Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The pancreas is a complex retroperitoneal gland with both endocrine (e.g., glucose homeostasis) and exocrine (e.g., nutrient digestion) functions. An adult human pancreas measures approximately 15 cm in length and weighs between 60 to 100 g; however, its size can vary because of aging or pathologic conditions (e.g., pancreatitis, neoplasia). , It is of endodermal origin and arises from two independent primordia: a ventral bud (derived from the hepatic diverticulum) and a dorsal bud (derived from the developing duodenum; see Chapter 1 ). Around the fifth week of gestation, the ventral bud rotates clockwise with the developing duodenum to fuse with the dorsal pancreatic bud. , Ultimately, the ventral bud will form the inferior pancreatic head and the uncinate process. The dorsal bud will constitute the majority of the pancreatic gland, representing the superior pancreatic head, the body, and the tail of the adult pancreas (see Chapters 1 and 2 ). During this process, the main ducts of the ventral and dorsal pancreatic buds fuse to form the main pancreatic duct (duct of Wirsung). The major pancreatic duct drains most of the organ’s secretions through the major duodenal papilla (ampulla of Vater). A separate draining duct, arising from the dorsal pancreatic bud, usually persists and forms the minor pancreatic duct (duct of Santorini). The minor duct drains a portion of the pancreatic head secretions into the duodenum through the minor papilla, located 2 cm anterosuperior of the major papilla.
The pancreas receives an abundant arterial vascular supply from branches of the celiac and superior mesenteric artery. The venous drainage follows the arterial supply, with venous effluents ultimately draining into the portal vein. Furthermore, the pancreas is supplied by several neural sources, including sympathetic fibers from the splanchnic nerves, parasympathetic fibers from the vagus nerve, and peptidergic neurons (releasing amines and peptides; see Chapter 2 ). ,
The islets of Langerhans are the functional units of the endocrine pancreas and have a paramount role in maintaining glucose homeostasis. In light of their complex cytoarchitecture structure and regulatory system, they are de-facto microorgan(s) within the pancreas. The pancreas of a healthy adult has approximately one million islets that are evenly distributed throughout the pancreatic gland and account for 1% to 2% of the organ’s mass. Each islet ranges in size from 50 to 300 μm in diameter and contains a few hundred to a few thousand endocrine cells.
There are at least five major cell types in each islet of Langerhans: α, β, δ, F, and ε cells. In humans, pancreatic α-cells, which principally secrete glucagon, represent approximately 35% of all islet cells. Pancreatic β-cells, which are responsible for the production and secretion of insulin and amylin, represent approximately 55% of islet cells. Pancreatic δ-cells, which principally secrete somatostatin, represent less than 10% of the islet cells, and pancreatic F cells, which secrete pancreatic polypeptide (PP), account for less than 5%. Finally, the ε cells, which secrete ghrelin, account for less than 1% of human islet cells.
The distribution and cellular composition of the different cell types within the islet vary among species. Previous animal models with rabbits, rats, and mice demonstrated that β cells occupy the core of the islet of Langerhans and that non–β-cells are distributed toward the outside of the islet. Recent studies in humans have demonstrated a different cytoarchitecture, where the majority of α-, β-, and δ-cells reside along the islet blood vessels without a specific order. Furthermore, approximately 70% of human β-cells appear to be in contact with non–γ-islet cells, suggesting a predisposition for paracrine interaction. The islet’s regional location within the human pancreas is also essential to islet cytoarchitecture. Islets located in the body and tail of the pancreas have a higher proportion of α cells and a lower proportion of F cells, whereas islets located in the uncinate process have a higher proportion of F cells and a lower proportion of α-cells. Notably, β-cells and δ-cells are present in nearly equal proportions throughout the pancreas.
