Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
As trainees and practicing vascular surgeons perform fewer open procedures, it becomes increasingly important to understand and become familiar with the established principles of open surgical technique. With advances in catheter-based technology, endovascular therapy is now the first option in many, if not most, clinical scenarios. However, open vascular surgery has traditionally been the “gold standard” with respect to durability and efficacy. Hybrid procedures which combine open surgery and endovascular techniques have been performed to achieve revascularization with limited exposures. Similarly, debranching procedures (i.e., iliorenal and iliomesenteric bypasses) in patients with suprarenal aortic aneurysms or carotid–subclavian and carotid–carotid bypasses in patients with thoracic aortic aneurysms can provide a landing zone during the endovascular treatment of a thoracoabdominal or thoracic aortic aneurysm. However, there remain clinical scenarios where open bypass may serve as the optimal mode of limb revascularization. Factors affecting this decision include life expectancy, degree of tissue loss, arterial anatomy, availability of autogenous conduit, and failed prior endovascular intervention. Despite advances in endovascular technology and the increase in the number of endovascular interventions, open vascular reconstructions will continue to play a significant role in the management of patients with vascular disease for the foreseeable future.
The key elements of a successful open vascular reconstruction involve choosing the optimal procedure at the optimal time point while selecting the appropriate method of vascular exposure and executing the selected procedure carefully and expeditiously. Appropriate choices of inflow, outflow, and bypass conduit are critical for the success of open revascularization. This success also requires the appropriate instruments, grafts, and suture materials. Exposure to the skin should be avoided while suturing and tunneling grafts, especially prosthetic conduits. The skin can be isolated with impregnated drapes or moistened laparotomy pads (see Ch. 49 , Graft Infection). All open vascular procedures should be performed under excellent lighting aided by magnifying loupes in most cases. Often overlooked, maintaining good ergonomic position during the reconstruction is essential for the surgeon to not only prevent significant work-related discomfort in the neck, shoulders, and back, but to also to allow the efficient execution of surgical maneuvers in awkward angles and limited spaces as is routine in open reconstructions.
A vascular instrument tray typically includes vascular clamps, needle holders, forceps, scissors, and various retractors. Depending on the size of the vessel and the location of the surgical reconstruction, the instruments used will vary.
