Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The human body possesses a tremendous healing potential. However, despite its innate restorative capacity, in many instances the body's ability to heal is limited. Musculoskeletal tissues such as tendon, ligament, and cartilage present challenges to clinicians because these tissues tend to heal slowly because of their limited blood supply and slow cell turnover. Furthermore, conservative management or surgical intervention alone may not reliably recapitulate the normal architecture and function of the injured tissue. Therein lies the potential benefit of biologic therapy, in which the addition of growth factors and reparative cells may not only augment the normal body healing process but also restore normal form and function.
The use of biologic therapy or regenerative medicine has grown exponentially in the field of sports medicine in recent years. Although these emerging therapies may be based on solid preclinical evidence, these therapies are still in the stages of building clinical evidence before they are incorporated into standards of care. This chapter reviews the basic principles and best available clinical evidence on two growing categories of biologic therapy in sports medicine: platelet-rich plasma (PRP) and stem cell therapy.
The use of human blood concentrates to treat various tendon, ligament, and cartilage disorders and to augment surgical repairs continues to grow within sports medicine. The availability of PRP and its autologous nature allow for easy clinical application without the risk associated with allogenic products. As a result, PRP has been used in several clinical studies for the management of pathologic conditions of ligament, tendon, bone, and cartilage ( Fig. 5.1 ). Although an extensive body of literature on PRP exists, a clear consensus for or against the use of PRP in the treatment of musculoskeletal diseases has not been achieved. What is clear is that not all PRP preparations are created equal, and our understanding regarding the various components of PRP, along with its optimal composition, timing, and delivery, continues to be refined.
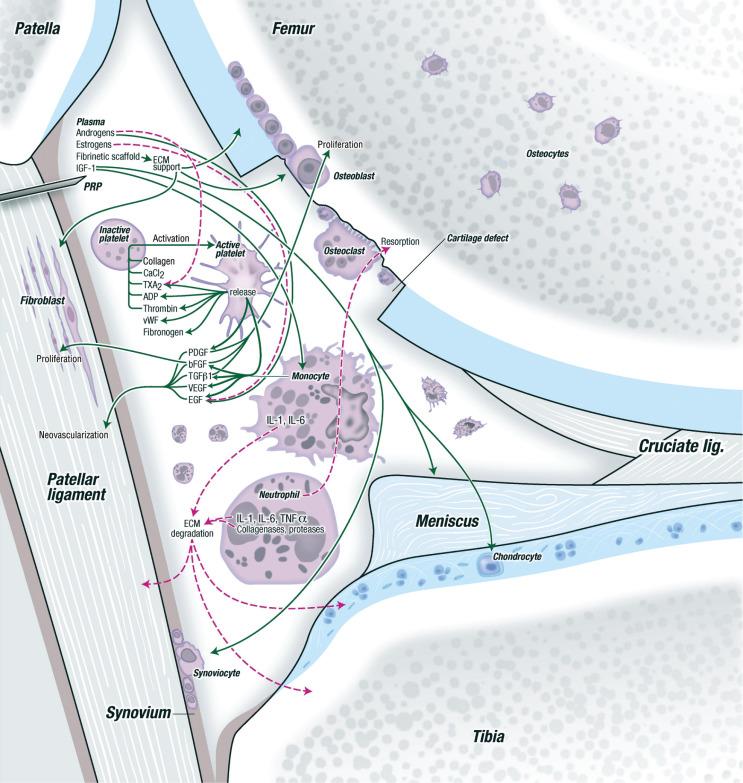
PRP is an autologous concentration of human platelets in a small volume of plasma produced from a patient's own centrifuged blood. The concentrated platelets contain increased amounts of growth and differentiation factors, which can then be delivered to an injury site to augment the body's natural healing process. The normal human platelet count ranges anywhere from 150,000 to 350,000 per µL. Improvements in bone and soft tissue healing properties have been demonstrated with concentrated platelets of 1,000,000 per µL, and thus it is this concentration of platelets in a 5-mL volume of plasma that has been suggested as one working definition of PRP. A more modern definition of PRP is any plasma fraction that concentrates platelets greater than baseline. A resultant three- to fivefold increase in growth and differentiation factors can be expected with PRP compared with normal nonconcentrated whole blood. PRP preparations are typically further categorized into leukocyte-rich PRP (LR-PRP) preparations, defined as having a neutrophil concentration above baseline, and leukocyte-poor PRP (LP-PRP) preparations, defined as having a leukocyte (neutrophil) concentration below baseline.
