in this case, they are phagocytosed by macrophages and other mononuclear phagocytic cells and proceed to multiply within these cells by binary fission. Promastigotes are only seen in skin specimens if there is a delay in processing the tissue, and no circulating motile blood form is present.
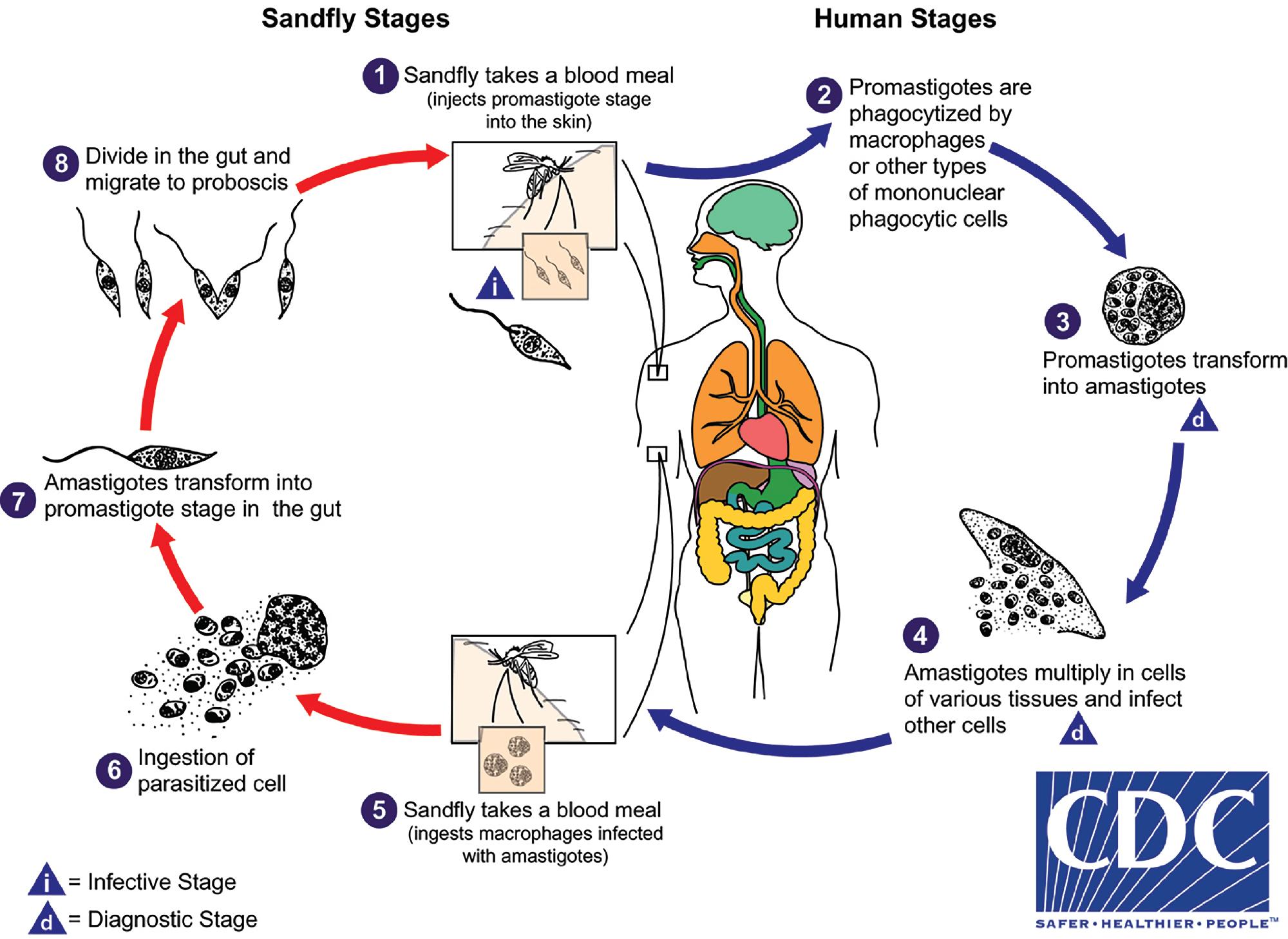
Most infected patients are asymptomatic, with only a small fraction of patients developing clinical disease. , CL, the most common form of infection, manifests as skin lesions on exposed parts of the body. The primary skin lesion usually appears 2 to 8 weeks after the sand fly bite, and commonly takes the form of a firm, painless papule at the bite site. , , Over time, the lesion generally progresses to an ulcer with an exudative necrotic center and raised border. The lesions usually heal over time, but satellite lesions may appear and merge with the initial ulcer, and sporotrichoid lymphatic spread can occur. Other subtle differences have been noted among diseases due to different Leishmania spp. CL is caused by members of the L. major, L. tropica, L. mexicana, L. braziliensis, L. guyanensis, and L. enrietii complexes. L. tropica may also cause VL, as was seen in military personnel who served in Operation Desert Storm. Leishmania aethiopica, an Old World species in the L. tropica complex, causes an aggressive form of CL, which may metastasize to other sites to cause diffuse CL, as well as mucosal and nodular cutaneous lesions resembling lepromatous leprosy. The forms of New World CL are very similar to those seen with Old World CL, with the important exception that some species are capable of metastasizing to the nasal, buccal, and pharyngeal mucosa and causing MCL, even after resolution of the initial cutaneous lesions. New World CL due to L. mexicana generally causes single ulcers that heal over a relatively short (i.e., 6-month) period. However, this species also involves the earlobe in up to 40% of cases and may cause destructive chronic lesions (chiclero ulcer). Cutaneous lesions due to L. braziliensis commonly take longer to heal than lesions due to other New World species and may be disseminated to the mucosa of the head and neck in approximately 1 to 3% of cases, resulting in MCL.
MCL, also known as espundia, may occur concomitantly with CL, or occur several months to years after the initial cutaneous infection. Mucosal involvement usually begins with edema, nasal stuffiness, and erythema and occasional bleeding. This presentation later progresses to erosion, ulceration, and eventually disfiguring destruction of the soft tissues and cartilage. Death is rare but can occur due to secondary bacterial infection or aspiration pneumonia.
VL is the most serious form of disease and may be fatal if untreated. Early infection is commonly subclinical but may progress as amastigotes within macrophages multiply in the liver, spleen, and bone marrow. In immunologically naïve patients, symptoms may be more acute, with high fever, weight loss, and diarrhea. The disease is called kala-azar in India due to the associated skin darkening that is commonly observed. Post–kala-azar dermal leishmaniasis (PKDL) is a special form of VL in which widespread cutaneous lesions develop 6 months to several years after treatment. The cutaneous lesions contain multiple parasites, and therefore individuals with PKDL are an important reservoir for disease.
VL may be contained, or even cured, by the cell-mediated immune response, but hosts with an increased risk for developing severe, symptomatic disease include young children, nonimmune individuals (e.g., travelers, soldiers), malnourished individuals, and patients with AIDS. Unfortunately, the response to treatment is generally poor in patients with concurrent AIDS, unless cell-mediated immunity can be restored.
The choice of therapy depends on multiple factors, including the type and severity of disease (CL, VL, MCL) and anatomic location, and must be individualized for the infected patient, ideally with expert consultation. , All patients with visceral and mucocutaneous infection should be treated. Furthermore, treatment is indicated for patients with CL acquired in Latin America, when the causative species is capable of causing MCL. In these cases, antimony-containing compounds including pentavalent antimonial (SbV; not licensed in the United States) and sodium stibogluconate (Pentostam, GlaxoSmithKline, Philadelphia, Pennsylvania) have been the mainstay of treatment, although liposomal amphotericin B deoxycholate and miltefosine are also FDA approved for VL treatment. Patients with CL due to species that do not cause MCL may be treated in a variety of manners. Options include doing nothing (waiting for self-healing), local therapy (cryotherapy, thermotherapy, intralesional administration of SbV, topical paromomycin), and systemic therapy (Sbv, amphotericin B deoxycholate, ketoconazole, fluconazole, miltefosine).
In many endemic areas, diagnosis is made primarily on clinical findings and epidemiologic factors. , However, the clinical differential diagnosis for leishmaniasis may be broad, and confirmation of infection is preferred whenever possible. The most common means of diagnosis is by visualizing amastigotes in aspirated or biopsied material. In CL, the most active lesion should be sampled, taking an aspirate or biopsy from the border of the ulcer. Giemsa is the most commonly used stain. When VL is suspected, blood (for buffy coat preparations), bone marrow, lymph node or splenic aspirates, and liver biopsies may be obtained. Splenic aspirate provides the highest rate of detection (>95%) but carries the risk for splenic laceration and is therefore not commonly performed in nonendemic settings. Antibody detection is commonly used for indirect detection of VL but reported sensitivities vary widely and it is not useful for CL or MCL (see below).
In addition to light microscopic examination and serology, culture and molecular testing may be performed in specialized centers. Culture offers increased sensitivity over microscopy alone, and provides ample material for isoenzyme and molecular studies. In one study, the sensitivity of hematoxylin and eosin (H&E)–stained histologic sections was only 14% for diagnosis of CL, while examination of imprints was 19% and culture of biopsies/aspirated material was 58%. Combining the methods yielded higher sensitivity (67%). Finally, molecular studies may be performed on culture material or directly on clinical specimens and offers the highest detection rates of all direct methods.
Due to their small size, Leishmania amastigotes are best appreciated on Giemsa-stained air-dried buffy coat, aspirate, and impression smears. They can also be seen on formalin-fixed, paraffin-embedded sections, but the diagnostic morphologic features are more difficult to identify (see the “Other” section later in this chapter). Amastigotes are round-to-oval, small (1 to 5 μm), and have a nucleus and rod-shaped kinetoplast (see Figs. 88.24 and 88.27 B). , , , , They are morphologically indistinguishable from the amastigotes of T. cruzi but are found only within macrophages and do not form pseudocysts in tissue. Less commonly, promastigotes may be seen in the scrapings taken from the superficial aspect of skin lesions. It is important to note that the various human-infecting Leishmania spp. are morphologically indistinguishable, and therefore additional techniques such as culture and PCR are necessary for species identification. This may be particularly important when evaluating patients with New World CL, in which there is a risk for progression to MCL.
Serologic detection of anti-leishmanial antibodies is an alternative approach for detection of VL but is of limited use for MCL and CL. Multiple methods have been described, including IFA, EIA, LFICA, and direct agglutination tests (DATs). EIAs are commercially available, with reported sensitivities ranging from 86 to 99%, depending on the causative species. LFICAs, specifically using the recombinant K39 antigen, are also widely available and have gained popularity due to their ease of use and ability to produce a rapid result. , They have reported sensitivities of more than 90% for VL due to multiple different Leishmania spp. It is important to note that, as with all parasitic infections, antibodies may remain detectable for months after disease resolution and therefore cannot be used for distinguishing between active and past infection. They may also be falsely negative in immunocompromised patients and exhibit cross-reactivity with other hemoflagellates.