The islets are rich in axonal terminals and blood capillaries that participate in extensive neurohumoral and nonneuronal paracrine regulation. Studies using three-dimensional reconstruction of the axonal terminal field revealed that the autonomic innervation to the human islet of Langerhans is different from that previously identified in rodents. Contrary to what was previously understood, human β cells receive minimal innervation from the parasympathetic cholinergic system. Instead, sympathetic neural terminals penetrate the human islet of Langerhans to innervate the smooth muscle cells of the blood vessels, allowing fine regulation of islet blood flow. Consequently, sympathetic nerves indirectly influence downstream endocrine cells by regulating the local blood flow containing secreted endocrine hormone.
The islets of Langerhans receive approximately 20% of the pancreatic arterial flow, with distribution significantly influenced by the different phases of digestion. Furthermore, an insuloacinar portal system responsible for draining blood and secreted hormones from the islets of Langerhans to the pancreas’ acinar element exists in several species, including humans. Hormones secreted by the islet of Langerhans are directly transported to the acinar cells, where they can exert a local regulatory function. Besides, several neuropeptides, including neuropeptide-Y, gastrin-releasing peptide, and calcitonin gene-related peptide (CGRP), exert a local regulatory effect on endocrine and exocrine pancreatic function.
In 1923 Banting and McLeod, two Canadian surgeons, were awarded the Nobel Prize in Physiology or Medicine for the discovery of insulin. This 51–amino acid polypeptide is primarily responsible for maintaining serum glucose between 4 mM and 8 mM (70–140 mg/dL) during periods of feeding and fasting. Moreover, insulin regulates lipid and protein metabolism. The gene responsible for encoding insulin is located on the short arm of chromosome 11 and leads to the translation of a preprohormone protein known as preproinsulin within the β-cell. Preproinsulin consists of a leading sequence of 24 amino acids, followed by three domains named “B,” “C,” and “A.” Successive cleavage processes take place starting at the time of translation until the final secretion. First, cleavage of the leading sequence in the endoplasmic reticulum leads to the formation of proinsulin. As the proinsulin is arranged into secretory granules in the trans - Golgi, additional proteases cleave the central 31 amino acid C-peptide. This process leads to the formation of a mature insulin peptide composed of an A-chain and B-chain held together by two disulfide bonds, ready to be released in the secretory vesicle. Cleaved C-peptide and other intermediate products, such as proinsulin, remain present in the secretory granules and are eventually released with mature insulin. The mature insulin peptide has a plasma half-life of 4 minutes. It is rapidly internalized by target organs expressing its receptor and degraded by the kidneys and liver. Notably, C-peptide has a plasma half-life of 30 minutes and is excreted unchanged by the kidneys, making it a clinically meaningful marker of endogenous insulin secretion.
The rise of islet cell transplantation has led to a renewed understanding of human β-cell regulation, building on earlier work completed in rodents (see Chapter 126 ). Insulin is released from β-cells through two mechanisms: unstimulated and stimulated secretion. Unstimulated secretion or basal insulin secretion occurs every 6 to 8 minutes. Stimulated secretion of insulin occurs in response to several stimuli, including glucose, amino acids (e.g., arginine), acetylcholine (ACh), glutamate, and incretins such as gastric inhibitory peptide (GIP) and glucagon-like peptide-1 (GLP-1). The change in extracellular glucose concentration, however, is the dominant factor controlling β-cell function.