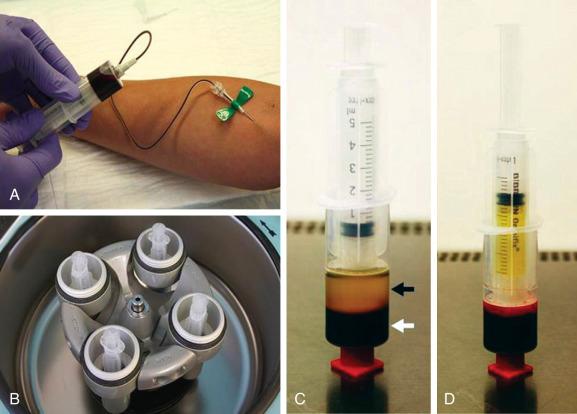
Currently more than 16 commercial PRP systems are available on the market, and hence quite a bit of variation exists in the PRP collection and preparation protocol depending on the commercial system being used ( Table 5.1 ). Each commercial system has a different platelet capture efficiency that results in different whole-blood volume requirements to achieve the necessary final platelet concentration for PRP. The commercial systems may also differ in their isolation method (one- or two-step centrifugation), the speed of centrifugation, and the type of collection tube system and operation. In general, whole blood is usually collected and mixed with an anticoagulant factor, such as acid citrate dextrose, sodium citrate, or ethylene diamine tetraacetic acid. Centrifugation is then required to separate red blood cells (RBCs) from platelet-poor plasma (PPP) and the “buffy coat,” which contains the concentrated platelets and ± leukocytes. The RBC and PPP layers may be discarded using various processing techniques to isolate the platelet-concentrated layer ( Fig. 5.2 ). The platelets can then be directly injected into the patient or be “activated” via the addition of either calcium chloride or thrombin, which then causes the platelets to degranulate and release the growth and differentiation factors. Approximately 70% of the stored growth factors are released within the first 10 minutes of activation, and nearly 100% of the growth factors are released within 1 hour of activation. Small amounts of growth factors may continue to be produced by the platelet during the remainder of its life span (8 to 10 days).
| System | Company | Blood Volume Required (mL) | Concentrated Volume Produced (mL) | Processing Time (min) | PPP Produced? | Gel Activator Available? | Increase in [Platelets] (Times Baseline) | Platelet Capture Efficiency (% Yield) |
|---|---|---|---|---|---|---|---|---|
| Leukocyte-Rich PRP | ||||||||
| Angel | Arthrex | 52 | 1–20 a | 17 | + | 10 a | 56–75% | |
| GenesisCS | EmCyte | 54 | 6 | 10 | + | 4–7 | 61 ± 12% | |
| GPS III | Biomet | 54 | 6 | 15 | + | + | 3–10 | 70 ± 30% |
| Magellan | Isto Biologics/Arteriocyte | 52 | 3.5–7 | 17 | + | 3–15 | 86 ± 41% | |
| SmartPReP 2 | Harvest | 54 | 7 | 14 | + | 5–9 | 94 ± 12% | |
| Leukocyte-Poor PRP | ||||||||
| Autologous Conditioned Plasma (ACP) | Arthrex | 11 | 4 | 5 | + | 1.3 | 48 ± 7% | |
| Cascade | MTF | 18 3 | 7.5 3 | 6 3 | + | 1.6 3 | 68 ± 4% | |
| Clear PRP | Harvest | 54 a | 6.5 a | 18 a | + | 3–6 a | 62 ± 5% a | |
| Pure PRP | EmCyte | 50 a | 6.5 a | 8.5 a | + | 4–7 a | 76 ± 4% a | |
a Data obtained from manufacturers' promotional literature or internal studies.