Culture should be attempted whenever species identification is desired for determining prognosis and selecting therapy. It is also more sensitive than microscopy alone. Specimens should be collected aseptically and cultured in NNN or other supportive media. , Cultures may have detectable promastigotes as early as 2 days after inoculation but should be held and examined for 4 weeks before reporting a negative result. The CDC provides culture services and can also perform isoenzyme or molecular analysis for species identification, and provides simple instructions for collection.
NAATs are increasingly used for primary detection and confirmation of leishmaniasis, and have been used on tissue specimens, whole blood, and buffy coat preparations. , Sensitivity and specificity are the highest for direct detection methods, with reported rates of 72 to 100% and 82 to 100%, respectively. Unfortunately, molecular testing is limited to specialized reference and research facilities and largely consists of laboratory-developed tests. The CDC offers species-level identification using PCR in combination with sequencing analysis. There is also an FDA-cleared Leishmania PCR (SMART Leish, Cepheid, Sunnyvale, California), but its use is limited to the US Department of Defense. ,
The leishmanin skin test, otherwise known as the Montenegro test, is a commonly used but highly variable test for detecting CL in endemic areas. It works on the same principle as the tuberculin skin test in which parasite antigens (killed promastigotes) are injected dermally and the site is observed at 48 and 72 hours after injection for evidence of a delayed-type hypersensitivity reaction. This test may be useful for epidemiologic studies but cannot differentiate between causative agents, and has no utility for diagnosis of VL. It is not widely available in nonendemic settings.
Histopathology is another commonly used method for diagnosis of leishmaniasis, particularly in nonendemic settings in patients with possible CL. Unfortunately, amastigotes shrink during formalin fixation, and therefore it is more difficult to identify the diagnostic nucleus and kinetoplast. Amastigotes must be differentiated from similar-appearing small intracellular objects, including small yeasts (e.g., H. capsulatum ). Leishmania amastigotes are negative using the Gomori methenamine silver fungal stain, which aids in this differentiation.
Malaria and babesiosis are potentially life-threatening diseases, and testing should be performed on a STAT basis.
Microscopic examination of thick and thin blood films remains the gold standard for detection of Plasmodium and Babesia spp. infections.
Rapid antigen detection methods are useful for preliminary malaria screening but should be confirmed by conventional microscopy when possible.
Serology is not recommended for diagnosis of acute malaria and babesiosis.
The species of the infecting organism ( Plasmodium only) and percent parasitemia are important for guiding therapy and predicting prognosis.
Trypanosomes can also be identified on conventional thick films, but concentration techniques are recommended to improve sensitivity.
Trypomastigotes of T. brucei may be found in the blood, CSF, and lymph node.
T. cruzi has both amastigote and trypomastigote forms; the latter are found in blood, while the former are only seen in tissue biopsies.
Leishmania spp. are only seen as amastigotes in humans, and can be identified in skin, spleen, liver, lymph node, and bone marrow biopsies/aspirates.
Toxoplasmosis is caused by a zoonotic protozoan parasite of cats, T. gondii, which infects a wide variety of animals including humans. It is one of five NPIs recognized by the CDC and is an important cause of morbidity and mortality in immunocompromised hosts (see Table 88.5 ).
T. gondii has a global distribution and can infect humans, in addition to a wide array of wild and domestic animals. Approximately 25 to 30% of the world’s population is estimated to be infected, with more than 40 million infected children and adults in the United States alone. , According to the CDC, toxoplasmosis is the second leading cause of death from a food-borne illness in the United States. Seroprevalence rates vary significantly from country to country and among socioeconomic groups, and some countries such as El Salvador and France have rates as high as 85%. , In the United States, it is estimated that 11% of individuals ages 6 or older have been infected with T. gondii.
Humans can become infected with T. gondii in a number of ways, and the predominant means of exposure may vary by country. The two most common means of acquiring infection are ingestion of meat containing infectious cysts, and infection of oocysts in food, water, and other environmental sources contaminated with cat feces ( Fig. 88.29 ). Transmission may also occur via receipt of an infected blood or organ donation and congenitally across the placenta.
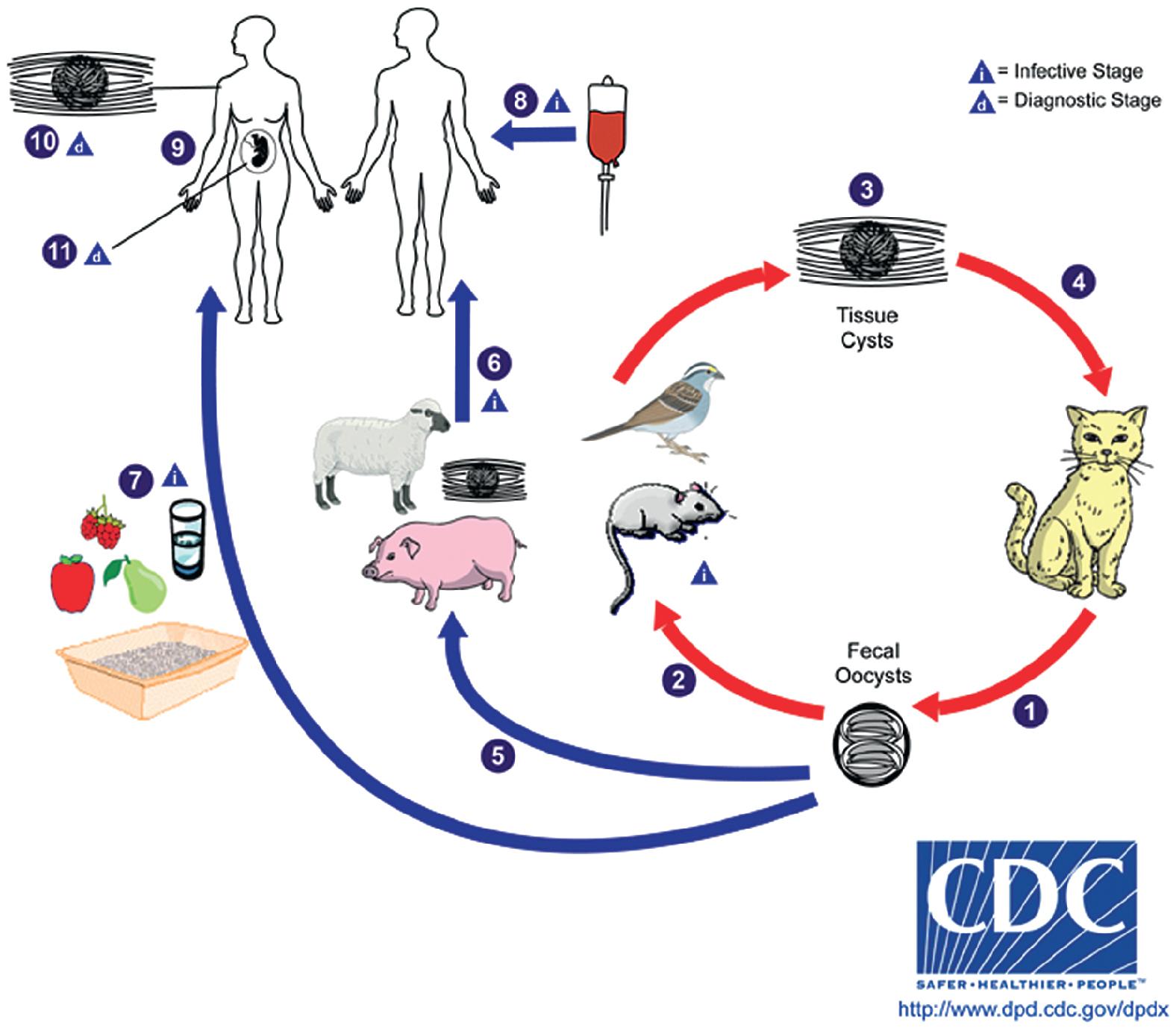
Infection is entirely asymptomatic in the vast majority (~90%) of patients, while the remaining 10% experience a self-limited mononucleosis-like syndrome with fever, malaise, and lymphadenitis. , Following acute infection, the parasite becomes dormant and patients remain asymptomatic unless they become immunocompromised later in life. Types of immunocompromised states commonly associated with toxoplasmosis are AIDS (CD4+ T lymphocytes <100 cells/mm 3 ) and receipt of immunosuppressive therapies after organ transplant or for cancer therapy. , In solid organ transplant recipients, the highest risk for infection is in seronegative recipients from a seropositive donor (D+/R−).
The most common form of toxoplasmosis in the immunocompromised host is encephalitis (see Table 88.5 ). Affected patients commonly present with motor or sensory deficits, mental status changes, lethargy, fever, seizures, and chorea. Other forms of disease seen in immunocompromised individuals are pneumonitis, myocarditis, chorioretinitis, cystitis, hepatitis, and multiorgan failure. , Congenital infection may also result in severe disease in the neonate, with blindness, epilepsy, developmental delays, and fetal demise. The risk and severity of disease is unrelated to the presence or absence of maternal symptoms, but correlates strongly with the gestational age at the time of maternal infection. The incidence of transmission to the fetus is low (9%) when maternal infection occurs during the first trimester, but severe fetal toxoplasmosis occurs in 78% of cases. , ,
Conversely, the incidence of transmission to the fetus is highest when maternal infection occurs during the third trimester (59%), but 90% of neonates are asymptomatic at birth. Maternal infection acquired prior to pregnancy does not usually pose a risk to the fetus, but infection may reactivate in significantly immunocompromised mothers during gestation. Infected infants may be asymptomatic at birth, but commonly develop symptoms later in life. Therefore infected infants should be treated regardless of symptoms. , ,
The preferred treatment varies by the type and severity of infection and the immune status of the host. , Immunocompetent patients with mild illness do not generally require treatment, whereas immunocompromised patients with visceral disease are usually given pyrimethamine and sulfadiazine plus folinic acid (leucovorin). Long-term treatment and/or prophylaxis is recommended for as long as a host remains severely immunocompromised. , When possible, immune restoration is an important part of therapy. In pregnant women, spiramycin is the recommended treatment for infection during the early stages of pregnancy (first and early second trimesters), while pyrimethamine and sulfadiazine with leucovorin are given during the later stages (late second and third trimesters). , , The prognosis depends on the type and extent of infection. Most congenital abnormalities are irreversible. ,
Serology, NAATs, microscopy, and rarely, in vivo culture, are used for diagnosis of toxoplasmosis. The preferred approach is based primarily on the patient’s immune status. , Serology is widely available and used as a first-line test for immunocompetent individuals, including pregnant women and neonates, who are likely to mount a detectable immune response. Serologic testing also allows for differentiation of other clinically similar infections such as acute HIV and mononucleosis. However, there are multiple caveats of serologic testing for toxoplasmosis that must be considered, which are described in more detail in the section on Antibody Testing below. NAAT and/or microscopic examination of involved tissues are generally recommended for evaluation of profoundly immunocompromised patients, particularly in the setting of negative or inconclusive serologic test results. NAAT of amniotic fluid is also recommended for prenatal diagnosis of congenital toxoplasmosis. Finally, T. gondii culture isolation may be attempted at specialized reference laboratories in cases of suspected prenatal congenital infection by inoculating mice or cell culture with amniotic fluid. In vivo and in vitro cultures of CSF, fresh tissue, and other specimens may also be helpful in detecting cases of toxoplasmosis in immunocompromised patients.
