Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Otorhinolaryngologic Procedures Represent a large segment of elective surgery in infants and children. Anesthetic management is provided by both pediatric and general anesthesiologists, commonly in ambulatory surgery centers and office practices. Additionally, anesthesiologists are often consulted to help manage potentially life-threatening pediatric otolaryngologic emergencies. These include airway obstruction caused by croup, foreign body aspiration, airway trauma, bacterial tracheitis, and, rarely, acute epiglottitis. In both the elective and emergent scenarios, it is essential to understand the pathophysiology, and to discuss the anesthetic plan in advance with the surgeon who will frequently be sharing the airway with the anesthesiologist. This ensures safe anesthetic management and ideal conditions for both children and surgeons.
Chronic serous otitis media is common in young children. If untreated or poorly managed, it can lead to hearing loss and formation of cholesteatoma. When conservative medical management fails, surgical drainage of accumulated middle ear fluid is indicated. Myringotomy creates an opening in the tympanic membrane through which fluid can drain. If only a myringotomy is performed, the drainage path is occluded when the incision heals. Therefore myringotomy is frequently accompanied by placing a small plastic tube (a variation of the grommet or the T -tube) in the incision in the tympanic membrane to serve as a stent for the ostium, facilitating continuous drainage from the middle ear until the tubes are naturally extruded in 6 to 12 months, or surgically removed at an appropriate time.
Children with cleft palate have a high frequency of middle ear disease compared with those without a cleft because of associated abnormalities of the cartilage and muscles surrounding the eustachian tubes. Surgical drainage and ventilation tube insertion is a standard treatment for chronic otitis media in these children; this is usually performed at the time of cleft repair.
Most young children require general anesthesia for tympanotomy tube placement, although occasionally older children tolerate the procedure with only topical anesthesia. This may be accomplished by iontophoresis or instillation of EMLA (eutectic mixture of local anesthetics) cream, which remains in the ear canal for an hour and is then suctioned out before the procedure.
Myringotomy with tube insertion is a very brief operation, usually performed in the ambulatory surgery setting using a potent inhalational agent (e.g., sevoflurane), oxygen, and nitrous oxide administered by face mask with spontaneous respiration. An oropharyngeal airway may assist in maintaining a patent airway when the head is laterally rotated and together with resting the forearm of the anesthesiologist on the table, reduces head movement during respiration (which is amplified through the microscope). Gentle manual assistance of ventilation can also reduce head movement. Occasionally, a laryngeal mask airway (LMA) may be used in children in whom the procedure is expected to be prolonged (e.g., children with narrow ear canals) or those with a difficult airway. Most children can be managed safely without intravenous (IV) access, but it is recommended to have IV equipment set up on standby. Some children with severe underlying medical or surgical conditions will require IV access despite the anticipated brief duration of the minor procedure. Although premedication is often omitted because its duration of effect exceeds that of the procedure, an anxious child may still benefit from a sedative premedication and/or having the parents present during induction of anesthesia.
In some instances, it is desirable to remove a retained tympanotomy tube. This can be easily accomplished in the surgeon's office without anesthesia; however, some stiff-flanged grommet tubes require general anesthesia for removal. If the incision does not heal spontaneously, a paper patch or fat graft may have to be applied over the ostium to stimulate healing of the tympanic membrane. The anesthetic would be the same as that for the tube placement, except that nitrous oxide is best avoided to minimize the chance of graft dislodgment (see later discussion).
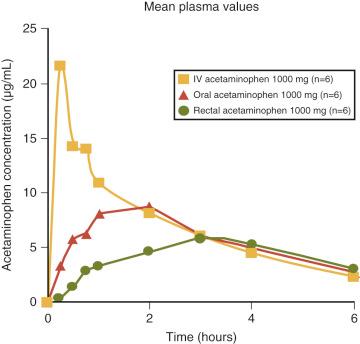
Discomfort after myringotomy and tube insertion is usually managed by the administration of nonsteroidal antiinflammatory drugs (NSAIDs) such as acetaminophen, ketorolac, or opioids. The recommended dose of acetaminophen to achieve therapeutic blood concentrations is 10 to 20 mg/kg when administered orally, and 30 to 40 mg/kg when administered rectally. Oral acetaminophen is very rapidly absorbed, achieving therapeutic blood concentrations within minutes, whereas rectal acetaminophen is slowly absorbed, with a time to onset of action of 60 to 90 minutes, and a time to peak effect of 1 to 3 hours ( Figs. 4.2 and 33.1 ). Consequently, the oral route is preferred for this procedure.
Preschool-aged children who receive sevoflurane without an analgesic for myringotomy and tube insertion may exhibit emergence delirium and postoperative agitation (see Chapter 4 ). Although pain may be partially responsible for these responses, their etiologies are not completely understood. Because the procedure is so brief and IV access is not usually established, intranasal (IN) fentanyl, 1 to 2 µg/kg, has been shown to provide analgesia and to reduce the frequency of emergence agitation. Other analgesics, including IV/intramuscular (IM) ketorolac (0.5–1 mg/kg), or rectal diclofenac or IN butorphanol (25 µg/kg), and IN dexmedetomidine (1–2 µg/kg), reduce the pain after myringotomy and tube insertion. However, larger doses of dexmedetomidine (2 µg/kg) significantly prolong the duration of stay in the postanesthesia care unit (PACU). A large retrospective pediatric study of bilateral myringotomy and tube insertion found that the combination of IM fentanyl (1.5–2 µg/kg) and ketorolac (1 mg/kg) was strongly associated with superior PACU analgesia and reduced the need for oxycodone rescue without clinically significant increases in recovery time or the incidence of emesis. This dual therapy appears similarly effective in children of European Caucasian, African ancestry, or those of Hispanic ethnicity. A nerve of Arnold block is also a reasonable alternative (see Chapter 42 ). Emergence delirium is often brief (lasting 10–20 minutes) and, provided that analgesia has been addressed, can be managed by maternal or nurse comforts (e.g., cuddling) without pharmacologic intervention. Voluntary rather than forced oral fluid administration reduces the incidence of postoperative nausea and vomiting (PONV).
Children with chronic otitis media frequently have persistent rhinorrhea and suffer recurrent upper respiratory tract infection (URI) (see Chapter 13 ). Eradication of middle ear congestion and improved fluid drainage often resolves the concomitant symptoms. The frequency of perioperative complications in children with mild URIs is similar to that in children who are asymptomatic. In general, morbidity is not increased in children who present for minor surgery with acute, uncomplicated mild URIs, provided tracheal intubation can be avoided. Canceling this surgery because of rhinorrhea or recurrent mild respiratory symptoms is not usually justifiable as the vicious cycle of repeated URIs and chronic ear infections require an intervention (e.g., myringotomy) to break the cycle. It is, however, recommended that children with respiratory symptoms have their oxygen saturation (Sp o 2 ) measured before induction of general anesthesia, and that supplemental oxygen be administered postoperatively to those whose Sp o 2 readings are less than 93%.