Platelets contain a milieu of growth factors and mediators in their alpha granules ( Table 5.2 ). However, the specific composition of PRP likely varies not only from person to person but also when the isolation process is repeated in the same individual. Several elements are known to influence the specific makeup of PRP, which includes patient-specific factors and different commercial system preparation methods. The variability in the cellular composition of PRP preparations creates challenges in interpretation of the literature regarding the clinical efficacy of PRP.
| Factor | Target Cell and Tissue | Function |
|---|---|---|
| PD-EGF |
|
|
| PDGF AB | Fibroblasts, smooth muscle cells, chondrocytes, osteoblasts, mesenchymal stem cells |
|
| TGF-β1 |
|
|
| IGF-I, II |
|
|
| bFGF |
|
|
| VEGF, ECGF | Blood vessel cells |
|
Our current understanding appears to suggest that PRP with elevated leukocyte content (i.e., LR-PRP) is associated with an acute inflammatory response. In an animal model, Dragoo et al. demonstrated this large inflammatory response in vivo with increased cellularity and vascularity in rabbit tendons 5 days after treatment with LR-PRP, compared with LP-PRP. Elevated leukocyte (neutrophil) concentrations present in LR-PRP are also associated with elevated catabolic cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and metalloproteinases, which may antagonize the anabolic cytokines contained within platelets. The clinical ramifications and cellular effects of these different PRP preparations, including leukocyte content, are still currently being elucidated but have begun to allow for more customized PRP treatments to target specific indications.
PRP has been most actively evaluated in the treatment of tendon and ligament injuries. Tendon and ligaments heal through a dynamic process with stages of inflammation, cellular proliferation, and subsequent tissue remodeling. Many of the cytokines found in PRP are involved in the signaling pathways that occur during this restorative process. PRP may also promote neovascularization, which may not only increase the blood supply and nutrients needed for cells to regenerate the injured tissue but may also bring new cells and remove debris from damaged tissue. Both these mechanisms of action are particularly attractive in chronic tendinopathy conditions in which the biologic milieu may be unfavorable for tissue healing.
Clinical studies on the use of PRP for tendon and ligament injuries have mainly focused on the areas of the elbow, knee, and ankle. Studies on lateral epicondylitis suggest that LR-PRP may be effective and a reasonable option for patients who have failed to respond to a physical therapy regimen. In a prospective cohort study, Mishra et al. evaluated 230 patients who failed to respond to at least 3 months of conservative treatment for lateral epicondylitis. Patients were treated with LR-PRP and followed up for 24 weeks. At 24 weeks, the patients who received LR-PRP reported a 71.5% improvement in their pain scores compared with a 56.1% improvement in the control group ( P = .019). The percentage of patients reporting significant residual elbow tenderness at 24 weeks was 29.1% in the patient group receiving PRP compared with 54.0% in the control group ( P = .009). There was a clinically meaningful and significant improvement at 24 weeks in patients treated with LR-PRP versus active control.
In terms of the sustainability of treatment effect, PRP may provide longer continuous relief of symptoms for lateral epicondylitis than do corticosteroids. Peerbooms et al. evaluated the efficacy of PRP versus corticosteroids in 100 patients who had a minimum 6-month history of recalcitrant chronic epicondylitis and had failed to respond to conservative management. Treatment success within this study was defined as, at minimum, a 25% reduction in the visual analog scale (VAS) score or Disabilities of Arm, Shoulder, and Hand score without a repeat intervention after 1 year. Although both groups improved in VAS scores from baseline, 73% (37 of 51 patients) in the PRP group versus 49% (24 of 49 patients) in the corticosteroid group were considered to have a successful response at 1 year ( P < .001). Furthermore, 73% (37 of 51 patients) in the PRP group versus 51% (25 of 49 patients) in the corticosteroid group noted improved Disability of Arm, Shoulder, and Hand scores at 1 year ( P = .005). Patients who received PRP also continued to report symptom relief 1 year after receiving the injection. In contrast, the short-term benefits of corticosteroids began to wane after 12 weeks. In a separate report, the improvement within this group of patients who received PRP continued to be noted 2 years after the PRP injection.
In addition to the use of PRP to treat lateral epicondylitis, results from randomized controlled trials support the use of LR-PRP to treat chronic refractory patellar tendinopathy. Dragoo et al. evaluated 23 patients with patellar tendinopathy on examination and magnetic resonance imaging (MRI) who had failed conservative management. Patients were randomized to receive ultrasound-guided dry needling alone or with injection of LR-PRP. Patients were followed for greater than 26 weeks. At 12 weeks, the PRP group had improved, as measured by Victorian Institute of Sport Assessment-Patella (VISA-P) score, significantly more than the dry needling group ( P = .02). However, the difference was not significant at greater than 26 weeks ( P = .66), suggesting that the benefit of PRP for patellar tendinopathy may be earlier improvement of symptoms. Vetrano et al. also reported the benefit of PRP injections for treatment of chronic refractory patellar tendinopathy. Forty-six patients with ultrasound-confirmed chronic unilateral patellar tendinopathy were randomized to received two PRP injections over 2 weeks or 3 sessions of focused extracorporeal shock wave therapy (ECSWT). Although there was no significant difference between groups at 2-month follow-up, the PRP group showed statistically significant improvement, as measured by VISA-P and VAS, over ECSWT at 6-month and 12-month follow-up, and as measured by Blazina scale score at 12-month follow-up ( P < .05 for all).
