Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The choice of imaging modality varies and depends on the location and nature of the disease process.
Computed tomography (CT) is fast and widely available and can be used as the first and often the only imaging modality in the majority of disease processes that affect the neck.
CT excels in demonstrating bone detail.
Intravenous (IV) contrast administration for CT scans improves the quantity and quality of information provided.
Radiation exposure from CT examination is substantial and should be taken into account, particularly in young patients who require repeat examinations.
Magnetic resonance imaging (MRI) provides excellent soft tissue contrast and is the modality of choice in most aggressive processes.
Appropriate use of fat suppression and contrast are essential for most MRI examinations of the head and neck.
The accuracy of positron emission tomography (PET) increases when it is used in conjunction with CT.
Successful primary staging of most malignant lesions in the neck can be accomplished with CT or MRI and clinical examination.
PET/CT is superior in identifying distant metastases and posttreatment recurrence.
Image interpretation should always be made in conjunction with clinical information.
To provide a practical differential diagnosis for neck mass lesions, an approach based on neck spaces defined by the cervical fascia is advised.
Diagnostic medical imaging has changed medical and surgical diagnosis in ways never imagined. Every area of clinical medicine has been affected in a profound way. Through their consultations, radiologists are able to assist otolaryngologists in a variety of ways: they can provide primary diagnosis, confirm a clinical impression, evaluate regional anatomy and extent of disease, assist in definitive treatment of patients, and assess response to treatment.
Neuroradiologists are subspecialty-trained radiologists who specialize in imaging the head and neck, skull base, temporal bone, brain, and spine. They are the primary imaging consultants for otolaryngologists.
This chapter provides an introduction and overview of head and neck imaging for the otolaryngologist. The various available imaging modalities are discussed, and imaging strategies for various regions and clinical questions are reviewed. The basic approach to the radiologist's image acquisition and interpretation is described so that the referring physician will gain a measure of understanding of this field. This is intended to maximize the usefulness of diagnostic imaging in the care of patients.
The scope of head and neck imaging is too broad a topic to be covered in one chapter. Here, we provide the clinician with only an outline and brief synopsis of the field. Definitive textbooks for each area of head and neck imaging are available.
Since the discovery of the x-ray, conventional radiology (CR) has been used in imaging the head and neck region. AP and lateral views of the neck exposed for soft tissue detail are useful for the overall contour of the soft issues of the neck, and are often used in the urgent diagnoses of upper airway infection or foreign body aspiration. However, CR in the head and neck region has been largely replaced by computed tomography (CT).
CT was developed for clinical use in the mid-1970s by Hounsfield. CT scanners have evolved over time such that the most advanced scanners now scan in a “helical” fashion, in which the scanner uses a slip-ring technique. This allows the table to move as the scan is performed, resulting in complete volumes of tissue being imaged without skipping tissue between slices. The ratio of the speed at which the table moves to the time it takes for a complete rotation of the CT tube is called the pitch. Multidetector CT allows scanning of multiple slices during one rotation of the CT tube, which significantly increases the speed of the examination and makes it possible to obtain much thinner slices in a short time. At present, CT scanners can obtain slices less than 1 millimeter thick, and a neck examination from skull base to the mediastinum can be performed in less than 30 seconds. More importantly, enhanced computer technology permits real-time manipulation of these data, allowing multiplanar and various forms of three-dimensional (3D) reconstructions without image degradation. Images can be reconstructed in any desired plane regardless of the plane used to acquire the images.
CT uses a tightly collimated x-ray beam that is differentially absorbed by the various body tissues to generate highly detailed cross-sectional images. The degree of attenuation of the x-ray photons for each voxel (smallest imaging unit) is assigned a numeric readout. These units of attenuation are known as Hounsfield units (HUs) and generally range from –1000 HU to 1000 HU. Water is assigned a value of 0 HU, whereas fat is approximately –80 to 100 HU. Calcium and bone are in the 100 to 400 HU range, and most fluids are in the 0 to 30 HU range.
To create images from the attenuation values, CT uses complex mathematical reconstruction algorithms also known as filters . Bone disease and bone trauma are best visualized with a bone detail algorithm, whereas a soft tissue algorithm is used to evaluate soft tissue structures ( Fig. 8.1 ).
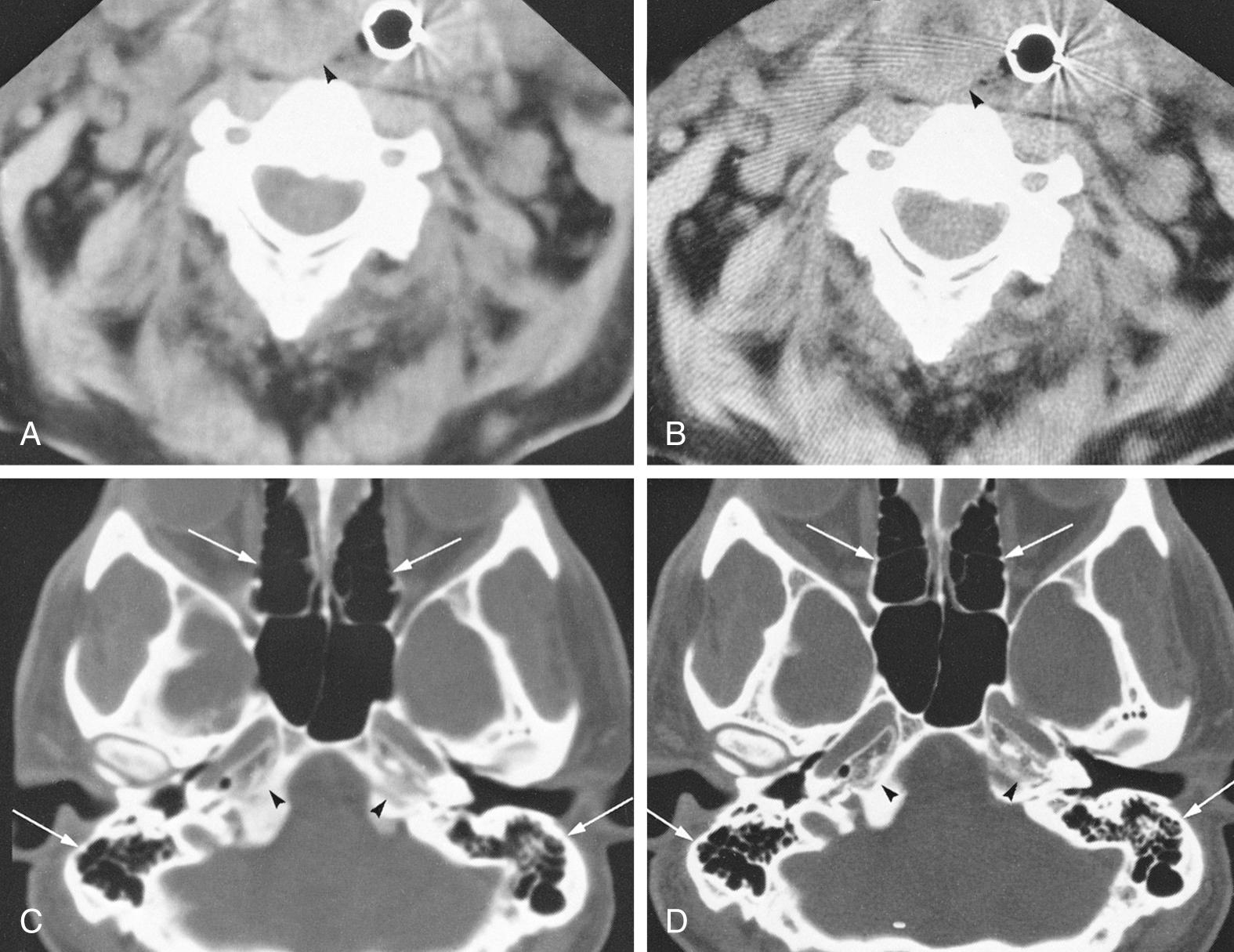
Images from a given reconstruction algorithm can be displayed in various ways to highlight differences in attenuation of different structures. The window width refers to the range of attenuation values in HU that make up the gray scale for a given image. The window level refers to the center HU value for that given window width. A narrow window width of 80 HU and a level of 40 HU is frequently used for brain imaging, because it centers the density at the common density of brain tissue, and it displays only those densities 40 HU greater than and 40 HU less than the window level. Thus any density greater than 80 HU will be displayed as white, and any density less than zero will be displayed as black on the gray scale. Any intermediate density will be spread out evenly along the gray scale. For imaging of the soft tissues of the head and neck, a window level of approximately 40 to 70 HU is usually chosen at a midpoint approximately equal to the density of muscle. The window width frequently is in the 250 to 400 HU range, thus displaying a wider range of densities that include calcification, intravenous (IV) contrast, muscle, and fat to best advantage. For imaging of bony structures, such as paranasal sinuses and temporal bone, window levels from 0 to 400 HU and a wide window width of 2000 to 4000 HU may be chosen. The reason for a wide bone window width is that a wide range of densities, from cortical bone (approximately 1000 HU) down to gas (–1000 HU), need to be displayed on the same image. However, structures of intermediate density between bone and gas occupy a narrow range on the gray scale at this window width and are poorly discriminated (appear washed out) at these settings. The terminology commonly used to describe the previously mentioned windows includes soft tissue windows (window width of 250 to 400 HU) and bone windows (2000 to 4000 HU). It is important to understand that these display windows are completely independent of the mathematical imaging algorithm chosen for creation of the image. In other words, an image created by a soft tissue algorithm can be displayed with soft tissue and bone window widths (see Fig. 8.1A and C ). Conversely, the image may be computer reconstructed using a bone algorithm and displayed with either a soft tissue or bone window width (see Fig. 8.1B and D ). To optimize imaging of the soft tissue lesion and adjacent bone, a soft tissue and a bone algorithm may be used to generate images with the appropriate soft tissue and bone windows.