Tympanoplasty and mastoidectomy are two of the most common major ear operations performed in children. General anesthesia usually consists of an inhalational anesthetic and IV opioids. Surgical identification and preservation of the facial nerve are necessary because of its proximity to the surgical field. To ensure the facial nerve can be identified using electrical stimulation, neuromuscular blockade is usually avoided. If a neuromuscular blocking drug (NMBD) must be used, a small dose should be given to facilitate tracheal intubation; if an NMBD is used for maintenance, suppression of the twitch response should not exceed 70%.
To gain access to the surgical site, the child's head is placed on a headrest, which may be positioned below the operating table. In addition, extreme degrees of lateral rotation may be required to visualize the middle ear anatomy. The anesthesiologist and surgeon must be especially vigilant to ensure that nerves, muscles, and bony structures are not injured as a result of this unusual positioning; the sternocleidomastoid muscles generally limit the safe degree of lateral head rotation. Left or right tilting (airplaning) of the operating room (OR) table minimizes the need for extreme lateral head rotation, which is an important consideration for children with Down syndrome. The laxity of the ligaments of the cervical spine, as well as immaturity of the odontoid process in children with Down syndrome, predisposes them to C1–C2 subluxation; 15% to 31% of children with Down syndrome or achondroplasia have atlantoaxial instability. Anteroposterior positioning requires the utmost care to avoid injury.
Positioning of the OR table to allow access to the respective middle ear and accommodate all the extra surgical equipment can also pose a challenge. Depending on the room configuration, the table may be rotated 90 degrees or even 180 degrees away from the anesthesia machine, necessitating the use of an extra-long breathing circuit ( Fig. 33.2 ). As a result of the limited access to the airway, very careful attention must be paid to securing the tracheal tube. Draping must allow immediate access to the airway should that be required.
Bleeding must be kept to a minimum during surgery on the small structures of the middle ear; relative hypotension (i.e., mean arterial pressure 10%–25% less than baseline) may help to reduce bleeding. Concentrated epinephrine solution (1 : 10,000) is frequently applied to the tympanic membrane to induce vasoconstriction of the blood vessels. Close attention should be paid to the dose of epinephrine used to prevent arrhythmias and wide swings in blood pressure. The maximum dose of topical epinephrine is 10 µg/kg, which may be repeated after 30 minutes. Alternatively, topical oxymetazoline 0.05% may be used to induce vasoconstriction.
The middle ear and sinuses are air-filled, nondistensible cavities; an increase in the volume of gas within these cavities increases the pressure. Nitrous oxide diffuses along a concentration gradient into air-filled middle ear spaces more rapidly than nitrogen moves out because nitrous oxide is 34 times more soluble in blood than nitrogen. The middle ear is vented through the opening of the eustachian tube. Normal passive venting of the eustachian tube occurs at 20 to 30 cm H 2 O pressure. Nitrous oxide increases the pressures within the middle ear such that they exceed the ability of the eustachian tube to vent the middle ear within 5 minutes, leading to pressure buildup. If the function of the eustachian tube is compromised during the surgical procedure, then pressure in the middle ear can further increase. Venting the middle ear occurs intermittently, and leads to constant fluctuations in middle ear pressure that cause movement of the tympanic membrane. During procedures in which the tympanic membrane is replaced or a perforation is patched, nitrous oxide should be discontinued before the application of the tympanic membrane graft to reduce the potential for pressure-related displacement. The omission of nitrous oxide does not significantly increase the requirements (minimum alveolar concentration) for the less-soluble inhaled anesthetics (desflurane and sevoflurane) in children. After nitrous oxide is discontinued, it is quickly reabsorbed, creating a void in the middle ear, with resulting negative pressure. This negative pressure may result in serous otitis, disarticulation of the ossicles in the middle ear (especially the stapes), and hearing impairment, which may last up to 6 weeks postoperatively. The use of nitrous oxide may increase the incidence of postoperative nausea and vomiting (PONV), as a direct result of negative middle ear pressure during recovery. The negative pressure created by the reabsorption of nitrous oxide stimulates the vestibular system by producing traction on the round window. Although all children are at risk for PONV, older children and adolescents, in particular, are at the greatest risk. Multimodal prophylactic administration of antiemetics (e.g., dexamethasone and ondansetron) is usually warranted. Local infiltration of the great auricular nerve can provide pain relief equivalent to that of opioids and may reduce the incidence of opioid-induced vomiting (see Chapter 42 ).
A smooth, quiet emergence is desirable. Deep tracheal extubation can be accomplished if the child breathes spontaneously during the last 15 to 20 minutes of surgery, the concentration of inhalational anesthetic is greater than 1.3 times the minimal alveolar concentration, and opioids are titrated to produce regular slow respirations. Gentle suctioning of the oropharynx and possibly the use of IV lidocaine (1–1.5 mg/kg) in children older than 1 year of age can minimize or even prevent coughing after the tracheal tube is removed. Alternately, total IV anesthesia may be used for middle ear procedures because nitrous oxide is avoided, postoperative vomiting is reduced and the frequency of emergence delirium is minimal (see Chapter 8 ).
The first multichannel cochlear implant was developed in Australia in 1978. After a series of clinical trials, the US Food and Drug Administration (FDA) approved the use of the Australian cochlear implant in adults in 1985, and subsequently in infants and children as young as 6 months of age. In recent years, the indications for cochlear implants have broadened and continue to evolve. With the application of universal neonatal hearing screening programs, a large pool of hearing-impaired infants has been identified. The benefits of early intervention with cochlear implants are being explored. Younger children with severe to profound hearing loss markedly improve their auditory, speech, and language skills after cochlear implants, and more of these children can be mainstreamed with their age-appropriate hearing peers when they receive an implant early in life. Experience has shown that cochlear implant surgery is safe in infants older than 6 months of age, provided that special attention is paid to the physiologic and anatomic differences present in this age group. The majority of children presenting for surgery have no significant health issues other than deafness. However, in a recent review, 40% had comorbidities that have been categorized into syndromes associated with deafness, problems associated with premature births, neurologic problems, and cardiac abnormalities. For these children, the availability of skilled postoperative nursing and a pediatric intensive care unit (ICU) are essential.
