Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The practice of neuropathology can be daunting. The spectrum of nervous system diseases is immense, spanning all ages and ranging from congenital anomalies of the fetus to degenerative diseases of the elderly. Molecular mechanisms of neurologic disease continue to be unraveled, giving rise to new laboratory-based tests that improve our diagnostic capabilities, yet also require continual updates of current diagnostic trends. Moreover, recent neurosurgical and neuroradiologic techniques have led to less invasive procedures that often result in modest amounts of diagnostic tissue for the pathologist. Above all, the challenges of diagnostic neuropathology remain steep because the diseases affect the human central nervous system (CNS), a structure often called the most complex system in the universe. A sound comprehension of basic CNS organization is absolutely required to understand its diseases. Among human organs, the brain is unrivaled in its intricacy, regional variation, and range of function ( Box 1.1 ). Details regarding the normal physiology of the brain that result in cognition, sensation, movement, and behavior remain incompletely understood. How, then, can we hope to understand and classify the entirety of pathologic conditions that affect the CNS?
The average adult weight of a human brain is 1300 to 1400 g; an adult gorilla’s weighs only 500 g.
The human CNS contains more than 100 billion neurons.
Each neuron forms an average of 1000 synaptic connections (some up to 10 5 ), resulting in 10 14 neuronal connections per brain (one hundred trillion).
Each synaptic vessel at the nerve terminal contains around 5000 neurotransmitter molecules.
Over 50 distinct types of neurons are present in the human brain; inhibitory neurons (most using GABA as a transmitter) account for 25%.
The length of myelinated tracts in the brain is 150,000 to 180,000 km—4 × the earth’s circumference.
85,000 neurons are lost from the brain per day (∼1/sec).
Glial cells are 10 to 50 times more numerous than neurons.
The brain expresses more genes than any other organ: >200,000 mRNA sequences.
Nerve impulses travel between 1 to 100 m/sec: an impulse starting in an anterior horn cell of the spinal cord travels to the big toe in 0.01 second.
Proteins and organelles are shuttled down nerve processes by axonal transport at a maximal rate of 400 mm/day: a protein produced in the anterior horn cell of the spinal cord reaches the big toe in 2 days.
Based on distinct layering patterns of the cerebral cortex, Korbian Brodmann subdivided 47 cytoarchitectonic regions (these are now thought to be overly simplified).
Cortical representation of sensory, motor, and higher cognitive function follows a general pattern in the human brain; however, cortical maps vary from person to person and can change in size and location with use or disuse.
Time to unconsciousness following complete cessation of blood flow to the brain: 8 to 10 seconds.
Fortunately, the historic fascination with the human brain among physicians and scientists has generated a fundamental understanding of its normal anatomy, histology, and ultrastructure. Often, but certainly not always, disease-related deficits correlate with structural alteration in the CNS. Recognition of the abnormal in the brain, whether by neuroimaging, gross inspection, or microscopy, rests on a firm knowledge of the normal, which can be learned and retained at a practical level. Current diagnostic neuropathology incorporates adjunctive laboratory-based studies to aid in diagnosis ( Table 1.1 ). Nonetheless, microscopic examination of hematoxylin and eosin (H&E)–stained tissue sections remains a central and dominant role in neuropathology. The following chapter introduces normal anatomy and histology of the human CNS at a depth necessary for routine practice. Most of the content describes the normal adult brain, but certain aspects of age-related phenomena, artifacts, and developmental considerations that are routinely encountered are also considered.
| Stain | Highlighted Structure |
|---|---|
| Immunohistochemistry | |
| Aβ amyloid | Amyloid in senile plaques, blood vessels |
| Aβ crystalline | Glial inclusions |
| α Synuclein | Lewy bodies |
| ATRX | Nuclear protein lost in IDH-mutant astrocytoma |
| Brachyury | Transcription factor in notochordal cells and chordoma |
| CAM5.2 | Epithelial intermediate filaments |
| CD3 | T cells |
| CD20 | B cells |
| CD31 | Endothelium |
| CD45 (LCA) | Lymphocytes |
| CD68 | Macrophage lysosomes |
| CD138 | Plasma cells |
| CD163 | Macrophages |
| Chromogranin | Neuronal vesicles |
| CMV | Cytomegalovirus |
| Cytokeratin (AE1/AE3) | Epithelial intermediate filaments |
| Cytokeratin 5/6/7/20 | Specific epithelial intermediate filaments |
| Desmin | Muscle intermediate filament |
| Epithelial membrane antigen (EMA) | Epithelial membranes |
| Estrogen/progesterone receptor | Nuclear hormone receptors |
| GFAP | Glial intermediate filaments |
| HMB-45 | Melanocytic differentiation |
| HER2 | Cell surface receptor |
| HSV | Herpes simplex virus |
| Inhibin | A marker of hemangioblastoma |
| INI1 | Tumor suppressor gene product |
| IDH1 | Mutant protein product |
| κ, λ Light chains | Immunoglobulin light chains |
| MIB-1 | Nuclear marker of proliferation (Ki67) |
| Neurofilament protein | Neuronal intermediate filament (axons) |
| NeuN | Neuronal nuclei |
| Oct3/4 | Nuclear transcription factors |
| Olig2 | Nuclear transcription factor expressed by oligodendroglia and astrocytes |
| p53 | Tumor suppressor protein accumulation |
| Polyoma virus | JC virus (progressive multifocal encephalopathy) |
| S100 | Melanocytic/neuroepithelial differentiation |
| Smooth muscle actin | Smooth muscle |
| Somatostatin receptor type 2 (SSTR2) | Meningioma marker |
| STAT6 | A marker of solitary fibrous tumor |
| Synaptophysin | Neuronal vesicle proteins |
| Tau (τ)/phospho-tau | Neurofibrillary pathology, tangles |
| TDP-43 | Neuronal inclusion |
| Thyroid transcription factor-1 (TTF-1) | Nuclear factor in lung/thyroid differentiation |
| Toxoplasmosis | Toxoplasmosis |
| Ubiquitin | Glial and neuronal inclusions |
| Histologic stains | |
| Acid-fast stains | Mycobacteria |
| Bielschowsky | Silver stain for neurofibrillary pathology |
| Bodian | Silver stain for neurofibrillary pathology |
| Iron | Iron |
| Congo Red | Amyloid |
| Gram | Bacteria |
| Hematoxylin and eosin (H&E) | General histology |
| Luxol fast blue | Myelin |
| Grocott methenamine silver (GMS) | Fungus |
| Oil red O | Lipid stain (frozen tissue) |
| PAS/PAS-D | Glycogen/basement membrane |
| Reticulin | Reticulin/basement membrane |
| Thioflavin S | Amyloid (fluorescence dye) |
| Trichrome | Collagen and fibrin |
| von Kossa | Calcium |
| Laboratory and molecular studies | |
| FISH or PCR/LOH | Chromosomes 1p, 19q, 10q, 9p, 7 |
| FISH for oncogene amplification | c-MYC, MYCN, EGFR, PDGFRA |
| Chromosomal microarray | Cytogenetic alterations |
| DNA methylation profiling | Methylation-based classification of disease |
| Gene fusion panel | RNA-based screening for gene fusion |
| Methylation-specific PCR (promoter methylation) | MGMT status |
| Flow cytometry | Lymphoma, hematologic disorders |
| Cytopathology | CSF examination |
| Microbiology | Viral, mycobacterial, bacterial, and fungal culture, cryptococcal antigen |
| Next Generation Sequencing (NGS) | Pathogenic mutations |
| PCR detection of pathogens in CSF/brain | HSV, CMV, EBV, HIV, enterovirus, mycobacteria |
| Immunologic studies | Protein electrophoresis for oligoclonal bands; syphilis IgG |
| Chemistry | Protein and glucose levels |
| Hematology | Differential cell count |
The nervous system contains central and peripheral divisions. The CNS, which is the focus here, is composed of the cerebrum, cerebellum, brain stem, spinal cord, and their meningeal coverings (see Chapter 11 for a discussion of peripheral nerve and skeletal muscle). The CNS is a complex three-dimensional structure that is normally viewed and described in two-dimensional representations. Most common for neuropathologic study is the coronal plane, a traditional view for brain cutting and gross examination. In today’s clinical practice, neuroanatomy in the sagittal and axial planes is also required because of the routine use of multi-planar neuroimaging (computed tomography, magnetic resonance imaging [MRI], and positron emission tomography). Adding slightly to the complexity of neuropathology, human neuroanatomy introduces terminology from the veterinary world that is not commonly encountered in the study of other organ systems. Anterior, posterior, superior, and inferior retain their meanings in the CNS and generally are in reference to a person in the standing position. Ventral is the anatomic side facing the floor when an individual is on all fours like a dog, with eyes fixed on the horizon; dorsal is the side facing skyward. Rostral indicates a direction toward the superior pole of the vertically oriented CNS (toward the frontal lobe), whereas caudal implies the direction toward the inferior pole (literally, “tail,” i.e., the filum terminale of the spinal cord).