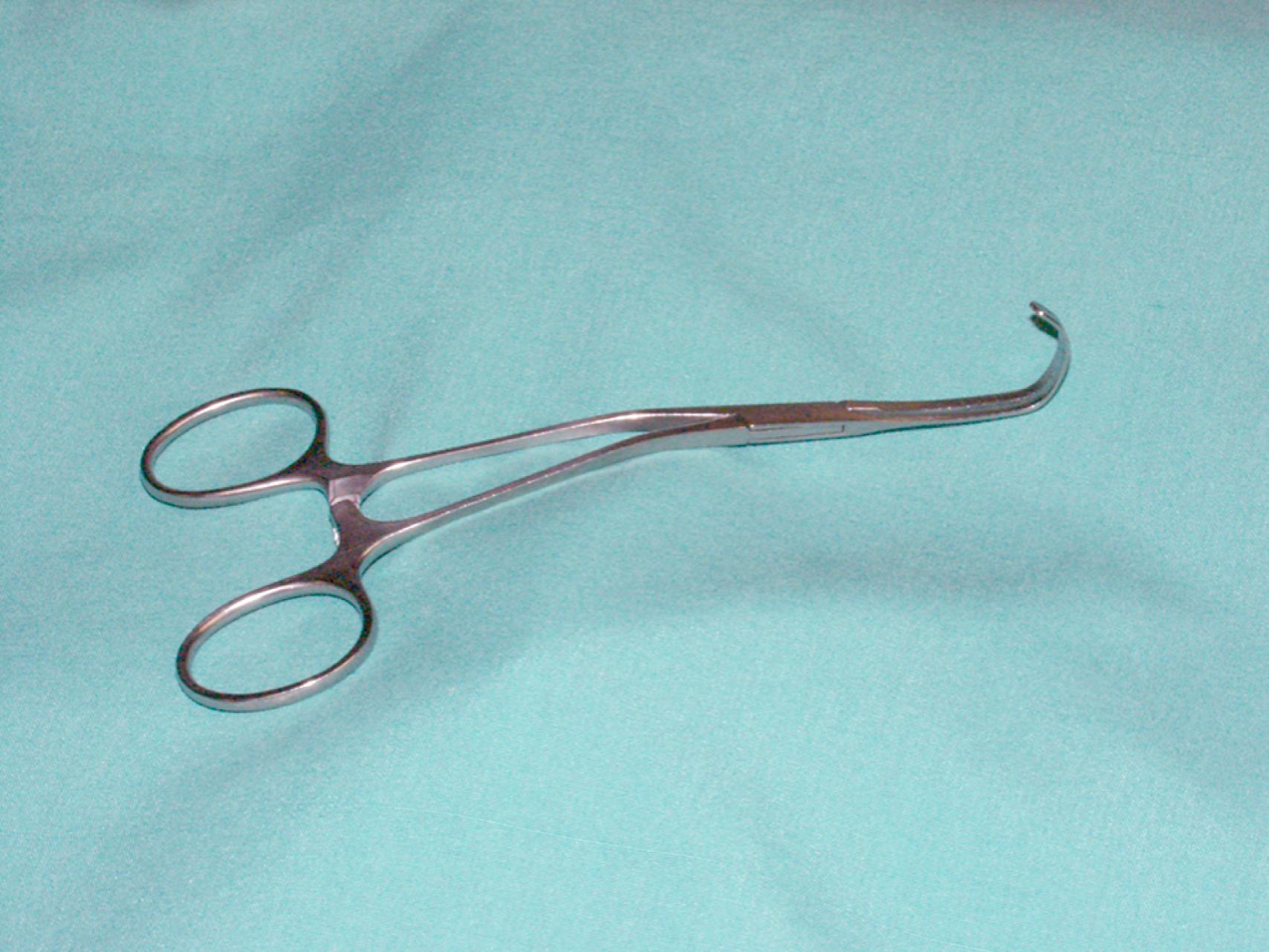
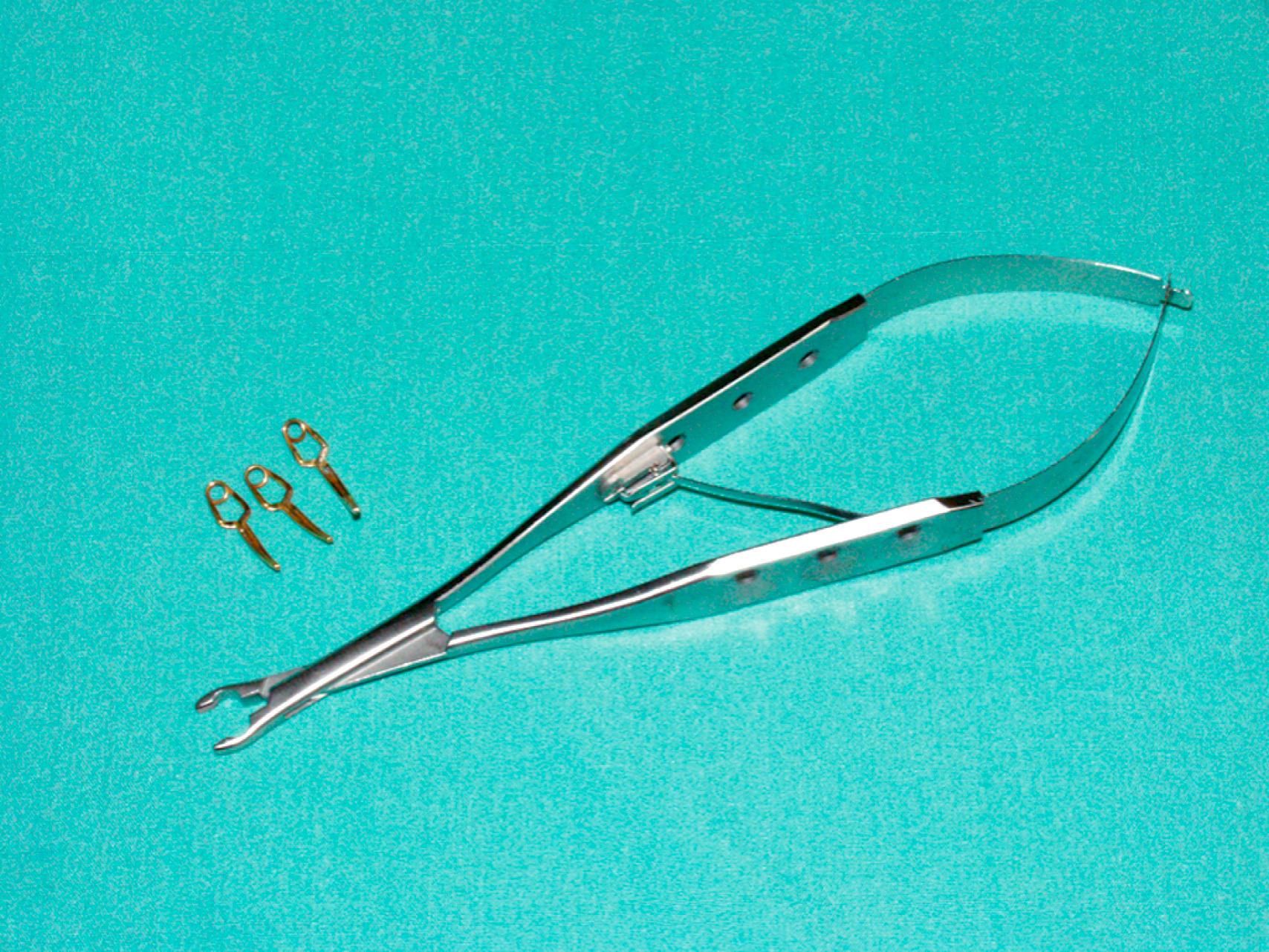
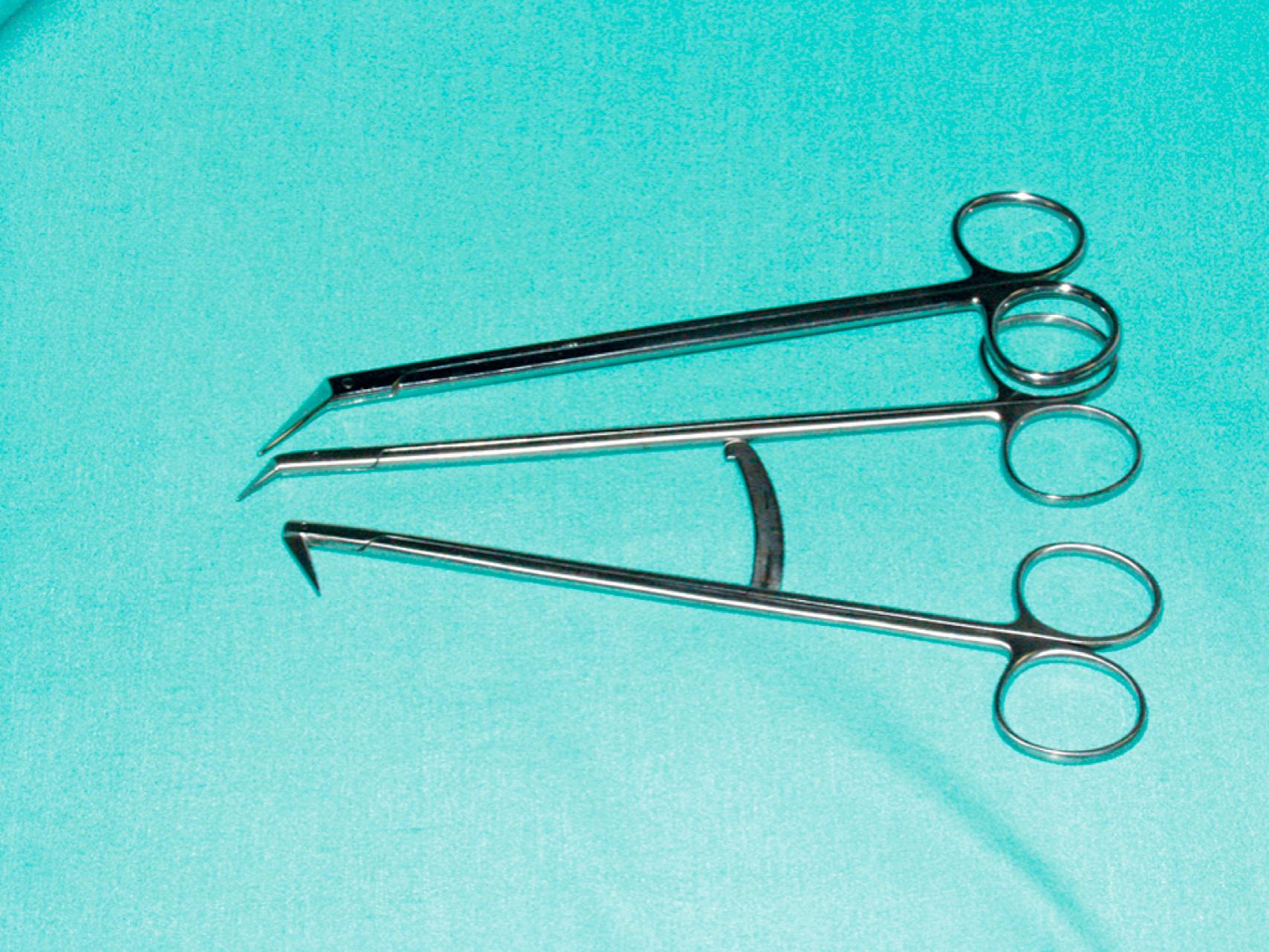
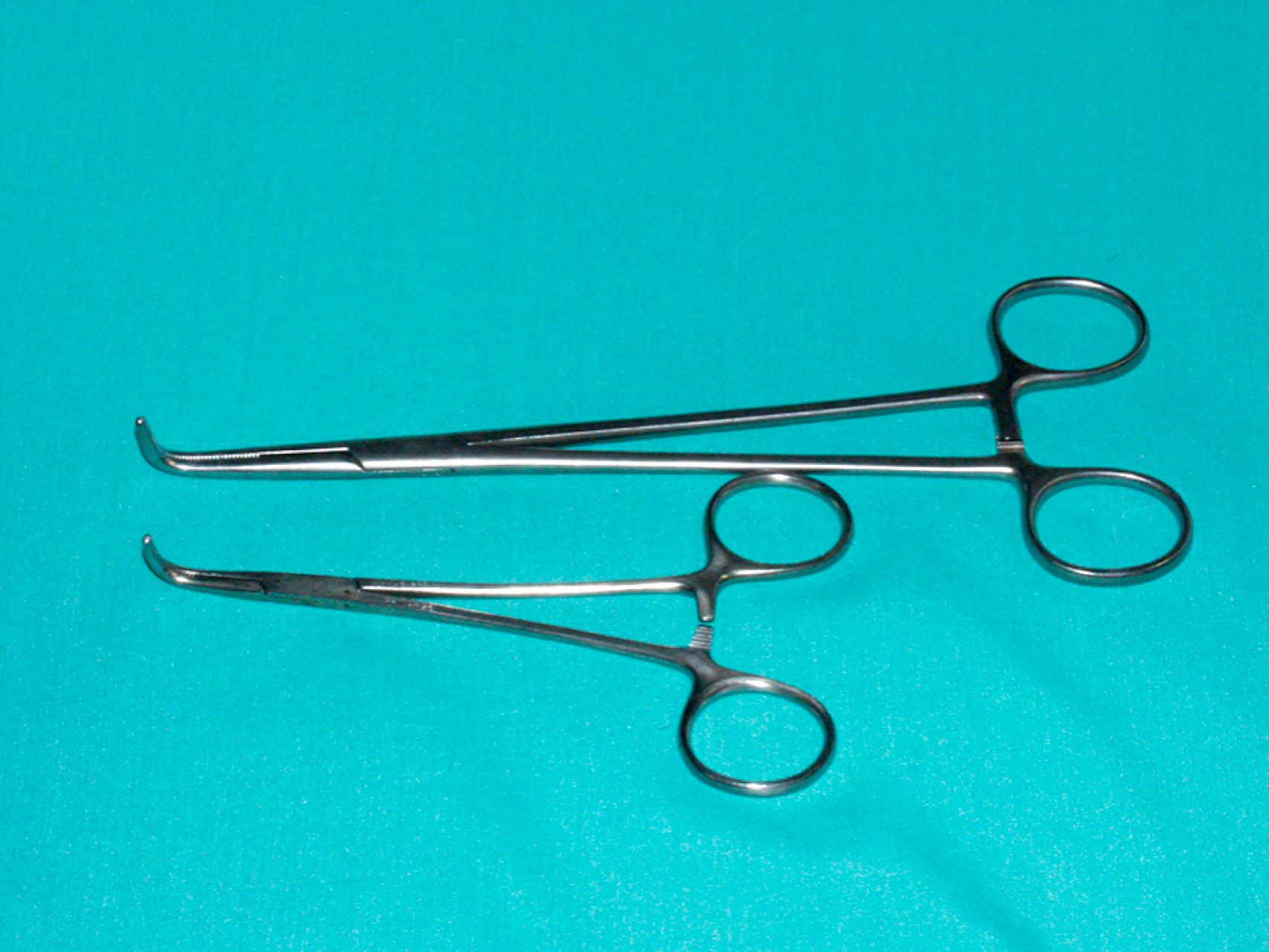
Vascular clamps typically have jaws with rows of fine interdigitating serrations that allow clamping of the vessel without slippage or significant crush injury. Although vascular clamps are considered atraumatic, a vascular clamp applied inappropriately can cause significant intimal damage or may tear the artery if placed over a plaque in an inappropriate manner. Even in a soft, minimally diseased artery, a clamp applied with excessive force can damage the arterial wall and intima. Palpation of the artery to locate the orientation of the plaque can be very helpful in proper placement of the clamp in the best geometric plane. Clamps vary in shape and angulation to fit into specific anatomic locations and for arteries of different sizes and can be used to fully or partially occlude the vessel. Clamps with special soft, atraumatic inserts (e.g., Fogarty soft jaw) are also available and especially important to allow clamping of prosthetic grafts without damaging the graft material. Types of vascular clamps and their suggested uses are outlined in Table 61.1 . For smaller vessels and branches, various sizes of bulldog clamps or intracranial aneurysm clips, such as the Yasargil or Heifitz clips, are available ( eFigs. 61.1–61.12 ).