Test interpretation can be challenging, particularly for T. gondii serology. Therefore consultation and testing at a T. gondii reference lab may be advised. In the United States, the CDC recommends consulting with the Dr. Jack S. Remington Laboratory for Specialty Diagnostics (formerly the Palo Alto Medical Foundation Toxoplasma Serology Laboratory; https://www.sutterhealth.org/pamf/services/lab-pathology/toxoplasma-serology-laboratory ) for additional testing options. Further information about the main testing modalities is presented below, and full information about testing in different patient populations is available through published reviews and guidelines. , , The Geneva Foundation for Medical Education and Research maintains a list of country-specific toxoplasmosis guidelines, programs, and publications at https://www.gfmer.ch/Guidelines/Maternal_neonatal_infections/Toxoplasmosis.htm .
T. gondii parasites may be seen as either dormant cysts or actively replicating tachyzoites ( Fig. 88.30 ). Tachyzoites are arc-shaped organisms measuring 4 to 5 μm in length that may be seen extracellularly or within infected cells. , Tissue cysts, on the other hand, range from 10 to 100 μm in diameter and contain hundreds of smaller bradyzoites, measuring 3 to 4 μm in length. The types of specimens that should be examined depend on the host and the clinical presentation. Parasites can be seen in amniotic fluid, CSF, blood, broncho-alveolar lavage (BAL) fluid, and numerous tissue types, although detection sensitivity depends on the parasite load, and a negative result may not reliably exclude infection. Giemsa-stained impression smears are preferred for demonstrating the parasite morphology, but organisms can also be seen using routine H&E and parasite-specific immunohistochemical stains. Identification of only cysts in the absence of an associated tissue inflammatory response may indicate latent rather than active infection.
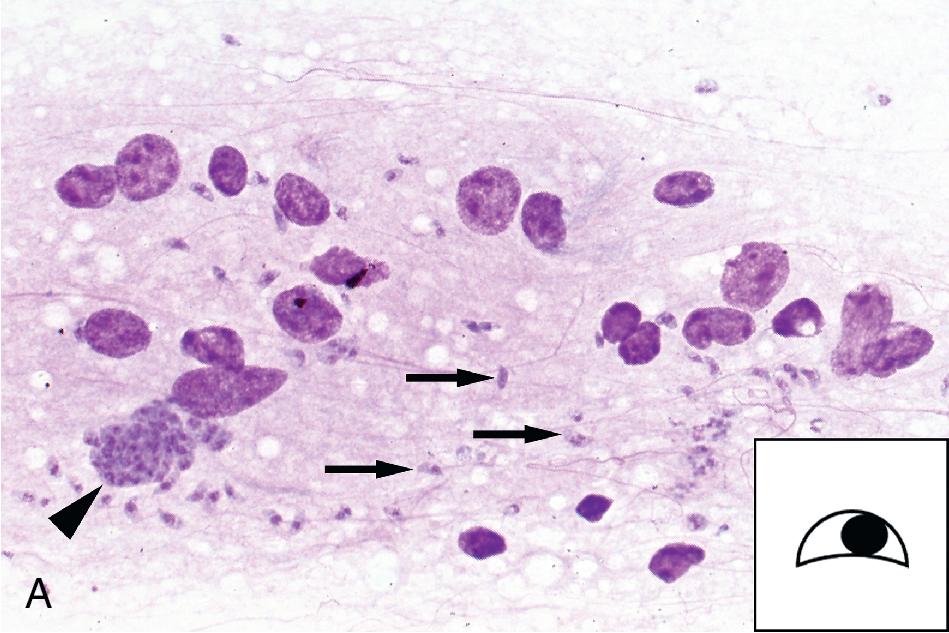
As mentioned earlier, serology is widely used for evaluating symptomatic immunocompetent patients, including pregnant women, for T. gondii infection. It is also used to screen organ donors and plays an important role in testing for congenital toxoplasmosis. Assays for IgM, IgG, IgA, and IgE are available. In general, anti– T. gondii IgM class antibodies develop within the first week of infection and slowly decrease over the next 6 to 18 months. , However, one study found that anti- T. gondii IgM was detectable for more than 2 years in women who had acquired toxoplasmosis during pregnancy. IgA also appears in the first week of infection and can be detected for up to 9 months. Given these long periods, detection of IgM and IgA antibodies cannot be used conclusively as evidence of acute infection. IgG antibodies become detectable 2 to 3 weeks after the initial rise in IgM and also decrease over time but are usually detectable for life. Specific IgE antibodies are also produced early in infection and become undetectable rapidly. Therefore the presence of anti- T. gondii IgE may suggest recent infection.
There are several methods available for detecting anti- T. gondii immunoglobulins. , The test most commonly used in the clinical laboratory setting is an ELISA for specific detection of IgM and IgG. Several FDA-cleared and CE-marked ELISAs are available for this purpose. Other test formats are employed primarily in the specialty reference laboratory setting, such as latex agglutination, indirect hemagglutination, indirect fluorescent antibody, western blot, and the Sabin-Feldman dye test. The Sabin-Feldman test was developed in 1948, and was considered the “gold standard” method for anti- T. gondii antibody detection for many years. However, it requires the use of live parasites which poses a hazard for laboratory staff and greatly limits its clinical availability. The test detects evidence of parasite lysis by antibodies in the patient’s serum in the presence of complement.
Another important serologic method is IgG-avidity testing. In the setting of a positive anti- T. gondii IgG result, IgG-avidity testing can be useful for determining how recently the infection was acquired. Antibody avidity is low after primary exposure but increases in the subsequent weeks to months after infection. , With avidity testing, the strength of antibody binding is evaluated by an ELISA in which a washing step with a dissociating buffer such as urea is added. The dissociating buffer will remove low-avidity antibodies; thus the avidity of the antibodies present can be assessed by comparing the IgG antibody titers of treated and untreated samples. FDA-cleared and CE-marked IgG avidity tests are commercially available. Finally, a differential agglutination test may be useful in differentiating acute from chronic infections in the setting of a positive IgM and/or IgG result. This test determines the ratio of antibody titers to two antigens: one expressed early in infection and one found later in infection. Avidity and differential agglutination testing are not widely available and are performed primarily at specialized Toxoplasma reference laboratories.
Given the number of serologic tests available, the preferred testing approach considers the characteristics of the patient and the disease setting. Immunocompetent patients are usually tested initially using a commercially available combined ELISA for anti- T. gondii IgM and IgG. If the result is positive and there is a concern for acute toxoplasmosis or the patient is eligible for chemoprophylaxis (e.g., to prevent reactivated infection in a patient undergoing immunosuppressive chemotherapy), then confirmatory testing at a specialty reference laboratory is recommended. Confirmation is particularly useful for evaluating IgM positive/IgG negative results, given the low specificity of many commercially available IgM tests and the long time that IgM is detectable after acute infection. , In the United States, the CDC recommends sending specimens to the Dr. Jack S. Remington Laboratory for Specialty Diagnostics.
For pregnant women, routine serologic screening may be performed in settings with a high prevalence of toxoplasmosis. In France, an IgM/IgG ELISA is performed during the first trimester of pregnancy. IgG positive, IgM negative results are considered evidence of past infection for which the risk to the fetus is low, and no further testing is generally recommended. , However, pregnant women without detectable IgM and IgG antibodies are considered nonimmune and may be at risk for acquiring and transmitting toxoplasmosis to the fetus. In this situation, repeat testing on a monthly basis is recommended until delivery, and then again at 2 to 3 weeks after delivery. If possible seroconversion is detected during this time period, additional confirmatory serologic evaluation (e.g., IgG avidity testing) is recommended at a specialized reference laboratory. Likewise, pregnant women who are both IgM and IgG positive at initial testing require additional serologic evaluation (e.g., IgG avidity and differential agglutination testing) in order to determine if infection occurred prior to or during pregnancy. When acute infection is suspected during pregnancy, molecular testing to determine the infection status of the fetus (e.g., PCR of amniotic fluid) is recommended along with ongoing ultrasound evaluation (see Molecular Diagnostics below).
Postpartum laboratory evaluation of a neonate with suspected congenital infection begins with serologic screening for anti– T. gondii IgM and IgA antibodies in conjunction with a comprehensive clinical and neurologic evaluation. Unlike IgG antibodies, IgM and IgA do not cross the placenta, and thus their detection would indicate local antibody production and infection in the neonate. Unfortunately, sensitivity for detecting neonatal IgM and IgA may be decreased following maternal treatment for toxoplasmosis. In this setting, qualitative western blot or enzyme-linked immunofiltration analysis of maternal and neonatal IgG may also be helpful, as it allows for comparison of band or precipitin patterns, respectively. As maternal and neonatal antibodies might recognize different antigens, differences between maternal and neonatal patterns would support the presence of locally produced IgG antibodies in both hosts. Finally, monitoring titers of neonatal anti– T. gondii IgG can aid in ruling out congenital toxoplasmosis, as they would be expected to disappear by approximately 12 months after birth in untreated infants without infection. Serologic evaluation for congenital toxoplasmosis should be performed in conjunction with PCR testing of placental tissue and/or cord blood (see below), clinical and radiologic features.
NAATs targeting the REP529 (preferred) and B1 repetitive genes of T. gondii have an increasingly important role in diagnosis of toxoplasmosis. , There are no FDA-cleared methods at this time, but CE-marked kits are available, and numerous laboratory-developed tests have been described. Reported sensitivities range from 64 to 100% using CSF, amniotic fluid, ocular fluid, and tissue. A positive T. gondii NAAT from amniotic fluid is considered diagnostic of congenital toxoplasmosis. , NAAT may also be helpful in establishing the diagnosis of disseminated toxoplasmosis in immunocompromised patients by testing CSF, tissue, respiratory specimens, and other body fluids. Molecular testing is usually used in conjunction with serologic testing (see section above for more information).
