Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
To address the future of neuromodulation, it is first important to recognize that I believe that evolutionary changes in neuromodulation devices do not play a critical role in this future. In the past, the field has identified that changes in the number of contacts per lead, the length of each lead, or the number of leads defined its future. Later, expanded stimulation parameters alone, such as stimulation at higher frequencies, higher pulse widths, higher current densities, and lower amplitudes, were used to define its future. These changes are clearly evolutionary and not revolutionary; it is the revolutionary idea, however, that will lead to the bright future of neuromodulation. I think of these revolutionary ideas as creating the paradigm shift necessary to change how we think about neuromodulation.
While the phrase “paradigm shift” has become incorporated in our everyday language, this important concept was introduced and defined by Thomas Kuhn in his sentinel 1962 work, “The Structure of Scientific Revolutions” subtitled in its paperback release “A Brilliant Original Analysis of the Nature, Causes and Consequences of Revolutions in Basic Scientific Concepts” [ ]. Kuhn refers to “paradigm” as the Greek word for pattern and “normal science” as the existing and accepted scientific paradigm for any given scientific field or set of questions. As experience grows, scientists recognize that “anomalies” are observed that cannot be well explained by normal science. As a result, a new paradigm is created to account for these anomalies. In what we have come to know as the Kuhn Cycle ( Fig. 18.1 ), early (or earlier) science has led to what is considered “normal science.” The model continues to drift as new and contrary data are collected until the model is in crisis; it can no longer explain the new scientific information that has been accrued. This demands a “model revolution” whereby a new model is created which can successfully incorporate and explain the newly collected data. This then leads to a process which involves a paradigm change resulting in the acceptance of the new model as normal science.
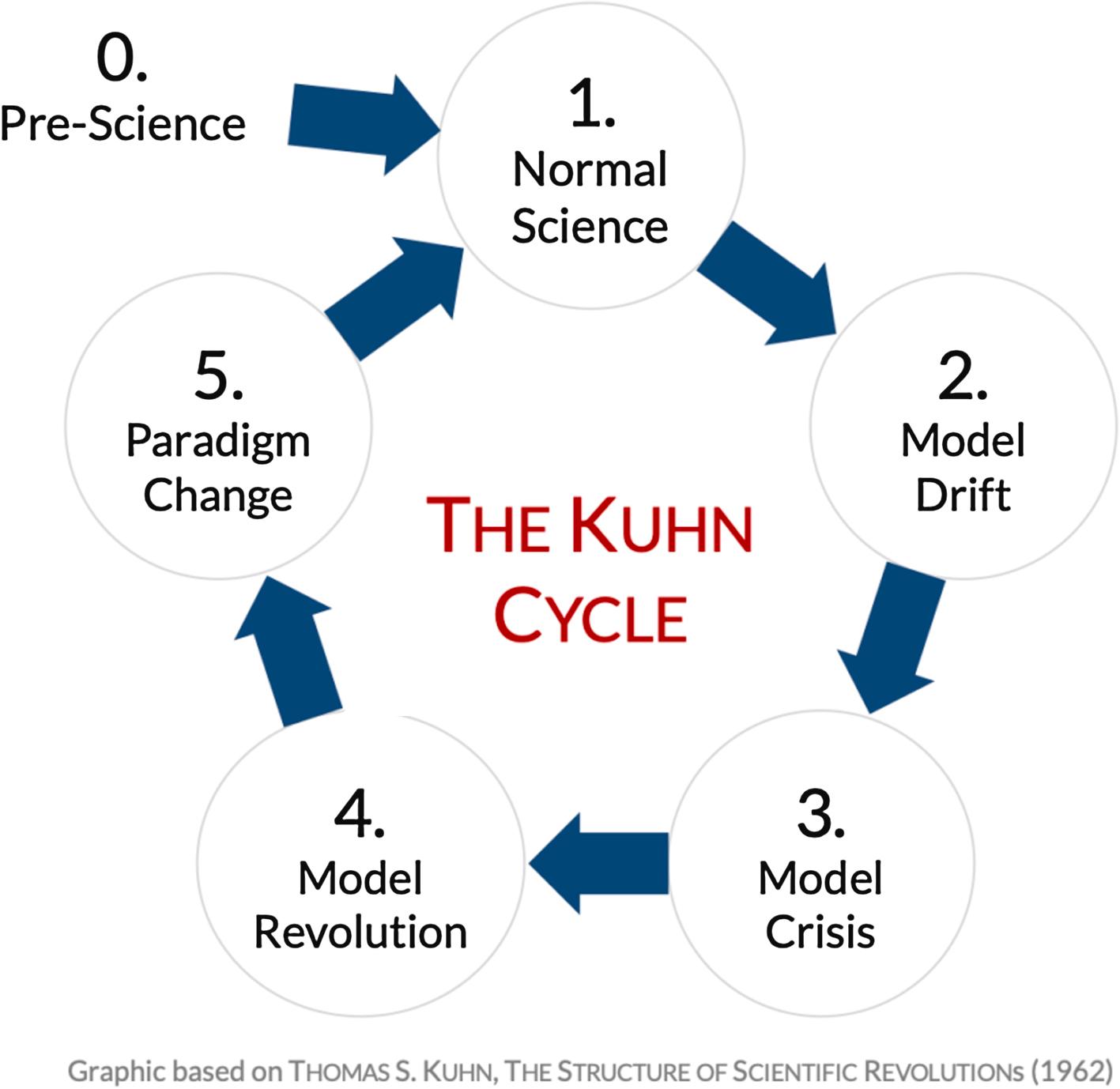
Despite what we might think, this transition from normal science to a new model is seldom smooth. There is much debate and profound controversy ensues. The latter part of the Kuhn cycle resulting in paradigm shift is usually violent. Science tolerates this violent process and over time, the new model is accepted as normal science. This occurs less often by changing the minds of believers in the old normal science and more often by the aging of elders leading to a group of younger scientists raised with the new model as being both truth and normal science.
