Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Breast reconstruction performed with a pedicled fasciocutaneous island flap from the back is a valuable tool in the armamentarium of the reconstructive surgeon. The two main options are: (1) latissimus dorsi musculocutaneous flap (LD-MC); or (2) its “modification”, the thoracodorsal artery perforator flap (TDAP). Both techniques have their advantages and disadvantages. The benefits of the TDAP flap include total preservation of the latissimus dorsi muscle, preservation of the functional motor nerves to the underlying muscle, and minimizing donor-site morbidity. The TDAP flap is becoming a common and viable option for volume replacement following conservative mastectomy reconstruction but can also be considered for total breast reconstruction following mastectomy in some patients. The “conventional” LD-MC requires less surgical expertise and allows for more flexibility in the design of the skin island. As previously mentioned, the donor-site morbidity following sacrifice of the latissimus dorsi muscle must be considered with this conventional flap.
The TDAP flap is one of the main surgical options in immediate or delayed partial breast reconstruction when a volume replacement technique is required. Volume replacement is performed with non-breast local or distant flaps offering both tissue for the filling of the glandular defect and the skin deficiency of the reconstructed breast. The resection of more than 15%–20% of the breast parenchyma in a small-volume (A- or B-cup) breast and more than 30% in a larger breast will result in volumetric deformities and bilateral asymmetries. Patients with small or non-ptotic breasts could require volume replacement. The TDAP flap also provides sufficient volume to reconstruct a B-cup sized breast. This is accomplished by totally or partially de-epithelializing the flap in patients following mastectomy. In the event that the volume obtained from the TDAP flap is insufficient, additional fat grafting may be performed to increase volume using the TDAP flap as an autologous scaffold.
The TDAP flap offers an alternative for patients in whom abdominal flaps are high-risk or unavailable options or even more as a primary option in patients with small to medium breast size. The TDAP flap is indicated when autologous reconstruction is required and the traditional abdominal donor site is not available or not preferred by the patient and/or surgeon. Based on the primary author’s experience, autologous breast reconstruction with the TDAP is the first choice when a volume similar to that on the contralateral side can be obtained in a single operative procedure. The benefits include avoidance of microsurgery, reduced operative time, reliability of the procedure, low morbidity of the donor area, and efficacy of the technique.
The use of autologous tissue from the posterior thorax was originally introduced by Tansini in 1896. It has been postulated that he originally performed a circumflex scapular flap that was later modified to include the thoracodorsal artery and vein, thus evolving into the latissimus dorsi musculocutaneous flap. His original technique was clearly described in subsequent publications and became popular in Europe during the 1920s.
Following a period of relative inactivity, the latissimus dorsi flap was rediscovered in the 1970s and became a widely used and popular flap that was safe and reliable for a variety of reconstructive applications that included breast reconstruction, chest wall coverage, head and neck reconstruction, and free flap transfer. There have been various modifications to the latissimus dorsi flap in order to increase flap volume such as the extended latissimus dorsi (LD) flap as well as modification of the skin pattern design i.e., fleur-de-lis . Lipofilling to the transferred muscle augments its volume and was reported as a valuable tool. In some patients, the thickness of the latissimus dorsi muscle posed a problem with flap inset and folding. Techniques to make this less problematic included reduction of the latissimus dorsi musculocutaneous flap that was reported by several authors. These included muscle thinning, muscle splitting, tailoring, and muscle reduction. In 1976, De Connick proposed the use of the thoracodorsal pedicle for a “pure skin flap” and J. Baudet may have been the first surgeon to perform what is currently known as the TDAP flap. This occurred in 1976, as he performed a free flap with the direct cutaneous branch of the thoracodorsal vascular pedicle. These initial flaps were in essence the origins of the perforator flap era that we are now immersed in. This would also include the deltopectoral free flap performed by Harii et al . in 1974.
As the perforator flap era continued to evolve, fasciocutaneous flap began to be harvested independently from the underlying muscles. Koshima led this technical advancement with the deep inferior epigastric perforator (DIEP) flap. Angrigiani was the first to report the “latissimus dorsi musculocutaneous flap without muscle” in 1992, commonly known today as the thoracodorsal perforator flap. Hamdi originally described its use for breast reconstruction. Future publications reassured the reliability of this flap. Total breast reconstruction with the TDAP flap was reported by Santanelli and Hamdi . The incorporation of fat compartments, in the same way as the extended latissimus dorsi described by Germann, was presented as the Extended TDAP.
Previous axillary or thoracic surgery with possible damage to the thoracodorsal vessels is a contraindication for latissimus dorsi flap or the TDAP flap. Previous scars and irradiation to the area may be considered as relative contraindications of local pedicled perforator flaps as they also might result in damaging the perforator.