Diagnosis of toxoplasmosis can be established through identification of cysts and tachyzoites in H&E-stained formalin-fixed paraffin-embedded (FFPE) tissue sections, although the parasites are more difficult to appreciate in these preparations than in impression smears (see Fig. 88.30 ). When seen, T. gondii cysts must be differentiated from other small intracellular objects such T. cruzi, Leishmania spp., and H. capsulatum. A number of commercially available monoclonal and polyclonal antibody immunohistochemical and immunofluorescence kits are available and may aid in the definitive identification of T. gondii in FFPE tissue sections.
The FLA are found widely in the environment and only rarely cause human disease. While many genera have been described, the primary human pathogens are N. fowleri, Acanthamoeba spp., and B. mandrillaris. Paravahlkampfia francinae and Sappinia pedata have also been implicated in rare cases of human infection. , , N. fowleri causes primary amebic meningoencephalitis (PAM), while both Acanthamoeba spp. and B. mandrillaris cause granulomatous amebic encephalitis (GAE). Acanthamoeba also cause a keratitis ( Acanthamoeba keratitis, AK). Both pathogenic and nonpathogenic amebae can also serve as an environmental reservoir for several intracellular pathogens. Acanthamoeba have been known to harbor Listeria monocytogenes, Legionella pneumophila, Burkholderia pseudomallei, E. coli serotype O157, and Vibrio cholerae .
The FLA have a worldwide distribution. Acanthamoeba spp. are the most prevalent and widespread of the FLA, having been found in water (fresh and brackish), chlorinated water, soil, sewage, air-conditioning units, heating systems, dental and medical equipment, and even in the nose and throats of healthy individuals. Several genotypes have been described (T1–T20), with species in the T4 genotype ( A. castellanii, A. lugdunensis, A. polyphaga, A. royreba, A. mauritaniensis, A. rhysodes, and A. triangularis ) being most commonly associated with human infection. As a result of the broad environmental distribution, humans are commonly exposed to Acanthamoeba spp., and this is supported by the approximate 80% seropositivity rate detected in otherwise healthy individuals. Less is known about the environmental niche of B. mandrillaris compared with Acanthamoeba; it is most commonly found in soil and less often in water sources. Most cases of B. mandrillaris infection in the United States have been reported from the Southwest, with Hispanic Americans having a relatively high incidence of infection. GAE due to Acanthamoeba spp. is primarily seen in immunocompromised individuals, whereas GAE due to B. mandrillaris may be seen in both immunocompromised and immunocompetent patients (see Table 88.5 ). , AK is seen primarily in contact lens wearers who improperly store, handle, or disinfect their lenses. Specific risk factors for infection include rinsing contact lenses with tap water, and wearing lenses while swimming, showering, or using a hot tub. , A conservative estimate of the incidence of disease in the United States is 1 to 2 cases per 1 million contact lens users.
N. fowleri is commonly found in warm freshwater sources, including lakes, rivers, geothermal springs, and untreated domestic water sources. , Historically, most cases in the United States occurred in southern states, but cases as far north as Minnesota have now been reported. Surveillance from the CDC detected 142 cases of PAM in the United States from 1937 to 2013, with 76% of the patients being male, and 83% being children (median 12 years, range 8 months to 66 years). Most cases are associated with recreational water use, but cases linked to nasal irrigation with inadequately treated domestic water have also been reported. ,
The life cycle of the FLA in humans is shown in Fig. 88.31 . N. fowleri enters the body through the olfactory mucosa and migrates along the olfactory nerve through the cribriform plate to the olfactory bulb into the adjacent meninges and cortex ( Fig. 88.32 A). From there, it spreads throughout the CNS, causing edema, necrosis, and ultimately brain herniation and death. In comparison, B. mandrillaris and Acanthamoeba enter the body through the respiratory tract or a break in the skin and disseminate hematogenously to the CNS. Acanthamoeba spp. can also enter the eye through contaminated contact lens solution or corneal trauma.
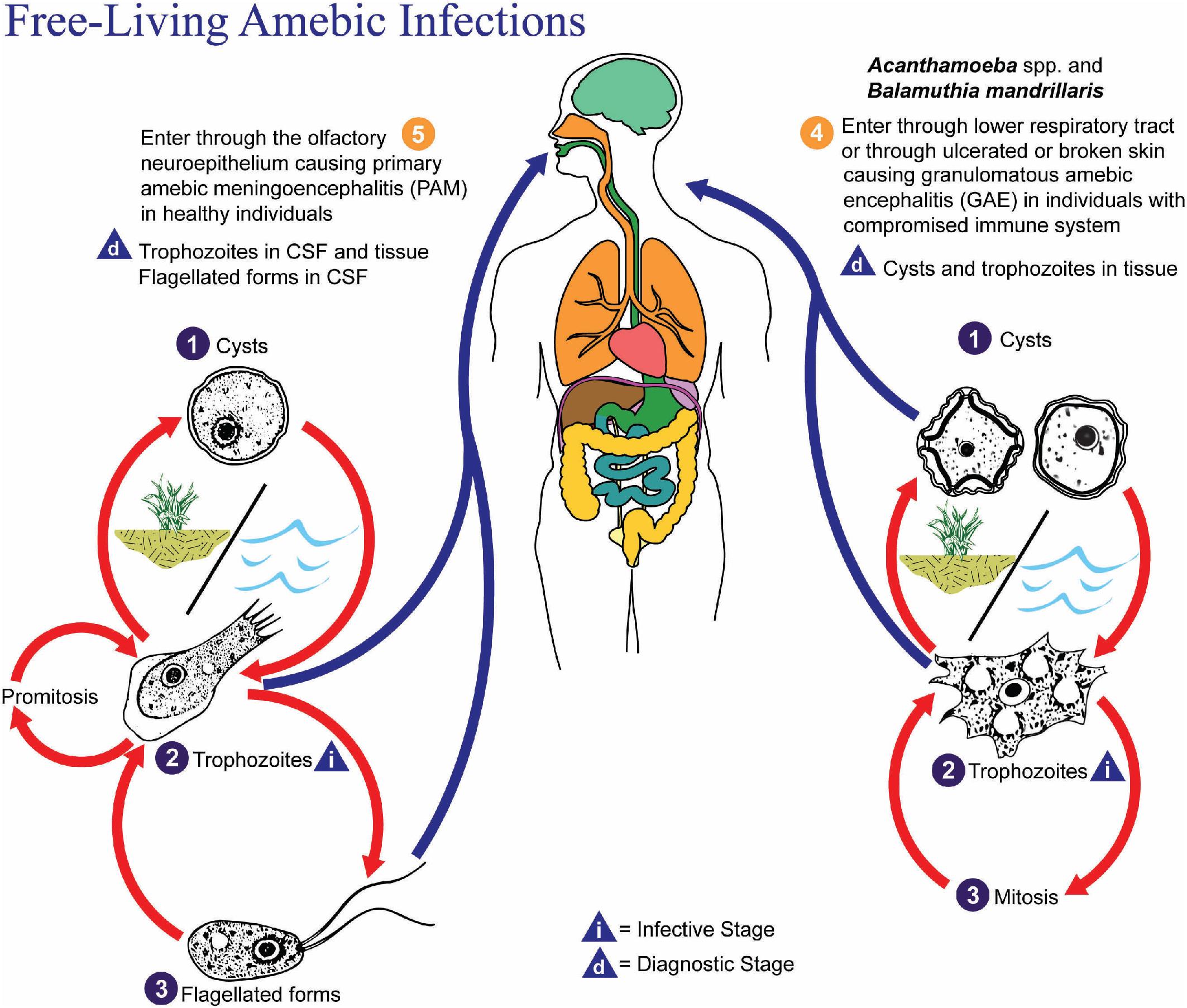
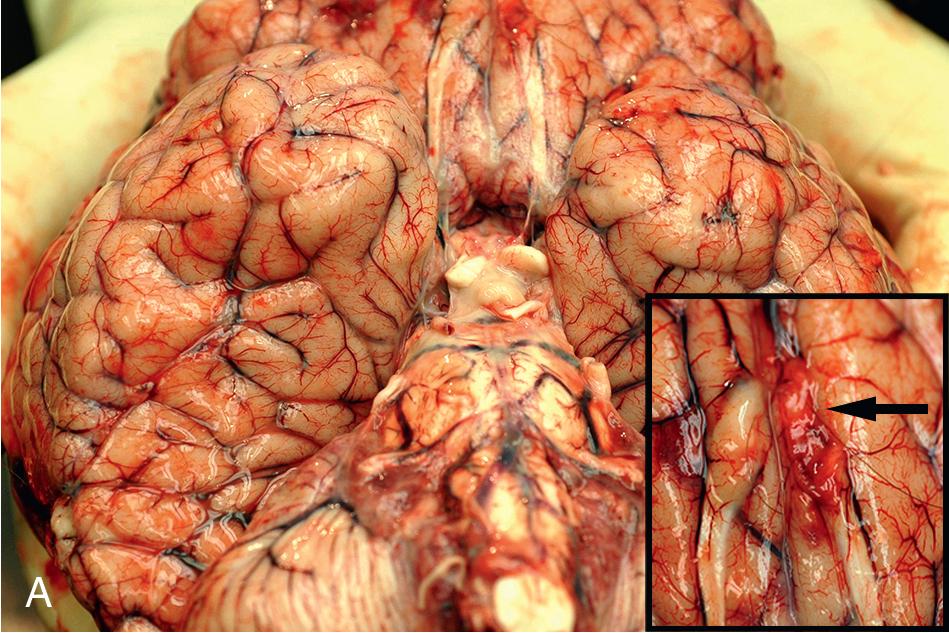
PAM usually begins with meningeal signs such as severe headache, stiff neck, fever, and vomiting and rapidly progresses over a period of several days to mental status changes, coma, and death. Several approaches have been used to treat patients with PAM, including administration of systemic and intrathecal amphotericin B, miconazole, rifampin, and miltefosine, and whole-body cooling (therapeutic hypothermia). Miltefosine was previously only available in the United States through the CDC for single-patient emergency treatment of FLA infections, but is now commercially available, as it has received FDA approval for treatment of cutaneous and muco CL. Despite aggressive interventions, fatality rates exceed 97%. ,
In contrast to PAM, GAE is a subacute to chronic infection presenting with headache, mental status changes, focal neurologic deficits, and eventually death, if untreated. , Acanthamoeba spp. and B. mandrillaris also cause granulomatous skin lesions, which may precede CNS involvement and serve as the source of subsequent hematogenous dissemination. The incubation period is unknown, but probably spans weeks to months. Likewise, the clinical course can span days to months. No specific treatment is recommended for GAE, although a number of agents including amphotericin B, paromomycin, azoles, metronidazole, macrolides, sulfadiazine, and miltefosine have been used with limited success. , Skin lesions can be successfully treated if the organism has not yet spread to the CNS.