Well recognized scientific paradigm shifts in history include the observations of Galileo which triggered the shift from belief in a geocentric to a heliocentric solar system. Historians have well outlined this violent paradigm shift in the perception of our solar system [ ]. More recently, Einstein's General Theory of Relativity triggered the paradigm shift from classical to quantum mechanics [ ] ( Figs. 18.2 and 18.3 ).


While we might well be too close to recognize it, several past paradigm shifts have affected our field as well. For decades, for example, normal science dictated that chronic opioid therapy is both safe and effective for the treatment of chronic pain. In fact, I may well have been practicing through two Kuhn cycles in which the first paradigm shift failed to explain the data that accrued after its adoption and science reverted to a revised version of its prior normal science.
First, how was the historical reticence to prescribe opioids, the normal science, replaced by a new paradigm favoring the use of chronic, high dose opioids for the treatment of chronic pain? The initial inconsistent “evidence” can be traced to a letter to the editor of the New England Journal of Medicine in 1980 authored by Porter and Jick [ ] ( Fig. 18.4 ).

In this one paragraph letter, the authors describe that in a series of 39,946 hospitalized patients, 11,882 patients received at least one narcotic preparation and only four later cases of addiction were identified in those patients without a history of addiction. Their conclusion was quite limited: “the development of addiction is rare in medical patients with no history of addiction.” Despite its limited observation and lack of relevance to chronic pain therapy, this paragraph has since been cited at least 608 times in the medical literature, often as a sentinel or landmark work, to support the use of chronic opioid therapy for intractable pain.
Following the Porter and Jick report, a series of papers reported successful management of patients with pain of nonmalignant origin using chronic opioid analgesics, the most influential being those by Russell Portenoy [ ] ( Fig. 18.5 ).

In this retrospective study of 38 patients, for example, Portenoy reported that 24 had acceptable pain relief while in 14 the relief was inadequate. Two patients had management issues with opioids; both had a prior history of drug abuse. The authors concluded “Opioid maintenance therapy can be a safe, salutary and more humane alternative … in those patients with intractable non-malignant pain and no history of drug abuse.”