Compared with findings for lateral epicondylitis and patellar tendinopathy, the higher-level data with regard to the use of PRP in patients with Achilles tendinopathy have been less promising. In a prospective randomized trial, de Vos et al. found no significant benefits with PRP versus a saline solution injection as an adjunct to eccentric exercises for mid-Achilles tendinosis. The authors reported no significant differences in Achilles tendon structure, the degree of neovascularization, and clinical outcome compared with the saline solution group. In a follow-up study on the same patients, de Jonge et al. similarly reported no significant benefit in terms of pain reduction, activity level, and tendon appearance on ultrasound at 1 year after injection of PRP for chronic Achilles tendinopathy.
Most studies on the use of PRP products as an adjuvant to tendon repairs have focused on rotator cuff repairs. Some studies used a fibrin matrix as a carrier for the PRP, whereas others injected PRP directly into the repair site. In a randomized controlled study, Castricini et al. reported outcomes based on Constant score and MRI-evaluated integrity of the repaired rotator cuff in 88 patients after 16 months follow-up. There was no significant benefit with PRP for small- (<1 cm) and medium-sized (1 to 3 cm) rotator cuff tear repairs. The authors found no significant differences between the PRP and the control group in terms of the Constant score, tendon thickness, and repeat rupture rate. In another randomized controlled trial, Weber et al. similarly reported no significant improvement using PRP in perioperative morbidity, clinical outcomes, or structural integrity. The study randomized 60 patients and found that structural results correlated with age and size of tear but did not differ between the PRP-treated group and control.
Randelli et al. evaluated use of PRP in a randomized control study of 53 single-tendon rotator cuff repairs. Patients in the PRP-treated group reported short-term pain reduction and increased Simple Shoulder Test scores at early postoperative periods, with all clinical scores superior for PRP at 3 months after surgery ( P < .05). In their subgroup analysis, patients who had undergone repairs of small-sized tears (grade 1 and 2 tears, with less retraction) had lower repeat tear rates (9 of 16 patients or 40% vs. 12 of 19 patients or 52%) and increased external rotation strength throughout the 24-month follow-up period ( P < .05). Jo et al. assessed the efficacy of PRP augmentation in a randomized controlled trial of 74 patients undergoing arthroscopic repair of medium to large rotator cuff tears. There was no difference between PRP and control in the Constant score at 3 months nor in VAS for pain, strength, or functional scores. However, the retear rate of the PRP group (3.0%) was significantly lower than the control group (20.0%) ( P = .032) and the PRP group had a lower decrease in the cross-sectional area (CSA) of the supraspinatus muscle ( P = .014), indicating an improved structural outcome. Finally, Pandey et al. used an only moderately concentrated LP-PRP preparation in a randomized study of 102 patients undergoing repair of medium and large degenerative tears. VAS scores were significantly lower in the PRP group at 1, 3, and 6 months ( P = .0005) but not later. Constant scores were significantly better in the PRP group at 12 and 24 months ( P < .01) and UCLA scores were significantly higher at 6 and 12 months ( P = .002). At 24 months, the retear rate in the PRP group was significantly lower than in the control group ( P = .01), with subgroup analysis showing significance only for large tears ( P = .03).
Data are limited on the use of PRP in the repair of acute Achilles tendon tears, and findings in the existing literature are conflicting. Schepull et al. evaluated the use of PRP in Achilles tendon repair in a randomized study ( n = 30). Although no differences were reported with regard to tendon elasticity and heel raise index between the PRP and control groups, the authors did note a lower Achilles Tendon Total Rupture score among the PRP group, which suggests a detrimental effect of PRP on subjective outcome after repair. However, Zou et al. enrolled 36 patients with acute Achilles tendon rupture in a prospective randomized controlled study using intraoperative LR-PRP injection versus repair without PRP. Patients were followed for 24 months. Patients from the PRP group had better isokinetic muscle at 3 months and had higher SF-36 and Leppilahti scores at 6 and 12 months, respectively ( P < .05 for all). Ankle range of motion (ROM) was also significantly better in the PRP group at all time points of 6-, 12-, and 24-months ( P < .001).