Picture archiving and communication systems (PACS) have become the norm for image display and storage.
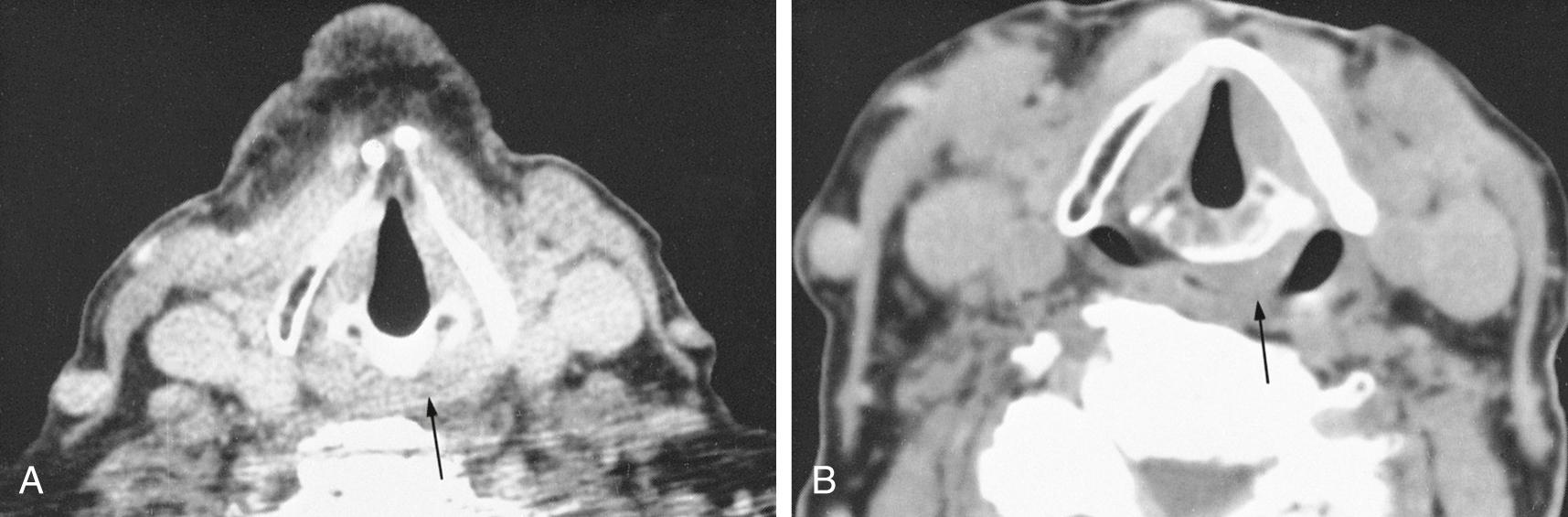
Patient cooperation is necessary to obtain optimal image quality. The patient is instructed not to swallow and to stop breathing or to maintain quiet breathing during each slice acquisition to minimize motion artifact from the adjacent airway and pharyngeal structures. Occasionally, provocative maneuvers may be necessary, such as blowing through a small straw or using a cheek-puffing (modified Valsalva) maneuver to distend the hypopharynx or phonating to assess vocal cord movement ( Figs. 8.2 and 8.3 ).
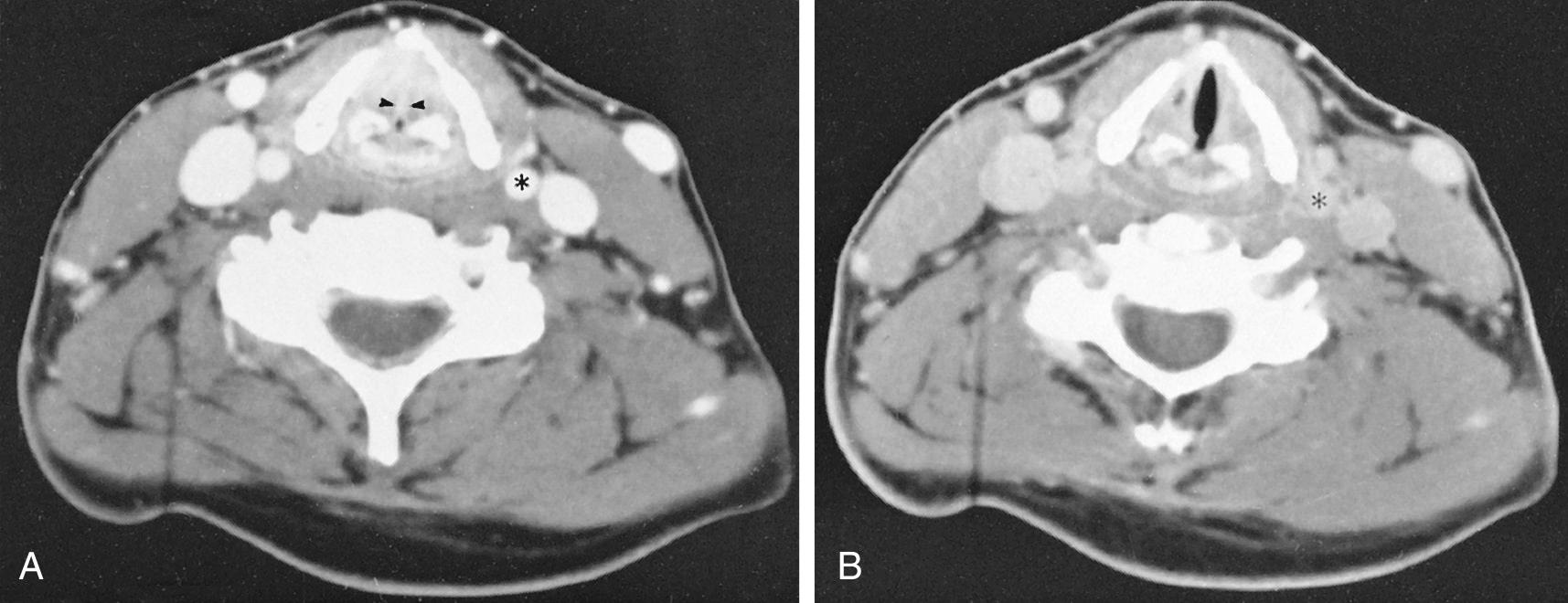
Contrast enhancement is often used to opacify blood vessels and to identify regions of abnormal tissue as identified by abnormal enhancement patterns ( Fig. 8.4 ). As it relates to head and neck imaging, contrast is particularly useful in CT scans of the neck and orbits. Contrast often is not needed to evaluate the temporal bones, although it can be necessary on occasion. CT of the facial bones and paranasal sinuses does not require IV contrast for most common applications. Iodinated contrast material is potentially nephrotoxic and may be harmful for patients with impaired renal function. Acute adverse reactions to iodinated contrast can be allergic-like or physiologic, and not uncommon but usually mild. They can rarely be in the form of anaphylactic shock.
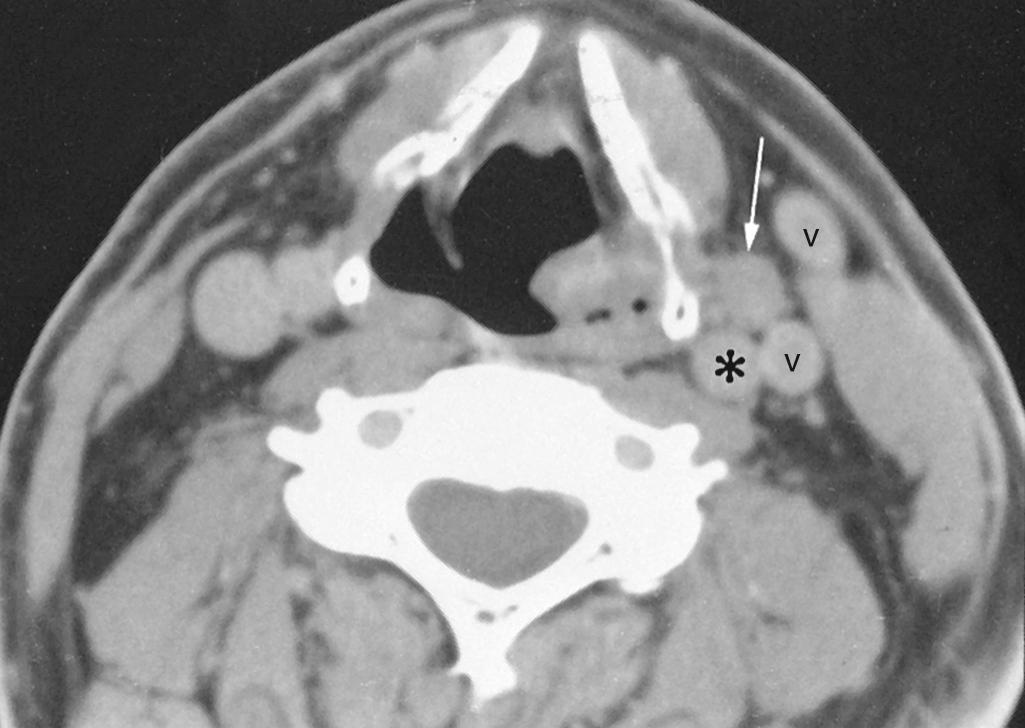
As a brief review, the radiation exposure (dose) that a patient receives is known as the radiation absorbed dose , a measure of the total radiation energy absorbed by the tissues; it is expressed in an international system (SI) unit known as the gray (Gy). One Gy is the amount of radiation needed to deposit the energy of 1 joule (J) in 1 kg of tissue (1 Gy = 1 J/kg). Formerly, the unit used to express radiation absorbed dose was the rad (1 rad = radiation needed to deposit the energy of 100 ergs in 1 g of tissue). The formula to convert rads to Gy is 100 rads equals 1 Gy.