Shortly thereafter, Zenz and coworkers reported a retrospective series of 100 patients with predominantly back or neuropathic pain treated with long acting opioids [ ]. Good relief was obtained in 51 patients, partial pain relief in 28 patients, and no relief in 21 patients; there were no cases of addiction to opioids. The authors concluded: “opioids can be effective in chronic nonmalignant pain, with side effects that are comparable to those that complicate the treatment of cancer pain.”
While there were many reports of chronic opioid therapy in patients with pain of nonmalignant origin concluding that such therapy was safe and effective, including the low risk of tolerance, addiction or overdose deaths, critical review of these studies indicates that such conclusions were made based upon insufficient data. By the early 2000s, systematic reviews of the safety and efficacy of chronic opioid therapy for noncancer pain were appearing and began to suggest the inadequacy of the existing data [ ]. The authors analyzed all randomized, double-blind placebo-controlled trials of WHO step three opioids for efficacy and safety in noncancer chronic pain. Of the 15 evaluable studies, four studies of 120 patients studied intravenous opioid testing while 11 studies of 1025 patients compared oral opioids to placebo ranging from 4 days to 8 weeks. They noted that only six of the trials had an open label follow-up of 6–24 months. The mean decrease in pain intensity with opioids was at least 30% regardless of whether the pain was neuropathic or musculoskeletal, while about 80% of the patients experienced side effects including constipation (41%), nausea (32%), and somnolence (29%). Of note is that less than half of the patients in the open label follow-ups remained on opioid therapy. The authors noted that any conclusions concerning problems such as tolerance or addiction were impossible based upon the available data.
In their 2006 report, Furlan, et al. [ ] performed a meta-analysis of the existing literature to compare the efficacy of opioids for chronic noncancer pain with other drugs and placebo, to identify types of noncancer pain that respond better to opioids and to determine the most common side effects of opioids in this setting. They identified 41 randomized controlled trials (RCTs) involving 6019 patients. Eighty percent of patients had nociceptive pain, 12% had neuropathic pain, and 7% had fibromyalgia. Despite reports of long-term efficacy, the average duration of treatment was only 5 weeks, with a range of 1–16 weeks. In all pain categories reported, opioids were better than placebo for both pain and functional outcomes and strong opioids provided significantly superior pain relief as compared to naproxen and nortriptyline. Only constipation and nausea were clinically and statistically significant side effects. It was noted that the short length of follow-up in all of the reported studies made relevant and important conclusions concerning long-term opioid complications including tolerance and addiction impossible. Of note is that despite the short length of these trials, one-third of patients discontinued opioid therapy.
Ballantyne and Shin performed yet another review of the then current evidence for the efficacy of opioids for chronic pain [ ]. They indicated that opioid therapy for chronic pain had become popular over the past few decades and that there was concern about the loss of analgesic efficacy over prolonged courses of treatment despite dose escalation and stable pain reports. They further raised the issue of potential serious adverse events of opioids, especially in vulnerable individuals. The authors concluded “That existing evidence suggests that analgesic efficacy, although initially good, is not always sustained during continuous and long-term opioid therapy.” They highlighted the important topics of pharmacologic tolerance, opioid-induced hyperalgesia, subtle and intermittent withdrawal, and psychological factors including the loss of the placebo effect over time.
The American Pain Society and the American Academy of Pain Medicine subsequently commissioned a systematic review of the evidence and convened a multidisciplinary expert panel to formulate recommendations for the use of opioids for chronic noncancer pain [ ]. The authors stated “although evidence is limited, the expert panel concluded that chronic opioid therapy can be an effective therapy for carefully selected and monitored patients with chronic non-cancer pain.” The panel then provided recommendations which “provide guidance on patient selection and risk stratification …”. Unfortunately, the potential significant financial contributions of the opioid industry to several panel members and one of the societies rendered these recommendations suspect.
This points to one of the potential flaws inherent to the Kuhn cycle. While a new paradigm must develop and replace normal science when new data are accrued that cannot be explained or supported by the existing science, we are dependent upon both the quality and comprehensiveness of these anomalous observations. In the case of chronic opioid therapy for pain of nonmalignant origin, the anomalous observations that chronic opioids were safe and effective came from studies that were too short to detect the severe problems of tolerance and addiction or where important outcomes were not assessed. These anomalous observations led to the development of a new paradigm and the field underwent a paradigm shift based upon incomplete, misleading and, in fact, patently false conclusions from a limited data set.
