Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Oxygen is the most frequently used ‘drug’ in emergency medicine.
Oxygen-delivery systems may be divided into variable-performance (delivering a variable concentration of oxygen) and fixed-performance (delivering a fixed concentration of oxygen, including systems that deliver 100% oxygen) types.
Fixed-performance systems are essential where precise titration of oxygen dose is required, as with chronic obstructive pulmonary disease (COPD) or where 100% oxygen is indicated.
Free-flowing circuits are least efficient at attempting to deliver 100% oxygen. A reservoir or demand system improves efficiency and a closed-circuit delivery system is most efficient.
One hundred percent oxygen is indicated when treating divers or patients with carbon monoxide or cyanide poisoning.
There is increasing evidence for goal-directed oxygen therapy, which should be regarded as a drug prescribed in therapeutic doses titrated to SaO 2 , rather than applied in a variable manner.
Titrated-dose oxygen therapy is required in treating patients with COPD, commencing with 24% to 28%. Response to therapy in these patients should be monitored with blood gases measurements.
Oxygen should never be abruptly withdrawn from patients in circumstances of suspected CO 2 narcosis.
Pulse oximetry provides valuable feedback regarding the appropriateness of oxygen dose provided to individual patients, but it should not be used as a measure of ventilatory adequacy; this should be monitored by end-tidal CO 2 , arterial blood gases, and the patient’s conscious state.
Oxygen was first discovered by Priestley in 1772 and was first used therapeutically by Beddoes in 1794. It now forms one of the cornerstones of medical therapy.
Oxygen (O 2 ) constitutes 21% of dry air by volume. It is essential to life. Cellular hypoxia results from a deficiency of oxygen regardless of aetiology. Hypoxaemia is a state of reduced oxygen carriage in the blood. Hypoxia leads to anaerobic metabolism, which is inefficient and may lead to death if not corrected. A major priority in acute medical management is the correction of hypoxia; hence oxygen is the most frequently administered drug in emergency medicine. There are sound physiological reasons for the use of supplemental oxygen in the management of acutely ill and injured patients.
To correct defects in the delivery of inspired gas to the lungs. A clear airway is essential
Where there is inadequate oxygenation of blood due to defects in pulmonary gas exchange
To maximize oxygen saturation of the arterial blood (SaO 2 ) where there is inadequate oxygen transport by the cardiovascular system
To maximize oxygen partial pressure and content in the blood in circumstances of increased or inefficient tissue oxygen demand
To provide 100% oxygen where clinically indicated
To titrate oxygen dose in patients with impaired ventilatory response to carbon dioxide
Oxygen proceeds from inspired air to the mitochondria via a number of steps known as the oxygen transport chain. These steps include
Ventilation
Pulmonary gas exchange
Oxygen carriage in the blood
Local tissue perfusion
Diffusion at tissue level
Tissue utilization of oxygen
The normal partial pressure of inspired air oxygen (P I O 2 ) is approximately 20 kPa (150 mm Hg) at sea level. If there is a reduction in the fraction of inspired oxygen (F I O 2 ), as occurs at altitude, hypoxia results. This is relevant in the transport of patients at 2400 m in commercial ‘pressurized’ aircraft, where ambient cabin pressures of 74.8 kPa (562 mm Hg) results in a P I O 2 of 14.4 kPa (108 mm Hg).
Hypoxia can result from inadequate delivery of inspired gas to the lung. The many causes include airway obstruction, respiratory muscle weakness, neurological disorders interfering with respiratory drive (seizures, head injury), disruption of chest mechanics (chest injury), or extrinsic disease interfering with ventilation (intra-abdominal pathology). These processes interfere with the maintenance of an adequate alveolar oxygen partial pressure (P A O 2 ), which is approximately 13.7 kPa (103 mm Hg) in a healthy individual.
An approximation of the alveolar gas equation permits rapid calculation of the alveolar oxygen partial pressures, as follows:
Oxygen diffuses across the alveoli and into pulmonary capillaries and carbon dioxide diffuses in the opposite direction. The process is passive, occurring down concentration gradients. The Fick law summarizes the process of diffusion of gases through tissues:
Where V̇O 2 = rate of gas (oxygen) transfer, ∝ = proportional to, A= area of tissue, T = tissue thickness, Sol = solubility of the gas, MW = molecular weight, P A = alveolar partial pressure and P pa = pulmonary artery partial pressure.