Initial inspection of the brain reveals its protective and functional coverings, the meninges ( Fig. 1.1 ). Outermost is the dura mater (“hard mother”), which is tightly adherent to the inner aspect of the skull and vertebral column. It is composed of a tough fibrous connective tissue (0.5 mm thick) with a glistening inner surface. Epidural and subdural spaces are defined by their locations outside and inside the dura, respectively. Embedded within the dura between its inner and outer layers are the large dural sinuses, vascular channels that receive blood from bridging veins exiting the brain and represent the organ’s main venous outflow. The superior sagittal sinus is the largest of the dural sinuses and runs the length of the cerebral hemispheres in the midsagittal plane. This vascular space—and to a lesser extent, the other large dural sinuses—contains numerous small inpouchings from the arachnoid space, the arachnoid villi (when larger, called arachnoid granulations). Arachnoid villi and granulations are lined only by endothelium and delicate connective tissue and function to resorb cerebrospinal fluid (CSF) from the subarachnoid space. Resorption occurs at a relatively brisk pace, giving a CSF half-life of only 3 hours. Four specific segments of dura deserve mention: the falx cerebri is a portion separating the two hemispheres in the midline, extending from the superior surface of the brain inferiorly to the corpus callosum; the falx cerebelli partially separates the cerebellar hemispheres in a similar manner; the tentorium cerebelli separates the posterior fossa from the middle cranial fossa, allowing passage of the midbrain and creating infratentorial and supratentorial compartments; and the diaphragma sellae is the dural covering of the sella turcica overlying the pituitary gland.
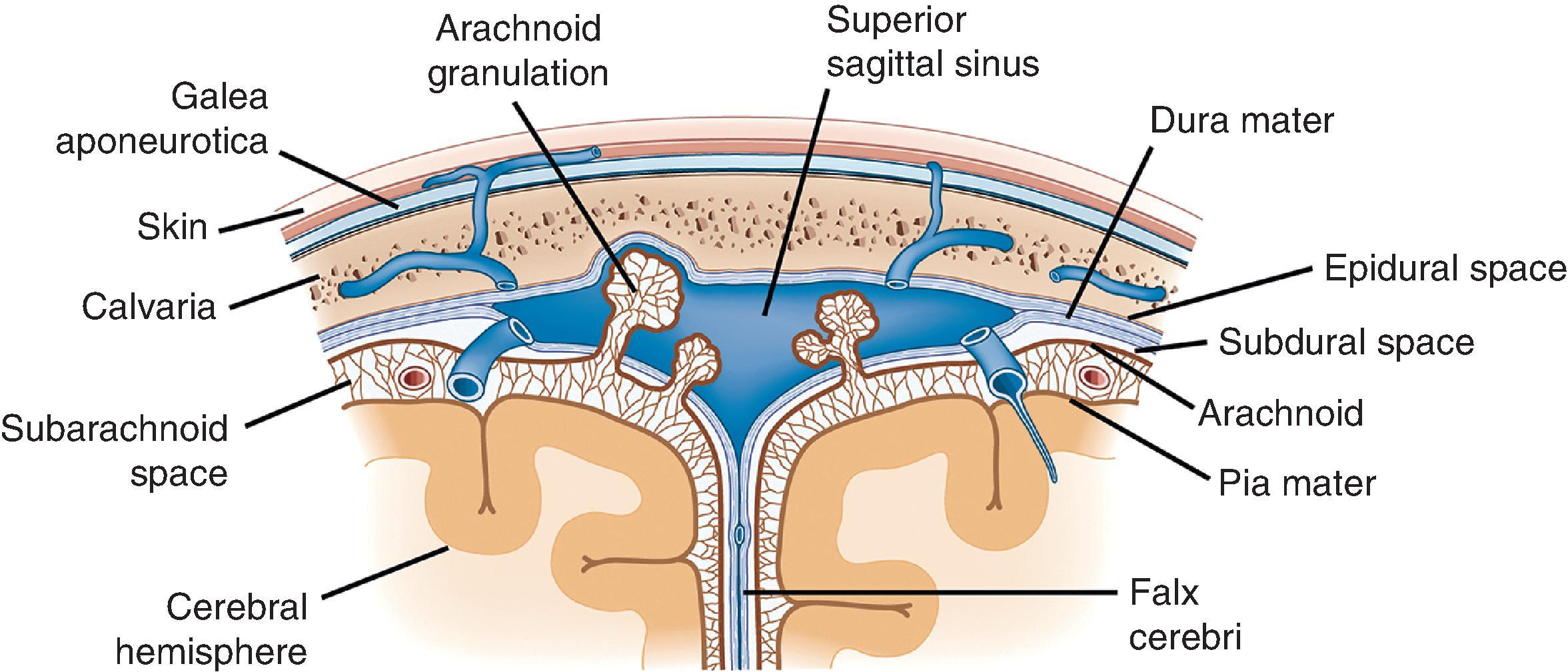
The web-like meshwork underlying the dura that is loosely adherent both to the dura and the CNS surface is the arachnoid membrane (“spider-like”). Within the webs of arachnoid is the subarachnoid space, which normally contains only CSF, arteries and veins, and scattered native cellular elements. It can be completely removed from the underlying brain (sometimes a long, tedious process) to view the surface of the cortex more clearly. Despite its porous appearance, the arachnoid membrane is relatively restrictive to fluid flow outside of its domain; blood contained within this space is largely confined and does not freely exit. Lastly, the pia mater (“soft mother”) is a thin, delicate coating that rests directly on the surface of the CNS. It cannot be seen grossly, much less removed or inspected, and is best evaluated microscopically. The delicate trabeculae that form the arachnoid membranes fuse with the pia to form a singular unit, the pia-arachnoid or leptomeninges.
The structure most often imagined as “the brain” is the cerebrum, the portion of the CNS occupying the supratentorial compartment and consisting of large left and right cerebral hemispheres and a smaller midline diencephalon ( Fig. 1.2 ). The outer view is dominated by numerous cerebral convolutions that greatly add to the human brain’s cortical areas. In contrast, the diencephalon is largely hidden from external view due to its deep location. Outwardly projecting folds forming the cerebral hemispheric convolutions are gyri, whereas infolded spaces between are sulci (or fissures when large). Each hemisphere is divided by surface landmarks into frontal, parietal, temporal, and occipital lobes. Frontal and parietal lobes are separated by the central (Rolandic) sulcus; frontal and temporal lobes are separated by the Sylvian fissure; temporal and parietal lobes are separated posteriorly by the continuation of the Sylvian fissure; and the parietal and occipital lobes are separated by parieto-occipital sulcus and calcarine fissures. At the intersection of the temporal, frontal, and parietal lobes, in the depth of the Sylvian fissure, lies the insular cortex, which cannot be seen from the surface. Cortical gyri (opercula) overlie the insula from each lobe.
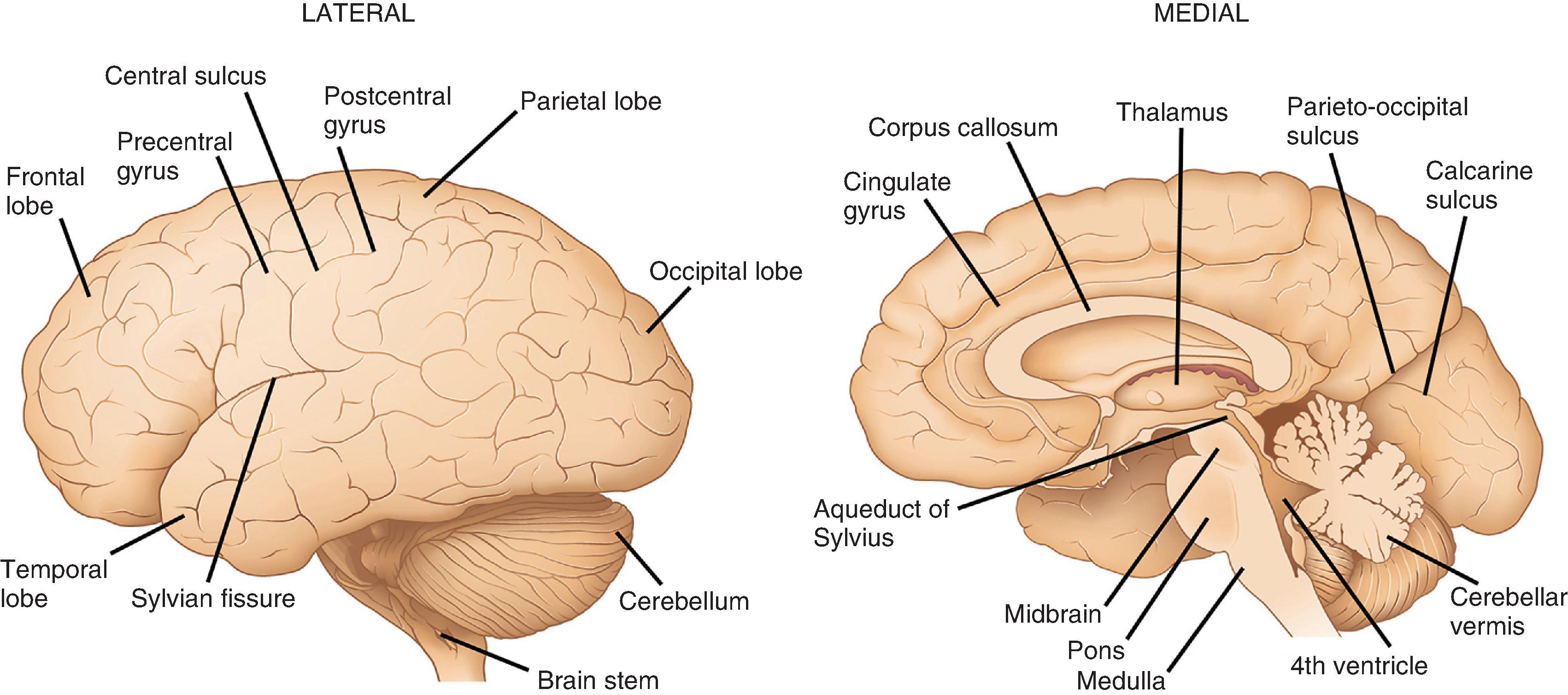
Gyri comprising each lobe have specific designations, largely based on anatomic location. Select gyri are better known for their functional significance. In the frontal lobe, the precentral gyrus, located anterior to the central sulcus, is the home of the primary motor cortex with its resident population of Betz cells (upper motor neurons). The cingulate gyrus, associated with limbic control of emotion and primal drives, is located on the mesial surface of the frontal, parietal, and occipital lobes, directly superior to the corpus callosum anteriorly. It follows a C-shaped path around into the temporal lobe, where it continues as the parahippocampal gyrus. Other frontal lobe gyri form premotor and prefrontal association regions as well as the specialized language area of Broca. On the inferior surface are the orbitofrontal gyri, the most medial of which is the gyrus rectus. Lateral to the gyrus rectus is the olfactory sulcus, where the olfactory nerve (cranial nerve [CN] I) and olfactory bulb are found ( Fig. 1.3 ). In the parietal lobe, the postcentral gyrus is the primary somatosensory cortex and has the same general body representation as the primary motor cortex: legs are represented on the mesial surface; the trunk and upper extremities are on the superior lateral surface; and the face and oral cavity are represented inferiorly on the lateral surface. Other parietal lobe gyri are sensory association cortices and those related to language comprehension. The main lateral subdivisions of the temporal lobe are simply referred to as the superior, middle, and inferior gyri, which together have functions in language, hearing, and vision. The medial surface of the temporal lobe is slightly more complex. It contains the parahippocampal gyrus, which is critical to both emotional and memory controls and is often included as a part of the functional limbic lobe. The anterior aspect of the parahippocampal gyrus contains the uncus, a small, medially projecting protrusion that has a slight horizontal notch on its surface due to its contact with the tentorium cerebelli. The parahippocampal gyrus houses the amygdala anteriorly and the hippocampus more posteriorly. In the occipital lobe, the calcarine sulcus is the most prominent and functionally relevant; gyri on either side of this mesially located sulcus form the primary visual cortex. Other occipital lobe gyri are part of the visual association cortex.