In light of the growing awareness of these issues, the American Academy of Neurology issued a position paper on opioids for chronic noncancer pain. Author Gary Franklin, MD stated: “Whereas there is evidence for significant short-term pain relief, there is no substantial evidence for maintenance of pain relief or improved function over long periods of time without incurring serious risk of overdose, dependence, or addiction ( Fig. 18.6 ).”

While there were a growing number of “anomalous” observations to the now “normal science” of using chronic opioids for nonmalignant pain, the nonscientific community was becoming aware of the opioid crisis which grew out of this paradigm shift. In his 2015 book, entitled “Dreamland: The True Tale of America's Opiate Epidemic,” Sam Quinones revealed that the unrelated single paragraph note written by Porter and Jick was the “foundation for a revolution in U.S. medical practice.” In other words, the foundation for a paradigm shift in medicine. Quinones noted that an article in Scientific American identified Porter and Jick's “extensive study” and Time Magazine labeled it a “landmark study” demonstrating that fears of prescription opioids leading to addiction were “basically unwarranted.” Incomplete or overinterpreted observations had led to an unwarranted paradigm shift. Of note is that Kuhn identified this risk and noted that in a further Kuhn cycle, paradigm shifts could revert to older normal science.
In a fitting example of symmetry, Leung et al. [ ] published a letter to the editor in the New England Journal of Medicine discussing the Porter and Jick letter to the editor 37 years prior. The authors evaluated the profound long-term implications of the Porter and Jick short report in the NEJM. While Porter and Jick's conclusion was limited to in-hospital patients given opioids only during their hospital stay, the letter was inappropriately generalized to suggest that addiction to prescription opioids were rare and, as a result, the aggressive use of opioids for chronic pain was warranted. As noted above, the authors revealed that the Porter and Jick letter had been cited 608 times in the medical literature and that frequently, these citations were inaccurate, uncritical, or overly interpreted and misrepresented its limited results.
Leong et al. cited articles in the medical literature that referred to Porter and Jick as evidence that “this pain population with no abuse history is literally at no risk for addiction” and that “medical opioid addiction is very rare”. The authors stated that “we believe that this citation pattern contributed to the North American opioid crisis by helping to shape a narrative that allayed prescribers' concerns about the risk of addiction associated with long-term opioid therapy …”.
To my mind, the scientific observation that gave clarity to the role of opioids for the treatment of pain of nonmalignant origin was the SPACE randomized clinical trial [ ]. This RCT evaluated the both the efficacy and adverse event rate of opioids versus nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain. As demonstrated in the figures below, there was no difference in pain scores between those treated with opioids and those treated with nonopioid medications while the adverse event rates related to opioids were significantly higher ( Figs. 18.7 and 18.8 ).