| Clamp Type | Vessel |
|---|---|
| Totally Occluding | |
| DeBakey aortic aneurysm clamp (side-to-side apposition of aortic wall) | Supraceliac, infrarenal aorta |
| DeBakey–Bahnson aortic aneurysm clamp | Infrarenal aorta |
| Howard–DeBakey aortic aneurysm clamp with reverse curve shafts (side-to-side apposition of aortic wall) | Infrarenal aorta |
| Fogarty aortic clamp (side-to-side apposition of aortic wall) | Infrarenal aorta; aortic grafts, calcified aorta |
| DeBakey aortic aneurysm clamp (apposition of anterior and posterior walls together) | Infrarenal aorta |
| Lambert–Kay aortic clamp (apposition of anterior and posterior walls together) | Infrarenal aorta |
| Wylie hypogastric clamp | Iliac arteries, especially hypogastric arteries |
| DeBakey peripheral vascular clamp (angled handle) | Iliac arteries |
| DeBakey peripheral vascular clamp (angled jaw, 45 degrees) | Iliac and common carotid arteries |
| Henley subclavian clamp | Subclavian and common femoral arteries |
| Partially Occluding (Side-Biting) | |
| Lemole–Strong aortic clamp | Aorta; aortic grafts |
| Satinsky clamp | Aorta, vena cava |
| Cooley anastomosis clamp | Aorta; aortic grafts |
| Cooley–Derra clamp | Graft limbs |
| Cooley pediatric clamp | Common femoral artery, saphenofemoral junction |
| Self-Compressing (No Applicator Required) | |
