Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Introduction to [CR] , Papillary Carcinoma Observation.
Recognizing that the incidence of papillary microcarcinoma (PMC) detected on autopsy and clinical screening studies far exceeded the prevalence of clinically apparent thyroid cancer more than 20 years ago, Miyauchi from the Kuma Hospital in Kobe, Japan, hypothesized that most PMCs would remain small and never develop into clinically significant disease. Furthermore, he posited that immediate surgery for all PMCs may be associated with more harm than good, as it was very likely that the few low-risk papillary thyroid cancers destined to become clinically significant disease would progress slowly and be very effectively treated at the time of documented disease progression. These hypotheses provided the scientific rationale for an observational clinical trial of PMCs that began at the Kuma Hospital in 1993 and then subsequently at the Cancer Institute Hospital in Tokyo in 1995.
Multiple publications from Japan since that time have demonstrated the safety and effectiveness of an observational management approach (also known as active surveillance) in PMCs. As Miyauchi posited, the incidence of having adverse events such as temporary and permanent vocal cord paralysis, temporary and permanent hyperparathyroidism, and the need for levothyroxine administration were all significantly higher in patients that underwent immediate surgery than in patients followed with active surveillance even though the surgeries were performed by well-experienced endocrine surgeons at Kuma Hospital, a center for thyroid care. Furthermore, active surveillance was also demonstrated to be a cost-effective alternative to immediate surgery. The calculated total cost of immediate surgery with postoperative management for 10 years, including the cost for reoperative surgery for recurrent disease, was 4.1 times the total cost of active surveillance for 10 years, including the cost for conversion surgery.
More recently, studies from the United States and Korea have reproduced and validated the Kuma Hospital observations. In 2011, the Japanese Association of Endocrine Surgeons and the Japanese Society of Thyroid Surgery guidelines endorsed active surveillance as a reasonable management option. Based largely on Japanese experience and an improved understanding of the natural history of low-risk papillary thyroid carcinoma, the American Thyroid Association (ATA) guidelines now endorse active surveillance as an acceptable management option for biopsy-proven, very low-risk thyroid cancer. Furthermore, the ATA guidelines also endorse an observational management approach without cytologic confirmation in subcentimeter thyroid nodules that demonstrate ultrasonographic characteristics that are highly suspicious for thyroid cancer. It is important to note that the active surveillance terminology can be applied to patients with either cytologically confirmed low-risk thyroid cancers or with thyroid nodules classified as having a high risk of being malignant without cytologic confirmation.
Consistent with the practice at the time, Kuma Hospital had previously recommended that thyroid nodules ≥ 5 mm with suspicious features on ultrasonography be evaluated with ultrasound-guided fine-needle aspiration (FNA). Today, at Memorial Sloan Kettering Cancer Center (MSKCC), patients with highly suspicious thyroid nodules who are deemed to be appropriate for active surveillance are given the option of a cytologic confirmation of malignancy before observational management or active surveillance without cytologic confirmation consistent with the ATA guidelines.
Thus an observational initial management approach is now considered to be a very viable alternative to immediate surgery. It is therefore incumbent on clinicians to understand (1) the low likelihood and pattern of disease progression in low-risk papillary thyroid cancer during active surveillance, (2) the approach to proper patient selection, (3) the characteristics of a successful observational management program, and (4) the criteria used to determine when transition from observation to surgical intervention is warranted.
In properly selected patients the vast majority of patients demonstrate tumor sizes that are stable or decreasing, whereas only 2% to 8% demonstrate an increase of ≥ 3 mm in maximal diameter (approximately 100% increase in tumor volume) and 12% to 14% demonstrate an increase in tumor volume more than 50% ( Table 21.1 ). An increase of ≥ 3 mm in maximal diameter is the minimum change in size that can reliably be measured on serial ultrasonography and has traditionally and effectively been used to be the primary determinate of whether or not a low-risk thyroid cancer has changed significantly. More recently, tumor growth has been described in terms of changes in volume, with a 50% increase or decrease in tumor volume being the smallest change that can be reliably detected. When analyzed based on tumor volume, 12% to 14% of tumors increase in size during the first 2 to 3 years of follow-up. Interestingly, the kinetics of tumor growth demonstrated classic log-linear exponential growth patterns, with rates of change that varied between tumors but were remarkably constant for an individual PMC. As described in subsequent sections (see indications to move from active surveillance to surgical intervention), although tumor volume measurements can reliably determine which small thyroid cancers are slowly growing, we do not consider a 50% increase in tumor volume as an indication for immediate surgery. Instead, we use tumor volume changes to help guide the frequency of subsequent ultrasound follow-up and view them as just one component in the description of disease progression that needs to be integrated into a decision about whether or not continued observation or immediate surgery is needed.