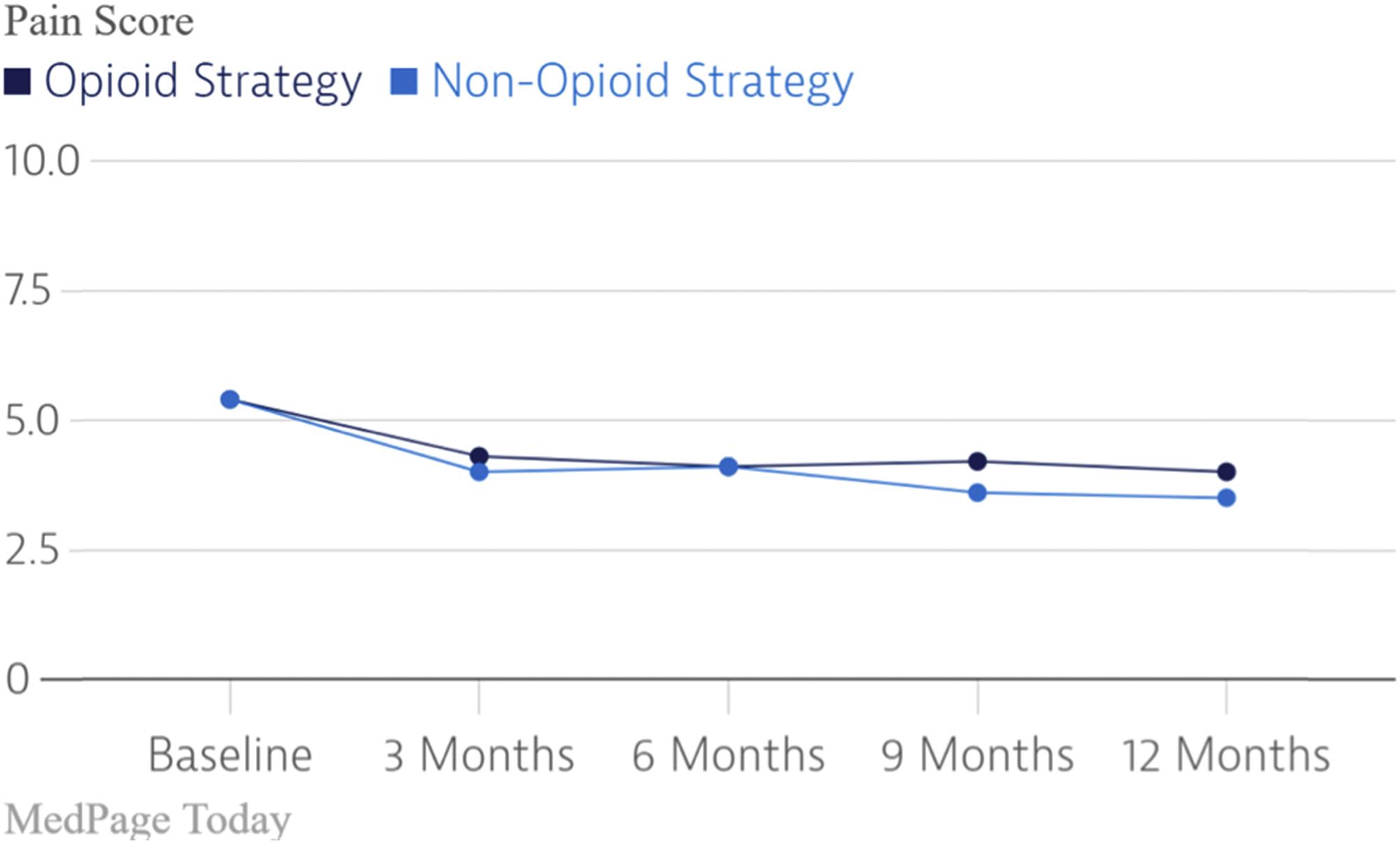
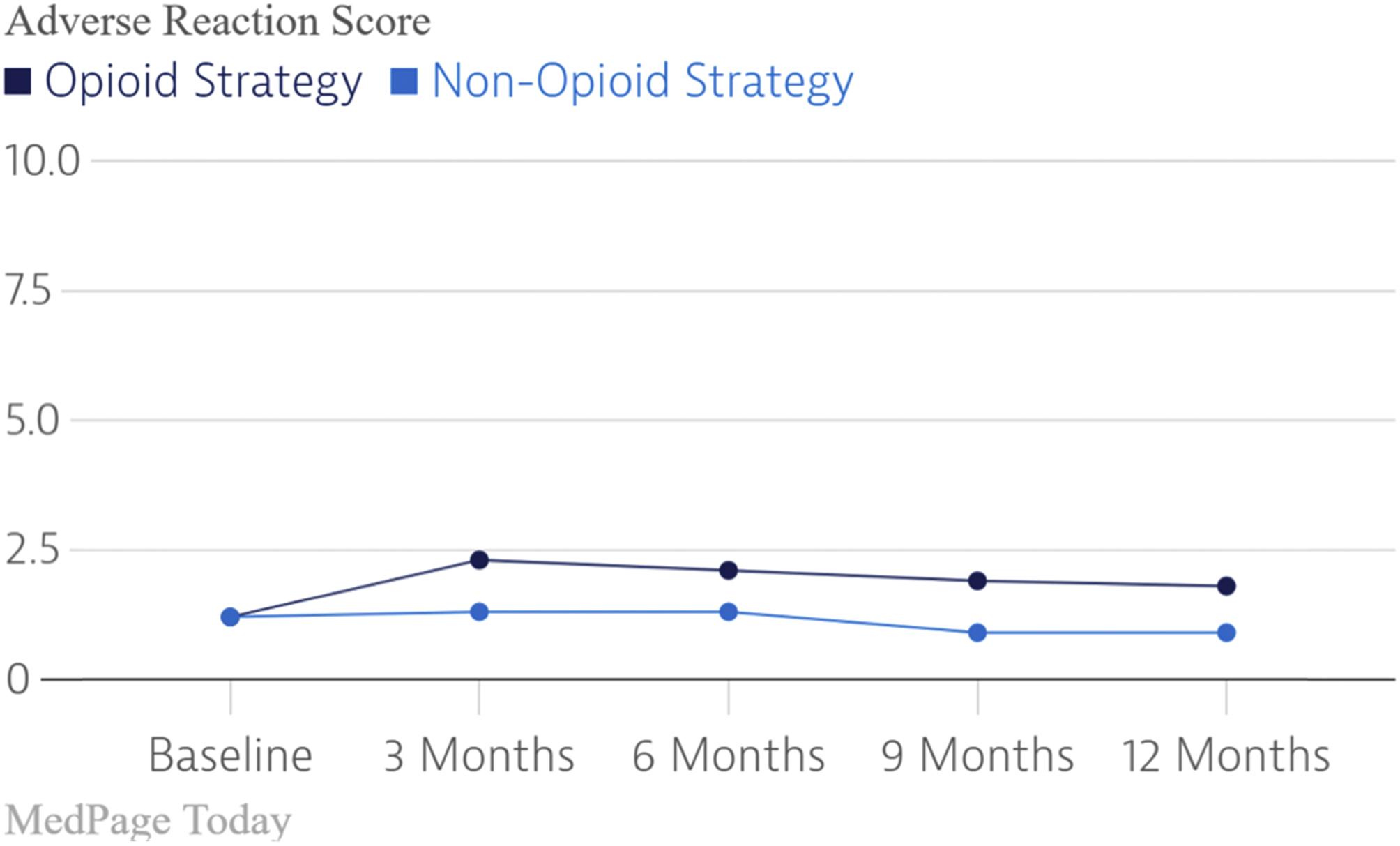
In parallel with the growing anomalous observations which could not be explained by the new paradigm supporting the safety and efficacy of chronic opioid therapy for pain of nonmalignant origin was a paradigm shift regarding the efficacy of spinal cord stimulation. First introduced in 1967 by Shealy and coworkers, dorsal column stimulation, now SCS, became widely used during the 1970s. Due to its overuse, the lack of clear patient selection criteria, the lack of clear indications, and the relatively primitive technology, the failure rate of SCS was high. The quality of the literature supporting SCS was also poor. As a result, SCS fell out of favor and the “normal science” dictated the lack of efficacy and the lack of proof of this efficacy for SCS.
During the same period during which anomalous observations suggested that chronic opioid therapy was ineffective and potentially dangerous, there was growing evidence supporting the safety and efficacy of neuromodulation therapies for these same chronic pain problems. First was the randomized clinical trial comparing physical therapy alone to spinal cord stimulation plus physical therapy in patients with reflex sympathetic dystrophy of the upper extremity [ ]. In this randomized trial, 36 patients were assigned to the SCS + PT while 18 patients were assigned to receive PT alone. 24 of 36 SCS trials were successful leading to implantation of SCS systems in 24 patients. Pain intensity, global perceived effect and health-related quality of life were significantly better in patients receiving SCS therapy and PT as compared to those receiving PT alone.
