Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Odontogenesis
Portions of the enamel organ
Odontogenic cysts
Nomenclature and other cysts of the oral region
Orthokeratinizing odontogenic cyst (keratinizing odontogenic cyst), separation from keratocystic odontogenic tumor (odontogenic keratocyst)
Odontogenic cyst of undetermined origin definition and discontinued use of primordial cyst term.
Glandular odontogenic cyst, definition, and problems with diagnosis
Odontogenic tumor
General treatment considerations of odontogenic tumors
Proper therapy for “simple unicystic ameloblastoma”
Keratocystic odontogenic tumor, re-reclassified as a cyst
Calcifying cystic odontogenic tumor, re-reclassified as a cyst
Avoiding overtreatment of calcifying epithelial odontogenic tumors
Odontogenic tumors are often intricate admixes of neoplastic and non-neoplastic lesions. Even the “simple” odontogenic lesions have their origin traced back to odontogenesis. But odontogenesis is far from simple. By understanding odontogenesis, the reader's appreciation for these lesions should be enhanced. In fact, it is the unique interaction of ectomesenchymal tissues with epithelial tissues during odontogenesis that make these cysts and tumors unique. These interactions of the mesenchyme and epithelium occur across very short distances and within the confined volume of the jaws. Complications in histologic interpretation include artifacts due to demineralization, sampling errors, separation of hard and soft tissues at the grossing bench, and general difficulties in sectioning disparate tissue types. Separation of disparate tissue at the grossing bench can make it impossible to analyze the relationship of tissues later under the microscope. Even when these background problems are not encountered, the result is often still a histopathologic “hodgepodge.” In the end, odontogenic lesions that contain both epithelial and mesenchymal components are often difficult to assess histologically.
A basic understanding of odontogenesis by the surgeon/clinician may help to predict behavior, enlighten the clinician on what information to give the pathologist, and guide proper treatment. Thus odontogenesis and the jaw's unique status within the body create a wide set of possibilities for clinical, radiographic, and histopathologic differential diagnosis lists. It is the goal of this chapter to review odontogenesis as well as odontogenic cysts and tumors. It is hoped by the end of this chapter that a better understanding of odontogenic cysts and tumors will lead to a more thorough appreciation of this intricate and complicated set of diseases.
Odontogenesis begins within generally well-defined areas of the stomodeum, then by maturational extension the oral cavity and alveolar processes of the jaws. Unfortunately, simply knowing location does not necessarily allow the clinician to always positively ascertain a lesion as being odontogenic in origin. Confounding elements include the embryologic nature of Rathke's pouch, extension of large odontogenic lesions outside the confines of the alveolar processes, and the propensity of products of odontogenesis to “wander.” Thus, reports of sinonasal ameloblastomas and craniopharyngiomas of the sella should not be surprising and still be considered odontogenic in origin. Simply put, the only prerequisite is that all odontogenic cysts and tumors must be derived from elements of stomodeal origin.
Histologically the rather unique epithelial portions of odontogenesis often remain distinct enough in cysts and tumors to allow for proper determination of origin. Unfortunately, the mesenchymal components of the dental papilla (as seen in myxomas) and cemental components (as seen in odontogenic fibromas) are impossible to definitively identify histologically as being of odontogenic origin. The only hope in identifying these as odontogenic is if they occur in association with other odontogenic epithelial elements. But generally, these mesenchymal lesions are identified as odontogenic based on location in the jaws.
Although this section will focus only on odontogenesis, remember that the oral and maxillofacial region is also associated with developmental remnants of salivary glands, seromucous glands, sinonasal epithelium, nasopalatine duct epithelium, and dermal epithelia. Due to these nearby, possibly confounding embryologic tissues, the true origin of any given lesion may be cloudy at best. However, by knowing the specific location, history, and histopathologic features, as well as being armed with knowledge of odontogenesis, the proper designation for any given lesion can almost always be filtered to arrive at a relatively short differential diagnosis list.