Surgery requires meticulous care with hemostasis, soft tissue dissection, and bone drilling because bleeding from bone can be difficult to control and can complicate the surgical outcome. The surgical procedure itself needs the facial nerve to be identified; thus if an NMBD is used for intubation, spontaneous recovery should be confirmed and communicated to the surgeon. Postoperative fitting of the externally worn speech processor is very important for successful use of the cochlear implant. However, this fitting process can be difficult, particularly in infants and young children, because of limited communication capabilities. Electrically elicited stapedius reflex thresholds (ESRTs) obtained intraoperatively are used to determine the maximum comfort level, which is defined as the loudest sound tolerated without pain. It has been used for postoperative speech processor fitting, although the influence of anesthetics on the threshold values must be taken into account. More reliable threshold values can be obtained by adjusting the dosage of hypnotics to achieve a lighter level of hypnosis during ESRT measurement. In most children, increasing the concentration of inhalational anesthetics increases the stapedius reflex threshold, whereas propofol and nitrous oxide have minimal effects on the ESRT. Consequently, total IV anesthesia (TIVA) is popular in some countries. Dexmedetomidine has been used to decrease inhaled anesthetic requirements, prevent intraoperative hypertension (and bleeding), and ensure a smooth emergence. As always, appropriate communication with the surgeon and audiologist will help ensure a successful outcome.
While cochlear implants are indicated in children with profound sensoneural or nerve deafness, many children with conductive hearing loss who cannot use traditional hearing aids (because of draining ears or chronic infection) can be candidates for bone-anchoring hearing aid devices (BAHA). The BAHA implant is a titanium device placed in the skull behind the ear and vibrates the bone to transmit sound directly to the inner ear. Although most children who are BAHA candidates are older than 5 years of age, some infants can now be fitted. The surgical and anesthetic approach is less challenging than that for cochlear implants.
Chronic sinusitis in children can be caused by antibiotic-resistant bacteria and is usually treated with broad-spectrum antibiotics. In some children with obstructive adenoid pads, adenoidectomy will improve the signs and symptoms of sinusitis. Functional endoscopic sinus surgery (FESS) using sharp biting instruments and/or a microdebrider has become the primary method of surgical therapy for chronic sinusitis. Current techniques aim to leave the mucosa intact to prevent scarring in the frontal recess. Although sometimes controversial, there is no evidence at present that FESS affects facial growth in children. Of interest to the anesthesiologist is that many children who require FESS have coexisting medical problems, such as asthma and cystic fibrosis. These conditions must be optimized before surgery (see Chapter 13 ).
An alternative to FESS is balloon sinuplasty. This technique uses ballooned catheters to dilate the maxillary, frontal, and sphenoidal natural ostia without bone or soft tissue removal. This should result in less bleeding and reduced anesthetic morbidity.
Anesthetic management usually requires tracheal intubation to secure the airway; the use of an oral preformed tracheal tube (e.g., the Ring-Adair-Elwyn [RAE] tube) allows secure fixation to the mandible and unobstructed access to the maxilla and sinuses. The use of a cuffed tracheal tube is particularly advantageous to eliminate a gas leak that could fog up the endoscopic instruments. A throat pack is frequently inserted to absorb blood in the oropharynx and limit the gas escaping around an uncuffed endotracheal tube (ETT). It is critically important that the pack is removed before tracheal extubation. Occasionally, an LMA may be used to facilitate a quick “second look.”
Because bleeding is inevitable with this surgery and can interfere with the surgical exposure, packing the nasal cavity with a vasoconstricting solution before surgery is common. Typically used topical vasoconstrictors include oxymetazoline 0.025% to 0.05%, phenylephrine 0.25% to 1%, and less frequently, cocaine 4% to 10%. It is important for the anesthesiologist to be aware of the type and dose of the vasoconstrictor and to ensure that no more than the maximum effective dose is applied. Application of topical phenylephrine or other potent vasoconstrictors to mucous membranes or open surgical sites can cause severe hypertension, reflex bradycardia, and even cardiac arrest. Hypertension induced by topically applied vasoconstrictors often resolves spontaneously and may not require aggressive treatment. The use of β-adrenergic blockers or calcium-channel blockers to control blood pressure in these circumstances can depress cardiac output, leading to pulmonary edema and cardiac arrest. It is recommended that the initial topical dose of phenylephrine should not exceed 20 µg/kg in children.
Corticosteroids, such as IV dexamethasone (0.25–0.5 mg/kg), are usually administered to reduce swelling and scarring. Frequently, the surgeon will want to leave an absorbable stenting material, such as MeroGel (Medtronic ENT, Jacksonville, FL), at the end of surgery. Unfortunately, this will interfere with nasal breathing and may increase the incidence of emergence delirium. An anesthetic technique that ensures adequate analgesia and rapid return of consciousness at the end of surgery is therefore desirable. One of the authors (RSH) has found that a combination of desflurane, fentanyl, and low-dose propofol works well in this regard. Alternatively, a pure TIVA technique (propofol + remifentanil) or desflurane + remifentanil can be used; the remifentanil dose is adjusted to the desired mean arterial pressure.
A unilateral or bilateral infraorbital nerve block can also be performed via the intraoral or extraoral route to provide analgesia (see Chapter 42 ). One further concern is the need to avoid NSAIDs in children with asthma and sinusitis secondary to nasal polyps (Samter triad).
Adenotonsillectomy is one of the oldest pediatric surgical procedures, yet its conduct and practice continue to evolve. In the past decade, notable changes have been introduced. These include the indications for adenotonsillectomy, the criteria for postoperative hospital admission, and recommended postoperative analgesic regimens. Additionally, the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) no longer recommends prophylactic antibiotics during adenotonsillectomy surgery.