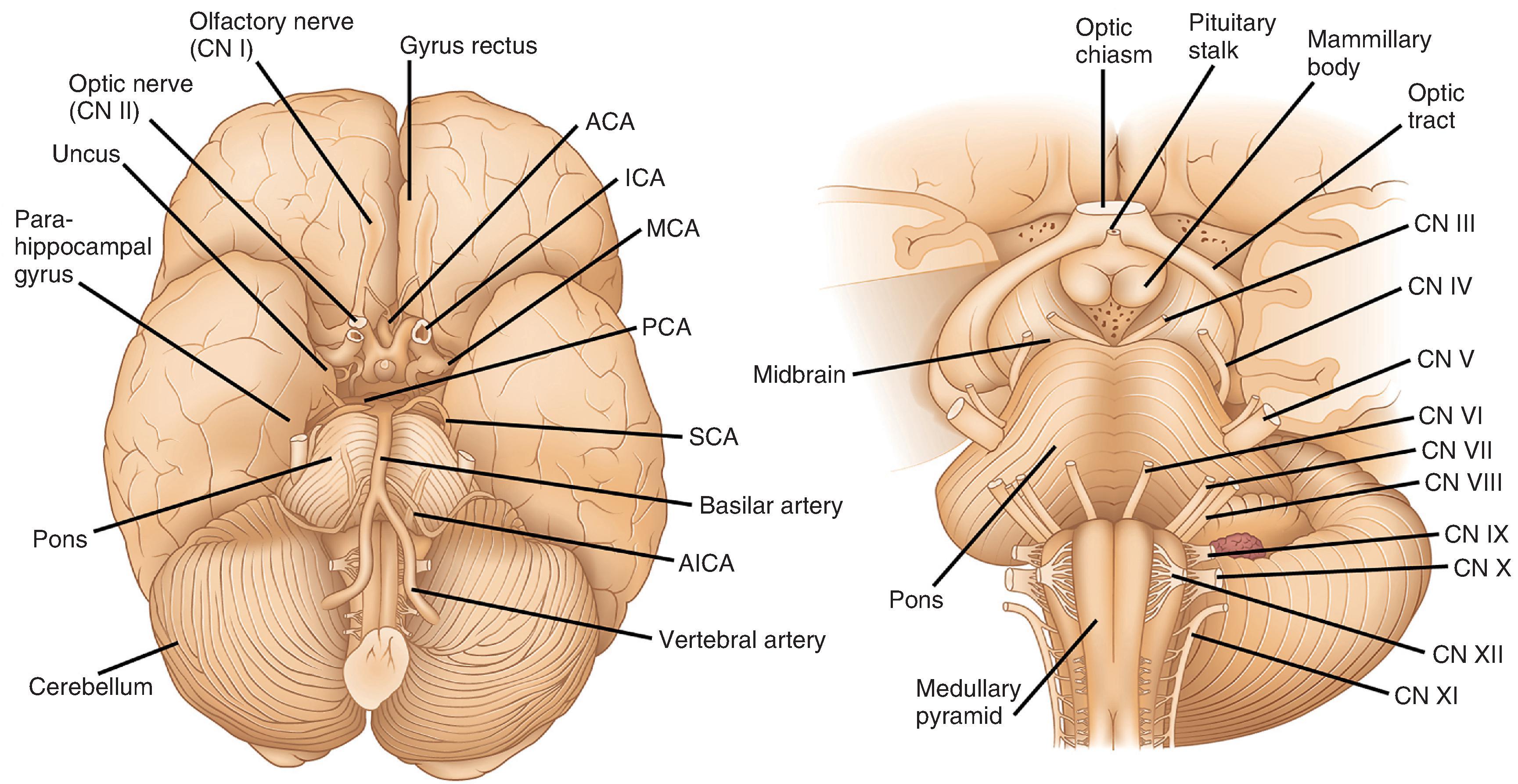
As viewed from the inferior surface of the brain, dorsal to the brain stem and in the midline is the pineal gland, a small (1 cm) piece of lobulated neural tissue. It sits immediately superior to the midbrain colliculi at the posterior aspect of the third ventricle. Ventral to the brain stem and riding the midline is the inferior aspect of the hypothalamus (see Fig. 1.3 ). This includes the mamillary bodies, which are small hemispheric protrusions just anterior to the cerebral peduncles and the midline infundibular stalk. The latter is composed of inferiorly projecting processes from hypothalamic nuclei that traverse the suprasellar space, penetrate the diaphragma sellae, and extend into the sella turcica to form the posterior lobe of the pituitary gland (neurohypophysis). The anterior lobe of the pituitary (adenohypophysis) is larger than the posterior lobe, slightly red and lobulated and, together with the posterior lobe, contained within the sella underneath its dural covering. Optic tracts emerge from the thalamus and extend centrally and anteriorly, merging to form the optic chiasm immediately anterior to the infundibular stalk. The optic chiasm separates into optic nerves (CN II) anteriorly, which course along the inferior frontal lobe a short distance before entering the orbital socket.
The inferior surface of the cerebrum gives the best view of the large arteries that supply blood to the brain (see Fig. 1.3 ). The two internal carotid arteries (ICAs) account for the anterior circulation which supplies 85 % of cerebral blood flow. Internal carotids give rise to the anterior cerebral arteries (ACAs), which course anteriorly between the frontal lobes, then loop back posteriorly along the corpus callosum, inferior to the cingulate gyrus, to supply the medial aspects of the frontal and parietal lobes. The left and right ACAs are interconnected near their origins from the ICAs by the anterior communicating artery, which completes the anterior segment of the circle of Willis, an arterial network critical for collateral circulation. The middle cerebral artery (MCA) also comes from the ICA and travels first laterally and then posteriorly within the Sylvian fissure to supply lateral aspects of the cerebral hemispheres. Within its territory is a large portion of the primary sensory and motor cortex. Importantly, the MCA also gives rise to smaller, perforating branches, the lenticulostriate arteries, which supply the basal ganglia. The posterior circulation includes the two vertebral arteries, which merge at the level of the pons as the basilar artery. Along their route, the vertebral and basilar arteries give rise to penetrating arteries that supply the brain stem as well as the superior, posterior inferior, and anterior inferior cerebellar arteries that supply the cerebellum. The basilar artery divides at the pons-midbrain junction to form two posterior cerebral arteries (PCAs). The PCAs supply most of the occipital lobes, the inferior aspects of the temporal lobes including the hippocampus, and much of the thalamus and hypothalamus. Connecting the posterior cerebrals to the internal carotids and completing the circle of Willis are the posterior communicating arteries.
For classic anatomic study at brain cutting, the brain stem and cerebellum are removed from the cerebrum by slicing across the superior midbrain in the axial plane. This can be done with the brain in its fresh state or following 7 to 10 days of fixation in 10 % buffered formalin. The brain is more easily handled and cut following fixation and its landmarks are better recognized. The cerebrum is traditionally cut in the coronal plane, beginning with a slice through the mamillary bodies and continuing anteriorly and posteriorly with 1.0- to 1.5-cm slices. Slices are placed with the anterior surface facing down so that the right side of the brain is on the examiner’s right side (this is opposite of neuroimaging studies, in which the brain’s right side is on the examiner’s left). Initial inspection reveals internal CNS structures that can be subclassified broadly as white matter, gray matter, or ventricular space ( Fig. 1.4 ). Gray matter structures, including the cerebral and cerebellar cortices, deep nuclei of the cerebrum, cerebellum, and brain stem, and the internal components of the spinal cord, are all characterized microscopically by their high density of neuronal cell bodies and their paucity of myelinated tracts. Together, these gray matter structures account for 40 % of brain mass (but consume 94 % of the oxygen). Grossly, gray matter is darker and tan-pink compared with adjacent white matter. By contrast, white matter contains few neuronal cell bodies and is composed almost entirely of myelinated and unmyelinated axonal processes in transit to their connections. Its white color on gross inspection is due to the high lipid content present in myelinated tracts. By neuroimaging studies, the higher lipid content makes white matter relatively hyperintense on T1-weighted MRI and hypointense on T2-weighted imaging (or FLAIR) similar to fat. The higher relative content of water in gray matter makes it T1 hypointense and T2 (FLAIR) hyperintense (see Fig. 1.4 ).
