Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lyme disease (LD), cutaneous borreliosis, borreliosis, borrelial lymphocytoma, lymphadenosis cutis benigna, erythema chronicum migrans, erythema migrans, acrodermatitis chronica atrophicans
This anthropozoonosis is endemic in North America and Europe.
It is caused by Borrelia burgdorferi , a spirochete.
The vector is the tick ( Ixodes ricinus complex ).
Clinical manifestations:
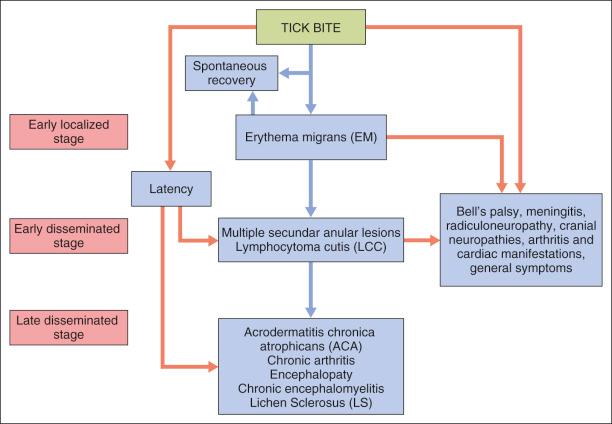
Early localized stage: erythema migrans (EM)
Early disseminated stage: wide spirochetal dissemination with or without extra cutaneous manifestations, lymphocytoma cutis (LCC)
Late disseminated stage: chronic arthritis, chronic neurologic manifestations and acrodermatitis chronica atrophicans (ACA).
Diagnosis: clinical findings; epidemiologic history (endemic area, tick bite), serologic tests (immunofluorescent antibody [IFA], enzyme-linked immunosorbent assay [ELISA], Western blot), histopathology, polymerase chain reaction (PCR).
Treatment: antimicrobials (doxycycline, amoxicillin, ceftriaxone, cefotaxime, penicillin G, cefuroxime axetil).
Lyme disease (LD) is an infectious syndrome caused by the spirochete Borrelia burgdorferi, transmitted by ticks. It is an anthropozoonosis that can cause a spectrum of disease from the initial skin lesion, through widely varied symptoms and signs, to chronic neurologic and arthritic disability. It has been widely reported throughout the USA, Europe, and many parts of the world.
The first erythema migrans (EM) cases were described in the early 20th century by Afzelius and Lipschutz. In 1977, Steere and colleagues in Lyme, Connecticut (USA) studied the association between EM and arthritis, and also non-specific symptoms, and cardiac and neurologic manifestations, which were conclusively linked in 1982 with the recovery of a previously unrecognized spirochete from the tick vector and from infected patients.
LD is the most common vector-borne disease in North America and Europe. In 2012, the Centers for Disease Control and Prevention (CDC) recorded approximately 20 000 confirmed and 10 000 probable cases of LD in USA, heavily concentrated in the northeast and upper Midwest. In Europe, LD is reported in middle Europe and Scandinavia, particularly in Austria, Czech Republic, Germany, Slovenia, and Sweden. The infection is also found in Australia, Canada, China, Japan, northeast Africa, and Russia. In South America, studies showed that antibodies to B. burgdorferi were present in the Argentinean population, and the disease was described in Bolivia, Colombia, Venezuela, Chile, and Brazil.
LD occurs in all age groups, in both sexes, and in all races. The evidence suggests that who becomes infected is more likely to be related to the frequency and intensity of tick exposure than to any genetic predisposition.
There is a bimodal age distribution, with the first peak at 5–19 years of age and the second at 55–69 years of age. Most cases of EM develop in the late spring and early summer, when the nymphs are actively searching for their blood meal.
The etiologic agent, B. burgdorferi sensu lato , is a Gram-negative bacterium; like other spirochetes, it is a helically shaped motile microorganism. Borrelial strains can be found around the globe ( Fig 27-1 ) . They have been subdivided into three main genospecies that cause the human disease: B. burgdorferi sensu stricto, B. afzelii , and B. garinii . Newer strains are being identified and will soon be included in the B. burgdorferi sensu lato complex. B. burgdorferi sensu stricto is the only known strain that causes disease in the USA, whereas B. garinii, B. afzelii , and occasionally B. burgdoferi sensu stricto cause LD in Europe.