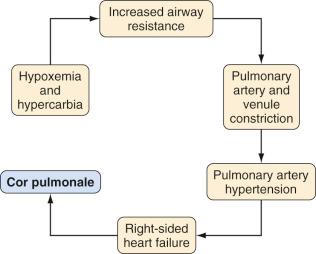
Adenotonsillectomy is one of the most commonly performed pediatric surgical procedures worldwide; one in eight American children will undergo adenotonsillectomy. Chronic or recurrent tonsillitis and obstructive adenotonsillar hyperplasia are the major indications for surgical removal ( Table 33.1 ). Surgical treatment is required when tonsillitis recurs despite adequate medical therapy, when associated with peritonsillar abscess, or when acute or chronic airway obstruction compromise breathing. Halitosis, persistent pharyngitis, and cervical adenitis may accompany chronic tonsillitis. Tonsillar hyperplasia may lead to chronic airway obstruction, resulting in obstructive sleep apnea (OSA), failure to thrive, swallowing disorders, speech abnormalities, pulmonary hypertension, right-sided heart failure, and eventually cor pulmonale ( Fig. 33.3 ). Certain children with cardiac lesions may be at risk for endocarditis caused by recurrent streptococcal bacteremia secondary to infected tonsils and will require prophylactic antibiotics (see Chapter 16 ).
| Infection |
| Acute tonsillitis or adenoiditis Recurrent tonsillitis or adenoiditis Chronic tonsillitis or adenoiditis Peritonsillar abscess Halitosis |
| Obstruction |
| Nasal airway (adenoids) Pharyngeal airway (tonsils) Obstructive sleep apnea/sleep-disordered breathing Cyanosis Failure to thrive Cor pulmonale due to airway obstruction |
| Mass Lesion |
| Tonsillar/adenoidal Benign Malignant |
Adenoidectomy is usually performed in conjunction with tonsillectomy, although in some situations it is performed as the sole surgical procedure. Indications for adenoidectomy alone include chronic or recurrent purulent adenoiditis, recurrent otitis media with effusion secondary to adenoidal hyperplasia, and chronic sinusitis. Advanced degrees of adenoidal hyperplasia may lead to nasopharyngeal obstruction, obligate mouth breathing, poor feeding resulting in failure to thrive, speech disorders, and sleep-disordered breathing (SDB). Long-standing nasal obstruction can result in orofacial abnormalities with a narrowing of the upper airway and dental abnormalities (so-called adenoid facies or long face syndrome).
Surgical techniques for adenotonsillectomy include guillotine and snare techniques, cold and hot knife dissection, suction, radiofrequency ablation, and unipolar and bipolar electrocautery techniques. Electrocautery dissection offers two advantages over the other techniques with less intraoperative blood loss and a reduced risk of postoperative hemorrhage, although these are offset, in part, by more pain and poor oral intake postoperatively.
A meta-analysis of 23 retrospective studies (N = 13,537 children) reported an overall frequency of postoperative complications after adenotonsillectomy of 19%. Respiratory compromise was the most frequent complication (9.4%) followed by secondary hemorrhage (5–10 days after surgery) (2.6%). Age has a major influence on these complications. Children older than age of 10 years more commonly have a secondary hemorrhage, whereas younger children more commonly have both poor oral intake and respiratory complications. The majority of children younger than 3 years of age experience airway problems after adenotonsillectomy for obstructive breathing.
Surgical complications after adenotonsillectomy are rare but include uvular amputation, uvular edema, velopharyngeal insufficiency, and nasopharyngeal stenosis. Atlantoaxial subluxation manifesting as neck pain and torticollis, mandibular subluxation and condylar fracture, cervical adenitis, and cervical osteomyelitis have also been reported. Bleeding, burns, and airway fires account for over one-third of malpractice claims associated with this procedure. The mortality associated with adenotonsillectomy is estimated at 1 per 16,000 to 1 per 41,000 procedures. Throat pain, otalgia, emesis, poor oral intake, and dehydration are common morbidities.
The general health of the child and the indications for surgery must be reviewed. URIs are frequent in these children and can interfere with the timing of adenotonsillectomy because the risk of respiratory compromise and hemorrhage is increased. Obese children often have SDB and they are at increased risk for postoperative hemorrhage. A history of bleeding tendencies requires investigation. Medications that interfere with coagulation include aspirin, NSAIDs, and valproic acid. Discontinuation of these drugs preoperatively is sometimes problematic, and preoperative consultation with neurology, cardiology, and hematology specialists may be indicated.
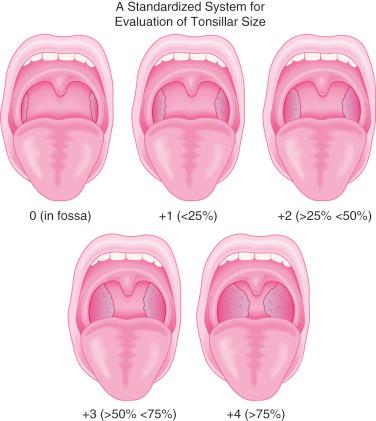
A careful cardiorespiratory history and physical examination is essential. Children with chronic tonsillar hypertrophy may have long-standing hypoxemia and hypercarbia, which can lead to cor pulmonale (see Fig. 33.3 ). The oropharynx should be evaluated and the tonsillar size classified ( Fig. 33.4 ). In some centers, a complete blood cell count and coagulation profile is required before adenotonsillectomy. There is no evidence that routinely performed preoperative coagulation studies are beneficial unless they are indicated by history or the presence of a disorder of hemostasis. The indications for the procedure should be clearly delineated in the surgical plan of care.
SDB describes abnormal breathing patterns during sleep. These abnormal patterns include obstructive breathing, including snoring, paradoxical chest wall motion, and increased respiratory effort, apneas, hypopneas leading to hypercarbia, and desaturation followed by arousals. The spectrum of SDB ranges from primary “benign” snoring to the obstructive sleep apnea syndrome (OSAS). The Childhood Adenotonsillectomy Trial (CHAT), a randomized controlled trial, compared watchful waiting for 7 months with early adenotonsillectomy in more than 400 children with OSAS. The primary outcome, attention and executive function, did not differ between the two groups. However, secondary outcomes for polysomnographic, behavioral, and quality-of-life metrics normalized in 79% of the children after adenotonsillectomy compared with 46% who did not undergo adenostonsillectomy.
The single most important task during the preoperative evaluation of the child for adenotonsillectomy is to distinguish the child with the OSAS from the child with isolated obstructive breathing (e.g., primary snoring) and chronic infectious tonsillitis, because the former children are at greater risk for developing severe perioperative respiratory adverse events (PRAEs), possibly including death, after adenotonsillectomy. Several recent studies have reported unexpected deaths after adenotonsillectomy from presumed sleep apnea after discharge from a monitored setting, including to home. A meta-analysis of 3 retrospective studies (N = 371 children) revealed that children with OSAS proven by polysomnography (PSG) criteria have a 5-fold increase in the odds for PRAEs compared with children without OSAS. In contrast, they were less likely to have postoperative hemorrhage (odds ratio 0.4, 95% confidence interval [CI] 0.2–0.7).