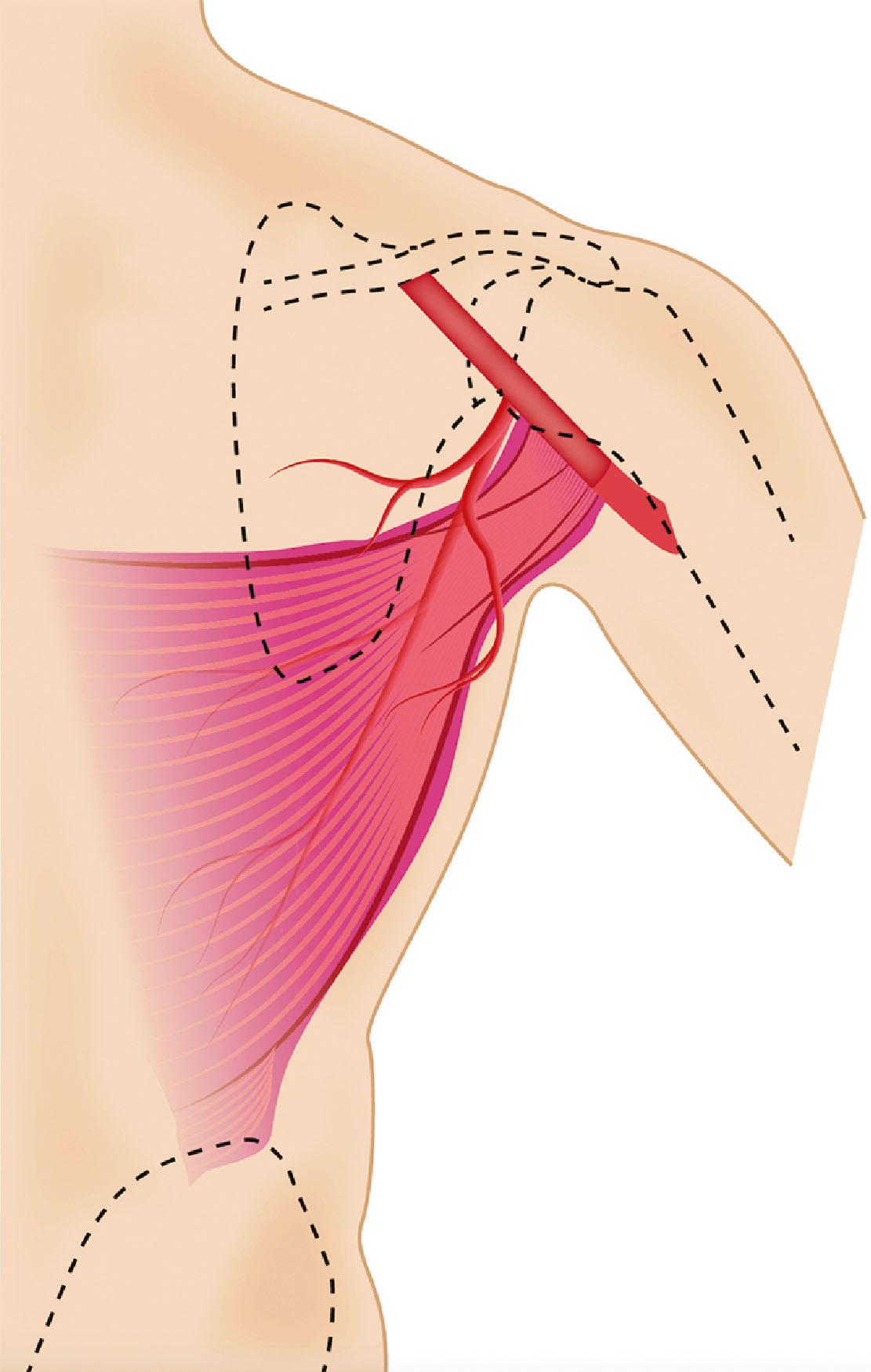
The TDAP flap is based on the perforators from the vertical (descending) or horizontal branches of the thoracodorsal vessels ( Fig. 46.1 ). Anatomic studies have demonstrated the presence of 2–3 musculocutaneous perforators from the vertical branch. The proximal perforator enters the subcutaneous plane obliquely 8–10 cm below the axillary fold and 2–3 cm posterior to the lateral border of the muscle. The second perforator is located 2–4 cm distally to the first one. Occasionally, a direct cutaneous perforator (septocutaneous) arising from the thoracodorsal vessel passes around the lateral border of the muscle, making flap harvesting easier. Due to anatomical variations, a single reliable perforator for the TDAP flap is not always identified. In this case the surgeon must be aware and be prepared to modify the flap dissection intra-operatively into a muscle-sparing LD flap.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here