Patients with AK commonly present with unilateral severe eye pain (out of proportion to the clinical findings), erythema, blurred vision, foreign body sensation, photophobia, and excessive tearing. , Less commonly, disease is present in both eyes. Physician exam findings include paracentral stromal ring infiltrate and nonhealing corneal ulcers. Amebic keratitis is a potentially sight-threatening infection and needs to be aggressively treated. Topical agents such as chlorhexidine and polyhexamethylene biguanide are usually prescribed for prolonged periods (6 months to 1 year or more), but corneal transplantation may be required for severe or refractory cases. The presence of residual cysts can lead to relapsed disease.
The first step in diagnosis is to have a high index of suspicion based on the clinical findings. Unfortunately, diagnosis of PAM and GAE is challenging and commonly made at autopsy. Microscopy of CSF, brain tissue, corneal scrapings, and corneal biopsies, in conjunction with culture, are the most commonly used methods for diagnosis. More recently, laboratory-developed PCR tests have been implemented at specialized reference and public health laboratories for testing of these same specimen types and provide more rapid diagnosis than culture. Culture and/or PCR are highly recommended, as they are the most sensitive methods for detection of the FLA from clinical specimens. Serologic testing is not routinely available and not clinically useful due to the high seroprevalence of antibodies to the FLA.
PAM is nearly always diagnosed at autopsy, but antemortem diagnosis can be rapidly made by identifying motile trophozoites in the CSF on direct wet mounts. Centrifugation of the specimen, with examination of the sediment, allows for improved sensitivity of CSF detection. Giemsa or Wright-stained cytospin preparations will also show the characteristic trophozoites, and less commonly, flagellated forms, usually in the presence of numerous associated neutrophils ( Fig. 88.32 B). Trophozoites measure 10 to 35 μm in greatest dimension and have a small nucleus with a large central karyosome ( Fig. 88.32 B and D). This nucleus is characteristic of the FLA and allows for their differentiation from host cells. If exposed to warm distilled water, the trophozoites will convert to a flagellated form in 2 to 3 hours. It is important to note that the cyst form of N. fowleri is not seen in tissues. ,
Direct wet preparations can also be made of brain tissue and examined for trophozoites and cysts of Acanthamoeba spp. and B. mandrillaris. Trophozoites measure 15 to 45 μm in greatest dimension and have a small nucleus with large karyosome, similar to that seen with N. fowleri ( Fig. 88.32 F). , In liquid culture, Acanthamoeba trophozoites can develop characteristic acanthopodia (spiny projections) that can help to differentiate them from other amebae. The cysts of Acanthamoeba are double-walled with an outer wrinkled wall (ectocyst) and an inner polygonal, round, or stellate inner wall (endocyst). Acanthamoeba spp. cysts measure 13 to 20 μm in diameter, while the cysts of B. mandrillaris are slightly larger (10 to 30 μm); however, it is not always possible to reliably differentiate B. mandrillaris from Acanthamoeba spp. by morphology alone. Fortunately, this differentiation is not important for treatment. , ,
The preferred specimens for diagnosis of AK are corneal biopsies or scrapings, and these should be submitted for culture and/or histopathologic examination.
Both N. fowleri and Acanthamoeba spp. will grow in culture; however, the turnaround time for culture may not be desirable for N. fowleri given the rapid progression and usually fatal outcome of the disease and therefore direct microscopic examination of CSF and/or PCR are recommended. The recommended culture method is to place the freshly collected specimen (CSF sediment, brain or corneal tissue, or contact lens) on non-nutrient agar (1.5% Difco agar made with Page’s ameba saline) that has been overlain with E. coli or Enterobacter aerogenes as a food source. It is important to note that N. fowleri is extremely temperature sensitive and may be killed during refrigeration. Therefore CSF specimens for amebic culture should not be refrigerated. After inoculation, culture plates are incubated at room temperature and examined daily using low magnification (10× objective) for 7 days, looking for characteristic trophozoites and cysts ( Fig. 88.32 E). Unfortunately, B. mandrillaris will not grow in this manner, but can be grown in lung fibroblast or other cell lines, such as brain microvascular endothelial cells. , The FLA can also be grown in axenic cultures, although this and cell culture are not commonly performed in the clinical laboratory.
Several conventional and real-time PCR assays have been described in the literature for detection of the FLA, including a triplex, TaqMan probe-based assay targeting the 18S rRNA gene developed by the CDC. , , , These assays can be used to test CSF, ocular, and CNS specimens, including fresh and FFPE tissues, and provide detection sensitivities as low as 1 ameba per reaction. Therefore PCR offers a sensitive and more rapid alternative to culture, when available. Since the FLA are widely found in the environment, practices must be in place to prevent contamination of PCR reagents and reactions. At this time, there are no FDA-cleared/approved or CE-marked assays.
Histopathology has limited use for antemortem diagnosis of PAM or GAE, but can be useful for identifying organisms in skin (and less commonly, lung) biopsies ( Acanthamoeba spp. and B. mandrillaris ) and corneal biopsies ( Acanthamoeba spp.). , In brain biopsies, trophozoites and cysts of Acanthamoeba spp. and B. mandrillaris are commonly found around blood vessels in the cortex (reflecting their hematogenous route of spread) and in necrotic foci ( Fig. 88.32 F). In comparison, N. fowleri trophozoites are usually concentrated around the meninges and can be seen invading surrounding brain parenchyma (see Fig. 88.32 C, D). , As mentioned earlier in this chapter, cysts are not seen in tissue with N. fowleri infection in humans. Both trophozoites (all three genera) and cysts ( Acanthamoeba and Balamuthia only) can be readily identified on routine H&E-stained sections, although organisms may be mistaken for host cells such as macrophages. , The cysts may stain with GMS and PAS stains.
According to the WHO global health estimates report, diarrheal diseases caused an estimated 81 million DALY in 2017, making them the fifth and seventh most common cause of DALY worldwide in females and males respectively. The majority of DALY due to diarrheal illnesses occurred in children between 1 and 5 years of age, with high associated morbidity and mortality rates. The enteric protozoa (specifically G. duodenalis, Cryptosporidium spp., and E. histolytica ), are responsible for more than 500 million cases of diarrheal disease, resulting in an estimated 3 million DALYs and over 60,000 deaths worldwide, thus underscoring the importance of this group of organisms as a cause of human disease.
Many different protozoal groups are capable of infecting the intestinal and urogenital tracts, including amebae, flagellates, coccidia, and ciliates. These organisms are traditionally identified by microscopic examination of stool specimens, although pathogen-specific antigen and molecular detections are increasingly used in the clinical microbiology laboratory. Microscopic identification of intestinal protozoans can be one of the challenging aspects of clinical parasitology as they are small organisms with subtle and overlapping morphologic features. The microscopist must be familiar with the appearance of both pathogenic and nonpathogenic species and be able to differentiate them from one another, and from a wide variety of host cells, yeasts, pollen, and other objects found in stool. Morphologic characteristics that are used for differentiating the intestinal protozoa include size, number of nuclei, chromatin characteristics, cytoplasmic features, and staining characteristics ( Tables 88.12–88.14 , Figs. 88.33–88.38 ).
| Organism | Usual (Observed) Size Range | Number of Nuclei | NUCLEAR FEATURES (CYSTS AND TROPHS) | Cytoplasm Features | |
|---|---|---|---|---|---|
| Peripheral Chromatin | Karyosome Characteristics | ||||
| Entamoeba hartmanni |
|
|
Fine, evenly distributed | Round, small, usually eccentric |
|
| Endolimax nana |
|
|
None | “Blotlike,” large, central; often red with trichrome stain |
|
| Dientamoeba fragilis a |
|
|
None | Irregularly shaped, “fractured” appearance (no single karyosome) |
|
| Iodamoeba buetschlii |
|
|
None | “Blotlike,” large, central |
|
| Entamoeba polecki |
|
|
Fine, evenly distributed, +/− clumping | Round, irregular or diffuse, small to large |
|
| Entamoeba histolytica/E. dispar/E. moshkovskii/E. bangladeshi |
|
|
Fine, evenly distributed | Round, small, usually central |
|
| Entamoeba coli |
|
|
Coarse, irregularly clumped | Round to irregular, large, usually eccentric |
|
a Dientamoeba fragilis is an ameboflagellate but resembles an ameba since its flagellum is internalized. The existence of a cyst stage has not yet been confirmed.