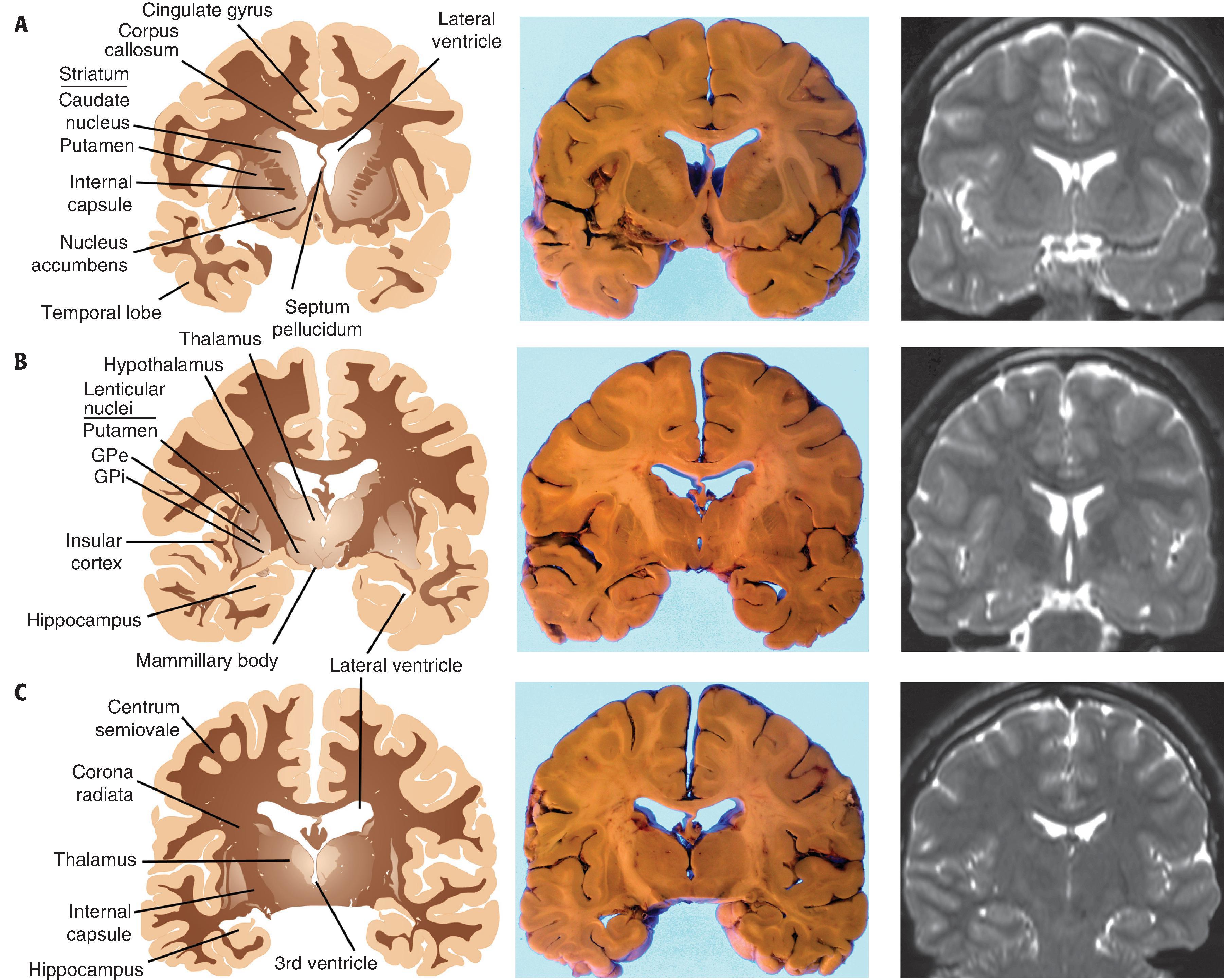
The ventricular system is a macroscopic network of large, interconnected channels within the CNS that contains CSF and is continuous with the subarachnoid space. The entire CSF compartment contains 125 to 150 mL. Lateral ventricles are located within the cerebral hemispheres near the midline anteriorly, and on each side they form a C-shaped path from the frontal pole into the temporal lobe. An occipital horn extends posteriorly into the occipital lobe at the atrium (trigone), which represents the confluence of temporal, parietal, and occipital components of the lateral ventricle. Anteriorly, the lateral ventricles are covered by the corpus callosum and separated from one another by the septum pellucidum, a single delicate translucent membrane that is occasionally split into two parallel membranes (cavum septum pellucidum, a normal variant). A foramen of Monro connects each lateral ventricle with the singular, midline third ventricle, located between the left and right lobes of the thalamus. In turn, the third ventricle is connected to the fourth ventricle by the aqueduct of Sylvius, a thin passageway that extends inferiorly from the posterior third ventricle into the midbrain, emptying into the superior aspect of the fourth ventricle. The latter is found between the cerebellum and brain stem and is bordered on its sides by the cerebellar peduncles. CSF maintains continuity with the subarachnoid space surrounding the brain stem through the fourth ventricle’s lateral foramina of Luschka and its midline foramen of Magendie. Within the ventricles at specific locations are patches of choroid plexus, the tufts of specialized vascular networks covered by choroid epithelium that produce CSF. Choroid plexus carpets the portion of the lateral ventricular surface from the foramen of Monro to the tip of the temporal horn, expanding to form a large aggregate (glomus) in the atrium. Choroid plexus also occupies the roofs of the third and fourth ventricles.
The cerebral cortex is the corrugated, outermost strip of brain parenchyma that covers the cerebrum and ranges from 2 to 5 mm in thickness. The number and degree of cerebral infoldings (sulci and fissures) become more fully apparent on coronal sectioning, with some inward extensions measuring over two inches. The subarachnoid space continues over the surface of the brain and deep into these infoldings. On gross examination, the cerebral cortex displays a fairly uniform thickness, texture, and color, with a sharp demarcation between it and underlying white matter. Two grossly specialized regions deserve attention. First, in the primary visual cortex (Brodmann area 17) of the occipital lobe, the cortex contains a thin white line that runs parallel to the gray-white interface. This “line of Gennari” represents thickened myelinated tracts that are more prominent in primary visual cortex than other cortical regions and can be reliably used to identify the visual cortex. Second, in the inferior temporal lobe medially, specialized cortical regions are the amygdala and the hippocampal formation . The amygdala is an almond-shaped region of homogeneous gray matter near the anterior tip of the lateral ventricle’s temporal horn that is medial and inferior to the lenticular nuclei and anterior to the hippocampus. The hippocampal formation is in the floor of the lateral ventricle’s temporal horn and extends in the anterior-posterior direction from the amygdala to the splenium of the corpus callosum. It is seen grossly as small wavy strips of gray and white matter anteriorly and as a curly structure of interlocking C shapes further posteriorly. The protrusion of the hippocampal formation outward is seen as the uncus on the surface of the anterior temporal lobe in the parahippocampal gyrus.
Underlying the cerebral cortex are large expanses of white matter, formed largely by efferent, afferent, and cortical-cortical white matter tracts and their supporting structures. As neuronal processes travel from the cortex inward toward the internal capsule, they take the shape of a funnel. The most peripheral portion of white matter, near the gray-white junction and corresponding to the mouth of the funnel, is called the centrum semiovale. The more central region of white matter, corresponding to the narrowing portion of the funnel and near the internal capsule, is called the corona radiata. The dominant white matter tract that connects the two hemispheres is the corpus callosum, which sits superior to the lateral ventricles and is subdivided in the anterior-posterior direction into genu, body, and splenium. Other interhemispheric white matter connections are the anterior commissure, located inferior to the basal ganglia and superior to the basal forebrain, and the posterior commissure, located near the rostral midbrain.
The basal ganglia are the deep gray matter structures within the cerebrum and brain stem that are involved in the regulation of movement and include the caudate nucleus, putamen, globus pallidus, subthalamic nucleus, and substantia nigra (SN). In the cerebrum are the caudate nucleus, putamen, globus pallidus. The caudate nucleus is located in the lateral wall of the lateral ventricle and follows it throughout its C-shaped course from the frontal pole into the temporal lobe. Although its head and body are readily noted grossly, the caudate tail in the temporal lobe is best identified microscopically across the lateral ventricle from the hippocampus. Lateral to the caudate and separated from it by the anterior limb of the internal capsule is the larger putamen. Together, the caudate, putamen, and internal capsule have a striated appearance and are called the striatum. At their anterior poles, the caudate and putamen form a continuum inferomedially with the nucleus accumbens, a functionally discrete nucleus with strong interconnections with limbic structures. Further posteriorly and medial to the insular cortex, the putamen is located peripherally to both external and internal segments of the globus pallidus (GPe and GPi, respectively). This triad of nuclei—putamen, GPe, and GPi—take the shape of a wedge and are referred to as the lenticular nucleus. The GPe is separated from the putamen by the lateral medullary lamina, while the GPe and GPi are separated by the medial medullary lamina. Peripheral to the putamen at the level of the insular cortex are the external capsule, the claustrum (a nucleus with a largely undefined function), and extreme capsule.