The OSAS encompasses a range of severity. At the most extreme, it includes pulmonary and systemic hypertension, cor pulmonale, ventricular hypertrophy, the metabolic syndrome, neurocognitive dysfunction, and life-threatening nocturnal hypoxemia. Because adenotonsillectomy is often the initial treatment for OSAS, the majority of these children may present with a spectrum of disease affecting multiple organ systems. Failure to thrive is common. Infections affecting the lower respiratory tract have been linked to chronic aspiration.
A high index of suspicion is required to identify the child with OSAS on clinical criteria. Clinical criteria do not always distinguish primary snoring from OSAS in children. There is a greater incidence of OSAS in Asian and African American populations. African American children desaturate more profoundly during sleep-related obstructive airway events than do Caucasian and Hispanic children. Obese children (BMI > 95th percentile) are at greater risk for OSAS and postoperative hemorrhage. Anatomic features may underlie the pathogenesis of OSAS; common medical conditions and syndromes that predispose to the development of OSAS are listed in Table 33.2 . Infants who have suffered an acute life-threatening event (ALTE) have a greater incidence of OSAS in childhood and adolescence.
| Craniofacial Syndromes |
| Crouzon syndrome Apert syndrome Pfeiffer syndrome Treacher Collins syndrome Pierre Robin sequence Goldenhar syndrome Larsen syndrome |
| Disorders of Cranial Base |
| Arnold-Chiari malformation Achondroplasia Syringobulbia |
| Neuromuscular Disorders |
| Cerebral palsy |
| Trisomy 21 |
| Infiltrative Disorders |
| Mucopolysaccharidoses Acromegaly Obesity Prader-Willi syndrome |
| Temporomandibular Joint Ankylosis |
Parents should be asked if the child snores loudly, if the snoring can be heard through a closed door, if there are gasps or pauses in respirations, if there is daytime somnolence, night terrors, nocturnal enuresis, attention deficit disorder, or poor school performance. However clinical features obtained from demography, parental report, and physical findings do not robustly identify OSAS severity and parental report of symptoms has a poor positive predictive value.
Although it is important to recognize the significance of OSAS in children who are scheduled for adenotonsillectomy, children who do not meet the criteria for OSAS but who have less severe forms of SDB, such as upper airway resistance syndrome (UARS) or obstructive hypopnea, may also be at increased risk for morbidity after surgery. Guidelines for the perioperative management of these children continue to be developed.
The obstructive events that characterize OSAS result in recurrent episodes of hypoxia, hypercarbia, and sleep disruption, a trilogy that has been linked to the development of medical sequelae that accompany severe OSAS. The severity of OSAS is assessed by the frequency and severity of the obstructive respiratory events during sleep; both occur most often during rapid eye movement (REM) sleep. The frequency and severity of obstructive events worsen after midnight.
The polysomnogram (PSG) is the gold standard diagnostic test for evaluation of SDB. The PSG simultaneously records the electroencephalogram, electromyogram, electrocardiogram, pulse oximetry, airflow, and thoracic and abdominal movement during sleep ( Fig. 33.5 ). Some cardiorespiratory studies limit the recording devices to pulse oximetry with sensors for thoracic and abdominal movement. A common definition of OSA in children is an obstructive effort that includes more than two obstructive breaths, regardless of the duration of the apnea. An obstructive apnea index of 1 is the cutoff for normality in children. The diagnostic criteria for pediatric OSAS according to the American Academy of Sleep Medicine are: mild OSAS corresponds to an apnea index >1 <5 events per hour; moderate OSAS corresponds to an apnea index >5 <10 events per hour, and severe OSAS corresponds to an apnea index ≥10 events per hour. Apneas are classified as central, obstructive, and mixed (see Fig. 33.5 ). A central apnea occurs when there is no apparent respiratory effort. An obstructive apnea is associated in the presence of apparent, often vigorous, inspiratory efforts that are ineffective because the upper airway is not patent. A mixed obstructive apnea is diagnosed when both central and obstructive components are present without interruption by effective respirations.
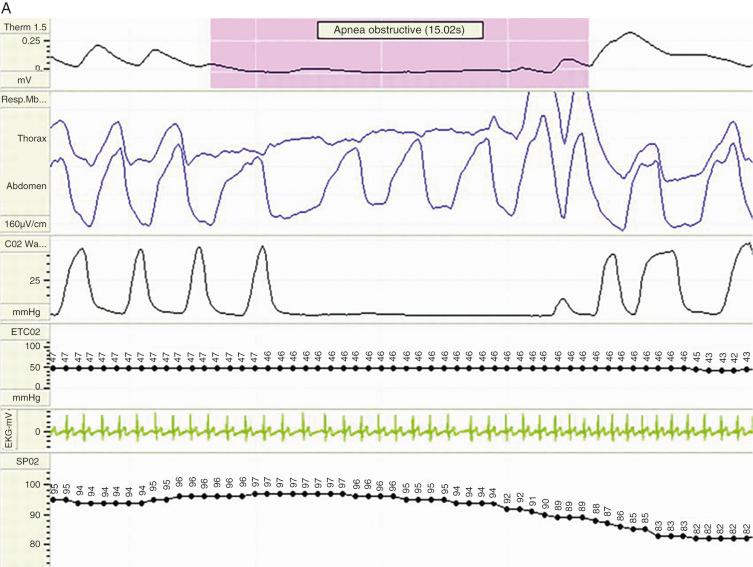
Hypopnea is defined as a reduction in airflow of more than 50%. The apnea hypopnea index (AHI) is the summation of the number of obstructive apnea and hypopnea events and is analogous to the respiratory disturbance index (RDI). The PSG study also records oxygen-desaturation indexes.
Metrics obtained during PSG predict the risk of PRAEs; a detailed history from the parents will also help to assess children likely to be at risk ( Table 33.3 ). An RDI of greater than 20 events per hour is associated with breath-holding during induction, whereas an RDI greater than 30 is associated with laryngospasm and desaturation during emergence. Ten obstructive events per hour during a screening polysomnogram is the threshold for severe PRAEs. A preoperative RDI, analogous to the AHI, above 19 events per hour may predict persistent OSAS in long-term follow-up.
|
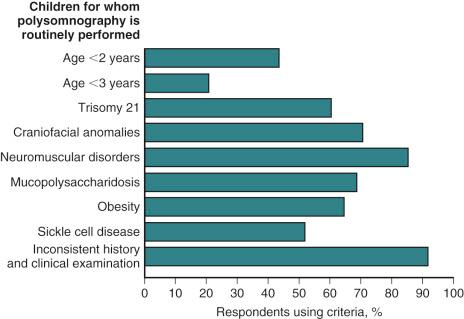
Fewer than 10% of children in North America are assessed with PSG before adenotonsillectomy. Many tertiary care children's hospitals in the United States do not have uniform criteria for routinely performing PSG before adenotonsillectomy ( Fig. 33.6 ). The notion that PSG should be reserved for children with medically complex conditions and evaluation of persistent SDB despite adenotonsillectomy is contentious. If PSG is not available, or is considered too costly, other diagnostic tests for OSAS may be considered. Many parents will record audio and/or videotapes of their child's breathing pattern during sleep. These have reasonably high positive and negative predictive values. Although home sleep apnea tests are increasingly used in adults, there are no published guidelines for their use in children. Nap PSG is an abbreviated study that records sleep and breathing in the laboratory during daytime naps. Because of the limited recording time and the likely absence of REM sleep, they are best regarded as screening tools.