| Microorganism | Usual (Observed) Size Range | Number of Nuclei | Shape | Additional Features |
|---|---|---|---|---|
| Retortamonas intestinalis |
|
|
|
|
| Enteromonas hominis |
|
|
|
|
| Chilomastix mesnili |
|
|
|
|
| Pentatrichomonas hominis |
|
|
|
|
| Giardia duodenalis |
|
|
|
|
| Species | Size (μm) | Shape | Modified Acid-Fast Staining | Condition Upon Passage in Feces |
|---|---|---|---|---|
| Cryptosporidium spp. | 4–6 | Round | Positive | Sporulated (infectious) |
| Cyclospora cayetanensis | 8–10 | Round | Positive | Unsporulated (noninfectious) |
| Cystoisospora belli | 25–30 × 10–20 | Oval | Positive | Unsporulated (noninfectious) |
| Sarcocystis spp. | 15–20 × 15–20 | Oval | Negative | Unsporulated (noninfectious) |
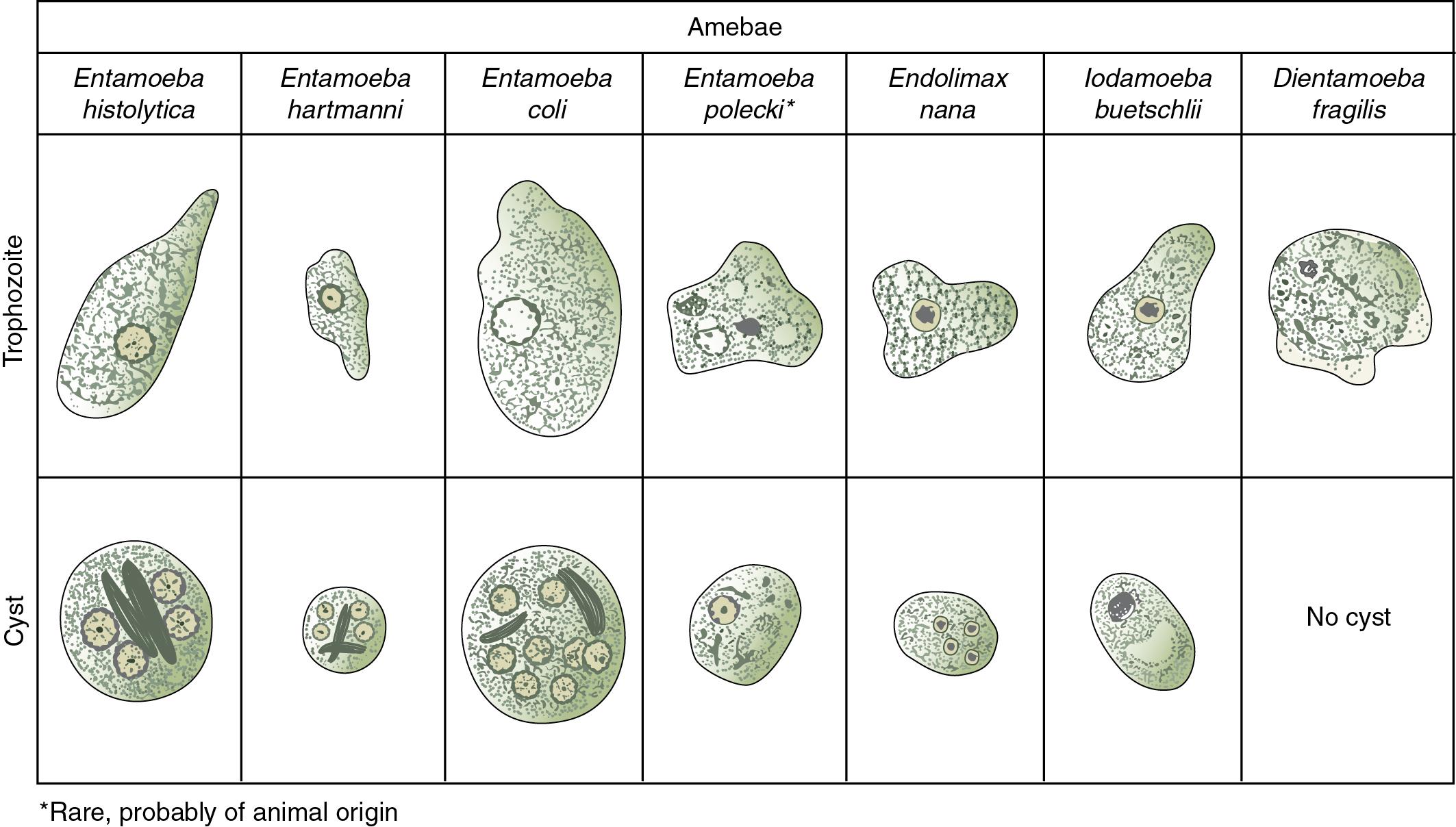
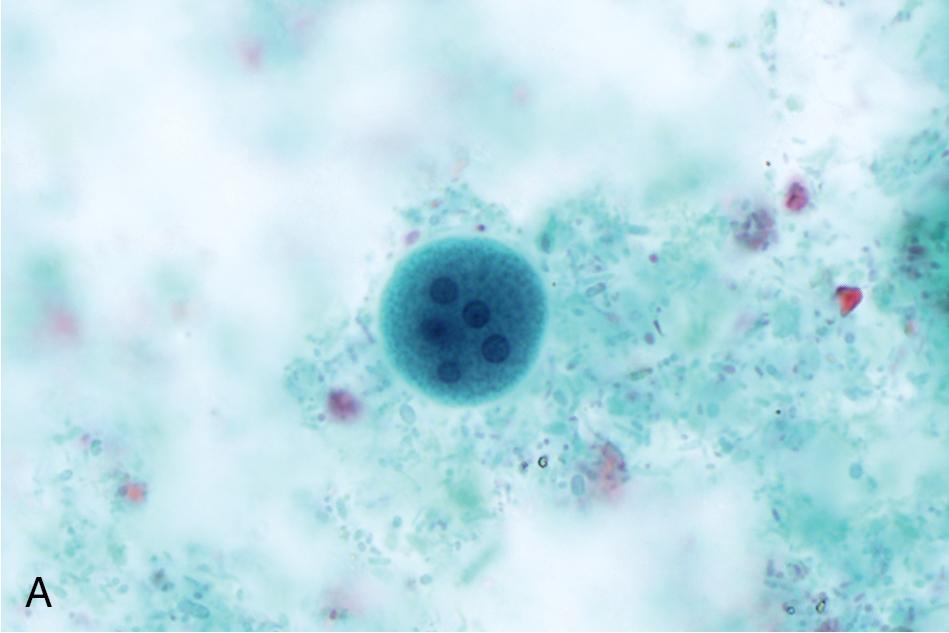
![FIGURE 88.36, Intestinal ciliate, coccidia, Blastocystis, and flagellates. (Publication No. [CDC] 848116. Washington, DC 1984. US Department of Health and Human Services. FIGURE 88.36, Intestinal ciliate, coccidia, Blastocystis, and flagellates. (Publication No. [CDC] 848116. Washington, DC 1984. US Department of Health and Human Services.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/Parasitology/35_3s20B9780323775724000979.jpg)
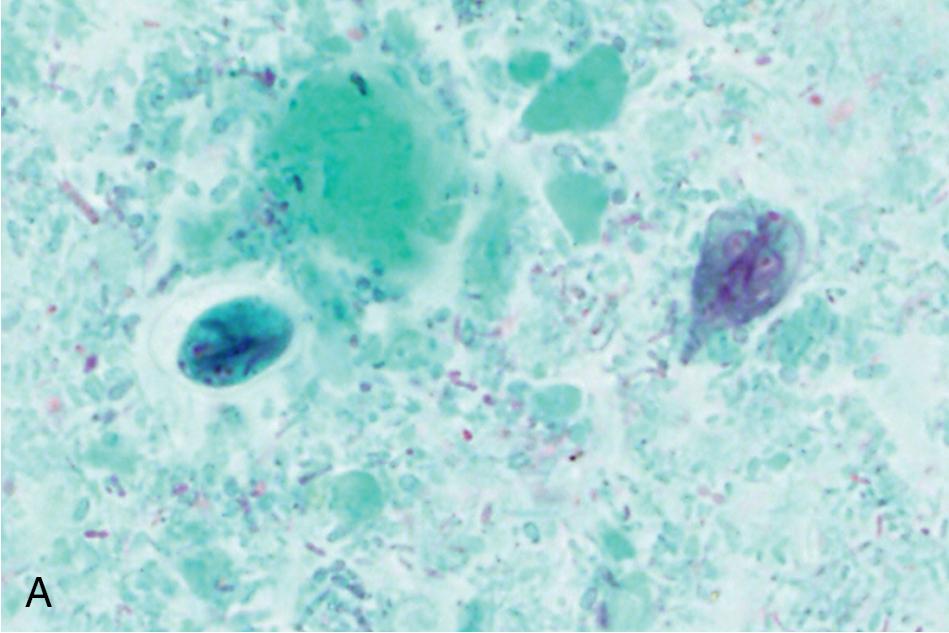
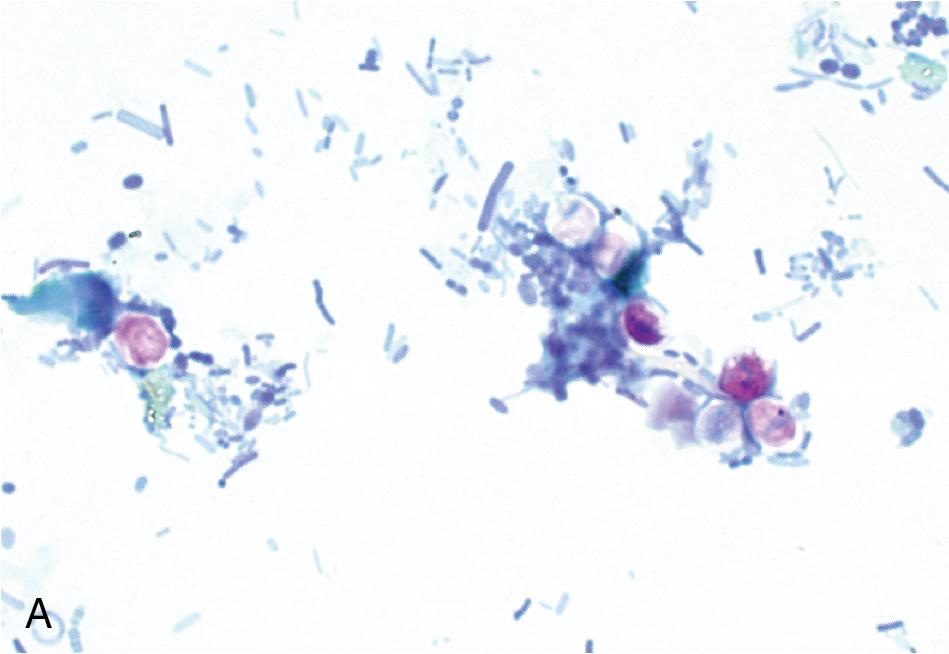
This section will focus on the most significant enteric protozoans to infect humans, including the amebae, flagellates, ciliates, coccidia, and Blastocystis spp. The urogenital protozoan, T. vaginalis , will also be covered.
The amebae that can inhabit the human intestinal tract are Entamoeba spp., Iodamoeba buetschlii, and Endolimax nana. D. fragilis is also sometimes grouped with the amebae due to its morphologic features, but it is actually a trichomonad with an internalized flagellum (see Table 88.12 ). This organism is discussed in the “Flagellates” section later but is found with the amebae in the tables and figures in this chapter due to its morphologic similarities to these organisms.
Of the intestinal amebae, only E. histolytica is a proven human pathogen. , The other Entamoeba spp. known to infect humans are E. hartmanni, E. coli, E. chattoni , E. polecki, E. dispar, E. moshkovskii, and E. bangladeshi . E. dispar is a common commensal organism that is morphologically indistinguishable from E. histolytica, which complicates laboratory diagnosis when only microscopy is used. , More recently, E. bangladeshi and E. moshkovskii have been described and are also morphologically indistinguishable from E. histolytica. The pathogenic potential of these two amebae is currently unknown. Finally, Entamoeba gingivalis is an oral protozoan that is most commonly found in patients with poor oral hygiene. I. buetschlii and E. nana are common commensal organisms.
All amebae share a similar life cycle; infection is acquired through ingestion of an environmentally resistant cyst, and trophozoites live in the intestinal lumen, feeding on bacteria. This is the same life cycle for the known pathogen, E. histolytica, which only rarely invades the intestinal mucosa and causes disease. , ,
E. histolytica is the protozoan responsible for amebic colitis, a common cause of gastroenteritis in developing countries. Less commonly, it causes extraintestinal disease, with the most common form being amebic liver abscess (ALA).