Located on the lateral aspects of the third ventricle are the components of the diencephalon, the thalamus, and hypothalamus. The thalamus is a major throughput for nearly all cortical functions and contains numerous subdivisions of nuclei that extend from the foramen of Monro anteriorly to the brain stem posteriorly. Laterally and inferiorly, the posterior limb of the internal capsule separates the thalamus from the putamen and globus pallidus. Beneath the thalamus on the lateral and inferior aspects of the third ventricle is the hypothalamus, which has elements that include bilateral mammillary bodies and a central region that extends inferiorly as the infundibular stalk. Lastly, the pineal gland is located in the midline, posterior and inferior to the third ventricle and immediately superior to the midbrain colliculi. The pineal gland, which is normally the size of a macadamia nut, is also referred to as the “third eye” because of its embryological relation to the retina and as the “seat of the soul” because of its deep-seated location and mysterious function.
The cerebellum is a fist-sized piece of neural tissue located in the posterior fossa, with functions that include muscle coordination and postural control ( Fig. 1.5 ). It is connected to the more anteriorly positioned brain stem by the superior, middle, and inferior cerebellar peduncles. Slicing across the cerebellar peduncles bilaterally reveals the fourth ventricle between the dorsal pons and the ventral surface of the cerebellum. The cerebellar surface contains numerous deep and superficial fissures that create lobular subdivisions. The smallest lobules are folia—the fundamental structural unit of the cerebellar cortex. Underlying the cortex in each folium are white matter tracts (medullary center) that extend from and to their cortical connections. Initial inspection reveals the dominant corpus cerebelli, which is formed by large left and right hemispheres, and a smaller midline lobe, the vermis (“worm”). The corpus cerebelli is separated from the smaller flocculonodular lobe by the posterolateral fissure. The two flocculi are located on the ventral surface inferior to the peduncles and are continuous in the midline with the nodulus, the vermian representation of the flocculonodular lobe. Opposite the flocculi are the tonsils of the cerebellar hemispheres; opposite the nodulus is the uvula. A major division of the corpus cerebelli is the primary fissure, which divides the anterior and posterior lobes of the cerebellar hemispheres and vermis.
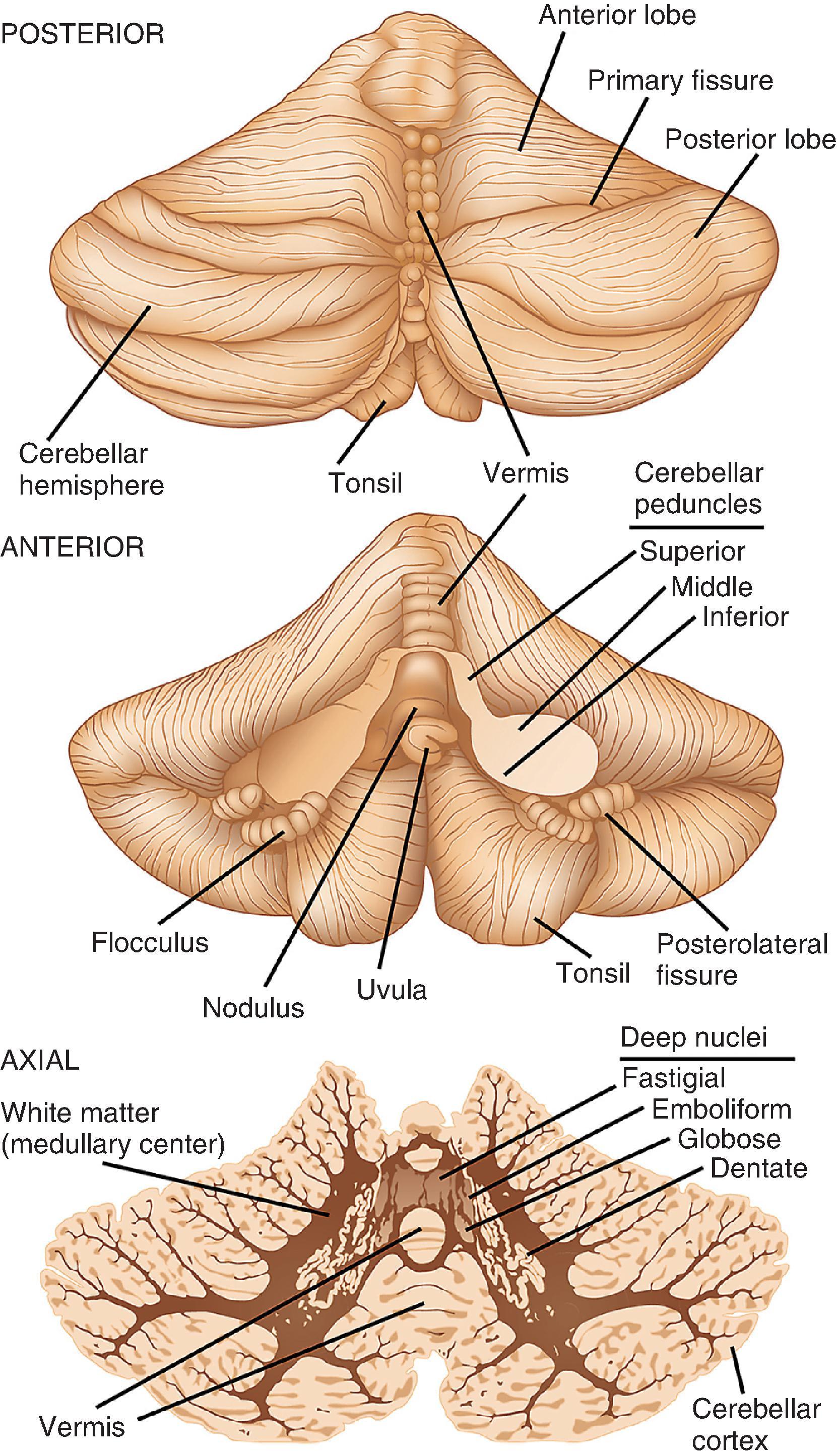
The cerebellum is usually cut for examination in either the sagittal or axial plane. In sliced tissue sections, the white matter is seen beneath the highly convoluted surface folia. Embedded deep within the white matter and toward the midline are four nuclei that are difficult to distinguish from one another on gross inspection. Together, they have a tortuous, ribbon-like appearance in the shape of a C, with the open mouth facing centrally and occupied by white matter. The largest and most peripherally located in each hemisphere is the dentate nucleus; more centrally placed, in order, are the emboliform and globose nuclei; the fastigial nucleus is a small nucleus located on either side of the midline in the vermis.
The brain stem consists of, from superior to inferior, the midbrain, pons, and medulla ( Figs. 1.3 and 1.6 ). Anterior in the midbrain are the cerebral peduncles, the main descending white matter tracts that run between the cerebral hemispheres, brain stem, and spinal cord. On the ventral surface, the oculomotor nerves (CN III) emerge from between the peduncles in the interpeduncular fossa. The trochlear nerve (CN IV) exits the midbrain on its dorsal surface immediately below the inferior colliculi. Gross inspection of axial slices of the midbrain reveals the pigmented SN immediately posterior to the large white matter tracts of the cerebral peduncles. This nucleus is normally brown-black in adulthood and composed of large dopaminergic neurons that project to the basal ganglia. Close to the midline in the most rostral midbrain and posterior to the SN is the red nucleus, a distinctly round and demarcated gray matter structure. Surrounding the aqueduct of Sylvius in the dorsal midbrain is the periaqueductal gray matter, a deep and primitive structure critical to pain perception and control. The superior and inferior colliculi are small hemisphere-shaped projections from the tectum that are seen on posterior view.