Sleep evaluation with oximetry alone offers a low-cost, easy-to-use alternate test to PSG. Published normative data in children indicate that the 2.5th percentile for the baseline Sp o 2 is 95%. The nadir saturation (nSAT) is the lowest Sp o 2 recorded during sleep and correlates inversely with the AHI. An nSAT of 92% is the minimum normal Sp o 2 in children ; however, an nSAT of <80% is a robust predictor of the risk for PRAEs and sensitivity to opioids.
A commonly reported index of desaturation events, the oxygen desaturation index (ODI), is the number of decreases in Sp o 2 ≥4% from baseline (ODI 4 ). In children, the 95th percentile for the ODI 4 is 2.2 episodes per hour. The ODI 4 is a sensitive metric used to evaluate the response to adenotonsillectomy in long-term outcome follow-up. As obstructive respiratory events in children usually occur during REM and stage 2 sleep, the desaturation events tend to cluster at 60- to 90-minute intervals. A cluster of desaturation is defined as 5 or more decreases in Sp o 2 (≥4%) within a 10- to 30-minute interval. At least 3 clusters of desaturation with at least 3 Sp o 2 decreases to less than 90% during a 6-hour nocturnal oximetry recording is diagnostic of OSA: positive predictive value for an AHI >1 event per hour of 97%; sensitivity 40%. For ≥2 clusters of desaturation along with at least 1 decrease in Sp≥ 2 to ≤90% increases the sensitivity of this measurement to predict OSA to 80%.
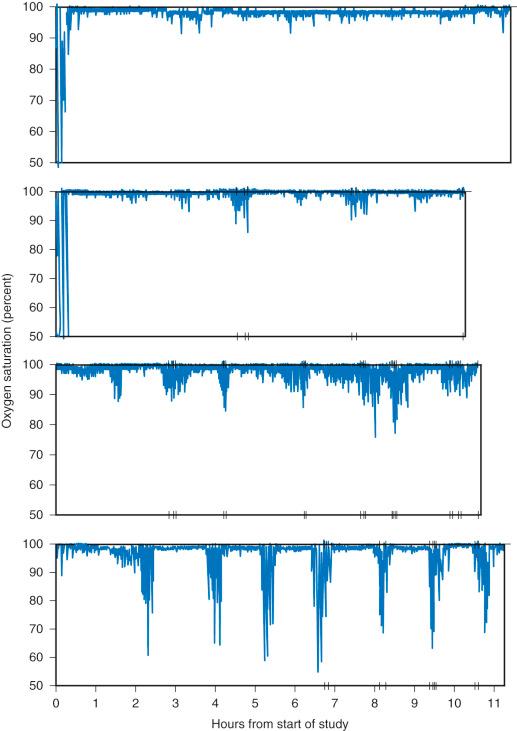
The McGill Oximetry Scoring (MOS) system further classifies the severity of nocturnal hypoxemia ( Fig. 33.7 ). The MOS correlates with both the AHI and the risk of PRAEs after adenotonsillectomy in children. The MOS4 is defined as >3 decreases in Sp o 2 to <80%; corresponding to a mean AHI of 40 events per hour. The risk for major PRAEs, including reintubation, for children with MOS4 managed with a standard opioid regimen was 20% to 24%.
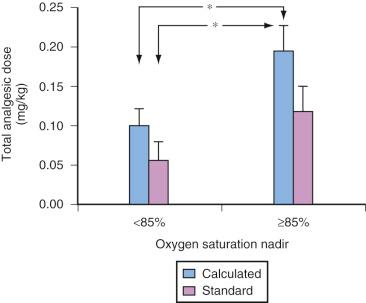
These findings are clinically relevant in at least two respects: the morphine (or morphine equivalent) dose required to achieve a uniform analgesic endpoint in children with OSAS who exhibited a preoperative nSAT <85% was half that required in controls ( Fig. 33.8 ). In updated statements from both the American Academy of Pediatrics (AAP) and the AAO-HNS, the criteria to admit children after adenotonsillectomy is an nSAT <80%.
Consultations to plan the perioperative care of children with severe OSAS are important. In contrast to children without OSAS, children with severe OSAS may require additional preoperative testing. A capillary blood gas sample collected in the morning may reveal an increased concentration of bicarbonate, consistent with a chronic respiratory acidosis. A preoperative electrocardiogram or echocardiogram may show evidence of right ventricular hypertrophy and/or pulmonary hypertension. A chest radiograph may demonstrate lower airway disease or cardiomegaly.
In a review of 9023 children with a mean age of 3.8 years evaluated over a 7-year period, 35 (0.4%) required urgent adenotonsillectomy within 48 hours of PSG. Urgent adenotonsillectomy for severe OSAS is associated with significant respiratory morbidity after surgery. On occasion, adenotonsillar hypertrophy may progress to compromise the upper airway during wakefulness. In some instances, the anesthetic considerations for obstructed and difficult airways may overlap; some may develop postobstructive pulmonary edema after intubation or surgery. Young children with profound oxygen desaturation during sleep, high obstructive indexes, and carbon dioxide (CO 2 ) retention may require admission to the pediatric ICU for optimization before and/or after adenotonsillectomy.
Although adenotonsillar hypertrophy is the primary cause of OSAS in children, those with comorbidities such as obesity, Down syndrome, and craniofacial abnormalities may experience airway obstruction that is unrelated to the tonsils and adenoids. Diagnostic, preoperative evaluation of these airways such as cine magnetic resonance imaging (MRI) and sleep endoscopy may be required before surgery; combinations of sevoflurane, propofol, remifentanil, dexmedetomidine, and ketamine anesthesia have been used. Dexmedetomidine, an α-agonist, mimics non-REM sleep, facilitating the maintenance of airway patency. However, as obstructive events in pediatric OSA occur primarily during REM sleep, dexmedetomidine may not replicate the breathing pattern during natural sleep.