| Gregory carotid “soft” bulldog | Small vessels |
| Potts bulldog – straight and angled jaw | Small vessels |
| DeBakey bulldog | Small vessels |
| Dietrich bulldog | Small vessels |
| Self-Compressing (Applicator Required) | |
| Yasargil aneurysm clip | Small vessels and branches |
| Heifitz clip | Small vessels and branches |
| Kleinert–Kutz clip – straight, angled, curved | Microvascular anastomoses |
| Louisville microvessel approximator | Microvascular anastomoses |
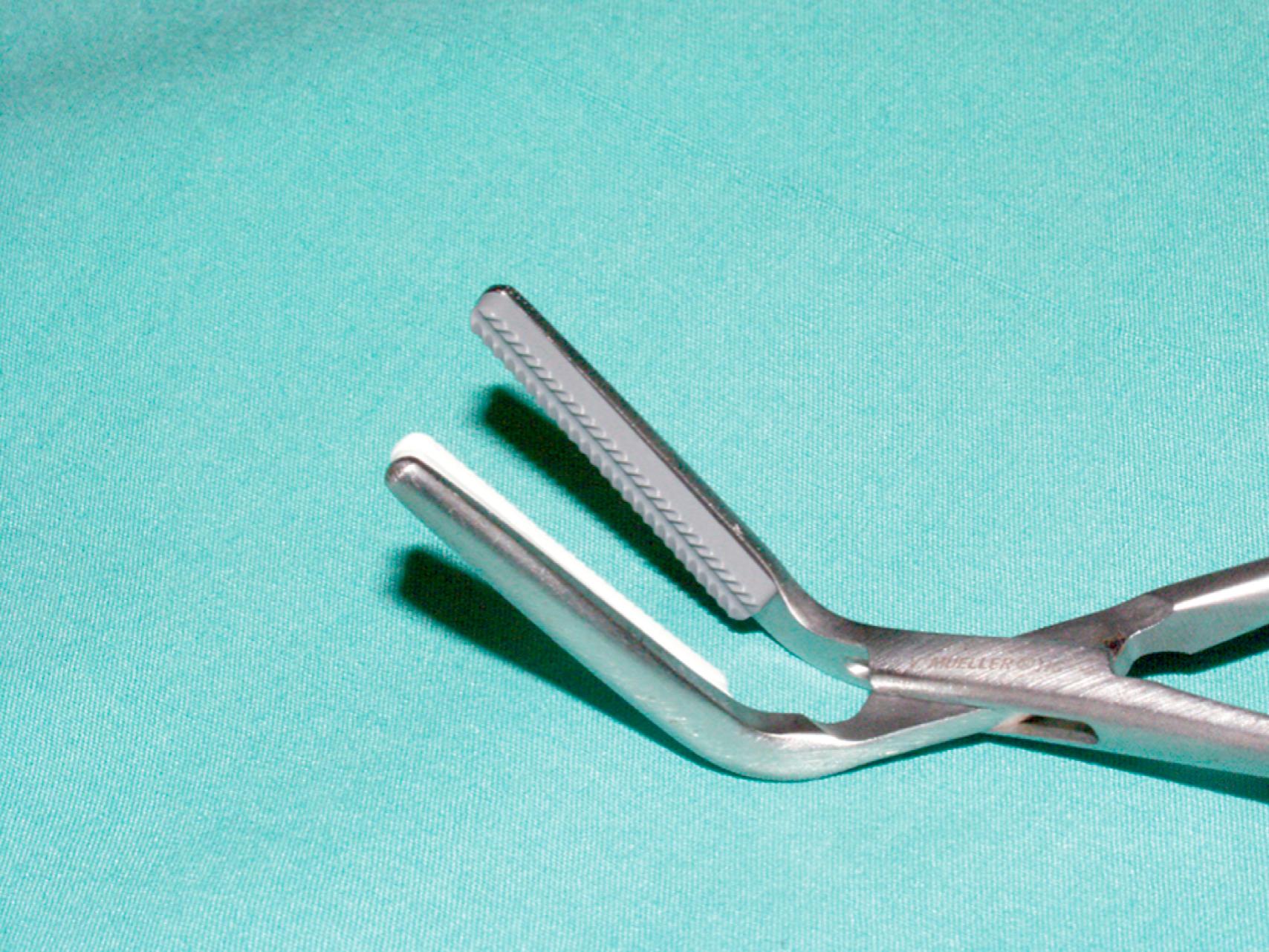
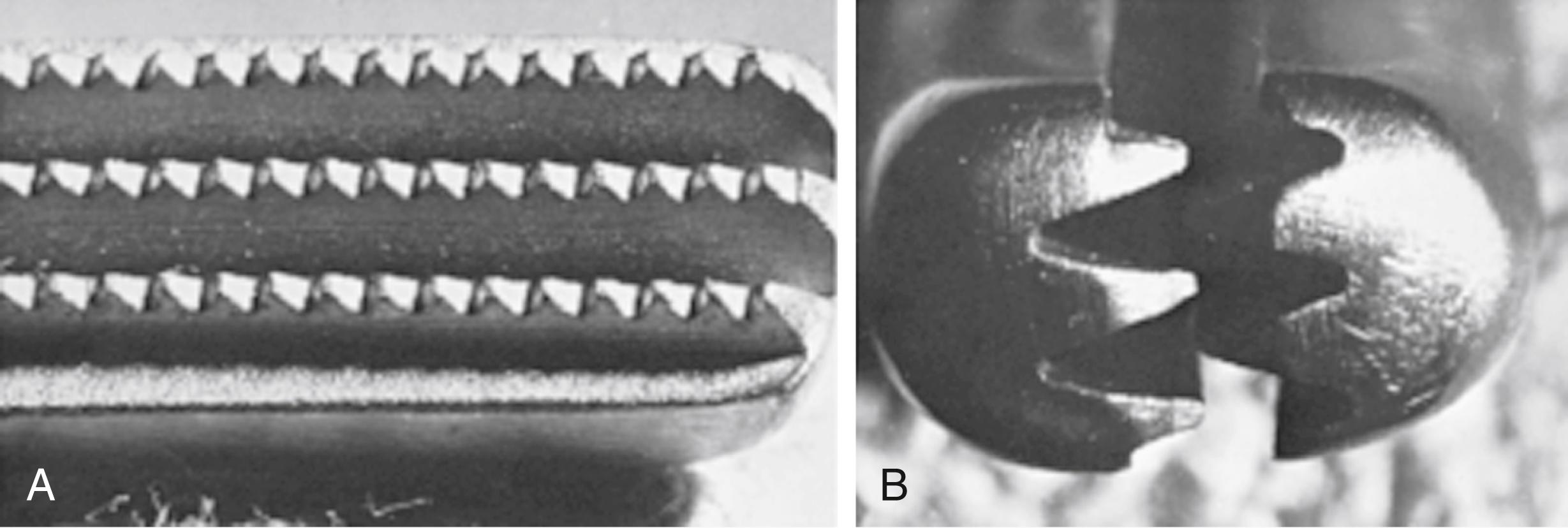
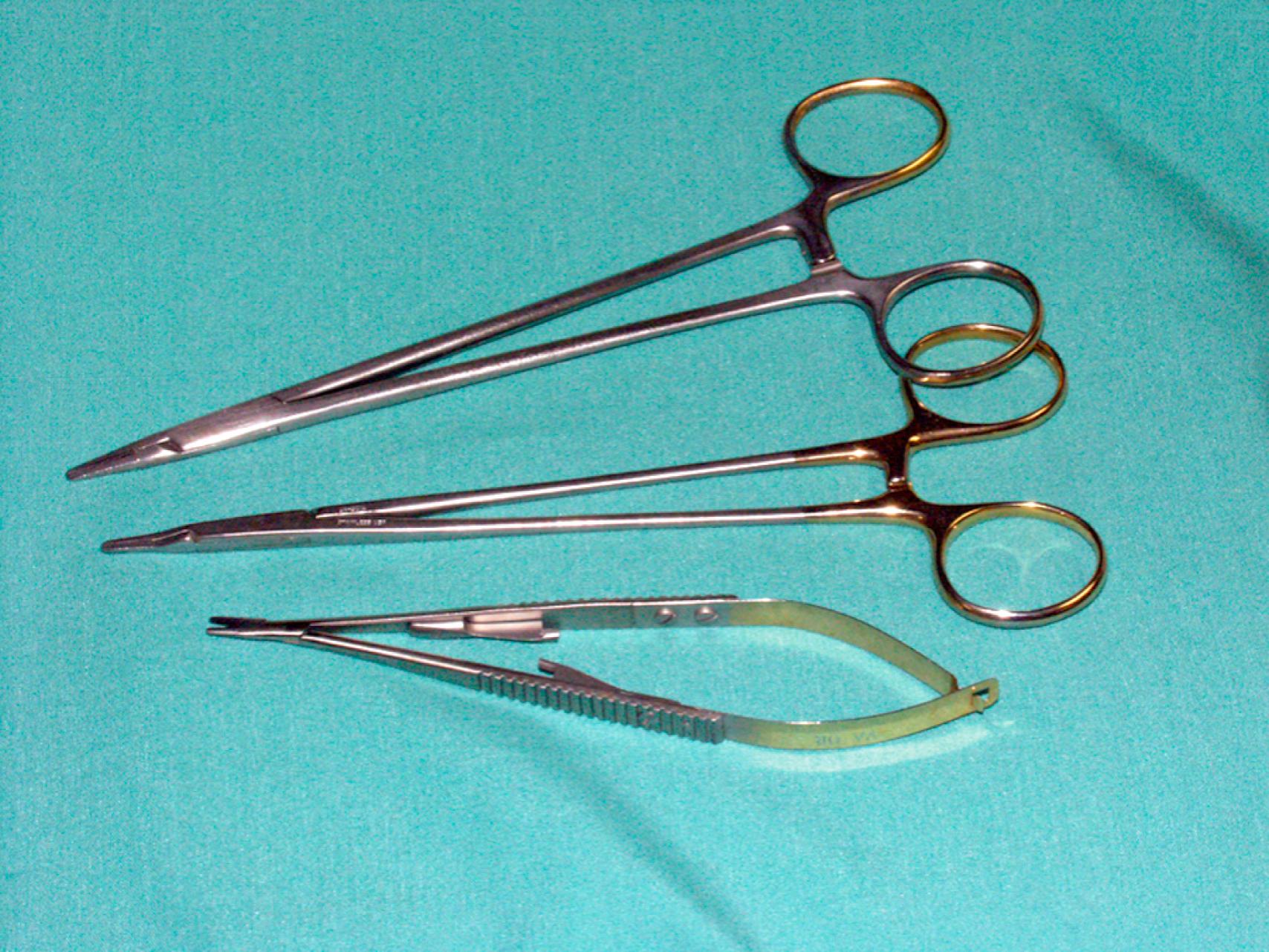
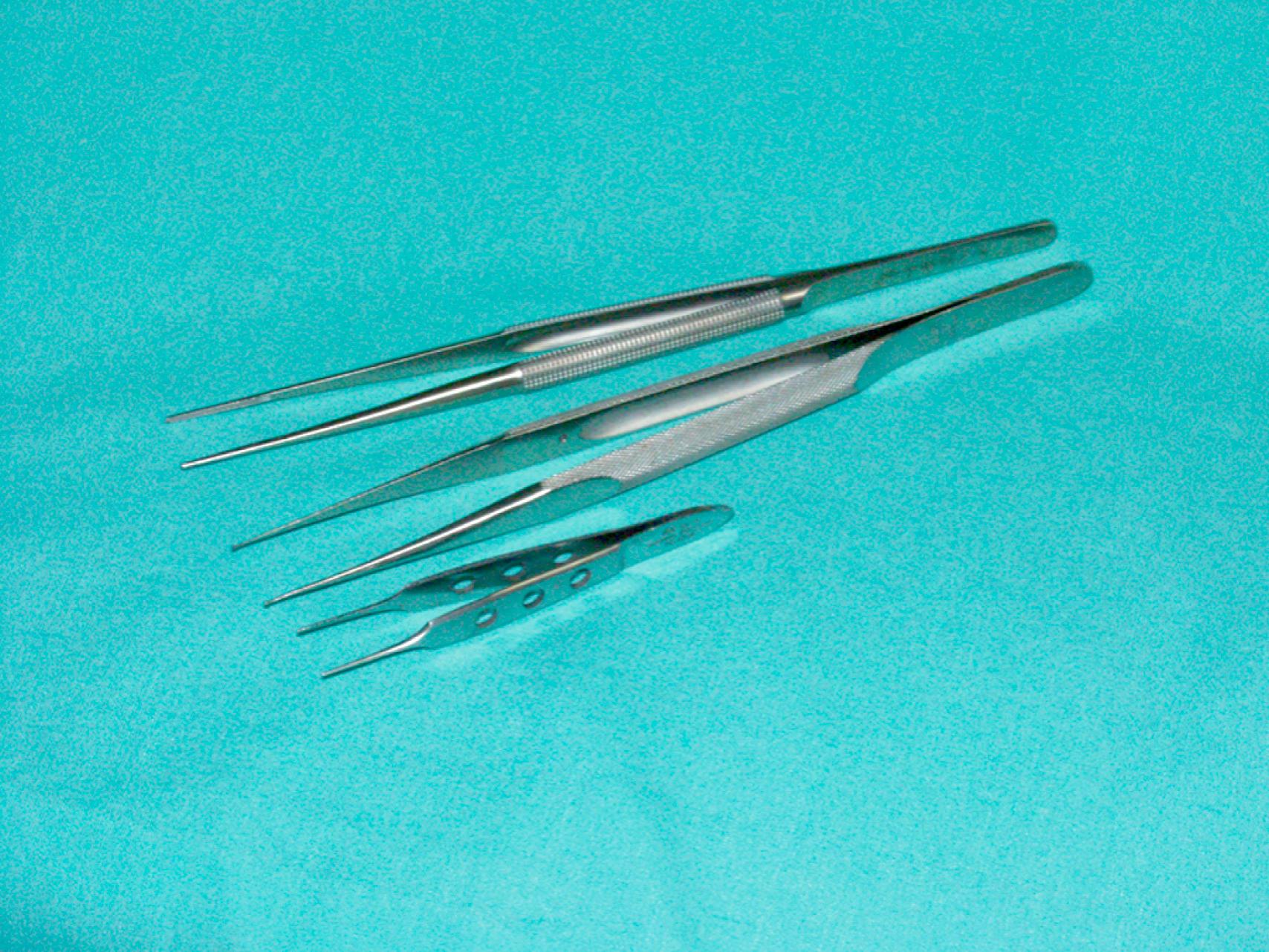
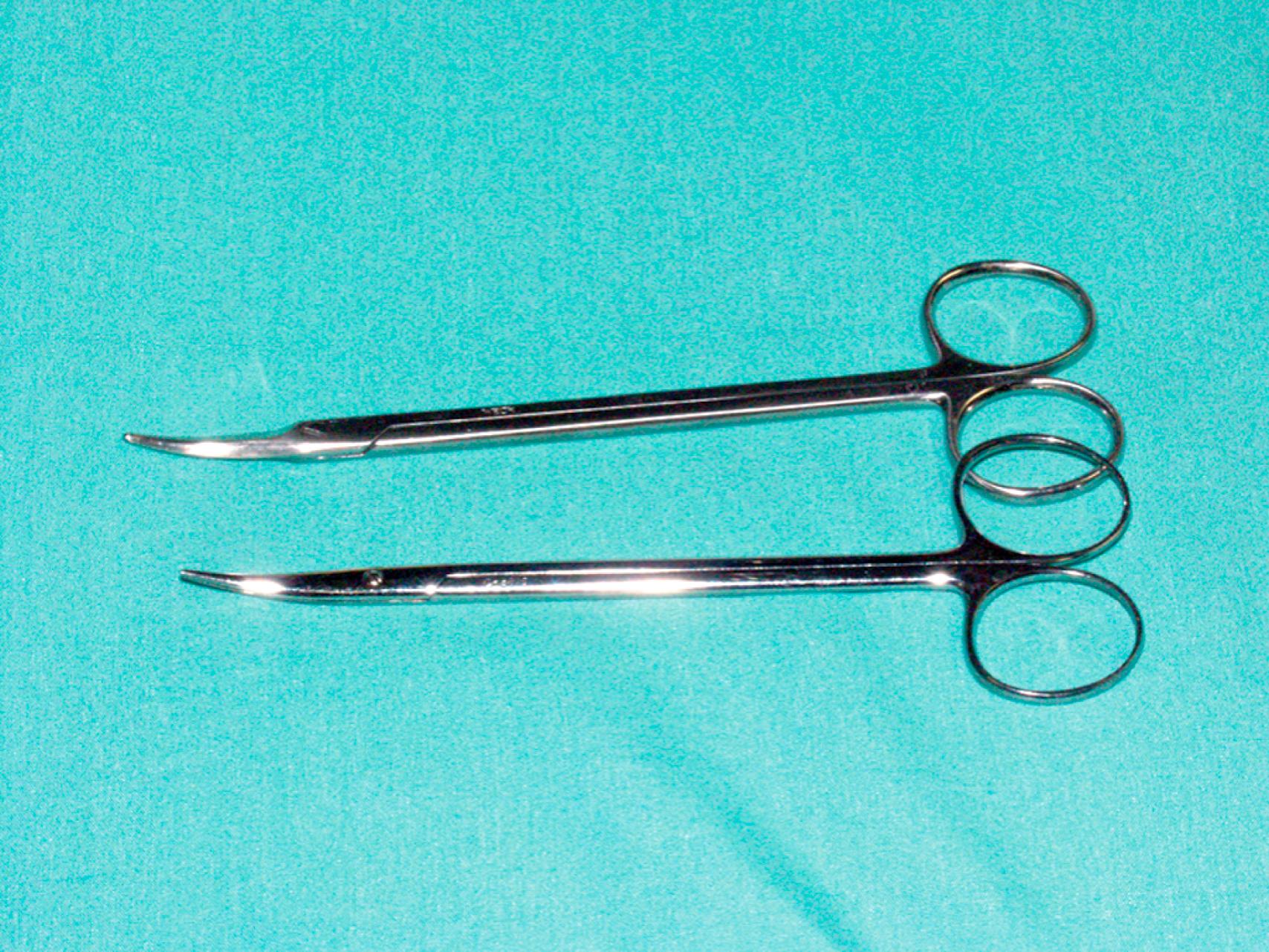
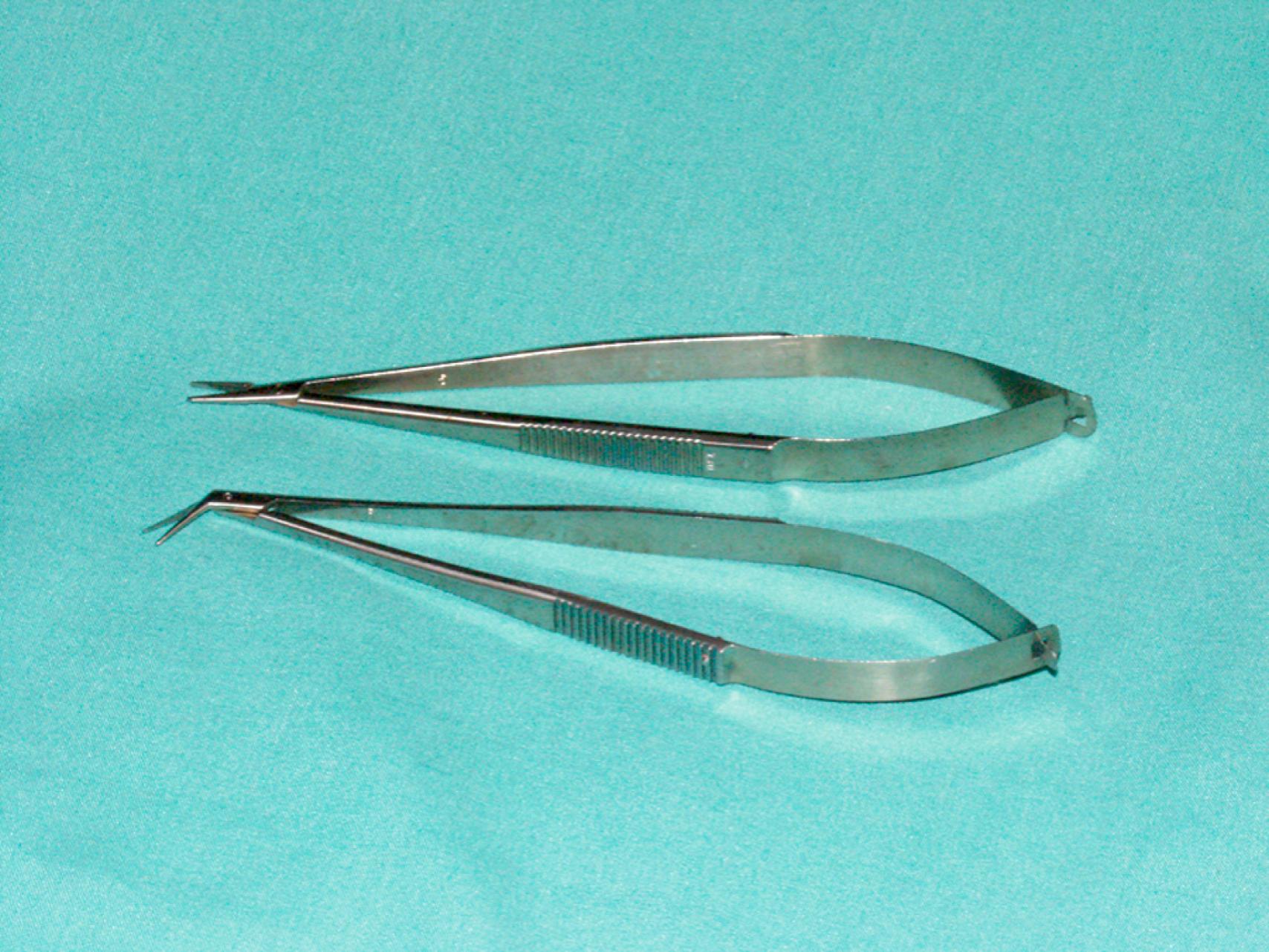
The choice of needle holder is often dictated by the size of the needle used; a Mayo–Hegar needle holder is typically used with large needles, and Castroviejo needle holders are typically used with small, fine needles ( eFig. 61.13 ). The forceps used during vascular procedures typically have very fine, non-crushing jaws, exemplified by the DeBakey or Gerald forceps. However, similar to clamps, vascular forceps can crush a vessel wall if they are not used appropriately and delicately. Fine-tip ring forceps, Jarrell forceps, are useful during the construction of vein bypasses to infrapopliteal vessels. Metzenbaum and Church scissors are used for the dissection of blood vessels while Stevens tenotomy scissors with sharp tips are used for dissecting smaller, tibial vessels. Potts scissors with various angulations are used to enlarge and shape arteriotomies and venotomies. Right-angle clamps with various tip sizes are used to encircle blood vessels and branches ( eFigs. 61.14–61.19 ).
Self-retaining retractors should be used when possible for stability and consistency without excessive pressure on retracted tissues. The Omni-Flex vascular retractor (Omni-Tract Surgical, St. Paul, MN) is frequently used for open aortic surgery, whether transabdominal or retroperitoneal ( eFig. 61.20 ). This retractor system consists of a wishbone that attaches to a post mounted on the operating room table rail, and it typically offers a selection of blades with different depths, widths, and shapes that can be clamped to the wishbone. Shallow, wide blades are typically used to retract the abdominal walls, whereas deeper splanchnic blades may be used to retract the splenic flexure and other parts of the colon. A wide, fence-shaped blade is typically used to retract the small bowel during aortic dissection, and a narrow, deep blade with a “lip” may be used to carefully retract the left renal vein cephalad. This retractor system is useful during abdominal and thoracoabdominal aortic aneurysm repair. Modifications of this retractor have also been designed for inguinal, carotid, and spine exposures.