The enamel organ is generally divided into the bud stage, the cap stage, and the bell stage. The bud stage begins as a proliferation of the basilar cells of the stomodeum. This proliferation occurs along the area of the future alveolar mucosa apical to and separated from the vestibular mucosa. In this early stage the ectoderm of the stomodeum is lined by two to three cells. It is during the sixth week that the ectoderm in the region of the future alveolar processes begins to proliferate and form two somewhat horseshoe-shaped epithelial bands. Before being differentiated into the epithelial surfaces of the alveolar processes each band is called a dental lamina. These dental laminae will eventually form the 20 separate proliferations necessary for the deciduous teeth. These 20 areas of proliferation are each called tooth buds. Each tooth bud then proliferates apically into the underlying ectomesenchymal tissue. Eventually at the bell stage the connection between the overlying stomodeum and the enamel organ will become separated. But the actual process of bud proliferation varies by arch as well as by tooth type (i.e., deciduous central incisor, deciduous lateral incisor, deciduous canine, deciduous first molar, and deciduous second molar). By the end of the eighth week all 20 buds have been produced.
During all phases of morphodifferentiation, histodifferentiation, and apposition the features of the enamel organ and the physiologic support of the enamel organ are changing. But with apposition of the dentin and vascularization near the outer enamel epithelium the enamel organ is further defined with a fourth cell layer termed the stratum intermedium. The cells of the stratum intermedium occupy an ill-defined area of flattened cells between the inner enamel epithelium and the stellate reticulum. Although the area of phenotypic change that defines the stratum intermedium must be present before enamel can be laid down, actually identifying these cells individually is extremely subjective.
In the tooth root, the dentin forms the huge majority of the root volume, which is then sheathed in a layer of cementum instead of enamel. But the odontoblasts still cannot differentiate and lay down dentin without induction by epithelium derived from the enamel organ. To accomplish this feat as the reduced enamel epithelium reaches the cementoenamel junction it becomes reduced to back-to-back inner and outer epithelial cells. This produces a collar of cells that separate from the reduced enamel epithelium. This separated collar is known as the Hertwig epithelial root sheath. As it moves apically to guide root formation, the Hertwig root sheath will “leave behind” epithelial rests known as rests of Malassez. These rests will reside in the region of the periodontal ligament. These rests provide a basis for several potential odontogenic cysts and tumors.
The periodontal ligament is actually a joint known as a gomphosis joint. Although the cementum cannot be distinguished except by location, the body, for instance during orthodontic movement, will resorb the bone on the lamina dura side of the periodontal ligament and not the cementum on the tooth side of the ligament. The tooth with the periodontal ligament will retain a certain amount of mobility within the joint. The reader is again referred to various texts for a more in-depth coverage of odontogenesis.
There are a number of odontogenic epithelial stages and each may provide a basis for odontogenic cysts or tumors. The four main component are (1) dental lamina, (2) enamel organ, (3) reduced enamel epithelium, and (4) Hertwig epithelial root sheath. The rests of Serres as well as Malassez are considered along with their respective progenitors of the dental lamina and Hertwig root sheath. In addition, the stomatodeal epithelium gives rise to Rathke's pouch, which retains odontogenic potential. Shafer et al. and others suggest that the original basal epithelium of the stomatodeum also retains potential. In the adult, this basal epithelium is represented by the gingival and alveolar mucosal surfaces. This concept seems to be supported when peripheral ameloblastomas appear to develop directly from overlying gingival epithelium. With that said, most would consider the adult basal epithelium to be a very rare source of odontogenic neoplasia. The rests of Malassez are common sources of inflammatory odontogenic cysts but retain little neoplastic potential. The rests of Serres, the enamel organ, and reduced enamel epithelium are generally considered the stages most likely to become neoplastic. All stages have the potential to form cysts to varying degrees. Dentigerous cysts with their origin from the reduced enamel epithelium and radicular cysts from rests of Malassez make up the overwhelming majority of odontogenic cysts.