In healthy persons, oxygen rapidly passes from the alveoli to the blood and, after 0.25 seconds, pulmonary capillary blood is almost fully saturated with oxygen, resulting in a systemic arterial oxygen partial pressure (P a O 2 ) of approximately 13.3 kPa (100 mm Hg). The difference between the P A O 2 and the P a O 2 is known as the alveolar to arterial oxygen gradient (A − a gradient). It is usually small and increases with age.
The expected A−a gradient when breathing air approximates to Age (years) ÷ 4 + 4.
An approximation of the actual value can be calculated as follows:
There is a defect in pulmonary gas exchange if the calculated value exceeds the expected value. The A−a O 2 gradient is increased if there is a barrier to diffusion, such as pulmonary fibrosis or oedema or a deficit in perfusion, such as a pulmonary embolism. An increased A−a gradient also reflects widespread ventilation/perfusion mismatch.
In circumstances of impaired diffusion in the lung, raising the F I O 2 assists oxygen transfer by creating a greater pressure gradient from the alveoli to the pulmonary capillary. The increase in F I O 2 may not be as helpful when lung perfusion is impaired as a result of increased intrapulmonary shunting.
Four steps are required to deliver oxygen to the periphery:
Uptake of oxygen by haemoglobin (Hb)
Cardiac output to carry the oxygenated haemoglobin to the peripheral tissues
Dissociation of oxygen from haemoglobin into dissolved oxygen in plasma
Diffusion of dissolved oxygen from blood to cells via plasma, extracellular fluid (ECF), and finally, intracellular fluid (ICF)
The haemoglobin – oxygen (Hb – O 2 ) dissociation curve is depicted in Fig. 2.2.1 , which also summarizes the factors that influence the position of the curve. If the curve is shifted to the left, this favours the affinity of haemoglobin for oxygen. These conditions are encountered when deoxygenated blood returns to the lung. A shift of the curve to the right favours unloading of oxygen and subsequent delivery to the tissues.
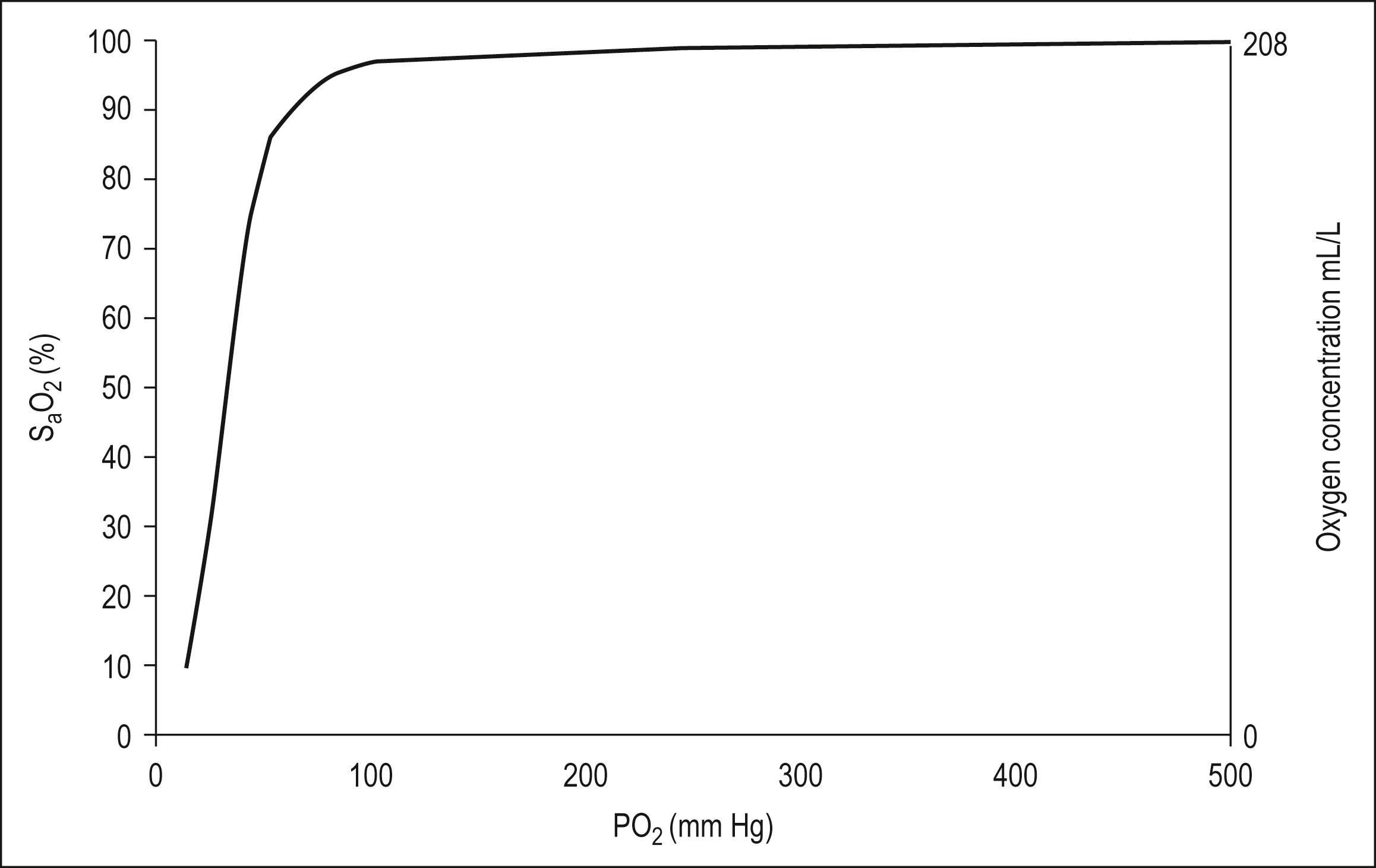
A number of advantages are conferred by the shape of the Hb – O 2 dissociation curve that favour uptake of oxygen in the lung and delivery to the tissues. They are as follows:
The flat upper portion of the curve allows some reserve in the P A O 2 required to keep the haemoglobin fully saturated; a reduction in P A O 2 of 20% will have minimal effect on the oxygen loading of Hb.