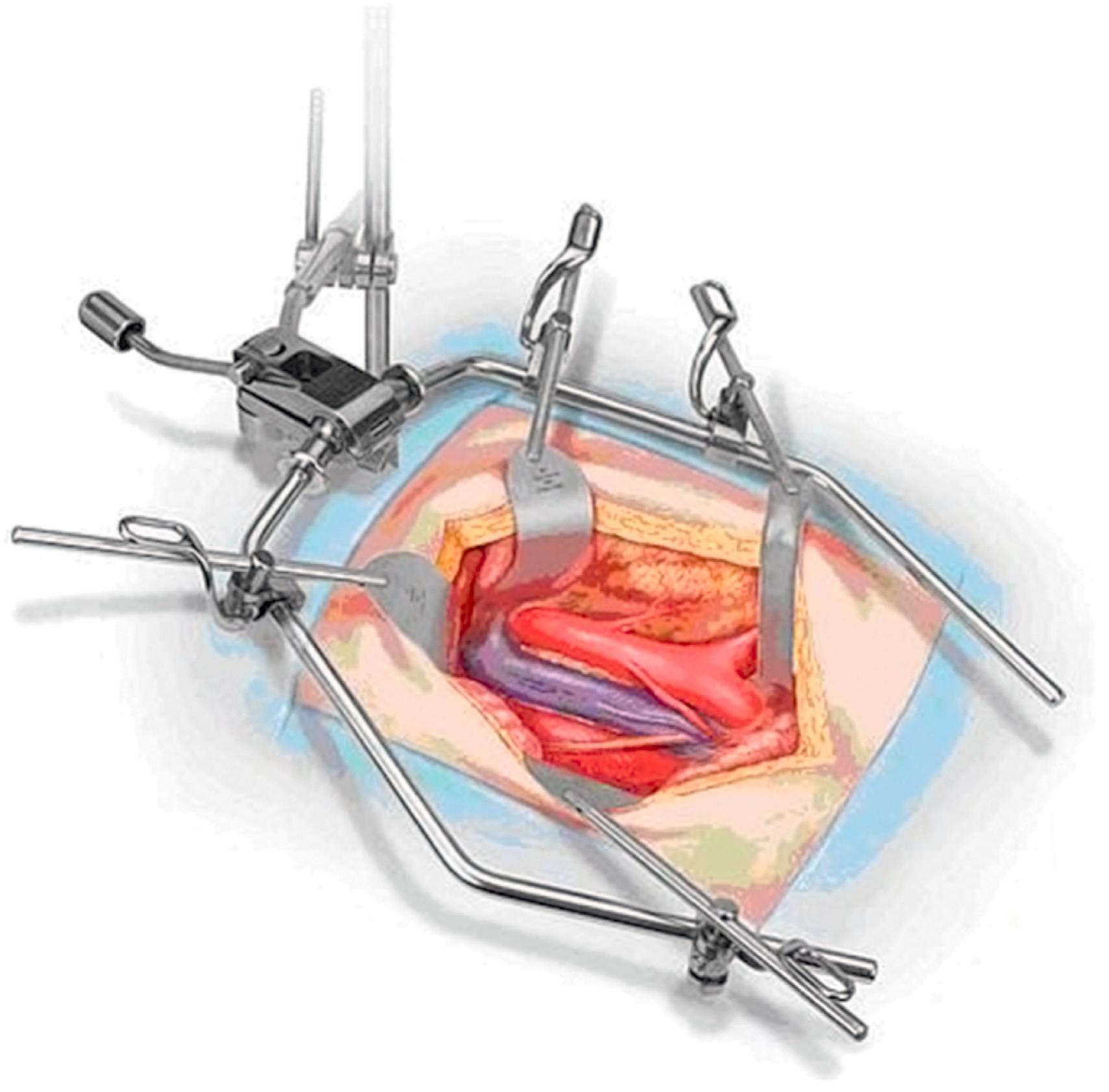
Another self-retaining abdominal retractor is the Bookwalter retractor (Codman Johnson & Johnson, Raynham, MA) ( eFig. 61.21 ). This retractor is often used when conducting abdominal aortic and iliac exposures especially through mini-laparotomy incisions. The retracting blades in the Bookwalter retractor attach to an oval metal ring placed around the abdominal incision instead of a wishbone. Several single-instrument self-retaining retractors are available for neck and extremity procedures ( eFigs. 61.22–61.24 ). The Weitlaner retractor is commonly used for inguinal, cervical, and popliteal incisions. Available with either sharp or dull retraction jaws, the sharp variety tends to hold its position in a more secure manner, thus requiring fewer adjustments than the dull version. The Adson cerebellar retractor is angled such that it can also be very useful in cervical, groin and below-knee exposures. In obese patients with significant inguinal pannus, a Miskimon retractor can be especially useful because of its deeper and wider blades, which provide a larger retracting area. Spring retractors are extremely useful when conducting infrageniculate vessel exposure because they tend to occupy little space. The Gelpi retractor is typically helpful when conducting a first rib resection through a transaxillary approach for thoracic outlet obstruction.

Nonabsorbable sutures are used for vascular anastomoses and repairs. These sutures typically provide the tensile strength necessary to juxtapose and secure the vessels together until healing occurs. When prosthetic conduits are used in vascular reconstruction, the tensile strength provided by the sutures is needed indefinitely to maintain vascular integrity. Monofilament sutures are used for vascular reconstructions, although braided silk or polyester multifilament sutures can be used for ligation of vessels. Vascular sutures are usually double armed with a needle on each end to allow continuous suturing in both directions from the initial knot. Commonly used monofilament sutures include polypropylene, polybutester, and polytetrafluoroethylene (PTFE). Polypropylene sutures (Prolene, Ethicon Inc., Somerville, NJ; Surgipro and Surgilene, Covidien, Mansfield, MA) are made of a monofilament strand of synthetic linear polyolefin. These sutures tend to maintain their tensile strength over time. Prolene has little friction and good handling characteristics, becoming widely popular and probably the most commonly used suture material for vascular reconstructions. It is important to moisten the suture and surgical gloves with saline prior to tying the suture in order to avoid microscopic tears and fraying of the material, and prevent suture breakage. Polybutester is another monofilament made of a copolymer of polyglycol terephthalate and polytrimethylene terephthalate coated with polytribolate to reduce drag and improve tissue passage. PTFE sutures were developed to minimize needle hole bleeding associated with suturing PTFE grafts or patches by optimizing the diameters of the needle and the suture to plug the needle hole with a slightly oversized suture diameter. This material has excellent handling characteristics with low friction and drag coefficient, but must be securely tied to with multiple throws of the knot to avoid slippage. The needle and suture sizes vary, depending on the vessel. Suggestions are provided in Table 61.2 .
| Vessel Size a | Needle Brand and Size | |||
|---|---|---|---|---|
| Ethicon | USSC | Davis and Geck | Gore-Tex | |
| Small (calcified tibial) | CC | DV-1 | CV-311/DTE-10 | PT-9 |
| Small (tibial, internal carotid) | BV-1 | CV-1 | CV-310/TE-10, 11 | TT-9 |
| Small (tibial) | BV | CV | CV-309/TE-9 | TT-12 |
| Medium (common carotid, femoral, popliteal) | C-1 | CV-11 | CV-301/TE-1 | TT-13 |
| Large (common iliac) | RB-1 | CV-23 | CV-331/T31 | TH-18 |
| Large (aorta) | V7 | V-20 | DV-305/T-5 | TH-26 |
| Large (posterior wall of aorta) | MH | V26 | CV-C00/T-10 | TH-35 |
a For arterial anastomoses, the following suture and needle sizes are recommended: 2-0 or 3-0, aorta; 4-0, iliac arteries; 5-0, axillary, common carotid, common femoral, and superficial femoral arteries; 5-0 or 6-0, internal carotid, popliteal, and brachial arteries; 7-0 or 8-0, tibial and inframalleolar arteries.