The primary glucose transporters on pancreatic β-cells are GLUT-2, highly expressed in rodents, and GLUT-1 and GLUT-3, both expressed at high levels on human β-cells ( Fig. 3.1 ). The GLUT transporters allow equalization of the intracellular and extracellular glucose concentrations. If the glucose concentration exceeds 5 mmol/L, the intracellular β-cell glucokinase enzymes activate to allow fine regulation of insulin secretion. Acting as a “glucose sensor,” these enzymes are responsible for the phosphorylation of glucose to glucose-6-phosphate. , Glucose-6-phosphate accumulates in the β-cell, is metabolized, and contributes to an increase in cellular adenosine triphosphate (ATP). The β-cell membrane is rich in ATP-dependent potassium channels, which subsequently undergo closure in response to the surge in ATP, resulting in membrane depolarization. , Depolarization activates voltage-gated L-type calcium channels, leading to an influx of calcium into the cell. , The increased intracellular calcium concentration leads to margination of secretory granules, their fusion with the cell membrane, and exocytosis of their content, including insulin and its intermediate products (e.g., C-peptide). This process characterizes the “first phase” of insulin release, in which insulin is rapidly secreted within 3 to 5 minutes of glucose administration and terminates within 10 minutes. The loss of the first phase of insulin secretion is one of the earliest metabolic defects identified in type 2 diabetes mellitus (T2DM). The “second phase” of insulin secretion is longer lasting (reaching a plateau in insulin secretion after 2–3 hours), and its regulation is not entirely understood. , Nevertheless, recent mathematic models suggest that the second phase is characterized by the recruitment and mobilization of intracellular granules containing insulin (as opposed to predocked granules as in the first phase) in a dose-dependent glucose response.
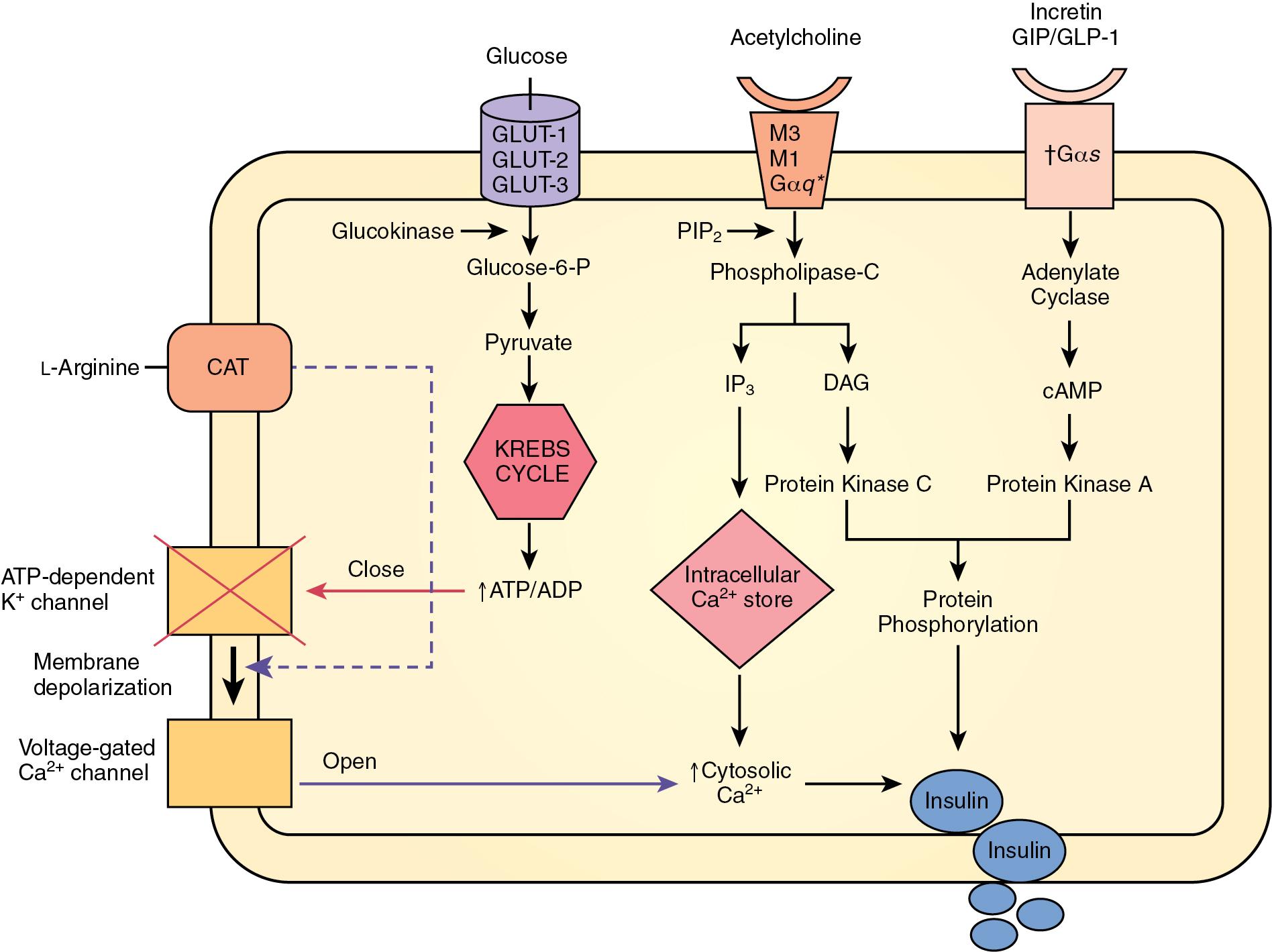
The amino acid arginine (l-arginine) is another well-known insulin secretagogue. After uptake into the β-cells through a cationic amino-acid transporter (CAT), arginine leads to depolarization of cell membrane, which triggers calcium influx. Furthermore, l-arginine can stimulate the release of GLP-1, which acts at its receptor (GLP-1R) to augment glucose-stimulated insulin secretion from pancreatic β-cells. ,
The incretin effect further potentiates insulin secretion from pancreatic β-cells through the enteroinsular axis. , The incretin effect is the phenomenon whereby the presence of nutrients, especially carbohydrates, in the duodenal lumen stimulates cells in the gut mucosa to release potent insulin secretagogues. The ingestion of nutrients stimulates duodenal and jejunal K cells to produce and release GIP, a well-studied incretin. Also, GLP-1 is produced and released by the L cells (also known as enteroglucagone cells), located in the distal small bowel, colon, and rectum. It has been shown that orally administered glucose can stimulate insulin secretion as much as 25% more than intravenously administered glucose, likely through the incretin effect.