Sleep endoscopy, also known as drug-induced sleep endoscopy (DISE), is a technique that enables the surgeon to evaluate the level of obstruction in an anesthetized child with suspected OSAS. The considerations for DISE are complex, requiring endoscopic evaluation of an obstructive airway in the supine position during spontaneous ventilation. Administration of lidocaine and oxymetazoline to the nasal mucosa may be useful. When DISE is indicated in children whose clinical evaluation is unremarkable or when OSAS persists after adenotonsillectomy, it should be performed by experienced otolaryngologists and anesthesiologists. A retrospective study determined that the frequency of oxygen desaturations to <85% during DISE with dexmedetomidine and ketamine was less than with either propofol alone or a combination of propofol and sevoflurane.
The anesthetic goals for adenotonsillectomy are to (1) provide a smooth, atraumatic induction; (2) provide the surgeon with optimal operating conditions; (3) establish IV access for volume expansion and medications as indicated; and (4) provide rapid emergence so that the child is awake and able to protect the recently instrumented airway. The need for a premedication is determined during the preanesthetic evaluation. Children with symptoms of SDB who require premedication should be closely observed , although the desaturation is transient and infrequent (1.5% of cases) after oral midazolam premedication. In children with severe OSA, premedication with short-acting, reversible drugs is advised and monitoring with pulse oximetry is indicated.
The anesthetic techniques for adenotonsillectomy are varied and include the choice of an inhalational or TIVA technique, the choice of an ETT or LMA, and the choice of spontaneous or controlled ventilation. Children who are scheduled for adenotonsillectomy have a greater incidence of airway reactivity and laryngospasm than those undergoing non-airway surgery. Of the currently available inhalational agents, sevoflurane provides a smooth induction of anesthesia. Maintenance of anesthesia with desflurane (for those whose airway is secured with an ETT) provides a rapid emergence and recovery. An infusion of dexmedetomidine (1 to 2 µg/kg IV over 5 to 10 minutes) combined with an inhalation agent can provide satisfactory intraoperative conditions for adenotonsillectomy without adverse hemodynamic effects. However, clinical experience and a recent study suggest a quicker time frame (0.49 µg/kg over 5 seconds) might be safe. Some authors have reported good results with a single IV dose of clonidine (1 µg/kg).
Cuffed ETTs have become increasingly used in children of all age groups, preventing the air leak and the consequent bubbling of gas in secretions and blood that can interfere with surgery. These tubes also minimize pollution by anesthetic gases and may decrease the risk of an airway fire when electrocautery is used.
Blood, secretions, and/or irrigation fluids may be present in the oropharynx at the conclusion of surgery and should be carefully suctioned by the surgeon or the anesthesiologist before emergence from anesthesia. Emptying the stomach with an orogastric tube, a maneuver frequently performed by surgeons under direct vision after completion of surgery, does not reduce the incidence of PONV.
Many anesthesiologists prefer to wait until the child is fully awake before removing the ETT as the presence of an intact airway and pharyngeal reflexes are of utmost importance in preventing aspiration, laryngospasm, and airway obstruction. However, with current surgical (use of electrocautery) and anesthetic (dexmedetomidine) techniques, a careful deep extubation is frequently chosen. The incidence of major respiratory complications requiring positive airway pressure, administration of drugs, airway manipulation, or instrumentation after deep or awake extubation is similar (~11%). At the time of extubation, a common practice is to position the child in the lateral “recovery” or “tonsil” position with the head slightly down to permit blood and secretions to pool in the dependent cheek and drain out of the mouth rather than accumulate at the laryngeal inlet. The child should remain in the tonsil position postoperatively, while being carefully observed and monitored during transport to the PACU. The lateral position has the additional benefit of increasing both the cross-sectional area of the upper airway and total volume of the upper airway compared with children in the supine position.
The use of the LMA for adenotonsillectomy was described in 1990, but it was not until the availability of a model with a flexible spiral, metallic reinforced shaft that made it practical for use in adenotonsillectomy ( E-Fig. 33.1 ). Advantages cited for the LMA over the ETT included a decrease in the incidence of postoperative stridor and laryngospasm ; recent evidence disputes these advantages.
Children experience significant pain and severe functional limitations for up to 7 days after adenotonsillectomy. Recovery from pain and return to normal daytime activities are considerably faster after isolated adenoidectomy. Surgical technique has a major impact on the speed of recovery from pain because electrocautery techniques are generally associated with greater pain, presumably owing to increased thermal injury, although this remains controversial. Intracapsular tonsillectomy causes less pain and morbidity than the conventional extracapsular techniques and the outcomes appear comparable ; this technique may provide an alternative that may reduce the risk for posttonsillectomy apnea because of the reduced pain.
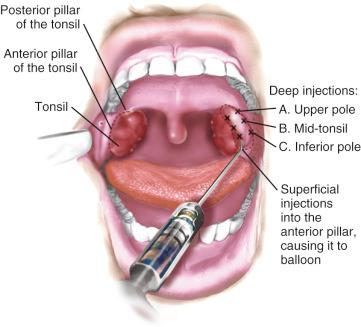
Infiltration of local anesthetics into the tonsillar fossa during tonsillectomy decreases postoperative pain, but the pain relief is brief ( E-Fig. 33.2 ). Life-threatening complications have been reported after local anesthetic infiltration in the tonsillar fossa, including intracranial hemorrhage, bulbar paralysis, deep cervical abscess, cervical osteomyelitis, medullopontine infarct, and cardiac arrest. The risks associated with injection of local anesthesia in the tonsillar fossa may outweigh its potential benefits, particularly in inexperienced hands.
Over the past decade, there has been a shift away from opioids as the mainstay of perioperative analgesia to nonopioid regimens, including dexmedetomidine, acetaminophen, NSAIDs, dexamethasone, and ketamine. An infusion of dexmedetomidine (2 µg/kg IV over 5 to 10 minutes followed by 0.7 µg/kg per hour) may reduce postoperative opioid requirements. After larger doses of dexmedetomidine (2 and 4 µg/kg), the opioid-free interval increases and the postoperative opioid requirements decrease; however, duration of stay in the PACU is markedly prolonged.