When reading various sources it will become quickly clear that what a cyst “is” varies by author, and that the classification schemata are in disarray as well. The modified classification scheme seen in Box 88.1 is my attempt at organization. The “odontogenic cyst of undetermined origin” is a new admittedly descriptive “diagnosis,” which is used by some oral pathologists. Unfortunately the descriptive nature of that term will not be available to look up in other texts or journals. However, it replaces the diagnosis of primordial cyst or when the history provided does not allow for categorization of cysts defined by relationship to the tooth. The need to use a descriptive term instead of primordial cyst is due to the ambiguous use of primordial cyst both as a keratocystic odontogenic tumor (KCOT) as well as simply a nonkeratinizing cyst, which cannot be classified in relation to the tooth. The descriptive role is to provide a pigeonhole in which to place lesions that are histologically ambiguous, or not directly associated with a tooth but are located in the alveolus and thus, presumably, odontogenic in origin.
Dental lamina cyst (gingival cyst of newborn)
Dentigerous cyst (follicular cyst)
Eruption cyst
Lateral periodontal cyst
Botryoid odontogenic cyst
Gingival cyst of adults
Orthokeratinizing odontogenic cyst
Glandular odontogenic cyst
Odontogenic cyst of undetermined origin (replaces the term primordial cyst that has been irretrievably confused in the literature; this new descriptor also serves as a useful designation for some lesions that do not classify well)
Periradicular cysts
Periapical cyst (apical periodontal cyst)
Lateralized periradicular cyst
Residual cyst
Dentigerous origin
Periapical origin
Paradental cyst
Mandibular infected buccal bifurcation cyst
Inflammatory collateral cyst
Nasopalatine duct cyst (incisive canal cyst)
Cyst of the incisive papilla
Nasolabial cyst (nasoalveolar cyst)
Palatal cysts of infants
Epstein's pearls (midline of hard palate)
Bohn's nodules (scattered on palates, especially at junction of hard and soft)
Lymphoepithelial cysts
Oral lymphoepithelial cyst
Cervical lymphoepithelial cyst (branchial cleft cyst)
Gastric heterotopic cyst
Thyroglossal duct cyst
Salivary duct cyst
Maxillary antrum associated
Surgical ciliated cyst
Soft tissue cysts
Epidermoid cyst
Thymic cyst
Bronchogenic cyst
Trichilemmal cyst (pilar cyst)
Pseudocysts
Idiopathic bone cavity (traumatic bone cavity, traumatic bone cyst, simple bone cyst, hemorrhagic bone cyst, etc.)
Aneurysmal bone cyst
Mucus retention phenomenon (mucous retention cyst)
Mucocele of the sinus
Cystic hygroma
Miscellaneous
Dermoid cyst
Polycystic disease of the parotid
HIV-associated lymphoepithelial lesion
Nonexistent/spurious cysts
The following cysts are generally considered embryologically impossible or have been reclassified in other cysts above.
Globulomaxillary cyst (dismissed on the basis of embryology)
Median mandibular cyst (dismissed on the basis of embryology)
Median maxillary alveolar cyst (subcategory of nasopalatine duct cyst)
There are several classification schemata for odontogenic cysts and for oral and maxillofacial cysts. The classification scheme seen here is a modified amalgamation from that of the World Health Organization (WHO). In 1992 that organization published the 2nd edition of Histological Typing of Odontogenic Tumors . Unfortunately the most recent WHO treatise on odontogenic tumors is contained within the Pathology & Genetics of Head and Neck Tumors . In that 2005 change odontogenic cysts were no longer covered within the text, and this also resulted in the reclassification of several cysts to tumors. Box 88.1 also contains selected nonodontogenic cysts for completeness and comparison. But to confuse things even more, the 2017 version of the WHO Classification of Head and Neck Tumours has now reverted back to the 1992 nomenclature. The organization within Box 88.1 will contain a parenthetical WHO 2017 nomenclature. The International Classification of Diseases and Related Health Problems-10 (ICD 10) also has a projected update to International Classification of Diseases and Related Health Problems-11 (ICD 11) projected in 2018. At the time of this writing it is unclear due to the fluidity of nomenclature exactly where the final decisions will occur. An online search of a draft version of ICD 11 ( https://icd.who.int/dev11/l-m/en ) showed that the term KCOT will still be employed and classified under odontogenic tumors but the term calcifying odontogenic cyst (COC) is being utilized but also classified under odontogenic tumors.