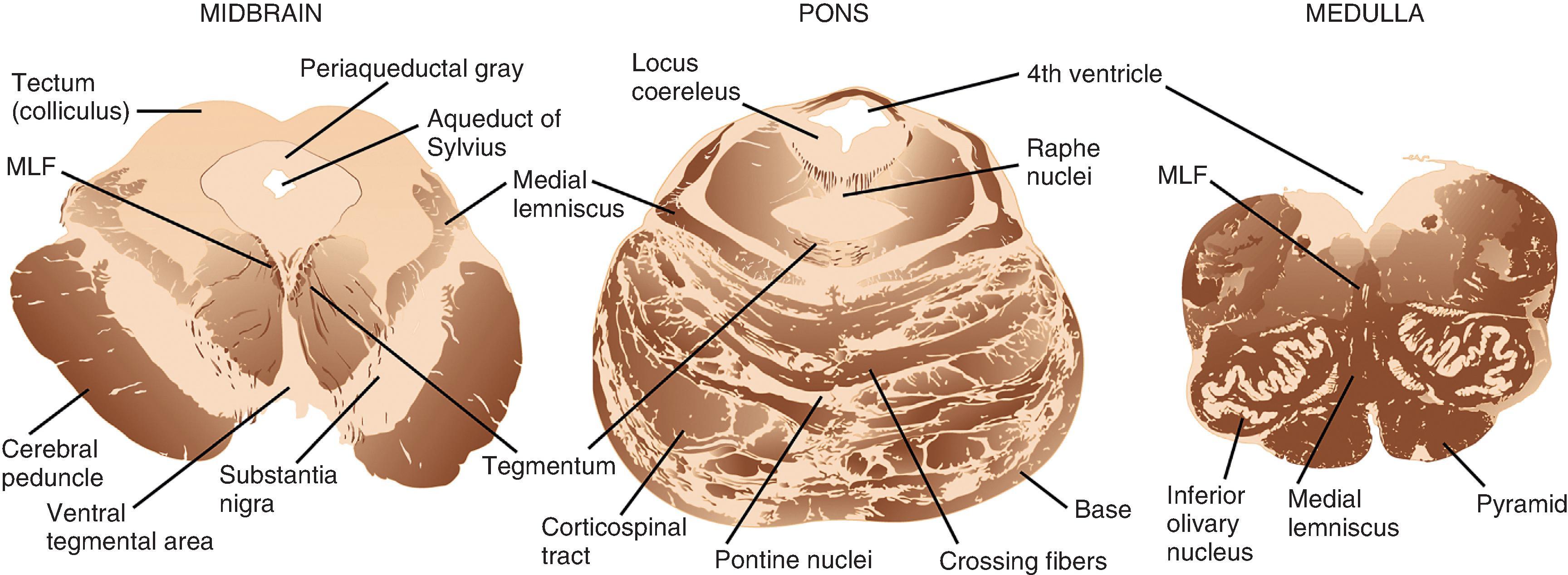
The pons has a larger circumference than the midbrain and medulla and bulges ventrally, giving the impression of a “bridge” over the brain stem. From its ventral surface in the midline, near its inferior border, emerges the abducens nerves (CN VI). From the lateral pons, the trigeminal nerve (CN V) exits superiorly and the facial (CN VII) and vestibulocochlear (CN VIII) nerves exit inferiorly in the region of the cerebellopontine angle. On cut section, the pons is dominated by its bulbous base region anteriorly, which is characterized by a mixture of ascending, descending, and crossing white matter tracts and pontine nuclei, giving the combined appearance of miniature tiger stripes in the axial plane. In the more posterior pontine tegmentum, within millimeters of the fourth ventricle, is the locus coeruleus, a small, darkly pigmented nucleus that houses large noradrenergic neurons. The cerebellar peduncles are large white matter tracts that solidly connect the posterolateral pons to the cerebellum, enclosing the fourth ventricle between them.
The medulla tapers in circumference from its superior junction with the pons to its inferior border where it merges with the spinal cord. On the ventral surface in the midline is a deep groove, the anterior median fissure, which separates the medullary pyramids, a collection of descending white matter tracts. More lateral and dorsal on each side are the olives, outward bulges of the inferior olivary nuclei. Between the pyramids and the olives is the hypoglossal nerve (CN XII). Arising from numerous thin filaments dorsal to the olive are, from superior to inferior, the glossopharyngeal (CN IX), vagus (CN X), and accessory (CN XI) nerves. On cut axial sections, the medulla is composed of the white matter tracts of the pyramids anteriorly; the more dorsolateral olivary nucleus, which is oval and consists of a peripheral convoluted ribbon of gray matter and central white matter; the tegmentum, which houses cranial and sensory nerve nuclei together with ascending and crossing white matter tracts; and the fourth ventricle, which is most dorsal.
The spinal cord extends from its junction with the medulla superiorly to its tapering inferior tip, the conus medullaris ( Fig. 1.7 ). The cord is divided into 31 segments on the basis of its spinal roots and consists of 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal segments. Men have a slightly longer spinal cord (45 cm) than women (43 cm). Along its length, the cord swells from its normal diameter in both the cervical and lumbar enlargements, where more neurons and fibers are present because of representation of the upper and lower extremities, respectively. The anterior surface of the cord is distinguished by its deep, single, midline anterior median fissure, which extends along its length, carrying the anterior spinal artery. Posterior in the midline, the less distinct posterior median sulcus separates the left fasciculus gracilis and the right fasciculus gracilis, white matter tracts that bulge posteriorly. In the cervical region, the fasciculus gracilis is joined by the more lateral fasciculus cuneatus to form two distinct bulges on either side. Both anteriorly and posteriorly at each of its 31 segments, the spinal cord gives rise to numerous small anterior and posterior rootlets that fuse to form anterior and posterior spinal roots. Anterior and posterior roots join together on exiting the spinal foramina to form functionally mixed spinal nerves. Because the dural sac is longer than the spinal cord, the more inferiorly located spinal roots course downward to their exit foramina. In the lumbar cistern, inferior to the spinal cord, the collection of roots is particularly dense, forming the cauda equina (“horse’s tail”). In the middle of the cauda equina, the filum terminale—an extension of the spinal pia mater—extends from the inferior tip of the conus medullaris and attaches to the most inferior aspect of the dural sac.
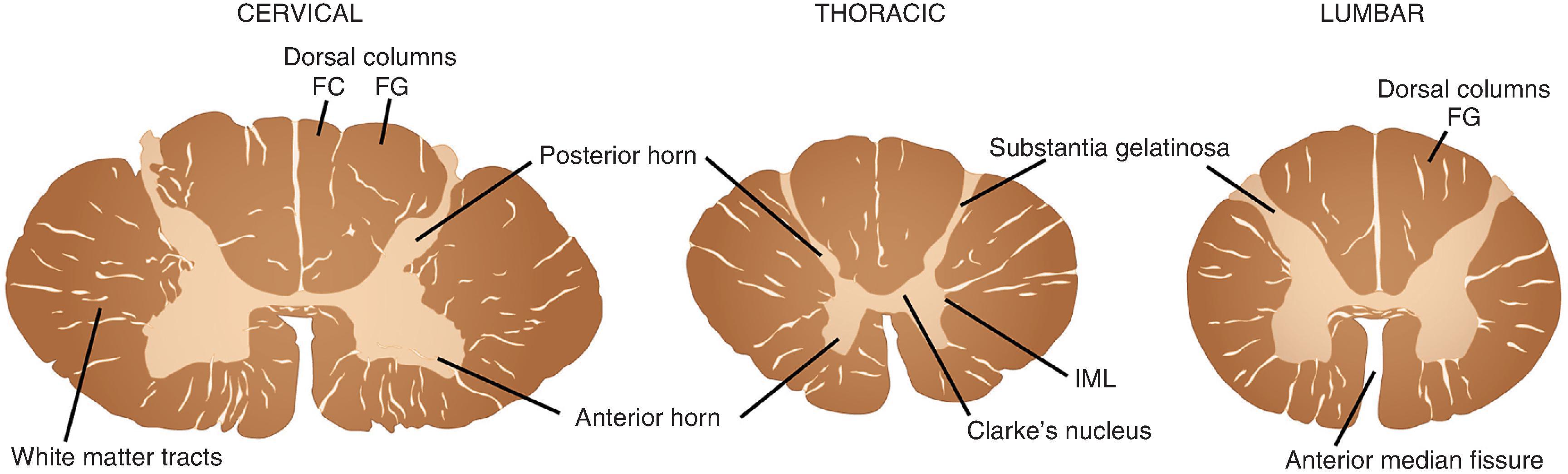
On cross section, the spinal cord is separated into central neural tissue (gray matter) and peripheral white matter tracts throughout its length. The anterior horn is home to a collection of motor neurons, interneurons, and projection neurons as well as the nerve terminals that end upon them. The white matter represents the collection of ascending and descending axons that communicate between the spinal cord and peripheral nerve and superior integrating networks of the cerebrum, cerebellum, and brain stem.
It may be somewhat surprising that an organ as complex as the brain would consist in large part of only two cell types, neurons and glia. Both are large families with many members having specific functions, and yet the underlying structure and cell biology of each retain some central features. Most imposing for the pathologist are the large morphologic range, the geographic diversity, and the functional capacities of neurons that are disturbed by specific diseases. Recognizing normal and reactive states of glia can also be a challenge.
Neurons are the integrating and transmitting cells of the nervous system, communicating by chemical and electrical means. The spectrum of their morphology, connectivity, and function is enormous. As a rule, neurons have a cell body, branching processes called dendrites for integrating incoming signals, and a longer cell process—the axon—with a terminal synapse for conveying a signal over space. Cell body shape and size, as well as the number and arrangement of branching processes, vary considerably. For practicing pathologists, recognizing the major forms of neurons, many of which are described later within their anatomic setting, is requisite because individual populations show differential vulnerability and pathologic reaction in specific disease processes.
Pyramidal neurons are a morphologic prototype that are best known for their presence in the cerebral cortex and subfields CA1, CA2, and CA3 of the hippocampus ( Fig. 1.8 ). In all cases, they have large, triangular cell bodies, a prominent apical dendrite extending toward the brain’s surface, and numerous finer branching basal dendrites. Measuring approximately 10 to 50 µm in greatest dimension, their cell bodies contain abundant cytoplasm, variable hematoxiphilic Nissl substance (rough endoplasmic reticulum) near the entry zone to processes, and a large nucleus with open chromatin and a prominent nucleolus. The pattern of loose chromatin and conspicuous nucleoli is typical of neurons and distinguishes them from resting glia.