A single intraoperative dose of dexamethasone reduces postadenotonsillectomy pain and edema when electrocautery has been used. These doses overlap the doses of dexamethasone to mitigate PONV (see later text). Dexamethasone (0.3–1 mg/kg) administration is associated with reduced parental- and physician-rated pain scores after adenotonsillectomy ( Table 33.4 ). The minimum morphine-sparing dose for dexamethasone is reported to be 0.5 mg/kg. The use of dexamethasone in adenotonsillectomy remains routine in most US centers. Single doses of dexamethasone have not been associated with aseptic necrosis of the hip or infections, but have been responsible for precipitating the acute tumor lysis syndrome.
| Source | No. of Children | Dexamethasone Dose | Electrocautery Technique | Effect on Pain |
|---|---|---|---|---|
| Catlin and Grimes | 25 | 8 mg/m 2 | No | No difference |
| Ohlms et al. | 69 | 0.5 mg/kg | No | No difference |
| Tom et al. | 58 | 1.0 mg/kg | Yes | Reduced |
| April et al. | 80 | 1.0 mg/kg | Yes | No difference |
| Hanasono et al. | 219 | 1.0 mg/kg | Yes | Reduced |
In 2008, a report suggested that posttonsillectomy bleeding was increased in children who had received dexamethasone up to 0.5 mg/kg (maximum 20 mg). A retrospective review of 31,934 children investigated whether a single IV dose of either dexamethasone or hydrocortisone administered on the day of surgery resulted in posttonsillectomy hemorrhage that required reoperation. The rate of reoperation for secondary hemorrhage in children who received either steroid, 1.2% (adjusted odds ratio 2.5, 95% CI 1.5–4.2), was significantly greater than that in those who received steroids, 0.5% (P < 0.001) ; this finding was not present in adults. However, several subsequent studies have refuted these claims. The consensus opinion and practice is that a single intraoperative dose of dexamethasone does not cause clinically important hemorrhage. Despite concerns that the routine use of NSAIDs for adenotonsillectomy might increase the risk for postadenotonsillectomy hemorrhage, the AAO-HNS now recommends their use for postoperative analgesia. An audit of more than 4800 pediatric tonsillectomies in which the NSAIDs diclofenac and ibuprofen were routinely used, reported a primary hemorrhage rate of 0.9%. Because the effects of ketorolac on platelet function are reversible, the effect depends on the presence of ketorolac within the body. Unlike the effect of aspirin on bleeding, the effects of these NSAIDs is short-lived. However, we recommend avoiding NSAIDs, especially ketorolac, during surgery. When used for postoperative analgesia, they should be administered after hemostasis is achieved. The use of ibuprofen in children who have been taking aspirin can result in severe posttonsillectomy bleeding.
A recent editorial suggested that perhaps we need a clean start with a new class of analgesic drugs such as selective cyclooxygenase-2 (COX-2) inhibitors for adenotonsillectomy patients. Although not available in the United States, parecoxib is a selective COX-2 inhibitor drug that reduces the production of inflammation-promoting prostaglandins. It spares platelet aggregation, bronchial tone, and gastric mucosal integrity unlike nonselective NSAIDs, and may reduce the need for rescue morphine and decreased the frequency of vomiting after adenotonsillectomy. Celebrex, a COX-2 available in the United States, reduces early postoperative pain and the need for supplemental analgesics. More evidence is needed before COX-2 agents can be recommended for children during adenotonsillectomy.
Acetaminophen is commonly used as a component of multimodal analgesic approach in these children. An IV formulation of acetaminophen is available in many countries, offering the theoretical advantage of greater predictability than the oral and rectal routes (see Fig. 33.1 ). Although the peak blood concentration is greater, the duration of analgesia after 15 mg/kg of IV acetaminophen is less than that after 40 mg/kg given rectally. Reports of 10-fold overdoses of IV acetaminophen with near-catastrophic outcomes in infants should alert clinicians to potentially fatal dosing errors with this medication.
Postdischarge pain management strategies vary among practitioners and institutions. Acetaminophen, dexamethasone, and ibuprofen are frequently prescribed in various combinations. Acetaminophen and NSAIDs (e.g., ibuprofen and diclofenac) have additive effects, prolonging the duration of analgesia when combined ; codeine is no longer indicated (see later text). Oxycodone may be used in older children. Scheduled dosing of any combination of these drugs is more effective than as-needed (pro re nata [PRN]) dosing in reducing pain intensity.
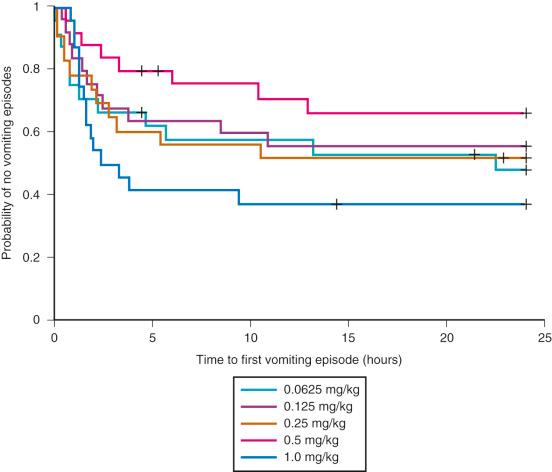
Emesis and poor oral intake are common comorbid conditions after adenotonsillectomy. Opioids increase the incidence of PONV, with two-thirds of treated children experiencing PONV. Propofol, ondansetron, and dexamethasone are widely used to reduce the incidence of emesis after adenotonsillectomy. A single intraoperative dose of dexamethasone reduces the incidence of emesis during the first 24 hours after adenotonsillectomy. The number of children needed to treat was only four, which means that the use of dexamethasone in four children undergoing adenotonsillectomy results in one less child experiencing PONV. In addition, children who received dexamethasone were more likely than those receiving placebo to advance to a soft diet on postoperative day 1, with a number needed to treat of five. Given the antiemetic and possible morphine-sparing advantages of a single dose of dexamethasone, and its low cost and safety profile, the evidence suggests that routine use of dexamethasone reduces morbidity after adenotonsillectomy in children. The smallest effective dose remains unclear. One study reported no difference in postoperative vomiting, pain scores, time to first liquid, and time to first analgesics among IV dexamethasone doses between 0.0625 and 1.0 mg/kg ( Fig. 33.9 ).
Postdischarge vomiting continues for days in some children. The use of at-home oral ondansetron disintegrating tablets may prevent emesis during the first 3 days after adenotonsillectomy. Acupuncture, acupressure at the P6 (Nei-Kuwan) point, as well as therapeutic suggestion, have also been used with variable results.
Children with OSA who require premedication should be closely observed because transient oxygen desaturation that did not require any intervention has been reported in 1.5% of children with OSA who received 0.5 mg/kg oral midazolam. This study excluded those with severe OSAS. Accordingly, caution should be exercised when premedicating children with severe OSAS.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here