Radiation dose equivalent is a more useful term because it considers the “quality factor” (Q) of the radiation involved (radiation dose equivalent = radiation absorbed dose Q). The quality factor considers the varying biologic activity of various types of ionizing radiation. For x-rays, Q = 1. Thus when discussing diagnostic x-rays, the radiation dose equivalent equals the radiation absorbed dose. The SI unit for the radiation dose equivalent is the sievert (Sv). The former unit was the Roentgen equivalent for man (rem). In summary, 1 Gy = 1 Sv, and 1 Sv = 100 rem.
The radiation dose equivalent depends on the choice of tube voltage and current settings (kilovolt peak [kVp] and milliamperes [mAs]), slice thickness, pitch, and gantry cycle time. For a given kilovolt peak, the radiation dose equivalent will vary linearly with the milliamperes; the actual dose will vary slightly among machines. The radiation dose equivalent for a CT examination can be considerably reduced using a low-milliampere technique.
The effective dose equivalent was developed as a means of representing the fraction of the total stochastic risk of fatal cancers and chromosomal abnormalities resulting from the irradiation of a particular body part. A system of weighting is used to consider the individual susceptibility of the body's major tissues and organs, although a full discussion of this is beyond the scope of this chapter. Suffice to say that, for a given examination, the effective dose to the patient is less than the dose (radiation dose equivalent) received by the area under examination. A list of common radiologic procedures and their effective dose equivalents is given in Table 8.1 .
| Examination | Effective Dose Equivalent |
|---|---|
| Chest radiographs | 20 mrem |
| CT, abdomen | 1,000 mrem |
| CT, chest | 1,000 mrem |
| CT, brain | 120 mrem |
| CT, sinus | 70–130 mrem |
Magnetic resonance imaging (MRI) is an imaging modality that uses the response of biologic tissues to an applied and changing magnetic field to generate images. It is not possible to completely describe the principles of MRI in an introductory chapter of all head and neck imaging; however, a brief summary follows.
Two types of magnets are used to perform clinical MRI: permanent and superconducting. Permanent magnets do not require continual input of energy to maintain the magnetic field. They are composed of large magnetic metallic elements set up to generate a uniform magnetic field between components. Superconducting magnets are electromagnets usually composed of niobium-titanium wire. They require an input of energy to start them, but once they are up to strength, they are maintained in a superconductive state by means of an encasing system of liquid nitrogen and liquid helium shells.
The earth has a magnetic field strength of 0.5 gauss (G). The tesla (T) is another unit of magnetic strength related to gauss by the Eq. 1 T = 10,000 G. Clinical MRI units usually operate at magnetic field strengths of between 0.3 and 3.0 T, although small-bore research scanners of strengths up to 9.0 T are in use.
Many MR pulse sequences are available to generate images. The most common pulse sequences in MRI are the spin-echo and gradient-echo techniques.
MRI is one of the most active areas of development and research within diagnostic radiology. MRI derives its signal from hydrogen protons, most abundant in tissue fat and water. When placed in a high magnetic field, the spinning protons are aligned in the direction of the magnetic field. Radiofrequency (RF) pulses are transmitted into the subject to excite the spinning protons, changing their orientation with respect to the magnetic field. As the protons realign with the magnetic field, they lose energy and give off a signal, which is picked up by coils and reconstructed into an image. The quality of MRI depends on a high signal/noise ratio, which is used to improve image contrast and spatial resolution. In general, the higher the field strength of the magnet, the higher the signal/noise ratio.
A surface coil is a receiving antenna for the RF signal emitted from the imaging subject after the initial RF stimulation. The standard head coil is usually adequate for studying head and neck disease above the angle of the mandible. A head coil allows imaging of the adjacent brain and orbits, an advantage when head and neck lesions extend intracranially. Neck coils cover a larger area from the skull base to the clavicles and come in various configurations, for example, volume neck coil and anterior neck coil. Surface coils significantly improve the quality of head and neck imaging by more effectively collecting the signal and hence increasing the signal/noise ratio, and they are able to collect signal from a smaller body part.
Slice thickness on MRI is most commonly 3 to 5 mm, with thinner sections used for smaller regions of interest. However, a thinner slice has a smaller signal/noise ratio. Occasionally, greater spatial resolution may be needed for small structures (e.g., facial nerves), requiring a volume acquisition technique. The number of slices is limited in MRI, as opposed to CT, by the specific sequence used. Covering the entire neck from skull base to superior mediastinum often requires that two separate acquisitions be obtained.
Motion artifact, chemical shift artifact, susceptibility artifacts from metallic implants (e.g., amalgam, orthodontic implants), and mascara degrade MRI ( Fig. 8.5 ). Motion artifact becomes more prominent with increased field strength, increased length of individual pulse sequences, and the total length of the imaging study. A typical imaging sequence may last from 2 to 8 minutes. To limit motion artifact, sequences of less than 4 minutes are preferred, and the patient should be instructed not to swallow and to breathe shallowly and quietly.
Chemical shift artifact arises from the differences in resonance frequencies of water and fat protons. The result is an exaggerated interface (spatial misregistration) in areas where fat abuts structures that contain predominantly water protons, such as the posterior globe or a mass. Chemical shift artifact may produce the appearance of a pseudocapsule around a lesion, or it may cause obscuration of a small-diameter structure, such as the optic nerve. Chemical shift artifact may be identified as a bright band on one side of the structure and a black band on the opposite side. This is usually most noticeable on T1-weighted images (T1WIs).
Metallic artifact from dental work varies in severity, depending on the amount and composition of the metal in the mouth as well as on the pulse sequence and field strength of the MRI scanner. Most dental amalgam causes mild distortion to the local magnetic field and results in a mild dropout of signal around the involved teeth. Extensive dental work, metallic implants, and braces may cause more severe distortion of the image, precluding visualization of the maxilla, mandible, and floor of the mouth. Mascara that contains metallic compounds can also cause localized signal loss in the anterior orbit and globe.
Numerous pulse sequences are available on clinical MRI units. The details of the physics of MRI may be found in most radiology-MRI textbooks. Commonly used imaging protocols include T1-weighted, spin (proton) density, T2-weighted, gadolinium-enhanced T1-weighted, fat-suppressed, and gradient-echo imaging ( Fig. 8.6 ). Magnetic resonance angiography (MRA) is infrequently obtained. The abbreviations used to identify sequence parameters are repetition time (TR), echo time (TE), and inversion time (TI) and are measured in milliseconds. The following description of pulse sequences is presented to help the clinician identify and understand the commonly performed sequences and to determine their respective uses in the head and neck.
T1-weighted (short TR) sequences ( Fig. 8.7A ; see also Fig. 8.6A ) use a short TR (500 to 700 ms) and a short TE (15 to 40 ms). T1-weighted imaging is the fundamental head and neck sequence, because it provides excellent soft tissue contrast with a superior display of anatomy and a high signal/noise ratio, and it uses a moderate imaging time (4 to 5 min), thus minimizing motion artifacts. Fat is high signal intensity (bright or white) on T1WIs and provides natural contrast in the head and neck. Air, rapid blood flow, bone, and fluid-filled structures, such as vitreous and cerebrospinal fluid (CSF), are low signal intensity (dark or black) on T1WIs. Muscle is low to intermediate in signal intensity on T1WIs. The inherent high contrast of fat relative to adjacent structures allows excellent delineation of the muscles, globe, blood vessels, and mass lesions that border on fat. The cortical bone is black, and the enclosed bone marrow is bright from fat within the marrow. The aerated paranasal sinuses are black, whereas retained mucous or mass lesions are of low to intermediate signal intensity. Most head and neck mass lesions will show a comparable signal to that of muscles on T1WIs. (To quickly identify a T1WI: fat is white, CSF and vitreous are black, and nasal mucosa is low signal.)
T2-weighted images (T2WIs; see Figs. 8.6C and 8.7B ) use a long TR (2000 to 4000 ms) and a long TE (50 to 90 ms) and are sometimes referred to as long TR/long TE images . T2WIs are most useful for highlighting pathologic lesions, because T2WIs show the vitreous and CSF as high in signal intensity (bright) relative to the low to intermediate signal intensity of head and neck fat and muscle. Fat loses signal intensity with increased T2 weighting. Nowadays, most radiologists use a fast spin-echo (FSE) T2WI for head and neck imaging, which provides a much faster acquisition with improved signal/noise ratio. Fat, however, remains bright on FSE images. Most head and neck masses are higher in signal intensity on a T2WI compared with their low to intermediate signal intensity on T1WI. The combination of the T1WI and T2WI is often useful for characterizing fluid-containing structures, solid components, and hemorrhage. Bone, rapid vascular flow, calcium, hemosiderin, and air-containing sinuses are black. Inflammatory sinus disease and normal airway mucosa appear very bright. (To quickly identify a T2WI: CSF, vitreous, and nasal mucosa are bright, whereas muscle is low to intermediate in signal.)