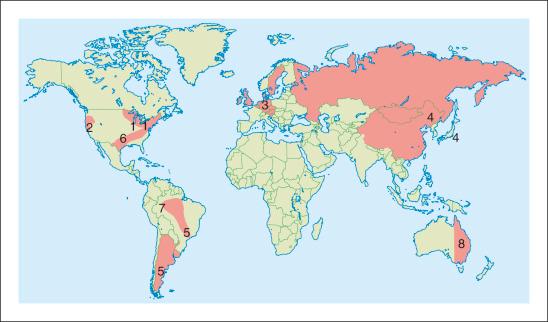
The Ixodes ricinus complex ticks transmit LD. These ticks have larval, nymphal, and adult stages, in a 3-year life cycle. All stages of ticks are capable of transmitting disease, but the nymphal stage is the most common because their small size permits them, once attached, to remain unnoticed for a long period of time. The risk of infection is largely dependent on the intensity of ticks, their feeding habits, and the animal hosts. Ticks are most commonly found attached to plants in grassy, wooded, bushy areas and are transferred by contact with animals or humans, who are incidental hosts. Other possible vectors include mosquitoes, deer flies, and horse flies.
In the northeastern and north–central USA, I. scapularis ticks are the most important vector, and in the west I. pacificus . In Europe, the main vector is I. ricinus , and in Asia it is I. persulcatus . Many mammals may serve as intermediate hosts for B. burgdorferi , but the major reservoir hosts are deer and white-footed mice. Domestic animals from endemic areas are also potential hosts.
After a tick bite, the spirochete may be transferred into the skin of the bitten host, and three outcomes are possible. First, the host defense mechanisms may overwhelm the spirochetal offense so that no disease develops. Second, the infection may become established, but remain localized to the skin around the bite. Third, the infection may spread through the lymphatic vessels or blood stream and become systemic. After the tick bite, the spirochetes migrate from the tick's midgut to the salivary glands, from where they find their way into the host. This fact probably explains the evidence from human and animal studies suggesting that tick bites of less than 24 hours duration rarely result in infection. In many cases, no symptoms occur. The host defense mechanisms may limit the infection, but pathogen-related factors may also play a role. There is evidence that genes coding the outer-surface protein C (Osp C) may play a role in the invasiveness of a B. burgdorferi strain. This may explain why some patients develop more invasive disease. Systemic invasion of the spirochete may occur early on in the course of LD. The spirochetes can change their outer antigenic structure and migrate into the intracellular compartment – both strategies may help the organism to evade the host's immune defenses. The last factor regarding pathogenesis is the phenomenon of coinfection, which may occur in 10–15% of cases. Other tick-borne pathogens, such as the agents of babesiosis, ehrlichiosis, and possibly others, can be transmitted by the same tick bite as the one that caused LD, and may modify the clinical manifestations and response to therapy.
The clinical manifestations of LD can be divided into three stages from the time of initial infection:
The early localized stage is characterized by EM and starts after a tick bite. It is caused by the responsible spirochete, which leaves a small macule or papule at the site, usually in the extremities, but it may be seen anywhere on the body. A total of 70–80% of patients are unable to recall the bite. A week later (range 1–36 days) there is expansion of the red papule, changing to annular erythema where the site of the bite may clear, or become vesicular or necrotic; the red peripheral ring has a mean of 10–16 cm of presentation ( Fig. 27-2 ). The central annular erythematous lesion shows elevation, or the erythema fades away and the border may be slightly raised, warm, or bluish-red ( Fig. 27-3 ), sometimes accompanied by a burning sensation or, rarely, becoming painful or pruritic. Usually the ring formation enlarges by as much as 3 cm per day. This typical EM happens in 85% of patients who are diagnosed with early LD. It is often accompanied by an acute flu-like illness. Untreated EM usually disappears in weeks to months.
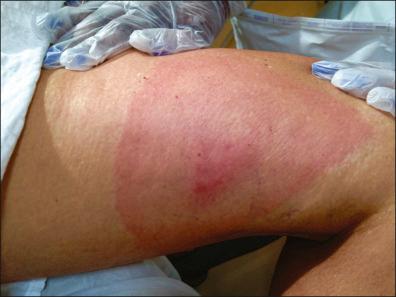
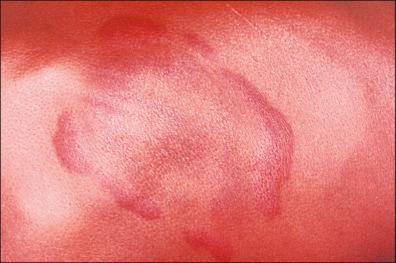
The early disseminated stage is characterized by multiple secondary annular lesions similar to the primary lesion, but smaller, and less migratory to the original lesion, which can be found in ≤50 of patients.