The success of anterior cruciate ligament (ACL) surgery not only hinges on technical factors (e.g., graft tunnel placement and graft fixation) but also biologic healing of the ACL graft. Given its potential for improving tissue vascularity and ligament healing, PRP has been used to augment ACL graft maturation and graft–bone tunnel incorporation after reconstruction. Within the research literature, ACL graft maturation tends to be assessed with MRI. The assumption is that a low homogenous intensity signal on T2- and proton density–weighted MRI is likely indicative of a healthy maturing ACL graft. In terms of the effect of PRP on ACL graft maturation, some studies have demonstrated improved graft maturation with PRP, whereas others reported a lack of significant differences. Authors of a recent systematic review of 11 controlled trials, which included studies in which statistical significance was not reached, concluded that PRP likely improves ACL graft maturation, by up to 50%. The authors pointed to insufficient sample size as a potential rationale for lack of statistical significance despite MRI improvement in some metrics measuring ACL graft maturation.
The other component to successful biologic healing of an ACL graft is graft–bone tunnel incorporation. The existing literature on the use of PRP to augment healing of the graft-bone interface is inconclusive at best. Vogrin et al. evaluated the effects of PRP gel treatment for hamstring autograft ACL reconstruction in a controlled double-blinded study. MRI was used after the operation to assess vascularization along the ACL graft-bone interface. The authors reported evidence of improved vascularization along the interface at 3 months with use of PRP, but the observed benefit dissipated by 6 months after the surgical procedure. Other studies have similarly reported limited to no evidence to support the use of PRP to augment ACL graft–bone tunnel incorporation. Of note is that nearly all of the studies used an LR-PRP formulation and LR-PRP formulations increase local tissue inflammation, which may delay or alter healing.
One final point of consideration is whether any of the observed benefit of PRP on ACL graft maturation or graft-tunnel healing would translate into improved clinical results. The current best available evidence seems to suggest no significant benefit for functional outcome with use of PRP. Ventura et al. found no differences in Knee Injury and Osteoarthritis Outcome score (83 vs. 84 points), KT-1000 (0.8 mm vs. 1.2 mm), or Tegner scores (0.9 vs. 0.8 difference preoperative to postoperative) between the PRP-treated group and control subjects at 6 months despite reporting a significant difference in graft appearance. Orrego et al. similarly noted no significant benefit in both Lysholm and International Knee Documentation Committee scores at 6 months after the operation despite identifying a positive effect of PRP on graft maturation. In summary, evidence from the current literature suggests that PRP may improve the rate at which ACL grafts achieve a low signal on MRI T2-weighted imaging but have little to no effect on graft-tunnel incorporation. A demonstrable benefit in patient outcome after use of PRP in patients undergoing ACL surgery is also lacking.
When considering biologic approaches to cartilage problems, it is important to understand that focal cartilage injuries differ from arthritis in terms of joint biology, homeostasis, and levels of metalloproteases and inflammatory cytokines. Therefore PRP applications and clinical results for cartilage lesions and osteoarthritis may be quite different for either condition. The idea of using PRP for cartilage repair is based on in vitro basic science literature that growth factors released by the platelet alpha granules may increase the synthetic capacity of chondrocytes through upregulation of gene expression, proteoglycan production, and deposition of type II collagen.
Although very few clinical reports or trials have evaluated the use of PRP for treatment of focal cartilage lesions, Dhollander et al. reported a pilot case series of five patients surgically treated with autologous matrix-induced autologous chondrocyte implantation (MACI) combined with a PRP gel. The authors reported that although VAS scores for pain decreased, the lesion was not filled after 12 and 24 months based on MRI evaluations, and intralesional osteophytes developed in three of the five patients.