Selection of a graft type is an integral part of any vascular reconstruction. Polyester grafts of different shapes and structures are available to replace the thoracic aorta. Polyester and PTFE grafts are available as conduits for the abdominal aorta and infrainguinal vessels. Autogenous vein grafts are favored in many small arterial reconstructions. These grafts are discussed in further detail in Chapter 66 (Prosthetic Grafts).
Open vascular surgery is conducted using the general principles of blood vessel exposure with proximal and distal control, along with the basic techniques of vascular reconstruction. These basic vascular techniques include thromboembolectomy, endarterectomy, creation and closure of an arteriotomy, and construction of bypasses or in-line graft replacement using end-to-side or end-to-end anastomoses.
Several excellent resources are available that describe the vascular anatomy and the variety of exposures available to the vascular surgeon (see Ch. 55 , Thoracic and Thoracoabdominal Vascular Exposure; Ch. 56 , Abdominal Vascular Exposures; Ch. 57 , Cerebrovascular Exposure; Ch. 58 , Lower Extremity Arterial Exposure; and Ch. 59 : Upper Extremity Vascular Exposure).
A basic concept of vascular exposure and dissection is to approach and expose the vessel by the most direct and expeditious route possible. Anatomic landmarks, skin characteristics, and location of a pulse are factors used to guide placement of the initial skin incision. For infrainguinal bypasses a palpable inflow pulse should always be prepped within the surgical field allowing for the possibility of a retroperitoneal extension. In most dissections, the vascular structures can be exposed between existing muscle bellies. If extensive muscle transection is required during dissection, the approach to the vascular structures should be reconsidered as an errant plane may have been encountered.
Following the incision, proper handling of the skin and soft tissue overlying the vessels is essential to prevent significant wound complications. Lymphatics are typically ligated and divided to avoid lymphorrhea and lymphocele. Use of electrocautery through lymph nodes should be avoided to prevent significant lymph leak or the need to excise larger lymph nodes, leaving dead space in the wound. Once the vascular sheath is identified and incised, the adventitia is retracted in one direction with dissection carried out in the tissue adjacent to the edge of the blood vessel wall. Maintaining intimate proximity to the arterial adventitia during dissection usually ensures an appropriate anatomic plane, thus enabling the vessel to be circumferentially skeletonized and encircled with a Silastic loop or umbilical tape (Dow Corning Corporation, Midland, MI). The basic surgical technique of gentle traction with counter traction is essential in vascular exposure. Traction on a vessel loop can be used to retract a vessel during dissection or mobilization. Gentle tension on the vessel loop is recommended to prevent injury to the intima.
Reoperative vascular exposure often poses a challenge, given the fibrous obliteration of the normal anatomic vascular planes. Sharp dissection is important with identification of side branches. When the surgeon is faced with a difficult “redo” operation, sharp dissection with a no. 15 knife blade may allow better management of the scar tissue than scissors and allow the surgeon to stay in the appropriate dissection plane. The belly of the blade can be angled away from the vessel at approximately 45 degrees to “carve” the scar away from the arterial wall. The principle of dissecting from “known to unknown” can also be useful in preventing vessel injury during re-exposure with proximal control of the artery outside of the scarred area – an important consideration should an inadvertent injury occur.
A significant concern with a reoperative femoral artery exposure is injury to the profunda femoris artery. To avoid this occurrence, the superficial femoral artery can be exposed in the distal part of the dissection, where minimal scarring exists. The dissection is then carried along the medial aspect of the superficial femoral artery and progresses proximally. Typically, the common femoral vein is identified during the process, revealing an important dissection plane. Once the inguinal ligament is reached, the common femoral artery is encircled with a loop. The direction of the dissection is then reversed and continued back distally toward the superficial femoral artery. The area of size transition between the common and superficial femoral arteries often identifies the location of the profunda femoris artery. The profunda femoris artery can be controlled at its origin by passing a vessel loop underneath the superficial femoral artery and then retrieving it just proximal to the common femoral bifurcation by passing a right angle above and below the profunda femoris from the same side (lateral) in order to avoid injury to deeper branches with a circumferential passing of the right angle. In cases of extensive scarring, the profunda femoral branch can be controlled with a balloon occlusion catheter after common femoral arteriotomy, to avoid potential injury during profunda dissection.
Before interrupting blood flow, the patient may be anticoagulated to prevent thrombosis or embolization due to a static column of blood created between the clamp and first major collateral branch. Unfractionated heparin at 75 to 100 U/kg is typically administered intravenously, approximately 3–5 minutes before blood flow interruption. Anticoagulation may be monitored by measuring the activated clotting time (ACT), aiming for a value of more than 250 seconds. While distal limb ischemia following aortic reconstruction often may result from distal embolization, unrecognized suboptimal anticoagulation may also be responsible. In patients with known heparin-induced thrombocytopenia, anticoagulation may be achieved with intravenous thrombin inhibitors such as argatroban (see Ch. 41 , Anticoagulant Therapy).
Blood vessel control can be achieved using vascular clamps, balloon occlusion, vessel loops, pneumatic tourniquet, Rumel tourniquet, or internal occluders.
Ideally, vascular clamps should be applied to a disease-free segment of the artery. Palpating the artery against a right-angle clamp can help determine the presence and extent of atherosclerotic plaque, which is often in a posterior location. In the presence of significant plaque, the artery should be dissected more proximally to identify a less diseased site for clamping. This situation is often encountered when clamping the common femoral artery, where plaque often extends to the level of the inguinal ligament. Further dissection proximal to the external iliac circumflex and inferior epigastric branches, however, usually reveals an external iliac artery that is more amenable to clamp placement. If clamping is necessary across an area of diseased artery, the clamp should be applied in a manner that opposes the soft part of the artery against the plaque without causing plaque fracture or vessel tear. Occasionally the plaque burden and calcification are so extensive that the only option is to identify a more proximal location for safe clamp placement. One example is supraceliac aortic control through the lesser sac, when clamping in the infrarenal or suprarenal location is not possible because of extensive calcification or scarring from previous surgery or an inflammatory process.
If plaque is circumferential or occupies more than 50% of the circumference, vascular clamps can fracture the plaque or tear the wall and may not provide adequate vascular control. This can be managed by occluding the artery from within using a compliant balloon occlusion catheter, such as the Fogarty catheter normally used to perform embolectomy, or a noncompliant balloon catheter used for angioplasty. There are also several compliant occlusion balloons that can be used to occlude the aorta during ruptured aneurysm repair, including Reliant (Medtronic, Minneapolis, MN), Coda (Cook, Bloomington, IN), and Equalizer (Boston Scientific, Natick, MA). Balloon occlusion can be used to control the external iliac artery in the presence of extensive calcification that extends beyond the most proximal part of the exposure. It is also useful for controlling the right common iliac artery during a left retroperitoneal abdominal aortic aneurysm repair, the profunda femoris artery during repair of a pseudoaneurysm of a femoral anastomosis, or the renal or visceral vessels during a thoracoabdominal aortic aneurysm repair.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here