Gadolinium-based contrast material is used in conjunction with T1WI sequences (gadolinium shortens the T1) and, with the dose used, it has little effect on T2WI. The advantages of contrast enhancement are increased lesion conspicuity and improved delineation of the margins of a mass relative to the lower signal of muscle, bone, vessel, or globe. However, gadolinium contrast enhancement (without concomitant fat suppression) has had limited usefulness within the head and neck, as well as in the orbit, because of the large amount of fat present within these regions (see Fig. 8.6D ). After gadolinium contrast injection, the signal increases within a lesion, often obscuring the lesion within the adjacent high signal intensity fat. Therefore, for head and neck imaging, gadolinium enhancement is optimally used with specific fat-suppression techniques that turn fat dark or black. Gadolinium contrast enhances normal structures, including nasal and pharyngeal mucosa, lymphoid tissue in the Waldeyer ring, extraocular muscles, and slow-flowing blood in veins, all of which may appear surprisingly bright, especially if combined with fat-suppression techniques. (To quickly identify a gadolinium contrast-enhanced T1WI: nasal mucosa is white, fat is white, and CSF and vitreous are black.) Gadolinium-based contrast material can induce nephrogenic systemic fibrosis in patients with kidney disease, which may be fatal secondary to multiorgan failure. Patients with an estimated glomerular filtration rate less than 30 mL/min/body surface area should not receive gadolinium-based contrast material. The risk of nephrogenic systemic fibrosis (NSF) in patients receiving some newer gadolinium-based agents has been demonstrated to be low to nonexistent, and routine screening of outpatients for renal failure may not be necessary when these contrast agents are used. The American College of Radiology publishes the ACR Manual On Contrast Media which can be referenced for more information.
Several sequences have been developed that suppress fat signal intensity. T2WIs, short tau inversion recovery (STIR), spectral presaturation inversion recovery (SPIR), and chemical shift selective presaturation (fat saturation) are some of the more common clinically available methods of fat suppression. One advantage of fat suppression is reduction or elimination of chemical shift artifacts by removing fat signal from the image while preserving water signal. Additionally, some fat-suppression techniques take advantage of gadolinium contrast enhancement by eliminating the surrounding high-intensity signal from fat while retaining the high-intensity enhancement produced by gadolinium. Most pathologic lesions have increased water content, and gadolinium contrast exerts its paramagnetic effects while in blood vessels and in the increased extracellular fluid of the lesion, but gadolinium contrast does not enhance fat. The fat signal can be manipulated as follows:
STIR (see Fig. 8.7D ) provides reliable fat suppression over large body parts. The inversion time (e.g., 140 ms) is individually “tuned” for each patient to place fat at the null point of signal intensity; thus it eliminates fat signal by turning it completely black. STIR images show the mucosa, vitreous, and CSF as very high signal intensity. Most mass lesions in the head and neck will have similar high signal intensity on STIR and T2WI. The disadvantages of STIR are image degradation secondary to a decreased signal/noise ratio and increased vulnerability to motion artifacts, including vessel pulsations. Additional disadvantages of STIR, such as increased scan time and fewer slices, are circumvented by the recently available fast sequences. (To quickly identify a STIR image: fat is almost completely black; CSF, vitreous, and mucosa are very bright. A T1 image is listed with the TR and TE times on the image.)
Frequency-selective presaturation sequences (see Fig. 8.7C ) typically used with a spin-echo technique selectively suppress fat signal. (Note that for the remainder of this chapter, the terms fat suppression and fat saturation are used interchangeably and refer to frequency-selective [chemical shift] presaturation techniques.) T1-weighted fat-saturation sequences take full advantage of gadolinium enhancement. A gadolinium-enhancing lesion within the head and neck retains its high signal intensity and is not obscured, because fat is suppressed to become low to intermediate signal intensity. Enhancing masses within the head and neck and orbit are particularly well imaged with this technique. Frequency-selective fat suppression is also highly complementary for FSE T2WIs. Fat-saturated T2WIs provide excellent fat suppression, optimizing the high signal from normal structures and lesions that are high in water content, contrasted against a black background of fat. The disadvantages of fat-saturation sequences are that non–gadolinium-enhancing lesions may be less well discriminated, these sequences are more susceptible to artifacts, and nonuniform fat suppression can occur. Also, fewer slices are acquired than with T1WI, unless the TR time is lengthened, which prolongs imaging time. (To quickly identify a gadolinium-enhanced T1WI with fat saturation, mucosa and small veins are white, fat is low to intermediate intensity, and CSF and vitreous are black.)
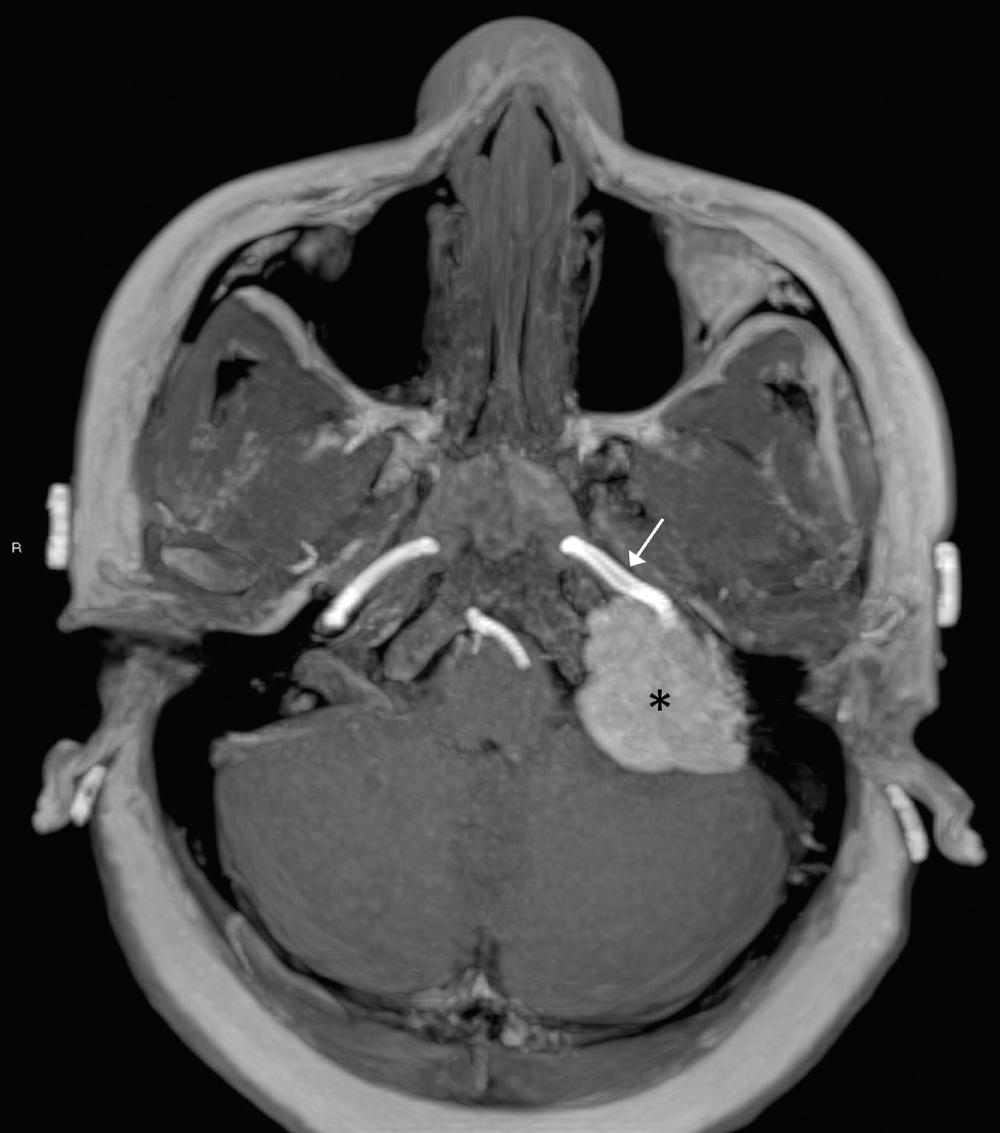
Numerous gradient-echo sequences are available that have a variety of applications. Gradient-echo scans have a very short TR (30 to 70 ms), a very short TE (5 to 15 ms), and a flip angle of less than 90 degrees. They have a variety of proprietary acronyms that include GRASS, MPGR, SPGR, FLASH, and FISP. Gradient-echo sequences take advantage of the phenomenon of flow-related enhancement. That is, any rapidly flowing blood will appear extremely bright. These sequences are useful for localizing normal vessels, detecting obstruction of flow in compressed or thrombosed vessels, or showing vascular lesions that have a tubular, linear, or tortuous bright signal that represents regions of rapid blood flow ( Fig. 8.8 ). Gradient-echo sequences may be obtained faster than conventional spin-echo techniques, although their increased susceptibility to motion artifact decreases the benefits of a short scan time. Gradient-echo techniques also permit volume, that is, 3D versus two-dimensional (2D) acquisition of images, allowing increased spatial resolution and computer workstation reconstruction of any imaging plane at various slice thicknesses. The disadvantage of gradient-echo sequences is the increased magnetic susceptibility artifact from bone or air, which limits their role near the skull base or paranasal sinuses. (To quickly identify a gradient-echo image, arteries and often veins are white; and fat, CSF, vitreous, and mucosa may have variable signal intensities, depending on the technique used.)
Diffusion weighted imaging (DWI) evaluates the random motion of water within tissue. It is routinely used in brain imaging for detecting acute stroke. Restricted water movement occurs with increased intracellular water or tissue cellularity, displayed as bright signal on DWI sequences. An apparent diffusion coefficient (ADC) is calculated from multiple DWI sequences acquired at different b-values to quantify diffusion and account for intrinsic T2 signal (T2 shine-through). Greater water restriction will have a lower ADC value, displayed as a darker signal on the ADC map.