GIP and GLP-1 play significant roles in the enteroinsular axis, mainly through activation of adenylate cyclase and subsequent increase in intracellular cyclic adenosine monophosphate (cAMP). The increase in cAMP leads to the activation of protein kinase A, with subsequent phosphorylation and activation of exocytosis-related proteins. Furthermore, cAMP activates L-type calcium channels, culminating in insulin release (see earlier).
Acetylcholine (ACh) maintains a pivotal role in glucose homeostasis. In human islets, ACh acts primarily as a nonneuronal paracrine signal released from α-cells rather than as a neural signal, as previously described in rodent islets. ACh stimulates the insulin-secreting β-cell via the muscarinic ACh receptors M3 and M5, causing insulin release. The activation of the M3 receptor leads to calcium release from intracellular stores through a phospholipase C–mediated increase in inositol-1,4,5-triphosphate (IP3). Additionally, ACh stimulates the somatostatin-secreting δ-cell via M1 receptors. Because somatostatin is known to inhibit insulin secretion, it appears that endogenous cholinergic signaling provides a direct stimulatory and indirect inhibitory input to β-cells, allowing further regulation of insulin secretion.
The islets of Langerhans secrete many additional hormones, including glucagon (α-cells), somatostatin (δ-cells), pancreatic polypeptide (F cells), and ghrelin (ε-cells). Furthermore, islets contain a variable, but small, number of cells responsible for secreting pancreastatin, serotonin, and vasointestinal polypeptide (VIP). Glucagon, a 29–amino-acid peptide (molecular weight, 3.5 kDa), counteracts insulin’s effect by increasing blood glucose concentration through stimulation of glycogenolysis, gluconeogenesis, and ketogenesis. The secretion from islet α-cells directly into the portal system is mostly in response to protein ingestion. Glucagon at physiologic concentration exerts its function primarily in liver tissue through activation of cAMP pathways, where it promotes gluconeogenesis and, indirectly, ketogenesis. Besides, glucagon indirectly stimulates fatty oxidation through the carnitine acylcarnitine translocase system (CAT). This process increases the ketone bodies β-hydroxybutyric acid and acetoacetic acid, which constitute metabolic fuel for other tissues. Glucagon inhibition is caused by increased blood glucose concentration and by paracrine effects of insulin and somatostatin within the islet.