| First Author | n (Tumor Size) | Median Follow-Up | Maximum Diameter ± 3 mm | Tumor Volume ± 50% | Lymph Node Metastasis | ||||
|---|---|---|---|---|---|---|---|---|---|
| Increase | Stable | Decrease | Increase | Stable | Decrease | ||||
| Ito | 1235 (< 1 cm) | 5 yrs | 5% (5 yrs) 8% (10 yrs) |
95% (5 yrs) 92% (10 yrs) |
— — |
— | — | — | 2% (5 yrs) 4% (10 yrs) |
| Sugitani | 415 (< 1 cm) | 6.5 yrs | 6% | 91% | 3% | — | — | — | 1% |
| Tuttle | 291 (< 1.5 cm) | 2 yrs | 4% | 92% | 4% | 12% | 79% | 7% | 0% |
| Kwon | 192 (< 1 cm) | 2.5 yrs | 2% | 95% | 3% | 14% | 69% | 17% | 0.5% |
Interestingly, during active surveillance a decrease in tumor size of more than 3 mm is seen in 3% to 4% of the patients, and a decrease in tumor volume of more than 50% can be seen in 7% to 17% of the patients (see Table 21.1 ). The first report about active surveillance from Kuma Hospital also described a decrease in tumor size of more than 2 mm in 12% of the 58 patients who were surveyed for 5 years or more. The precise reasons for decreasing tumor size over time during active surveillance are unknown but could be associated with either damage to the tumor related to the FNA biopsy procedure, the natural history of these small nodules, or an immune response from the patient.
Identification of novel lymph node metastases during follow-up ranges from 2% to 4% over 5 to 10 years of follow-up. In properly selected patients, it is unusual to identify new lymph node metastases during the first 2 years of follow-up. But as these lymph node metastases usually grow quite slowly, consistent with the primary tumor, they can be identified with high quality ultrasonography during long-term follow-up. Distant metastases have not been identified in any of the patients being followed with observation.
Many published studies have looked for possible predictors of disease progression during active surveillance. They uniformly find that age is a significant predictor of disease progression such that patients < 40 years old at the time of a PMC diagnosis have about a 6% risk of having an increase greater than 3 mm, whereas patients ≥ 60 years old only had a 2.2% risk (p = 0.0014). Similarly the likelihood of identifying lymph node metastases was higher in the younger patients than the older patients (5.3% versus 0.4%, p < 0.001). Interestingly, most of the tumors that showed disease progression had either tumor enlargement or the appearance of lymph node metastasis. The risk of disease progression (increase in tumor size greater than or equal to 3 mm or novel lymph node metastasis) was significantly lower in older patients than in younger patients. After 10 years of active surveillance, patients get 10 years older. Miyauchi et al. calculated disease progression rates at 10 years of active surveillance for patients in each age decade. The lifetime disease progression rates estimated with these values were 48.6%, 25.3%, 20.9%, 10.3%, 8.2%, and 3.5% in patients diagnosed in their 20s, 30s, 40s, 50s, 60s, and 70s, respectively. The estimate indicates that more than a half of the patients in their 20s, about 75% of the patients in their 30s, and the vast majority of the older patients will not require thyroid surgery in their lifetime.
Although there is uniform agreement that excess thyroid-stimulating hormone (TSH) stimulation (TSH levels above the normal reference range) should be avoided during active surveillance, it is less certain whether mild TSH suppression would be beneficial. Although Sugitani did not find a correlation between TSH levels and PMC disease progression, a study from Korea did demonstrate that sustained TSH elevations more than 2.5 mIU/mL were associated with PMC disease progression. In the Kuma Hospital series, none of the 50 patients that were treated with levothyroxine to achieve a low normal TSH demonstrated disease progression. Further research on this topic is needed; in the meantime it is certainly reasonable to maintain TSH levels in the low normal reference range during active surveillance.
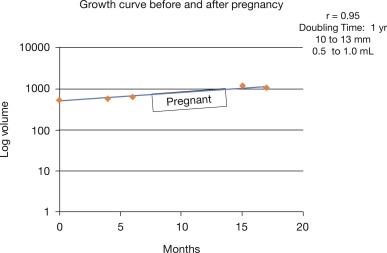
Pregnancy is known to be a mild growth stimulant to papillary thyroid carcinoma. Although the initial small case series suggested that papillary thyroid microcarcinoma enlargement during pregnancy was common, a follow-up study of 51 pregnancies demonstrated that only 8% of the patients had enlargement of the papillary thyroid carcinoma during pregnancy. After delivery, two patients underwent surgery, and two other patients had disease stabilization again after pregnancy and continue to follow with observation. It is also important to remember that PMCs are more likely to grow in the younger age group, so an increase in the size of a PMC during pregnancy may be due to the underlying growth kinetics of the tumor rather than to the influence of pregnancy ( Figure 21.1 ). Thus we consider women with childbearing potential to be appropriate patients for active surveillance, provided the patient understands the potential for pregnancy to increase the tumor size.
Currently, molecular profiling of PMCs cannot accurately identify the few tumors that are destined to progress and therefore is not used in the selection of patients for active surveillance. Although present in more than 50% of PMCs, BRAF V600E mutation was not predictive of tumor progression or identification of nodal metastases and TERT promoter mutations were not found in any of these tumors in a small series that went to surgery after a period of observation.
MSKCC is exploring the role of both functional and anatomic characteristics of neck magnetic resonance imaging (MRI) as a noninvasive biomarker to predict disease progression. In small pilot studies, lower apparent diffusion coefficient, diffusion coefficient, and higher perfusion fraction were associated with histologic features of tumor aggressiveness in small thyroid cancers.
A histopathological study of PMCs that were resected due to tumor enlargement, appearance of nodal metastases, and other nonprogression-related causes revealed high Ki-67 labeling index being associated with tumor enlargement. Although this was evaluated after surgery, these findings indicate the potential for analyzing aspirates of the tumors to predict tumor progression.
Other factors such as multifocality, family history of differentiated thyroid cancer, gender, and tumor size were not significantly associated with disease progression.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here