There is increasing interest in extracranial application of DWI techniques. Studies of DWI tumor characterization demonstrate lower ADC values in malignant tumors compared to benign lesions, and in lymphomas compared to carcinomas. Cholesteatomas in the temporal bone typically restrict diffusion which is useful in postoperative evaluation for recurrent or residual disease (see Fig. 8.74 ). Parotid gland tumor subtypes can be predicted with DWI, with pleomorphic adenoma demonstrating relatively high ADC values. Staging and follow-up of head and neck squamous cell cancer may benefit from DWI which has shown promise in the detection of nodal disease and disease recurrence. Significant obstacles currently limiting the use of DWI in head and neck include patient motion and susceptibility artifact from air-filled spaces.
MRA is most commonly generated by time-of-flight or phase contrast techniques, which rely on the flowing blood within the vessels as the signal source. Contrast-enhanced MRA uses the enhancement of blood with gadolinium-based contrast as the primary source of the signal. MRA can be obtained with 2D and 3D algorithms and currently provides the most pertinent information in many clinical scenarios, although it typically lacks the temporal resolution (ability to image a vessel over time, as the contrast material passes through) necessary to evaluate some of the vascular malformations. There are newer time resolved contrast enhanced MRA techniques with improved spatial and temporal resolution that may have value in the evaluation of head and neck hemodynamics and the workup of vascular malformations.
Several disadvantages of MRI of the head and neck bear consideration. MRI frequently requires 30 to 45 minutes for scanning, during which time the patient must remain motionless, a process difficult for a sick patient to accomplish. Motion artifacts are more frequently encountered than with CT, although dental artifacts may be less problematic. Although no harmful effects are known to occur during pregnancy, MRI should not be used during the first trimester. Absolute contraindications to MRI include patients with cardiac pacemakers and ferromagnetic intracranial aneurysm clips (select patients with “MR-conditional” pacemakers may be eligible for MRI under specific conditions). With the ever-increasing variety of implants with electronic components, the clinician must follow the manufacturer's recommendations as to the safety of MRI. Those patients at risk for metallic orbital foreign bodies should be screened with plain films or CT before MRI. Generally, ocular prostheses and ossicular implants are safe. Unfortunately, MRI is one of the more expensive imaging modalities.
High-resolution diagnostic ultrasound uses the properties of reflected high-frequency sound waves to produce cross-sectional images, which are obtainable in almost any plane. The transducer, a high-frequency 5- or 10-MHz probe, scans over the skin surface of the region of interest. Fat has a moderate degree of internal echoes (echogenicity), skeletal muscle is less echogenic than fat, and a solid mass has a variable echogenicity but is usually less echogenic than fat. A cyst has few, if any, internal echoes, a strongly echogenic back wall, and strong through-transmission of sound behind the cyst. Both calcium and bone are strongly echogenic and thus obscure adjacent structures by an acoustic shadow. Ultrasound has no known harmful effects and no contraindications. High-resolution ultrasound is quick and accurate, and Doppler ultrasound provides information on blood vessels and flow patterns. Furthermore, ultrasound is relatively inexpensive compared with CT or MRI. Disadvantages include limited field of view, lack of easily identifiable anatomic landmarks, and operator dependence.
As opposed to the imaging modalities already discussed in this chapter, which allow detailed anatomic information, positron emission tomography (PET) imaging provides physiologic and biochemical data. A positron-emitting radiopharmaceutical is intravenously injected, and its distribution in the body is measured. Positron-emitting radiopharmaceuticals can be developed from naturally occurring substances such as 15O water, 11C carbon monoxide, 13N ammonia, or radioactive analogues of other biologic substances such as 18F-fluorodeoxyglucose (FDG). After being emitted from the atom, the positron travels in the tissue for a short distance, until it encounters an electron and forms a positronium, which is immediately annihilated and converts its mass to energy, forming two 511-keV photons. These annihilation photons travel away from each other at approximately 180 degrees and are picked up by the detectors placed around the patient. Simultaneous detection of these photons relates them to the same annihilation event and allows spatial localization. Annihilation coincidence detection can be accomplished by expensive dedicated PET scanners, thus yielding superior spatial resolution and sensitivity. Less costly gamma camera–based hybrid systems allow for utilization of PET imaging outside academic centers.
Attenuation of the photons in the tissues they travel through decreases the apparent activity picked up by the detectors. Attenuation correction methods provide improved anatomic detail and better lesion localization, but they result in “noisier” images. The effect of attenuation correction on visual image quality is controversial and, in many centers, the images are generated both with and without attenuation correction. For semiquantitative and quantitative evaluation, however, attenuation correction is necessary.
Depending on the radiopharmaceutical chosen, PET imaging can provide information regarding blood flow, ischemia, deoxyribonucleic acid (DNA) metabolism, glucose metabolism, protein synthesis, amino acid metabolism, and receptor status. Radiopharmaceutical development requires sophisticated knowledge and equipment, which, combined with the very short half-life of most of these substances, limits its clinical utility. The relatively long half-life of FDG (110 minutes) accounts for its widespread use. In addition, FDG can be delivered to PET imaging facilities through commercial vendors, obviating the need for an on-site cyclotron.
Glucose metabolism in growing neoplastic cells is enhanced and accounts for the increased uptake on FDG-PET studies. Molecular studies have revealed that several genetic alterations responsible for tumor development also have direct effects on glycolysis. It has also been shown that increased tumoral FDG uptake is strongly related to the number of viable tumor cells, but it is not clearly associated with their proliferative rate. The glucose analogue 2-deoxy-D-glucose is transported into the cell and is metabolized in the glycolytic cycle. After phosphorylation with hexokinase to DG-6-phosphate, the compound is metabolically trapped in the cell. Because of this trapping mechanism, FDG concentration steadily increases in metabolically active cells, yielding high contrast between tumor and normal tissue. Bear in mind that increased glucose metabolism is not unique to malignant cells and can be seen in benign tumors, inflammatory or infectious lesions, and even normal tissues. Also, some malignant cells may not have increased glucose metabolism for a variety of reasons.
A typical PET scan is started 30 to 60 minutes after IV administration of approximately 10 mCi of FDG. A 6- to 12-hour period of fasting is required before injection, and patients are encouraged to drink water before the FDG injection to minimize collection in the urinary system. Because normal FDG uptake in muscle may mimic tumor, muscle relaxants such as benzodiazepines are used in some centers. Scanning takes 30 to 60 minutes and is performed in the supine position at multiple table positions to cover the entire body.
Qualitative evaluation of FDG-PET images is sufficient for most clinical purposes, but quantitative measurement of FDG concentration is possible. Several approaches of different complexity can be applied for this purpose. Some of these require complex computation, data acquisition, and arterial blood sampling during scanning. The most commonly used method, standardized uptake values (SUVs), is simple and is confined to the measurement of radioactivity concentrations at a single time point. The activity concentration is normalized to the body weight or body surface area. SUV calculation may allow differentiation of malignant tissue from benign causes of increased uptake and can be used to measure the response to treatment. A downside of SUV calculation in therapy monitoring is that it only allows comparison of two measurements obtained at the same time point after tracer injection.
A major disadvantage of PET is lack of anatomic information that results in poor lesion localization. A number of software applications are used to “fuse” PET images with CT scans or MRIs, which are obtained at different time points. Fusion of anatomic and functional images significantly improves lesion localization, but it is still subject to many technical difficulties and errors. Combined PET/CT units permit acquisition of both CT and PET images using a single piece of equipment in the same session without the need to move the patient. Errors in lesion localization are minimized, although they do occur in certain body regions where physiologic or involuntary motion is unavoidable.
Another major limitation of PET is poor spatial resolution. However, PET is an evolving technology, and improvements in spatial resolution will surely be accomplished. Because of fundamental limitations inherent to the method, however, the maximum achievable spatial resolution is 1 to 2 mm. Therefore PET is incapable of showing microscopic disease.
Scintigraphy has several applications in the head and neck. In salivary gland imaging, technetium-99m (Tc-99m) pertechnetate imaging may be useful for assessing salivary gland function in autoimmune and inflammatory disease of the salivary glands. If the salivary glands are obstructed, the degree of obstruction, as well as the follow-up of obstruction after treatment, can be assessed. In evaluating neoplasms of the salivary glands, the findings of the Tc-99m pertechnetate scan are almost pathognomonic of Warthin tumor and oncocytoma. Spatial resolution is approximately limited to 1.5 cm, so accurate localization of the mass within the gland is difficult. Single-photon emission computed tomography (SPECT) may be useful in some cases in providing more precise anatomic localization compared with planar images.
Techniques of thyroid imaging and thyroid therapy are described in several textbooks. Many centers use indium-123 (I-123) to obtain a thyroid update determination, and Tc-99m pertechnetate is used to obtain whole gland images. These images determine whether thyroid nodules are “hot” or “cold.” I-131 is used for therapy of hyperthyroidism and in follow-up to detect and treat residual, recurrent, and metastatic thyroid cancers.
Medullary carcinoma of the thyroid is difficult to visualize, but Tc-99m succimer (Tc-99m dimercaptosuccinic acid [DMSA]) has been used, and In-111 pentetreotide has also been used with some success.
Identification of parathyroid adenomas has been accomplished for several years with a subtraction technique using Tc-99m pertechnetate and thallium-201 (Tl-201). The basis of this test is that thallium is taken up by thyroid tissue and parathyroid tissue, whereas thyroid is the only tissue that takes up Tc-99m pertechnetate. Therefore the subtraction of the Tc-99m pertechnetate image from the Tl-201 image should leave only parathyroid tissue. The sensitivity of this technique is believed to be very high for lesions larger than 1 g. Sensitivity decreases for smaller lesions, and the subtraction technique can be hampered by patient motion. Tc-99m sestamibi is now the favored agent in many institutions. A double-phase imaging protocol is used with improved identification of parathyroid adenomas ( Fig. 8.9 ).