Somatostatin, which acts primarily as an inhibitory hormone, is secreted by the islet δ-cell and by several other organs, including the hypothalamus and the D cells of the gastrointestinal (GI) tract. Among others, somatostatin inhibits insulin, glucagon, gastrin, and VIP. Its broad inhibition makes somatostatin and its pharmacologic analogs (e.g., octreotide) useful therapeutic agents in the medical management of secreting pancreatic neuroendocrine tumors (e.g., insulinoma; see Chapter 65 ) along with other medical diseases (e.g., Cushing’s disease, acromegaly, carcinoid). Furthermore, somatostatin analogs are used in the treatment of some surgical complications. For example, pasireotide, a multi-somatostatin receptor ligand, significantly decreased pancreatic leak complications after pancreatic surgery (see Chapter 117 ).
Pancreatic polypeptide (PP) is produced and released by islet F cells; however, its physiologic role remains under investigation. Some studies suggest that the absence of PP secretion, resulting from the removal of the uncinate process (rich in islet F cells) during pancreaticoduodenectomy, can lead to pancreatogenic diabetes (type 3c).
Ghrelin, produced by the ε-cells, also known as the “hunger hormone,” is a centrally active neuropeptide that participates in metabolic regulation, growth hormone release, and energy balance. , In particular, ε-cells participate in the regulation of various function of β-cells, including control of blood glucose levels as well as cellular growth. An increased understanding of ε-cells function and regulatory mechanism could lead to new therapeutic options for patients with diabetes mellitus.
Pancreatic endocrine insufficiency is a dreaded consequence of acute pancreatitis (AP). Recent studies suggest that after hospitalization for the first episode of AP, there is as much as a 40% risk of prediabetes or diabetes mellitus (see Chapters 55 and 56 ).
Approximately 15% of newly diagnosed diabetes mellitus occurs within 12 months from the first episodes of AP, and the risk remains high, increasing significantly with time. It appears that the development of endocrine insufficiency is at least partially related to the severity of the episode of AP with an estimated 2-fold increase in the prevalence of endocrine pancreatic insufficiency after an episode of necrotizing pancreatitis. The rate of insulin use within 5 years of the first episode of AP approaches 14%. Pancreatic necrosis and ethanol etiology represent strong risk factors for the development of pancreatic endocrine insufficiency. Alternative mechanisms have been proposed and are currently under investigation, including both patients and disease-related factors, such as age, body mass index, family history, duration of pancreatic disease, presence of exocrine insufficiency, and pancreatic surgery. It is worth mentioning that the risk for diabetes mellitus can be as high as 80% in patients with chronic pancreatitis (CP; see Chapters 57 and 58 ). , Although pancreatogenic diabetes mellitus (type 3c) is most commonly the result of CP, it can also occur secondary to pancreatic cancer. In fact, the prevalence of diabetes mellitus in pancreatic ductal adenocarcinoma at the time of diagnosis is approximately 50%, and importantly, 75% of these patients are diagnosed with diabetes mellitus within 2 years before the diagnosis of cancer (see Chapter 62 ). Pancreatic polypeptide deficiency represents a distinctive feature of pancreatogenic diabetes and has been associated with reduced hepatic sensitivity to insulin. ,
The exocrine pancreas constitutes 80% to 90% of the gland mass and secretes the majority of digestive enzymes, as well as approximately 2000 mL of colorless, odorless, and isosmotic alkaline protein-rich fluid (pH, 7.6–9.0) daily. It is mainly regulated by the neuroendocrine system, and it is integrated anatomically and physiologically with the endocrine pancreas, which helps modulate its function.
The exocrine pancreas consists primarily of two distinct but integrated units: the acinus and the ductal network.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here