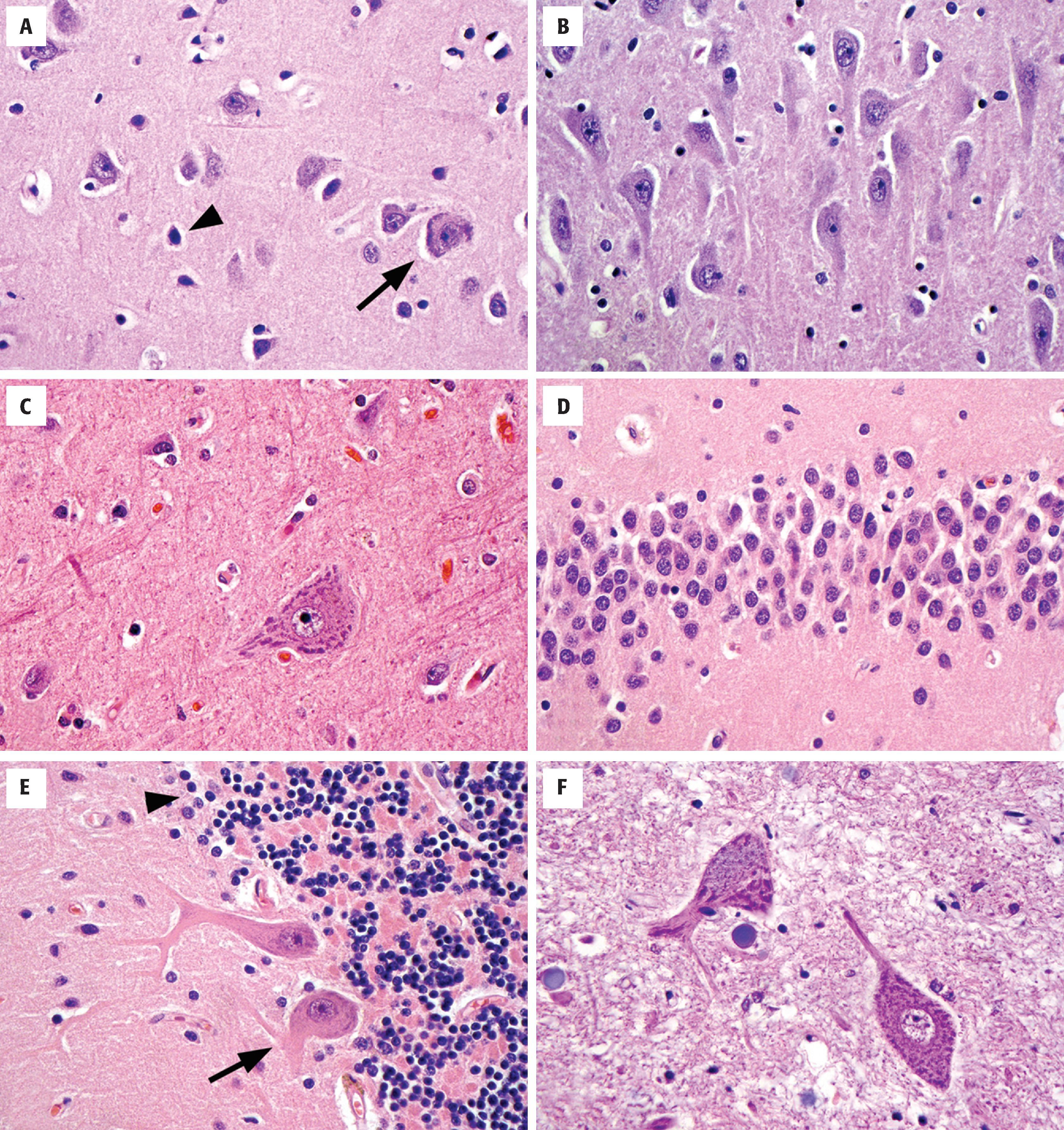
Cortical granular (stellate) neurons are the smaller counterparts to pyramidal neurons in the cortex, typically 15 µm or less. As interneurons, they have numerous shorter processes that remain within the confines of the cortex.
Betz cells, which function as upper motor neurons, are the largest neurons of the cerebral cortex (70 to 100 µm) and are found in the primary motor cortex where they dwarf their neighboring cortical pyramidal cells. Both the amount of cytoplasm, the amount of Nissl substance, and the number of visible processes far exceed normal pyramidal cells.
Small, tightly packed granular neurons form the stratum granulosum of the dentate gyrus in the medial temporal lobe, intimately connected to the hippocampus proper. These neurons are nearly as small as cerebellar granular cells and have an extensive dendritic arbor that forms the adjacent molecular layer of the dentate gyrus.
Purkinje cells are large (50 to 80 µm), histologically distinctive neurons of the cerebellum with cell bodies that sit at the interface of the molecular and internal granular cell layers. Each neuron has a prominent pink cell body and an expansive dendritic tree with thick processes that extend into the molecular layer, as well as a large axon that travels centrally out of the cerebellar cortex.
Granular neurons of the cerebellar granular cell layer are tiny and densely packed, often displaying a linear arrangement or loose rosettes around delicate neuropil. Perinuclear cytoplasm is sparse, giving the appearance of only nuclei on H&E stains. This population can cause confusion on frozen section or cytologic preparations because they resemble small round blue cell lesions.
Anterior horn cells are large, lower motor neurons (alpha motor neurons) that populate all levels of the spinal cord in the anterior horns and send long axonal processes via the anterior roots for their eventual termination on peripheral skeletal muscle endplates.
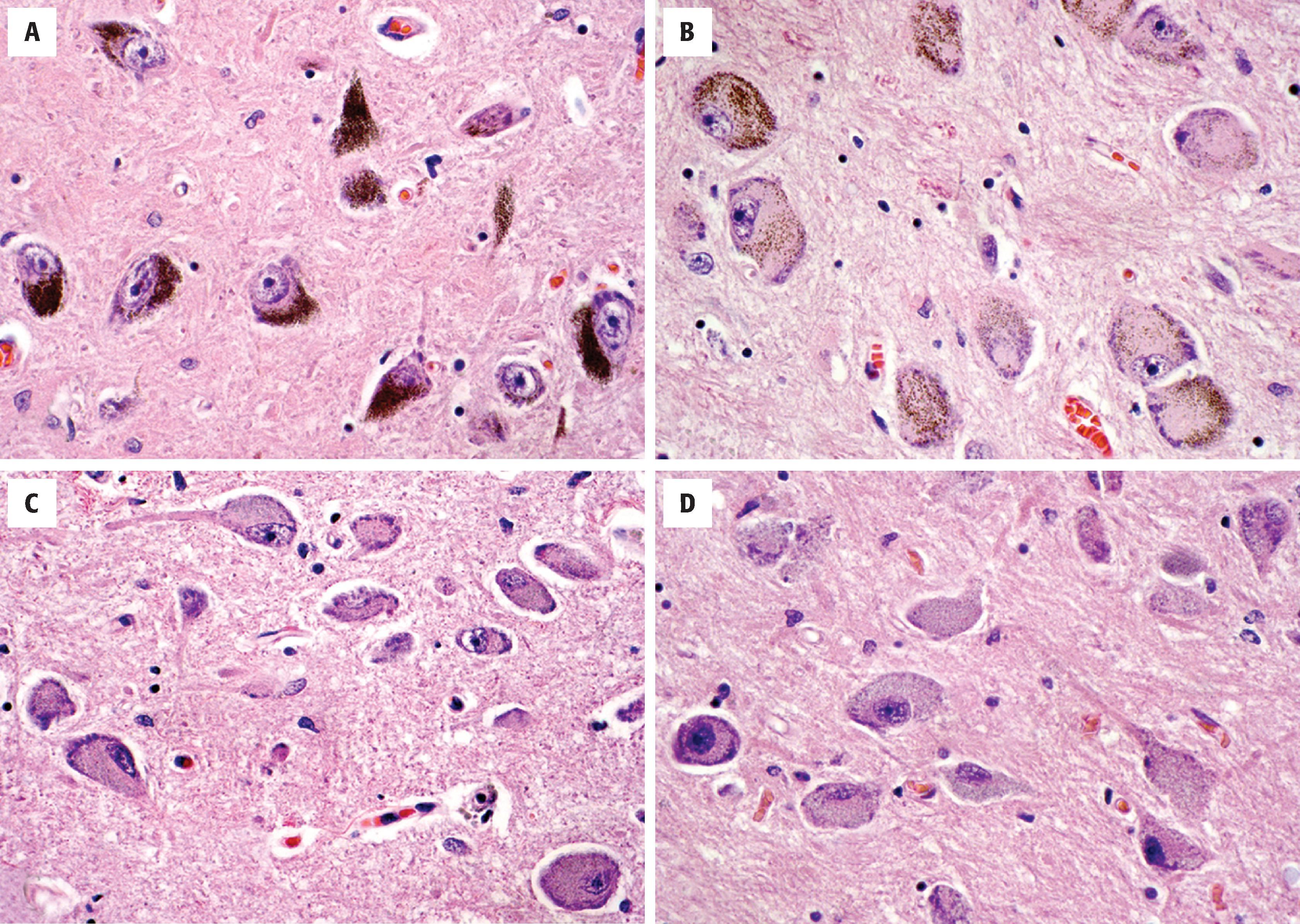
The CNS contains a small number of highly specialized nuclei that contain neurons that produce specific bioaminergic neurotransmitters and project diffusely throughout the brain to affect global or regional tone. These include the SN, locus coeruleus, raphe nuclei, and nucleus basalis of Meynert ( Fig. 1.9 ). The dopaminergic cells of the SN pars reticulata (and the ventral tegmental area) are large, heavily pigmented neurons with neuromelanin (not to be confused with melanin of melanocytes). This pigmented material accumulates in the cytoplasm as coarse brown granules and represents a combination of oxidized and polymerized dopamine within lysosomal granules. Similarly, the locus coeruleus, located near the fourth ventricle in the rostral pontine tegmentum, contains a population of large, pigmented neurons that serve as a major source of norepinephrine in the brain. Neurons located in the raphe nuclei, located along the midline of the brain stem, are similar in size and shape to the noradrenergic neurons of the locus coeruleus but lack the pigmentation. These cells produce serotonin and have diffuse projections throughout the nervous system, but most heavily innervate limbic and sensory regions. Within the basal forebrain, inferior to the anterior commissure in a region called the substantia innominata, is the nucleus basalis of Meynert, a collection of large cholinergic neurons that projects throughout the cerebrum.