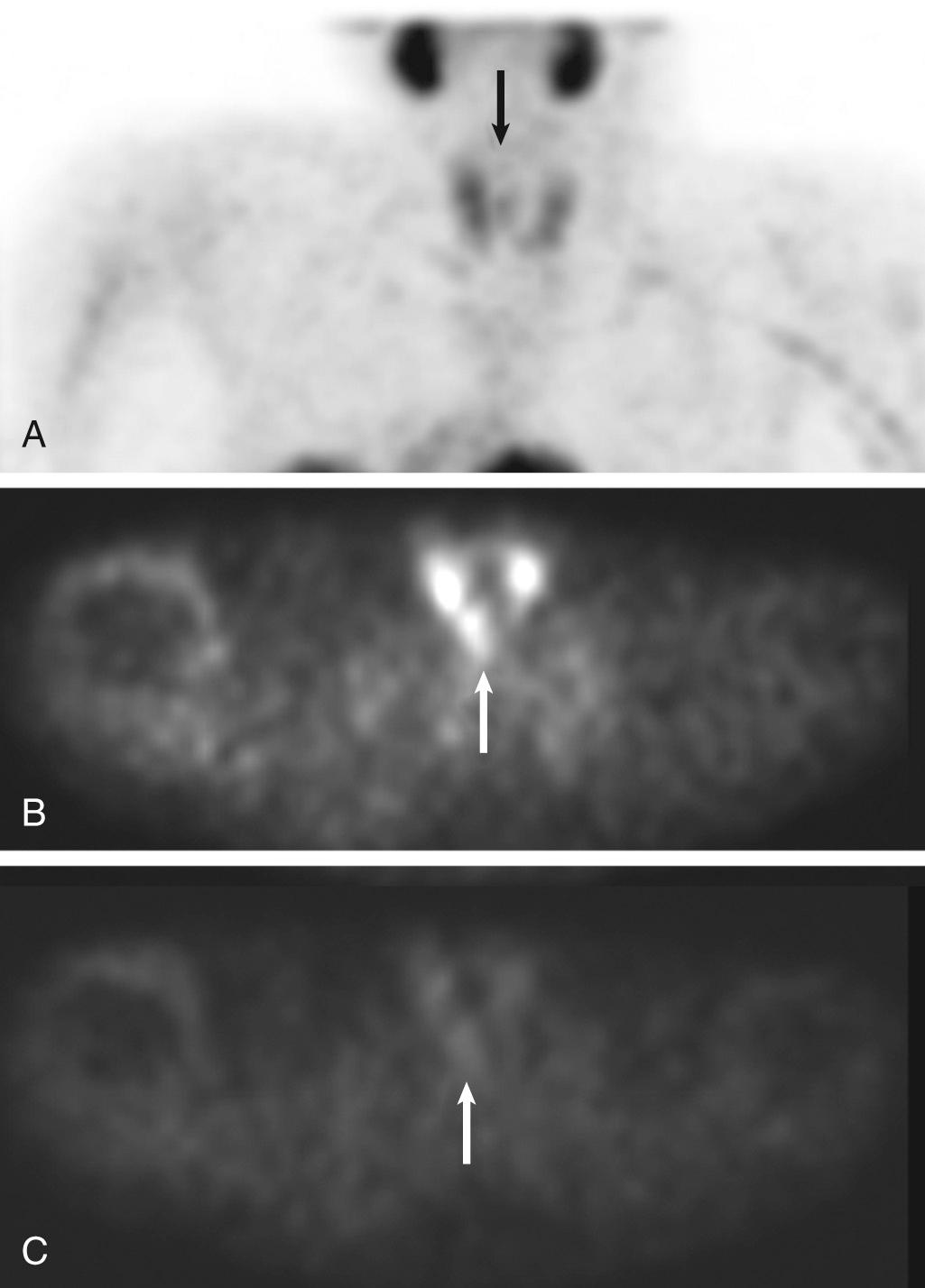
CSF leaks can be detected with In-111 pentetate (In-111 DTPA) placed into the subarachnoid space.
Image data from either CT or MRI can be processed to create 3D reconstructions. The state-of-the-art PACS now available in most institutions obviate the need for a separate workstation to perform these reconstructions.
CT data are loaded as a stack of contiguous 2D slices that define the scanned volume. Reconstructions are created either by choosing a specific range of densities for display or by manually tracing the outline of the desired structure. Improvements offered by multislice-multirow CT scanners and enhanced computational capacity of imaging workstations have led to a paradigm shift in radiology, in which volume imaging has replaced axial imaging. CT data from a large body part can be gathered in a very short time as a whole, and the obtained volumetric dataset can be displayed in various planes and as 3D reconstructions.
MR data for image analysis are best acquired using a volume acquisition method, in which data are acquired as a complete 3D block rather than as individual slices. Because volume acquisition takes longer, gradient-echo techniques are usually required to reduce the imaging time. Once acquired, the data are displayed in any desired plane, and by selecting a range of signal intensities, or by tracing specific structures with a cursor, 3D surface models are created.
The utility of 3D reconstruction is best appreciated with craniofacial reconstructions. Directly visualizing the 3D relationships of the facial structures aids surgical planning, and instructors find 3D models of the face and orbital structures useful for teaching medical students, residents, and anatomy students. Virtual endoscopy is a computer-generated simulation of endoscopic perspective. The virtual endoscopic images of the trachea, larynx, pharynx, nasal cavity, paranasal sinuses, and ear have demonstrated clinical utility ( Fig. 8.10 ).
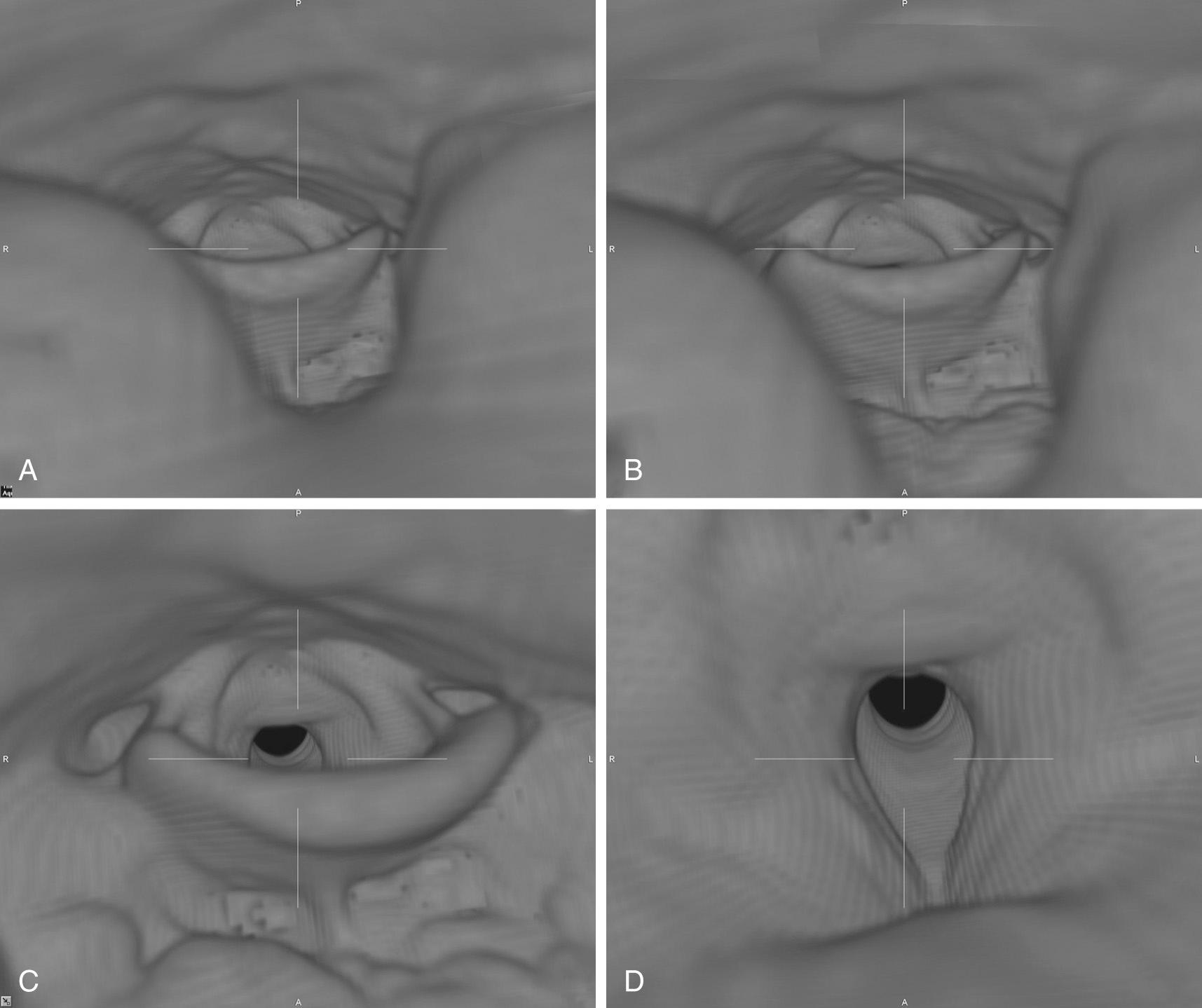
At present, the spatial resolution of CT is superior to MRI in the head and neck for displaying bony relationships. However, MRI provides a superior display of transcranial soft tissue structures, such as the entire visual pathway, and it has better tissue contrast resolution than CT. Thus CT and MRI will likely have complementary roles in 3D image display.