The flat upper portion of the curve also ensures that a large difference remains between P A O 2 and the pulmonary capillary oxygen partial pressure (P pc O 2 ), even when much of the haemoglobin has been loaded with oxygen. This pressure difference favours maximal Hb – O 2 loading.
The lower part of the curve is steeper, which favours offloading of oxygen in peripheral tissues with only small falls in capillary PO 2 . This maintains a higher driving pressure of oxygen, facilitating diffusion into cells
The right shift of the Hb – O 2 curve in circumstances of increased temperature, fall in pH, increased PCO 2 and increased erythrocyte 2,3 diphosphoglycerate (2,3-DPG) assists in further offloading of oxygen, even when the driving pressure has fallen and PO 2 has reached 5.3 kPa (40 mm Hg) (i.e. venous blood which is still 75% saturated with oxygen).
Oxygen is carried in the blood as dissolved gas in combination with haemoglobin. At sea level (101.3 kPa), breathing air (F I O 2 = 0.21), the amount of oxygen dissolved in plasma is small (0.03 mL oxygen per litre of blood for each 1 mmHg P a O 2 ). Hence at a P a O 2 of 100 mm Hg, 3 mL of oxygen is dissolved in each litre of plasma. Dissolved oxygen is important because it is the first available oxygen to diffuse into the tissues. The dissolved component assumes greater significance in the hyperbaric environment, where, at 284 kPa and an F I O 2 of 1.0, up to 60 mL oxygen can be carried dissolved per litre of blood.
Haemoglobin carries 1.34 to 1.39 mL of oxygen per gram when fully saturated. Blood with a haemoglobin concentration of 150 g/L carries approximately 200 mL oxygen per litre.
The total amount of oxygen delivered to the body per minute is known as oxygen flux.
where Hb = haemoglobin concentration g/L; S a O 2 = arterial oxygen saturation (percentage); P a O 2 = partial pressure of arterial oxygen (mm Hg); Q = cardiac output (L/min).
A healthy individual breathing air transports approximately 1000 mL of oxygen per minute to the tissues, based on a cardiac output of 5 L/min; 30% or 300 mL/min of this oxygen is not available, because at least 2.7 kPa (20 mm Hg) driving pressure is required to allow oxygen to enter the mitochondria. Therefore approximately 700 mL/min is available for use by peripheral tissues. This provides a considerable reserve above the 250 mL/min consumed by a healthy resting adult.