Glia account for approximately 90 % of all CNS cells and have been generally regarded as “glue,” providing structural and functional support for neuronal elements. It is now known that the role of glia is not so limited. Functions being elucidated include glia-neuronal signaling, extracellular buffering of electrolytes and metabolites, and turnover of neurotransmitters. Glia are divided into the macroglia—astrocytes, oligodendrocytes, and ependyma—and the microglia.
Astrocytes are the multipolar, star-like glial cells of the CNS ( Figs. 1.10 and 1.11 ). They are typically subdivided into protoplasmic and fibrillary families on the basis of their location and morphology, although overlap between these divisions may outweigh their distinctions. Protoplasmic astrocytes reside in the cortex, whereas fibrillary astrocytes populate the white matter. In addition to similar cell shapes and numerous processes, all astrocytes contain abundant cytoplasmic intermediate filaments, largely composed of glial fibrillary acidic protein (GFAP). In the resting state, astrocyte nuclei are recognized on H&E-stained sections, but the scant, delicate cytoplasm and processes are not readily seen because they blend with surrounding neuropil. Nuclei are oblong and can be slightly angulated with a chromatin pattern that is lighter and looser than those in oligodendrocytes. Nucleoli that are typically seen in neuronal nuclei are not present in most resting astrocytes. Many astrocytes have processes that terminate as end-feet on blood vessel walls, where they contribute to the blood-brain barrier. Others have processes that extend end-feet to the pial surface of the brain, contributing to the glial limitans of the brain-CSF barrier. At these locations, astrocytes provide laminin, fibronectin, and proteoglycans for the limiting basement membrane.
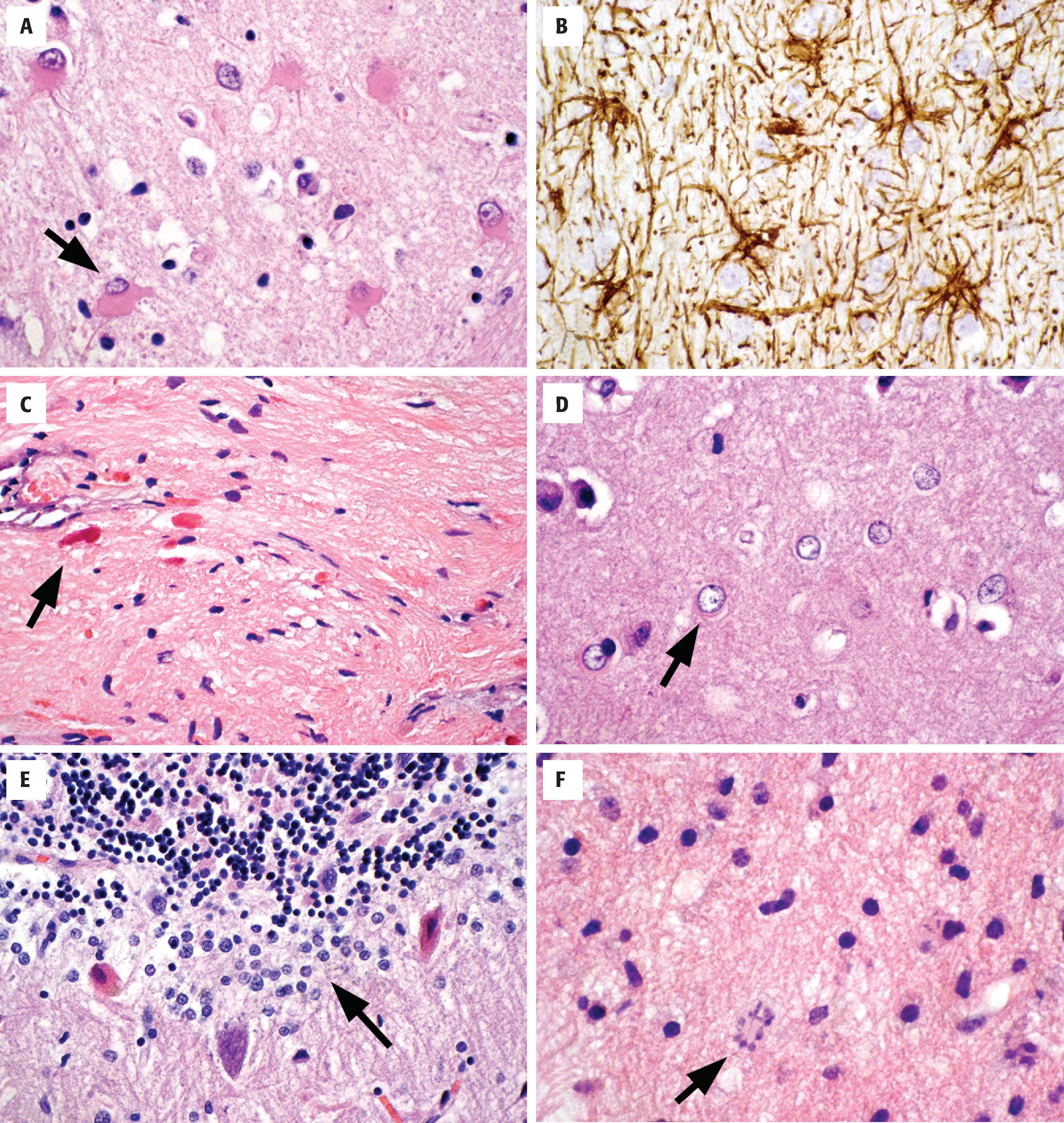
Astrocytes are activated in response to a variety of pathologic conditions (see Fig. 1.11 ). The morphologic spectrum of reactive astrocytosis is critical to recognize because (1) it brings one’s attention to pathologically affected tissue for further evaluation; (2) it serves as validation that a disease process is present in the CNS (i.e., rather than artifact); and (3) reactive astrocytosis often causes diagnostic dilemmas because of its morphologic similarity to neoplastic conditions. Reactive astrocytosis involves both proliferation and hypertrophy of astrocytes, and its appearance varies with the chronicity and severity of insult. The initial response is enlargement of cell body, processes, nuclei, and nucleoli. In H&E-stained sections, the presence of visible astrocytic cytoplasm and processes is almost always a pathologic finding. Immunohistochemistry for GFAP will highlight the reactive nature of these astrocytes, demonstrating the extensive arborizing of their processes and the orientation of the reactive cells to the underlying injury. Reactive astrocytes of longer duration often take on a gemistocytic appearance, with large amounts of brightly eosinophilic cytoplasm in their cell bodies. Chronic reactive astrocytosis is more fibrillar in nature, with numerous long astrocytic processes forming a layer of dense gliosis adjacent to injury (often called piloid gliosis). Rosenthal fibers, which are large, flame-shaped or globular proteinaceous deposits, are often seen in this type of long-standing reactive astrocytosis.
Alzheimer type II astrocytes are a reactive form seen in states of elevated blood ammonia, usually related to renal or hepatic disease. They are present in highest concentration in the basal ganglia where cells show nuclear swelling, marked chromatin clearing, and micronucleoli. Cytoplasmic hypertrophy is not prominent in this form of astrocytosis.
The Bergmann glia are a layer of astrocytes located between the molecular and granular layers of the cerebellum. Cells are only one to two layers thick and can go unnoticed in resting states. In response to cerebellar injury, especially to individual Purkinje cell loss, the reactive proliferation of this cell layer is referred to as Bergmann gliosis.
Creutzfeldt cells are another form of reactive astrocyte that have abundant cytoplasm and “granular mitoses,” the fragmenting of nuclear material that gives the impression of multiple micronuclei. They are seen in active inflammatory diseases (classic in demyelinating disease) and require recognition so that they are not mistaken for the mitoses of an infiltrating astrocytoma.
Markedly enlarged and atypical astrocytes can be seen in non-neoplastic conditions, including reactions to radiation (radiation atypia) and progressive multifocal leukoencephalopathy. In both conditions, nuclei can be wildly atypical with hyperchromatic nuclei that have irregular outlines. The context of additional microscopic changes and the clinical history are often required to avoid a misdiagnosis of neoplasm.
Oligodendrocytes are the myelinating cells of the CNS and are therefore more numerous in white matter than gray (see Fig. 1.10 ). With their cellular processes extending in all directions, oligodendrocytes provide internodes of myelination to multiple axonal processes in their environment. In H&E-stained sections, only the nucleus of oligodendrocytes is usually visible because of the blending of cellular processes with the neuropil. A clear zone surrounding the nucleus—the so-called perinuclear halo—often highlights oligodendrocytes and tumor derived from them (oligodendrogliomas) and in either case appears because of an artifact of fixation. Nuclei are generally round and regular but vary from small and darkly basophilic (accounting for a majority) to slightly larger with pale vesicular nuclei. Nucleoli are not usually noted by standard light microscopy. In the white matter, oligodendrocytes are disposed along the length of axonal processes, whereas in the cerebral cortex, they are scattered within the neuropil and concentrated immediately surrounding neuronal cell bodies (satellite cells). In the latter location, they may serve as a progenitor population. Approximately 10 % to 15 % of non-neuronal cells in the cortex and white matter are NG2 glia that express NG2 chondroitin sulfate proteoglycan and platelet-derived growth factor receptor alpha. These stellate-shaped cells are distinct from other glial cell types, and they function as an oligodendrocyte progenitor. These cells undergo morphologic changes in a variety of disease processes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here