Each anatomic region requires a different imaging approach to optimize the detection and characterization of the structure or lesion of interest. The following is a description of the indications for using CT, MRI, and ultrasound in specific head and neck regions, plus a general imaging approach is given relevant to each anatomic region in terms of imaging planes, slice thickness, contrast agents, and pulse sequences. Whenever possible, CT and MRI are performed before a biopsy is taken and before resection of lesions because the resulting edema may obscure the true margins of a mass.
Multichannel CT scanners have revolutionized head and neck imaging: the entire neck can now be scanned in less than 30 seconds at a slice thickness of less than 1 mm; these data can then be reconstructed in any plane with a desired slice thickness. This breakthrough obviated the need for site-specific imaging protocols. A typical neck CT using a multidetector scanner uses a 1-mm or less slice thickness and a pitch of approximately 1, with contiguous axial scanning performed from the sella turcica down to the thoracic inlet. Then, typically 3- to 5-mm-thick axial, sagittal, and coronal images are reconstructed for view. The use of IV contrast is critical for interpretation of the study. Determination of extent of disease and vascular invasion, compression, and discrimination of vessels from nodes and small muscle bundles can be extremely difficult (see Figs. 8.3 and 8.4 ). Evaluation of the normal mucosa-submucosa interface and mucosal tumors cannot be accomplished without contrast enhancement. Optimally, contrast should be present in both arteries and veins during image acquisition, and enough contrast should be allowed to diffuse from vessels to the tissue interstitium for tumors to enhance. This is particularly important for high-end multidetector CT scanners, which tend to finish image acquisition before optimal tumor enhancement is achieved, unless a delay between injection of contrast and scanning is used. To maintain good opacification of vessels after this delay, a biphasic contrast injection scheme is used. The delay time and the rate at which contrast material is injected vary depending on the specifications of the scanner. Contrast is best administered with a mechanical pump infusion, although a drip-infusion technique may also be effective. Frequently, image reconstruction using a soft tissue algorithm suffices. If a suspicion of bone erosion or destruction by tumor or inflammation exists, sections of the skull base and mandible need to be reconstructed using a bone algorithm.
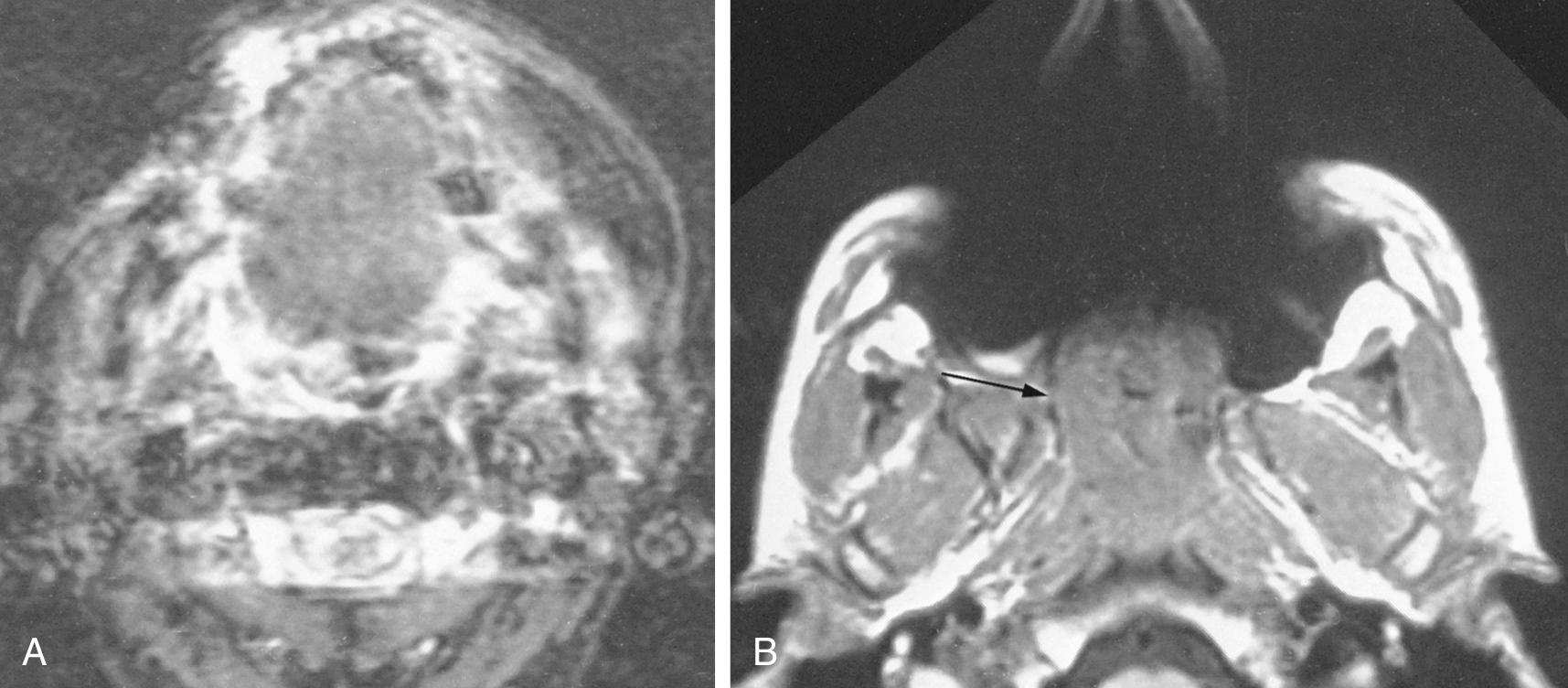
Suprahyoid neck CT is often performed for simultaneous evaluation of the deep extent of mucosal-based tumors and to evaluate associated metastatic disease to the cervical lymph node chains. Because streak artifacts from dental fillings frequently obscure the oropharynx and nasopharynx, it is usually necessary to obtain additional angled sections to assess the pharynx directly posterior to the dental work ( Fig. 8.11 ).
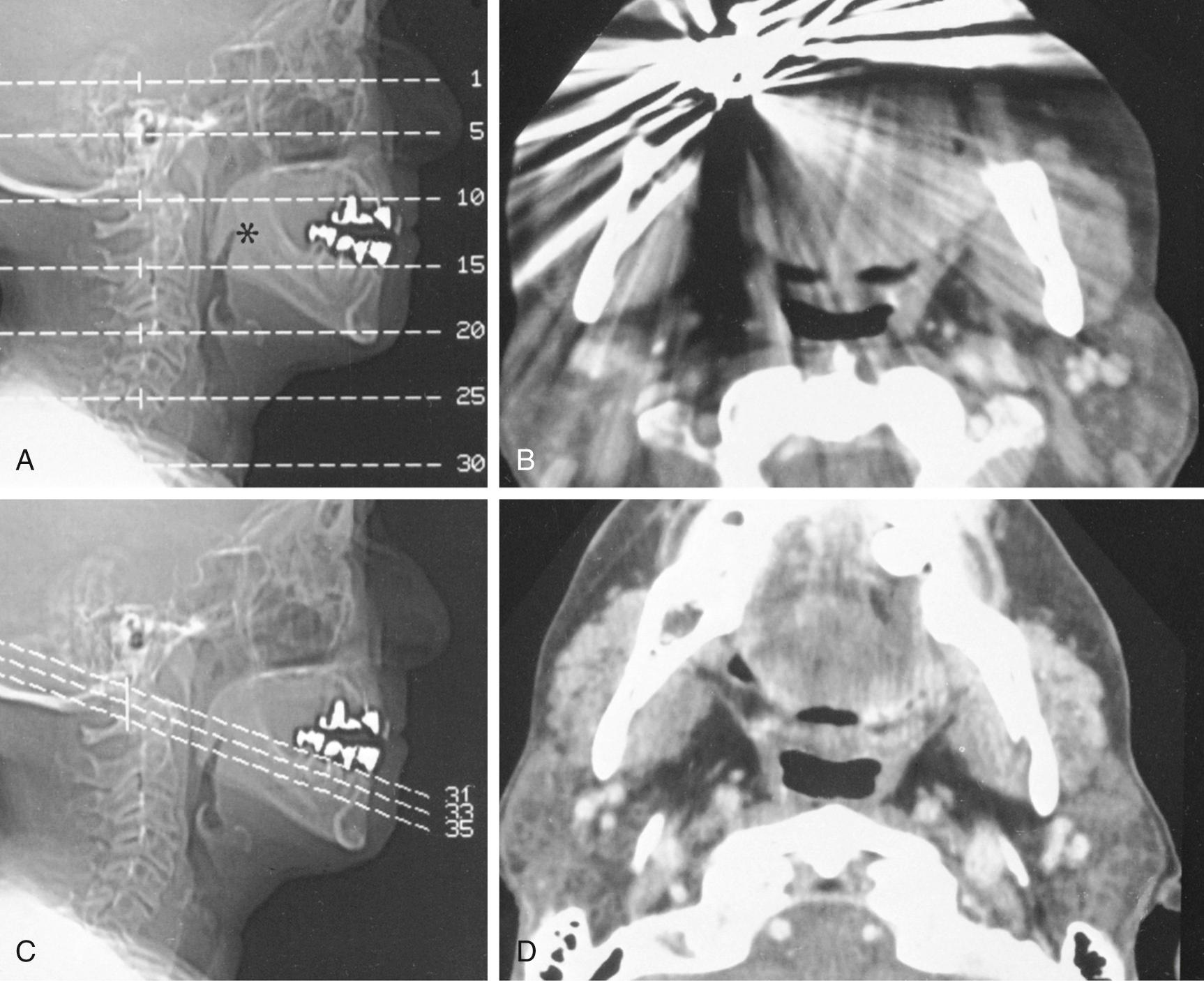
Lymph node CT evaluation is concomitantly performed during CT investigation of most suprahyoid and infrahyoid tumors or inflammation. The quality of lymph node assessment depends very much on the success of achieving a high concentration of contrast in the arterial and venous structures of the neck. Otherwise, nodes and vessels may appear remarkably similar.