In illness or injury, this reserve may be considerably eroded. Factors that reduce oxygen flux include a fall in cardiac output of any aetiology (including shock states), anaemia or a reduction in functional haemoglobin (carbon monoxide poisoning) and a drop in the S a O 2 . These situations are frequently encountered in the emergency department. Supplemental oxygen is required in addition to specific therapy, such as volume replacement, transfusion, and measures to improve cardiac output.
Cellular hypoxia results if there is impairment of perfusion to local tissues. Oedema associated with medical illness or local injury increases the diffusion distance between blood and the cell, thus mandating a higher P a O 2 to ensure adequate tissue oxygen delivery.
Increased oxygen flux is required if either
Tissue demands for oxygen are higher than normal
Tissue utilization of oxygen is impaired
Elevation of cardiac output increases oxygen flux in these circumstances, but frequently this too is significantly impaired by the disease state.
Tissue demands for oxygen increase by 7% for each degree Celsius elevation in body temperature and considerably greater increases in demand occur in seizures, sepsis, severe dyspnoea, restlessness and shivering.
Tissue extraction of oxygen is impaired in sepsis and by poisons, such as carbon monoxide or cyanide. In all cases, oxygen therapy must be combined with general measures, such as reduction of fever and specific treatment of the primary disease process.
Oxygen delivery systems are classified into three groups ( Box 2.2.1 ) :
Variable-performance systems
Fixed-performance systems
100% oxygen systems
Nasal cannulae
Hudson mask with/without reservoir
T pieces and Y connectors
Venturi mask
Oxygen blenders
Non-rebreathing circuits
Free-flowing circuits
Self-refilling circuits
Soft reservoir bags
Oxygen-powered resuscitators
Partial rebreathing circuits
Closed-circuit systems
These systems deliver a variable F I O 2 to the patient, which is altered by the inspiratory flow rate, the minute volume of the patient, and the physical characteristics of the delivery system.
These systems deliver a specified F I O 2 to the patient that is not altered by changes in ventilatory pattern, volume, or inspiratory flow rate.
This is a subgroup of fixed-performance systems wherein 100% oxygen is delivered to the patient.
The oxygen source in most Australian and New Zealand emergency departments consists of a wall-mounted flowmeter capable of delivering oxygen up to 15 L/min, with most available oxygen delivery systems connecting to this apparatus. A flow rate of 15 L/min limits the delivery of high F I O 2 to adults for the following reasons:
The quietly breathing adult has a peak inspiratory flow rate (PIFR) of approximately 30 to 40 L/min, which exceeds the oxygen supply. Hence a free-flowing system, such as a Hudson mask, must entrain air into the system in order to match the patient’s PIFR, with a resultant reduction in F I O 2 to a maximum of 0.6.
The quietly breathing adult has a respiratory minute volume (RMV) of 4 to 8 L; in a child, this value is approximately 150 mL/kg. Oxygen is stored during expiration by incorporating a reservoir into the circuit for use during inspiration, with a considerable improvement in the economy of oxygen use. This system is limited by the patient’s minute volume. If the minute volume exceeds 15 L, there is a danger of the patient asphyxiating due to insufficient gas supply or, if safety valves allow air into the system, the F I O 2 falls.
If higher flows are needed, some oxygen ports provide up to 25 L/min. More efficient control of flow is achieved via higher-output or dial-up flowmeters. An extra source of oxygen flow may cause variable-performance systems such as the Hudson mask to become fixed-performance systems. Hence the terms variable performance and fixed performance are loosely applied and are largely dependent on whether or not the gas flow delivered is sufficient to match the patient’s ventilatory requirements.
An example of this is in paediatric oxygen delivery. A high F I O 2 can be delivered using a standard 15 L/min oxygen source because the child’s ventilatory requirements are smaller in proportion to the available oxygen supply.
The oxygen delivery systems available for use in emergency medicine, summarized in Box 2.2.1 , can be further subdivided according to economy of oxygen use and whether or not the system can be used to ventilate the patient manually.
The F I O 2 delivered by these systems is summarized in Table 2.2.1 . Options available for use in emergency medicine include
Nasal cannulae
Face masks with air inlets with or without reservoirs
T pieces and Y connectors
| Apparatus | Oxygen flow (L/min) | Oxygen concentration (%) |
|---|---|---|
| Nasal catheters | 1–4 | 24–40 |
| Semi-rigid mask | 6–15 | 35–60 |
| Semi-rigid mask and double O 2 supply | 15–30 | Up to 80 |
| Semi-rigid mask and reservoir bag | 12–15 | 60–90 |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here