E. histolytica infection is found worldwide, but is most commonly reported from resource-poor countries with poor sanitation, especially in the tropics and subtropics in Latin America, Africa, and Asia. Unfortunately, the accuracy of available prevalence data is dependent on the methods used for detection, with rates calculated using microscopy-based studies inflated due to morphologic similarities among E. histolytica and morphologically identical Entamoeba spp. ( E. dispar, E. moshkovskii, and E. bangladeshi ) (see Table 88.12 ). , E. dispar is the more common of these Entamoeba spp., and may be more than ten times more common than E. histolytica in endemic regions. Newer studies using methods capable of identifying and differentiating the various Entamoeba spp. have estimated that there are 28 million illnesses each year, with as many as 1470 associated deaths.
The actual prevalence rates vary significantly by geographic location. Studies from South Africa, Egypt, and Cote d’Ivoire using methods capable of specifically identifying E. histolytica show prevalence rates ranging from 0% to greater than 21% for asymptomatic carriage. In Northern India and China, seroprevalence and PCR data showed overall prevalence rates of approximately 11.1%, , while Northwestern Saudi Arabia reported rates of 16.5%. E. histolytica infection is much less common in developed countries. The reported prevalence rates range from 0.2 to 12.5%, with the higher rates being from outbreak settings. In these countries, cases of E. histolytica are more commonly detected in patients with travel history to developing countries, recent immigrants, and immunocompromised patients, particularly those with HIV infection (see Table 88.5 ). Data from the International GeoSentinel-associated clinics showed that most returned travelers diagnosed with E. histolytica infection from 2007 to 2011 had traveled to India, Indonesia, Mexico, and Thailand. Tourism was the most common reason cited for travel in this group.
As with the other intestinal amebae, E. histolytica infection is acquired following ingestion of food or water contaminated with mature cysts ( Fig. 88.39 ). In the intestinal lumen, the cyst matures into a trophozoite through a process known as excystation. Colonized individuals may shed mature and immature cysts that can be detected in stool by microscopy. Noninvasive, asymptomatic infections occur when the trophozoites attach to the surface of the cells lining the lumen. Trophozoites may also be shed in stool, but unlike the cysts, are susceptible to environmental factors and therefore are not always detected using the O&P examination. Rarely, trophozoites can invade the intestinal epithelium and cause gastroenteritis. Extraintestinal disease occurs when invading trophozoites enter the portal bloodstream from the bowel and migrate to the liver (primarily) and other organs such as the lung and brain. , ,
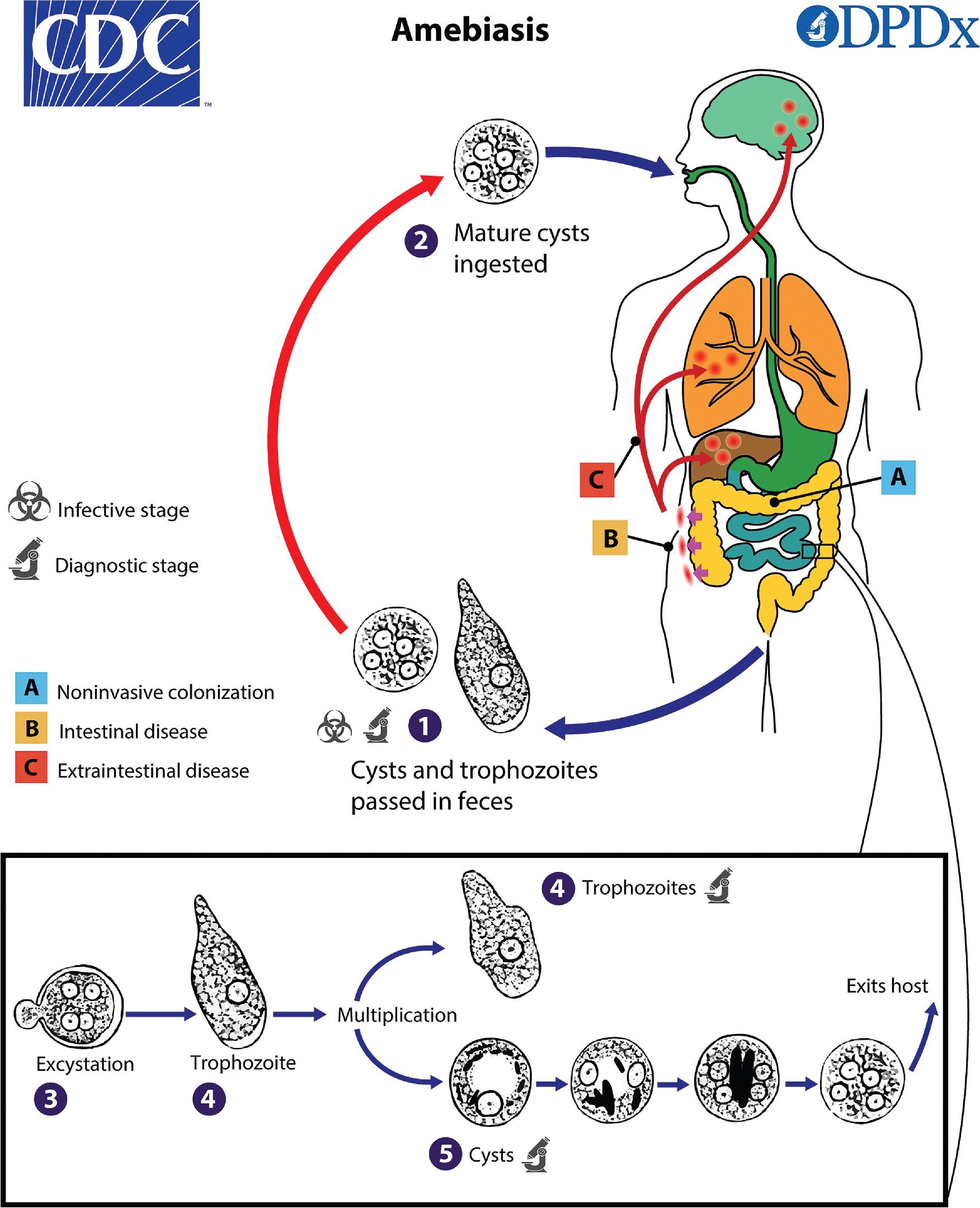
Most individuals with confirmed E. histolytica infection are asymptomatic, and only a minority goes on to develop symptomatic disease. Longitudinal studies revealed that approximately 4 to 10% of infected patients develop symptomatic intestinal disease within 1 year of acquiring the infection if left untreated. , When present, symptoms range from self-limited diarrhea to amebic dysentery, with the latter causing profuse bloody diarrhea and abdominal pain. In severe cases, complications such as extensive ulceration ( Fig. 88.40 A), toxic megacolon, ameboma (formation of an inflammatory mass in the intestine), and intestinal perforation may ensue.
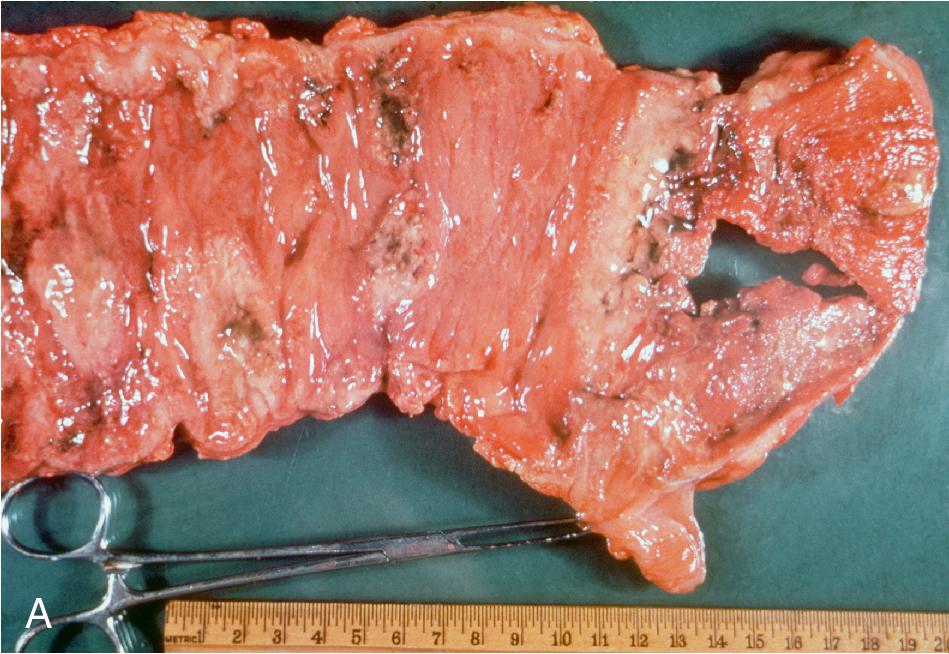
ALA is the most commonly reported extraintestinal form of amebiasis caused by E. histolytica, occurring in approximately 5% of patients with a previous history of intestinal amebiasis ( Fig. 88.40 B). , Patients with ALA commonly present with fever, right upper-quadrant abdominal pain, and acute liver tenderness. Diarrhea may be present, although stool O&P examination is negative for E. histolytica in most cases. , Laboratory findings include leukocytosis, increased alkaline phosphatase, and increased liver transaminases. Disease often progresses relatively quickly and can be fatal if untreated. One of the most important complications of ALA is rupture and extension into the peritoneum or across the diaphragm to the lung and heart. Although rare, hematogenous dissemination to other organs may also occur, and cerebral amebiasis must be differentiated from infection with the FLA. ,
To prevent progression to symptomatic disease and contamination of the environment, asymptomatic carriers of E. histolytica are routinely treated with drugs that effectively concentrate in the intestinal lumen such as paromomycin or diloxanide furoate. , Amebic colitis and extraintestinal disease are treated with a course of a nitroimidazole drug such as metronidazole followed by a luminal drug as prescribed for asymptomatic carriage. Percutaneous drainage of liver abscesses is not generally recommended unless patients do not respond to therapy within 5 to 7 days and there is a risk for abscess rupture. Antibacterial therapy is warranted in cases of ALA with bacterial superinfection.
The most common methods of E. histolytica laboratory detection are microscopy, stool antigen detection, and more recently, NAATs. , Serology is most useful for detection of extraintestinal and invasive disease. Culture is not routinely used for clinical diagnosis but may be performed in specialized research and public health laboratories. ,
E. histolytica is most commonly diagnosed by microscopic examination of stool specimens. , , Depending on the stage of the infection, both cyst and trophozoite forms may be detected in stool. Trophozoites can also be detected in material aspirated from abscesses. The last portion of aspirated material is most likely to contain diagnostic trophozoites since they are usually concentrated at the invading edge of the abscess. Cysts are not seen in extraintestinal specimens.