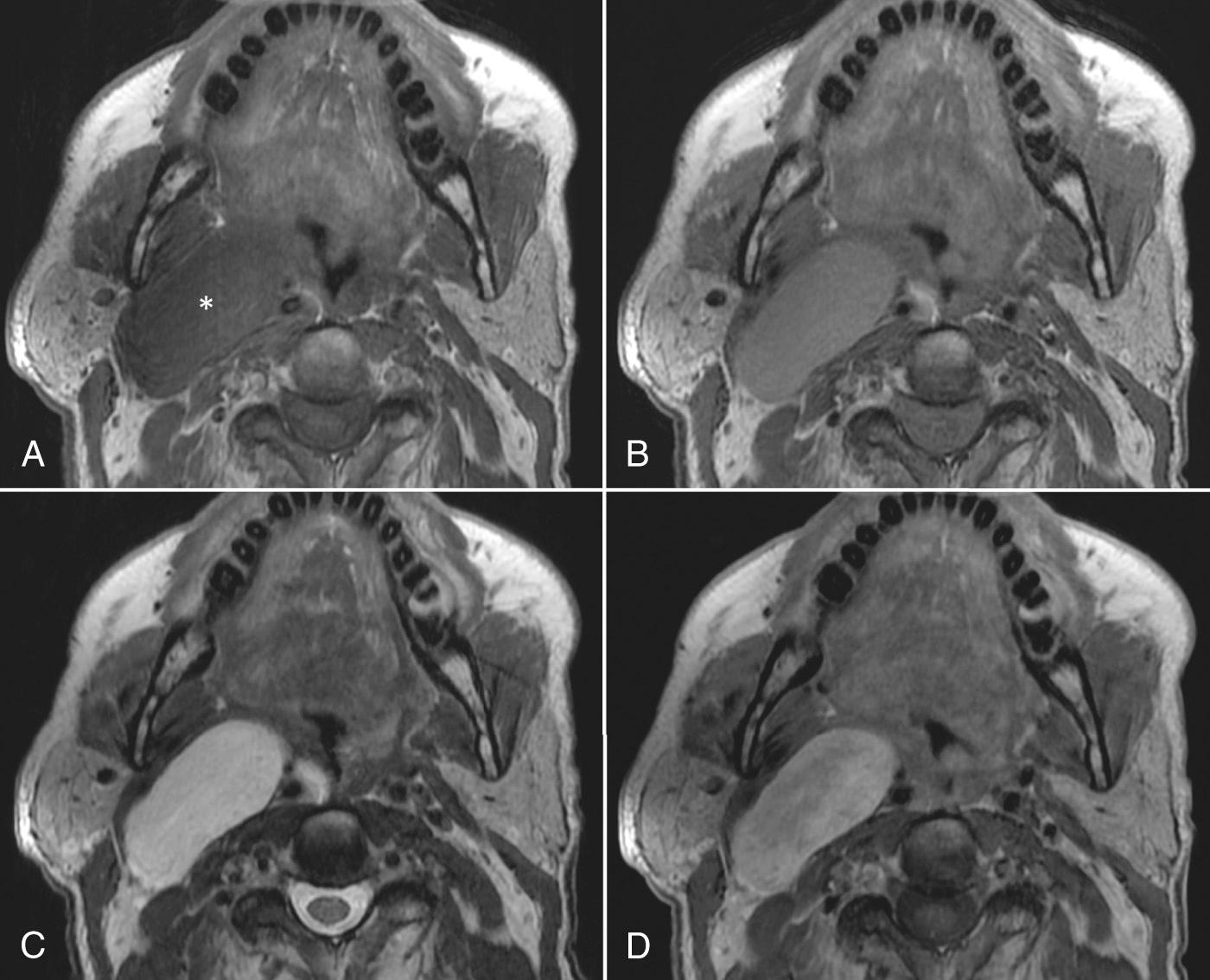
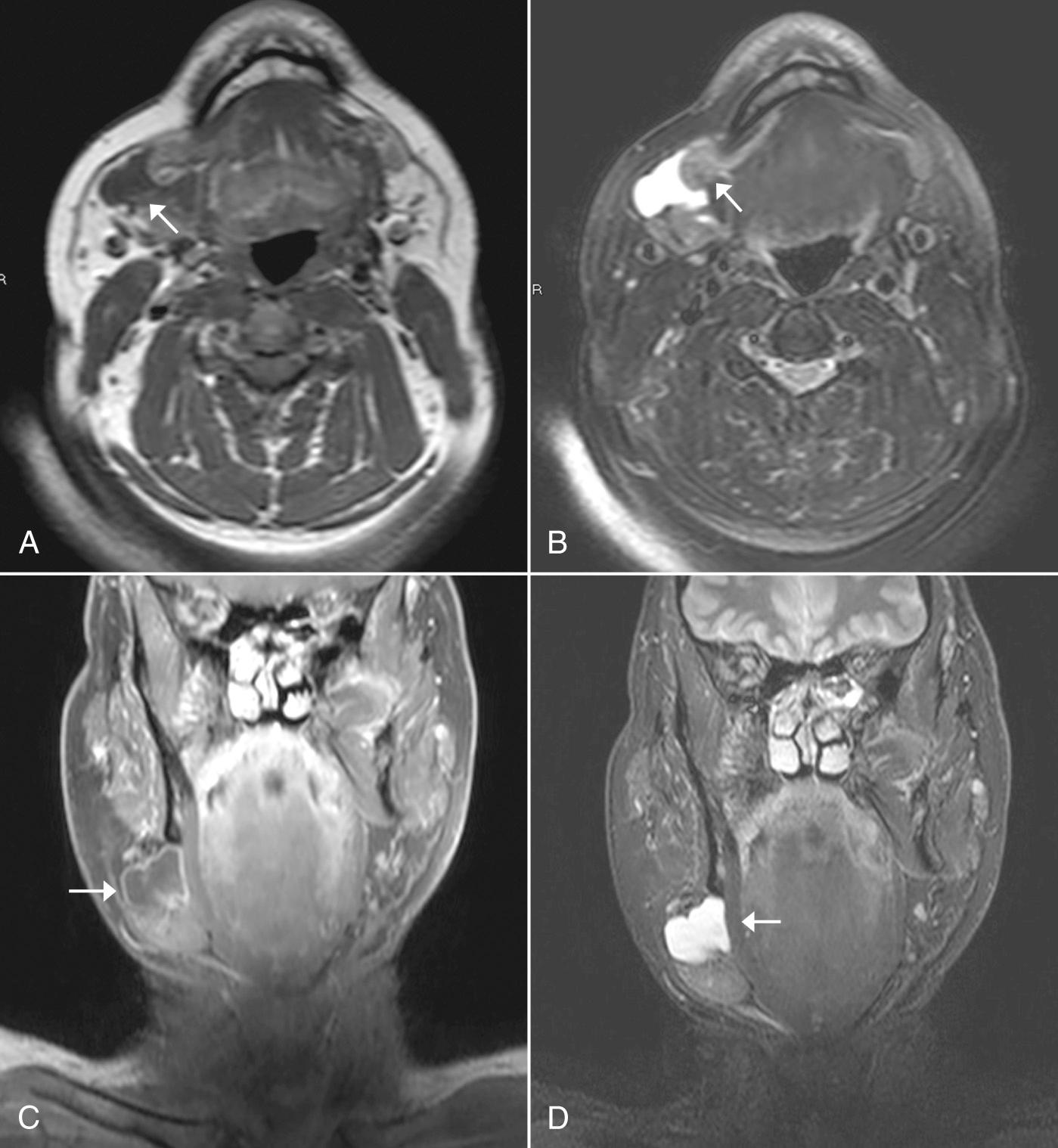
Dental amalgam can cause significant streak artifacts that obscure the parotid or submandibular gland parenchyma. If the dental work is identified on the lateral scout view (scanogram), dental artifacts can usually be avoided if an oblique semiaxial projection is chosen with the scanner gantry angled in a negative direction, between a coronal and an axial plane, to avoid the teeth. This plane has the advantage of visualizing both parotid and submandibular glands in the same slice and is parallel to the posterior belly of the digastric muscle. Contrast administration is required for both neoplastic and inflammatory conditions of the salivary glands. Precontrast scan is not typically needed for detection of stones in suspected sialolithiasis. The CT attenuation of a normal parotid gland is variable and depends on the proportion of fat and glandular tissue present, which varies with age. Submandibular glands have a more predictable attenuation similar to that of muscle. Any difference in attenuation values of the right and the left submandibular glands should be suspicious for an obstructing lesion, such as cancer of the floor of the mouth.
Although rarely needed, conventional sialography remains the best radiographic method for evaluating duct anatomy in obstructive, inflammatory, and autoimmune salivary gland diseases. Supplemental CT-sialography may be performed in patients who cannot have MRI to evaluate a dense gland suspected to harbor a mass. CT-sialography may be obtained at the time of intraductal injection of fat-soluble or water-soluble contrast or after a routine sialogram (the gland may be reinjected during the CT with the catheter left in place). The plane of study is the same as that used for noncontrast CT (NCCT) and should be similarly angled to avoid dental filling artifacts. The use of concentrated sialographic contrast material may cause significant streak artifacts if too much contrast collects in dilated ducts, acini, or large pools—all of which can obscure smaller masses in the gland. For optimal CT scan, the injection is extended into the acinar phase to maximize parenchymal opacification and thereby silhouette mass lesions within the parenchyma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here