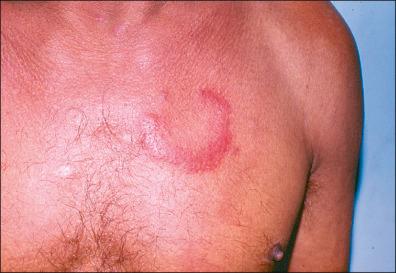
The lesions fade away at about a month, but some of them may be present or recur over the following months. In Europe, during this early period, lymphocytoma cutis (LCC) may occur, which is most commonly found as bluish-red papular nodules in the earlobes of children or on the anterior chest of adults ( Fig. 27-4 ). Hematogenous dissemination may cause frequent complications: arthritis (mono- or oligoarthritis involving large joints in early and late disease, migratory joint pains, 10% of these in the knees), neurologic symptoms (stiff neck, meningitis, Bell's palsy, cranial and peripheral neuropathies), and cardiac manifestations (myocarditis, congestive heart failure, atrioventricular partial or complete block). Non-specific findings are related: mild anemia, elevated erythrocyte sedimentation rate, elevated immunoglobulin M (IgM) level, and altered liver function. Other general symptoms may be present. Transplacental transmission has been reported, but it is unclear whether transmission has any effect on malformation or fetal demise if the mother receives the appropriate antibiotic treatment.
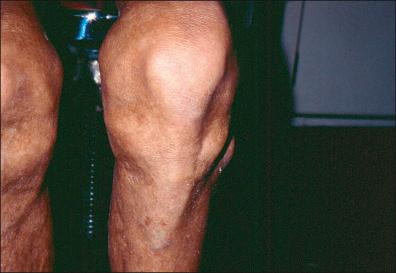
The late disseminated stage may happen in approximately 60% of patients with untreated infection and may begin with intermittent bouts of arthritis, with severe joint pain and swelling. Large joints are most often affected, particularly the knees. Arthritis caused by LD manifests differently than other causes of arthritis and must be distinguished from arthralgias. Up to 5% of untreated patients may develop chronic neurologic complaints months to years after the infection. These include paresthesias, radiculopathy, sleep disturbance, and cognitive and sensory deficits. Another manifestation of this stage is acrodermatitis chronica atrophicans (ACA), found only in Europe and most often associated with B. afzelli disease. ACA has been observed, involving most commonly the cutaneous lower extremities, causing a bluish, erythematous swelling and edema ( Fig. 27-5 ) that atrophies and permits vizualization of the underlying blood vessels ( Fig. 27-6 ). ACA is insidious in onset, occurring from months to years after the initial tick bite.
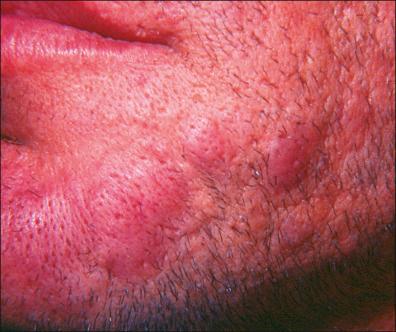
In Europe, some patients develop morphea ( Fig. 27-7 ), scleroderma-like plaques, or lichen sclerosus (LS), occurring concomitantly with or after ACA, but it remains unclear whether borrelial infection has a causative role in the development of these manifestations.
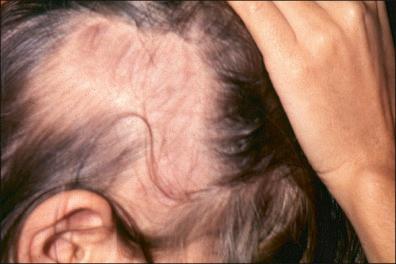
Some cases of LD begin with asymptomatic seroconversion, and remain asymptomatic, appearing to have been spontaneously cured, or they develop manifestations of disseminated or late LD as their initial clinical manifestation ( Fig. 27-8 ).
Approximately 10–20% of patients with LD have symptoms that last months to years after treatment with antibiotics. These symptoms can include muscle and joint pains, cognitive defects, sleep disturbance, or fatigue. The cause of these symptoms is not known, but there is no evidence that these symptoms are due to ongoing infection with B. burgdorferi . There is some evidence that PTLDS is caused by an autoimmune response, in which a person's immune system continues to respond, doing damage to the body's tissues, even after the infection has been cleared. Studies have shown that continuing antibiotic therapy is not helpful and can be harmful.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here