Kumar et al. [ ] then published the results of a multicenter RCT comparing spinal cord stimulation to conventional medical management (CMM) in patients with failed back surgery syndrome (FBSS). One hundred FBSS patients with predominant leg pain of neuropathic radicular origin were randomized to receive either spinal cord stimulation plus CMM alone for at least 6 months. Cross-over at 6 months was permitted and all patients were followed for 1 year. At 6 months, 48% of the SCS patients and 9% of the CMM patients obtained 50% or more pain relief ( P < .001). Compared to the CMM group, the SCS group experienced improved leg and back pain relief, quality of life, functional capacity, and treatment satisfaction ( P < .05). Of note is that 5 SCS crossed to CMM while 32 CMM patients crossed to SCS ( Fig. 18.9 ).
North et al. [ ] published their complimentary RCT comparing SCS to repeat lumbosacral spine surgery for chronic pain. Self-reported pain relief and patient satisfaction was greater in the SCS group as compared to the surgical group ( P < .01). While 14 of 26 surgical patients chose to cross over to SCS, only 5 of 24 SCS patients chose to cross over to surgery ( P = .02). Patients randomized to reoperation required increased opioid analgesics significantly more than those randomized to the SCS group ( P < .025) ( Fig. 18.10 ).

Thus, during the same time frame, anomalous observations concerning both the risk of tolerance and addiction with opioid use for chronic pain and the safety and efficacy of spinal cord stimulation for the many of the same conditions led to two paradigm shifts in our field. The existing normal science stated that chronic opioid therapy is safe and effective for the treatment of chronic pain that neurostimulation is less effective in this setting and that chronic opioid therapy should come before neurostimulation in a chronic pain treatment algorithm. With the multiple, parallel anomalous observations noted above which were inconsistent with the normal science, new models were developed and the paradigm shifted to a new model in which chronic opioids are considered ineffective and potentially dangerous for the treatment of chronic pain and that neurostimulation is safer, more effective, and should be used prior to the consideration of chronic opioids.
While I have abbreviated the discussions for the sake of brevity, there is evidence equal to that of the paradigm shifts discussed above for the past paradigm shifts in neuromodulation now presented. For decades, the normal science dictated that the gate control theory proposed by Ref. [ ] fully explained the mechanism of action of spinal cord stimulation induced analgesia. Epidural spinal cord stimulation activated the A-beta fibers in the dorsal horn. Anterograde conduction produced the epiphenomenon of stimulation-induced paresthesias while retrograde conduction inhibited the pain gait, resulting in decreased output of wide dynamic range neurons and less pain information being sent to the brain.
Starting with the work of [ ], other potential mechanisms of action at both the segmental and supraspinal levels were reviewed. A series of studies were then performed which suggested even more potential mechanisms of SCS action. In the SENZA RCT [ ], randomized 198 subjects with back and leg pain to either novel high-frequency SCS or traditional low-frequency SCS. At 3 months, 84.5% of implanted HF10 therapy patients were responders for back pain and 83.1% for leg pain while 43.8% of traditional SCS subjects were responders for back pain and 55.5% for leg pain ( P < .001). The superiority of HF10 therapy was sustained through 12 months ( P < .001). Apropos to the current discussion, and despite their better pain relief, HF10 therapy subjects did not experience paresthesias [ ]. investigated the potential mechanism of action of 10 kHz SCS and determined that 10 kHz stimulation selectively activated nonadapting neurons in vivo and more readily activates GABAergic or tonic-firing neurons than presumed excitatory neurons ex vivo. They suggest that 10 kHz SCS inhibits pain sensory processing in the spinal dorsal horn by activating inhibitory interneurons without activating dorsal column fibers, resulting in paresthesia-free pain relief. That work, and other work implicating stimulation-induced proteomic changes in glia involved in the neuropathic process, however, examined dorsal horn circuitry under the electrodes and not in the lower portions of the cord where back and lower extremity pain is processed. Moreover, changes in only GABAergic (i.e., inhibitory) interneurons per se does not necessarily translate into suppression of WDR cells (the cells carrying pain signals) and may result in a dorsal horn circuit dynamic that actually favors excitation, by overinhibiting some of the other inhibitory pathways.