Wet mount preparations of stool or abscess fluid are useful to detect motile trophozoites (which characteristically exhibit “directional” motility), but they are not ideal to visualize detailed morphologic characteristics. Further, their sensitivity decreases if the sample is not analyzed within 1 hour of collection. , Wet preparations made from a concentrated specimen improve detection (see Fig. 88.34 ). Permanently stained smears (e.g., trichrome or iron hematoxylin) allow for improved detection and characterization of both cysts and trophozoites over wet mount preparations. The cyst form of E. histolytica measures between 10 and 20 μm in diameter and is characterized by the presence of four nuclei in the mature cyst and one to two nuclei in the immature cyst (see Table 88.12 and Figs. 88.33 and 88.34 ). , , , In a mature cyst, each nucleus contains a central karyosome and chromatin evenly distributed on the nuclear membrane periphery. The cytoplasm may be fine and granular and contains a rectangular or oval-shaped chromatoid body with blunted or rounded ends (see Fig. 88.34 C). The immature cyst is characterized by the presence of large vacuoles in the cytoplasm and clumps of glycogen (see Fig. 88.34 E). The trophozoite varies in size, ranging between 10 and 60 μm (noninvasive forms tend to be smaller) and shape (round to ovoid) and is characterized by the presence of a single nucleus (see Fig. 88.34 D–F). Similar to the cyst form, the nucleus of the trophozoite contains a small, centrally located karyosome and peripheral chromatin. The cytoplasm is finely granular, and ingested bacteria or debris may be present. The presence of ingested red blood cells within trophozoite cytoplasm constitutes definitive identification of E. histolytica (see Fig. 88.34 F). , Also, detection ofmorphologically consistent trophozoites from any extraintestinal site is diagnostic of E. histolytica. Otherwise, this species cannot be differentiated from E. dispar, E. moshkovskii, and E. bangladeshi. Differentiation from other Entamoeba spp. must also be considered (see Table 88.12 ). In addition to these limitations, microscopy has lower sensitivity and specificity than antigen and molecular amplification methods, especially in low-prevalence areas. , ,
Several commercial assays are available for detection of E. histolytica antigens in unpreserved stool samples (see Table 88.7 ). Formats vary and include EIA and immunochromatographic (LFICA) assays. E. histolytica antigen assays have reported sensitivity and specificity ranging from 90 to 100% according to manufacturer studies. However, it is important to note that sensitivities and specificities vary widely between assays and settings (e.g., endemic versus nonendemic), and not all assays are capable of differentiating E. histolytica from E. dispar. The TechLab E. histolytica II EIA and LFICA tests (Abbott Laboratories, Chicago, IL) are currently the only assays that are FDA-cleared for the specific detection of E. histolytica from stool specimens, although cross-reactivity with E. dispar and E. moshkovskii have been observed. The TechLab E. histolytica II EIA and the Entamoeba CEIA Path (Cellabs, Brookvale, Australia) detect the galactose adhesin antigens of E. histolytica/E. dispar through binding of polyclonal antibodies immobilized to wells of a microtiter plate. A monoclonal antibody conjugated to peroxidase is used specifically to bind to adhesin epitopes unique to the E. histolytica assay. A study performed in Australia compared the two EIAs to a laboratory-developed PCR assay for species-specific detection of E. histolytica, E. dispar, and E. moshkovskii using 279 fecal samples positive for any of the three species of Entamoeba spp. by microscopy. The performance of both EIA assays was suboptimal, with sensitivity and specificity of 28 and 100%, respectively, for the CEIA Path and 0 and 99.1%, respectively, for the TechLab assay. Therefore the utility of these EIA tests in low-prevalence areas must be considered when interpreting patient results.
Several commercial EIA assays are available for detection of E. histolytica antibodies with sensitivities and specificity ranging from 92.5 to 100% and 90.9 to 100%, respectively. The detection of E. histolytica antibodies in serum samples is primarily used to support a diagnosis of ALA, since greater than 95% of patients with liver involvement are seropositive. This is especially helpful in the large number of cases in which stool samples are negative for E. histolytica cysts or trophozoites by conventional microscopy. The seropositivity rates drop significantly for patients with intestinal infection; seropositivity rates of 70 to 85% have been reported for patients with invasive intestinal disease, and only 10% in asymptomatic carriers. , Therefore antibody detection should only be used in conjunction with microscopy, antigen detection, or molecular diagnostics in these patients. It should be noted that only E. histolytica (and none of the other Entamoeba spp.) elicits an antibody response. Hence, a positive serology confirms patient exposure to the pathogenic ameba, although in areas of high prevalence this information may be of limited value.
A recent development in the diagnosis of E. histolytica amebiasis is the introduction on the market of several commercial FDA-cleared molecular assays (see Table 88.8 ). Detection of E. histolytica DNA has previously been performed using laboratory-developed tests, and most published studies showed a significant increase in sensitivity and specificity for PCR compared to both microscopy and antigen assays. All currently available FDA-cleared assays detect E. histolytica as part of the gastrointestinal pathogens panel alongside other parasites and bacterial and viral pathogens (see Table 88.8 ). The complexity of these assays varies from simple, completely automated sample-to-answer tests to high-complexity assays that require specialized laboratory settings to ensure accurate performance. The sensitivity and specificity of molecular assays have been reported in a few multicenter and head-to-head comparative studies. However, many of these studies were performed in countries with low prevalence of E. histolytica, and sensitivity could only be evaluated on rare positive samples. Most molecular assays are designed to specifically detect E. histolytica DNA; however, false-positive results may occur in the presence of high concentrations of E. dispar , and thus this is an important limitation of these tests. Another major limitation of molecular diagnostics testing is the cost of testing, which is still significantly higher than other diagnostic tests. Therefore it may not be readily applicable in developing countries where the impact for diagnosis of parasitic infection would be greatest.
Culture is no longer routinely used for clinical diagnosis and is now only available in limited research and public health settings. Histopathology is not generally used for primary diagnosis but may be useful for detecting infection in atypical cases. On intestinal biopsy, mucosal erosion and ulceration are commonly observed, and characteristic “flask”-shaped ulcers may be seen ( Fig. 88.41 A). , , Trophozoites are best identified at the leading edge of the ulcer in the submucosa ( Fig. 88.41 B) and demonstrate morphologic features that are similar to those seen in stool preparations, including ingested erythrocytes in the cytoplasm. Trophozoites may also be seen at the edge of extraintestinal abscesses but are seldom detected in aspirated abscess fluid. Cysts are not seen in tissue.
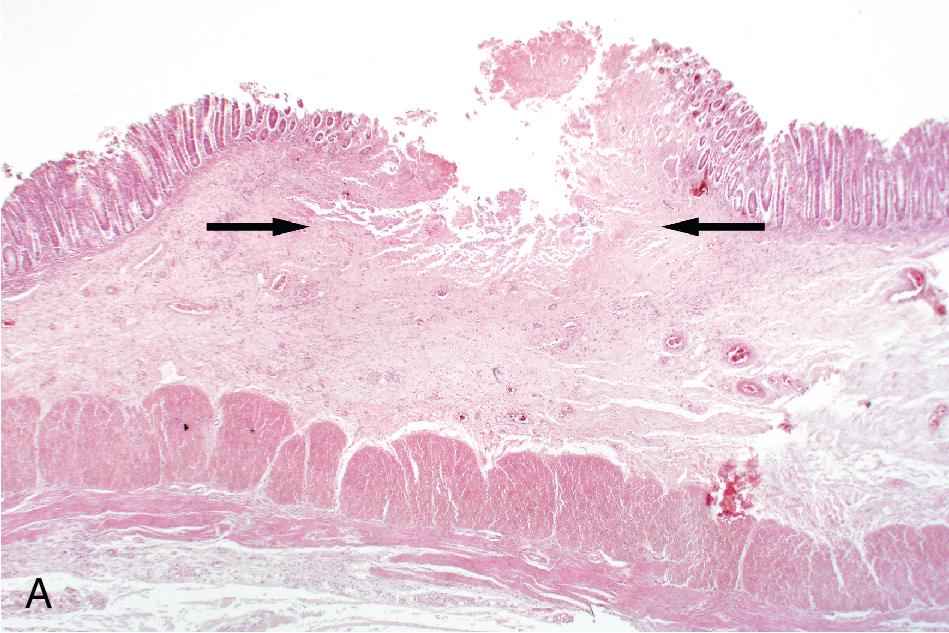
Several flagellates may be found in the human intestinal tract, while T. vaginalis is a genitourinary flagellate that causes disease in men and women. Of the flagellates found in the human intestinal tract, only G. duodenalis and D. fragilis are believed to be true pathogens. Chilomastix mesnili, Pentatrichomonas hominis, Enteromonas hominis, and Retortamonas intestinalis are commensal organisms that may also be seen in stool specimens . These commensals have not been definitely associated with clinical disease but are worth mentioning due to their morphologic similarity with pathogenic flagellates described in this section (see Tables 88.12 and 88.13 ). They are also transmitted to humans in a similar manner, and therefore their presence in stool may indicate that the patient is at heightened risk for harboring other parasites. ,
Giardia duodenalis (previously known as G. lamblia or G. intestinalis ) is one of the most commonly detected gastrointestinal parasites worldwide. It is capable of infecting a wide variety of animals including other mammals, birds, and reptiles. ,
Giardiasis is found in both resource-rich and resource-poor settings and causes epidemic and endemic patterns of infection. Infections are commonly endemic in tropical regions with poor sanitation, where infection is transmitted largely person-to-person and clinical immunity develops over time. Children are frequently infected and shed large numbers of organisms into their stool. In contrast, sporadic epidemics can occur in resource-rich settings with good sanitation and are usually transmitted through consumption of Giardia cysts in contaminated food, municipal water supplies, or untreated groundwater. There is no preexisting immunity in the population, and therefore symptomatic disease occurs in both children and adults. , Immunocompromised patients, including those with HIV/AIDS and selective IgA deficiency (sIgAD) disorder, are at risk for persistent and chronic infections.
Global epidemiology data show a prevalence rate as low as 0.4% in developed countries and as high as 30% in developing countries. Giardiasis is a nationally reportable disease in the United States, and therefore good data exist for its incidence. During the 2011 to 2012 reporting period, the rates of giardiasis were 6.4 and 5.8 per 100,000 populations in 2011 and 2012, respectively, with higher rates reported in the northwestern states compared to the southwestern states (4.6 to 4.8 versus 8.5 to 9.4 per 100,000 population). In Canada, an average rate of 19.6 per 100,000 population per year (1999–2002) has been reported. The rate of infections in North America peaks in the summer months, which reflects its association with ingestion of contaminated surface water by campers and hikers. Other populations with a high incidence of infection are travelers, children attending day care (and their guardians), and men who have sex with men. A number of large water-borne outbreaks have been associated with contaminated municipal water supplies.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here