Most clinical reports on the use of PRP for cartilage injury involve patients with osteoarthritis of the knee. A recent meta-analysis by Riboh et al. compared LP-PRP and LR-PRP in the treatment of knee osteoarthritis and found that LP-PRP injections resulted in significantly improved WOMAC scores compared with hyaluronic acid (HA) or placebo. Patel et al. performed a prospective randomized trial comparing single- or double-injection LP-PRP with saline solution in 78 patients with early osteoarthritis. They concluded that a single injection of PRP was as effective as a double injection. On the other hand, Filardo et al. enrolled 192 patients in a randomized controlled study and found no difference between LR-PRP and HA, providing further evidence that LP-PRP may be an effective choice for treatment of osteoarthritis symptoms and LR-PRP appears not to be. The biological basis for this may be in the relative level of inflammatory versus antiinflammatory mediators present in LR-PRP and LP-PRP. Inflammatory mediators TNF-α, IL-6, interferon-γ (IFN-γ), and IL-1β are increased significantly in the presence of LR-PRP, whereas injection of LP-PRP increases IL-4 and IL-10, which are antiinflammatory mediators. IL-10 specifically was found to be helpful in the treatment of hip osteoarthritis and may also suppress the release of the inflammatory mediators TNF-α, IL-6, and IL-1β, and block the inflammatory pathway by neutralizing nuclear factor–kB activity. In addition to its deleterious effects on chondrocytes, LR-PRP may also fail to help treat osteoarthritis symptoms due to its effect on synoviocytes. Braun et al. found that treatment of synovial cells with LR-PRP or erythrocytes resulted in significant proinflammatory mediator production and cell death.
The idea of augmenting meniscus repair with growth factors is not a recent one. In 1988 Arnoczky et al. proposed the use of an exogenous fibrin clot to stimulate a reparative response in the avascular portion of the meniscus. In 1990 Henning et al. reported that the failure rate for an isolated meniscus tear repair was 41% without a fibrin clot versus 8% with a fibrin clot. Bhargava et al. demonstrated that platelet-derived growth factor increases the number of cells and tissue formation in a meniscus defect explant model. Several other in vitro and in vivo studies have demonstrated the potential for cytokines found in PRP to improve meniscal cell growth and meniscus repair healing. Ishida et al. performed a combined in vivo and in vitro study demonstrating that PRP led to improved meniscal repair in full-thickness 1.5-mm diameter defects in the avascular zone of the rabbit meniscus.
Augmenting meniscal repair with PRP is logical because it may deliver growth factors to the healing tissue, but a paucity of human clinical data exists. Pujol et al. evaluated 34 young patients (age 13 to 40, median age 28) with symptomatic grade 2 or 3 horizontal meniscus tears in a case-control study. PRP was injected prior to skin closure in an open meniscal repair versus open meniscal repair alone. One year after surgery, KOOS scores for pain, symptoms, daily activities, sports, and quality of life were superior in the PRP-treated group, reaching statistical significance for pain and sports parameters ( P < .05). Five out of the 17 cases in the PRP group had complete disappearance of any abnormal signal within the repaired meniscus on MRI, compared with none in the open repair alone group ( P < .01).
The use of PRP in the treatment of muscle injuries has attracted a significant amount of interest in recent years. Similar to tendon healing, the steps in muscle healing involve the initial inflammatory response, which is then followed by cell proliferation, differentiation, and tissue remodeling. Hamid et al. conducted a single-blind randomized study of 28 patients with grade 2 hamstring muscle injuries comparing an injection of LR-PRP with a rehabilitation program versus rehabilitation alone. The group treated with LR-PRP was able to return to play (RTP) in a significantly shorter amount of time compared with control (mean time in days, 26.7 vs. 42.5, P = .02), but structural improvement was not achieved. In a double-blind randomized controlled trial, Reurink et al. evaluated 80 patients comparing PRP injections to placebo saline injections, with all patients receiving standard rehabilitation. The patients were followed for 6 months and there were no significant differences in RTP time or with reinjury rate.
Although clinical studies have not found PRP to be efficacious in treating muscle injuries, advances in laboratory research may lead to improved understanding of treatment modalities. In vitro studies have found that PRP is capable of leading to myoblast proliferation but not to myoblast differentiation, a requisite step in producing muscle tissue. Furthermore, growth factors contained in platelets, specifically myostatin (MSTN) and transforming growth factor-β1 (TGF-β1), are actually detrimental to muscle regeneration. Miroshnychenko et al. found in vitro that treatment with PPP or PRP with a second centrifugation step to remove the platelets induced myoblasts into muscle differentiation. This suggests that perhaps the most beneficial treatment of muscle injuries may be with PPP, although in vivo animal studies followed by human clinical trials will be necessary to further explore this treatment option in the future.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here