A more recently compelling mechanism has been suggested in computational modeling work from Ref. [ ] wherein axonal thresholds in the dorsal column fibers change based on stimulation. The larger fibers (e.g., A b vibration-carrying), which normally have the lowest thresholds and typically carry the paresthesia signal of tonic stimulation, have a higher and more rapid rise in their thresholds than medium and smaller fibers. When this threshold accommodation is taken into account, many waveforms now being used (Burst, frequencies higher than 200 Hz, longer pulsewidths) are shown to lead quickly to no firing in these larger paresthesia-carrying fibers, but instead causes activity in the much more numerous medium-sized fibers. Such a shift in the population of fibers being recruited by these waveforms, even at the clinically low amplitudes used, can account for pain relief without paresthesia and the potential for utilization of other ascending pathways that might be additive in the overall tolerance of pain.
Burst spinal cord stimulation has also been demonstrated in an RCT to be superior to traditional tonic SCS [ ]. This study compared a proprietary pulse sequence of a group of five 1 ms pulses separated each by 1 msec, with this burst being repeated 40 times per second to traditional tonic SCS in 100 subjects. Subjects received both Burst and tonic SCS and were randomized to which type of stimulation they received first. After receiving the first pulse sequence for 12 weeks, they were switched to the second pulse sequence for 12 weeks. Noninferiority of Burst stimulation ( P < .001) and superiority of Burst stimulation ( P < .017) over tonic stimulation was demonstrated. Again and apropos to the current discussion, patients treated successfully with Burst stimulation did not experience stimulation induced paresthesias.
To explain the mechanism of action of Burst stimulation and how it differs from tonic stimulation, it is important to recognize that there are at least two ascending pain processing pathways. The lateral discriminatory pathway ascends in the spinothalamic tract, synapses in the sensory thalamic nuclei (VPM for the face, VPL for the body) and then project to the primary and secondary somatosensory cortices. This pathway carries the sensory aspects of pain: its location, intensity, and type. Both tonic and Burst stimulation modulate this lateral discriminatory pathway and reduce pain to a similar degree and through a common mechanism.
The second is the medial affective/attentional pathway which addresses the affective component of the pain. The medial affective pathway ascends the spinal cord and projects to the medial thalamus, where it synapses with neurons in thalamic nuclei including MDvc and VMpo. These fibers then project to structures of the limbic system, including the anterior cingulate, the anterior insula, and the amygdala. Burst and not traditional SCS modulates this medial affective pathway; this results in a decrease in the suffering component of the pain. When the somatosensory and affective components of pain are both modulated by Burst stimulation, this results in improved patient satisfaction with SCS therapy.
We began this section with two normal sciences related to neuromodulation. First, that the mechanism of action of SCS was fully explained by the pain gate theory. Second, and related to the first, was that in order to produce pain relief, SCS must create stimulation-induced paresthesias. Over the past decade, several anomalous observations emanating from high-quality RCTs demonstrated several other potential mechanisms of SCS action and that superior pain relief could be obtained in the absence of stimulation induced paresthesias. This has led to the introduction of new science and resulted in a paradigm shift in neuromodulation. We now recognize that there are several different mechanisms underlying the analgesic effect of SCS and that stimulation induced paresthesias are not required for this effect. In fact, these stimulation induced paresthesias are epiphenomenal at best.
Clearly from my perspective, the evolutionary changes in neuromodulation technology are unlikely to create the future of our field. For any neuromodulation technique, whether it be deep brain stimulation (DBS), SCS, peripheral nerve stimulation (PNS), or sacral nerve stimulation, simply increasing the number of leads or the number of electrodes will not be sufficient to have a major impact on our field. Similarly, novel pulse trains, currently erroneously referred to as “novel waveforms” may well contribute to the asymptotic improvement in efficacy that the field has experienced but these improvements must be limited in impact as we already approach 90% efficacy for many of our therapies. Smaller batteries, increased battery capacity, power saving strategies may result in improved patient comfort and satisfaction but are unlikely to drive our field in the future. Even demonstrating efficacy for previously unproven applications in pain, movement disorders, psychiatric disorders, bowel, and bladder dysfunction will be limited in terms of their impact on the future of our field.
Research that has the potential to disrupt current normal science and lead to a paradigm shift, however, is not as uncommon as might be thought. I present here a list of potential disruptive technologies that may well be the short and longer term future of neuromodulation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here