By definition, a cyst will be considered a “pathologic cavity at least partially lined by epithelium.” To be an odontogenic cyst the epithelial lining must be derived from odontogenic epithelium. The best advice to the reader is that all classification schemata are artificial to some extent. The key is to organize them the way that is most useful to you. This modification is an attempt at self-clarification and will hopefully be useful for others either in its pure or modified form.
Many odontogenic cyst lumina are surfaced by nonkeratinized epithelium. Histologically the following nonkeratinizing cysts can look identical and cannot be separated except by clinical, historical, and/or radiographic means. These include dentigerous cyst (follicular cyst), eruption cyst, odontogenic cyst of undetermined origin, periapical cyst (apical periodontal cyst), lateralized periradicular cyst, residual cyst, and paradental cyst.
These cysts make up the vast majority of odontogenic cysts. As a group they are known as the “common odontogenic cysts.” For proper diagnosis of these common odontogenic cysts the clinician must provide sufficient information for the diagnosis to be made. The reader is urged to closely note the necessary or significant features when each of these cysts is specifically discussed later in this section.
The other odontogenic cysts will display histologic features to allow for the proper diagnosis, although history and communication is of course always appropriate and often essential. It is important to note that the presence of a specific type of keratin is not pathognomonic for a KCOT and may be seen with other odontogenic cysts. However, parakeratin is indeed the most common form of keratin in a KCOT.
Cyst expansion occurs due to a number factors, including accumulation of inflammatory cells, fibrin, serum, and desquamated epithelial cells. As these products enter the cystic cavity, it is the accumulation of the intraluminal products that spurs the cystic expansion of the wall. Alternatively, cyst expansion may be spurred on by the inherent mitotic activity of the cyst wall itself. If this mitotic activity is the major component of the cyst expansion, it may be better to consider the lesion a cystic neoplasm rather than a simple cyst. This debate lies at the center of how to name and classify the KCOT (odontogenic keratocyst) as well as the calcifying cystic odontogenic tumor (CCOT). In the case of the odontogenic keratocyst the term the KCOT will generally be used in this chapter and discussion of the entity will be in the odontogenic tumor section. The CCOT as well as the closely related more solid dentinogenic ghost cell tumor will also be considered under the tumor section.
Multilocularity may in itself be a signal that the lesional growth is mitotically or multifocally driven rather than hydraulically driven. For this reason the potentially multilocular odontogenic cysts, such as the botryoid odontogenic cyst and glandular odontogenic, may arguably have a neoplastic potential as well. However, in the case of the botryoid cyst the possibility of multifocality cannot be ignored. Cell regulation protein studies to determine cell inhibition and division activities may be helpful in future classifications. In addition, the ability of epithelia to break down elements of the connective tissue wall could be important.
However, even simple cysts like the periradicular cyst derived from rests of Malassez must possess some mitotic activation or growth would be impossible. Activation of the inflammatory cysts is thought to occur as a result of inflammatory production within the periodontal membrane. In the skin and gingiva, it has been shown that inflammation leads to the release of inhibitors, which then allow the renewal of mitotic activity. Once a solid epithelial sphere has been formed it is thought that it eventually outgrows its vascular nourishment and the central area degenerates to form a lumen. Following the formation of the central lumen, transepithelial flow of fluid is sustained by osmotic forces. Thus hydrostatic pressure plays a role in the development of the classic unilocular appearance of most cysts. How the pressure results to produce osteoclastic resorption is less clear.
Those cysts, which are derived from the more neoplastic dental lamina or are in themselves “cystic neoplasms,” probably occur as a result of self-sustained or unregulated mitotic activity. Even in neoplastic cysts, luminal expansion may occur through degenerative effects, debris accumulation, hydraulic forces, as well as mitotic activity.
The periapical cyst must be associated with a nonvital tooth. The tooth may be rendered nonvital by trauma, caries, developmental defect, or periodontal space extension. As such, these cysts may be seen at any age, although permanent teeth are more likely to be involved than deciduous teeth. They are thought to be derived from rests of Malassez.
These cysts will present as a unilocular radiolucency at the apical portion of the tooth. Although well defined, the border will vary from corticated to sclerotic to merely well defined. The variations will depend on the amount of inflammation present. Long-standing neglected lesions can get quite large, although most are less than 1 cm in diameter ( Fig. 88.1 ).
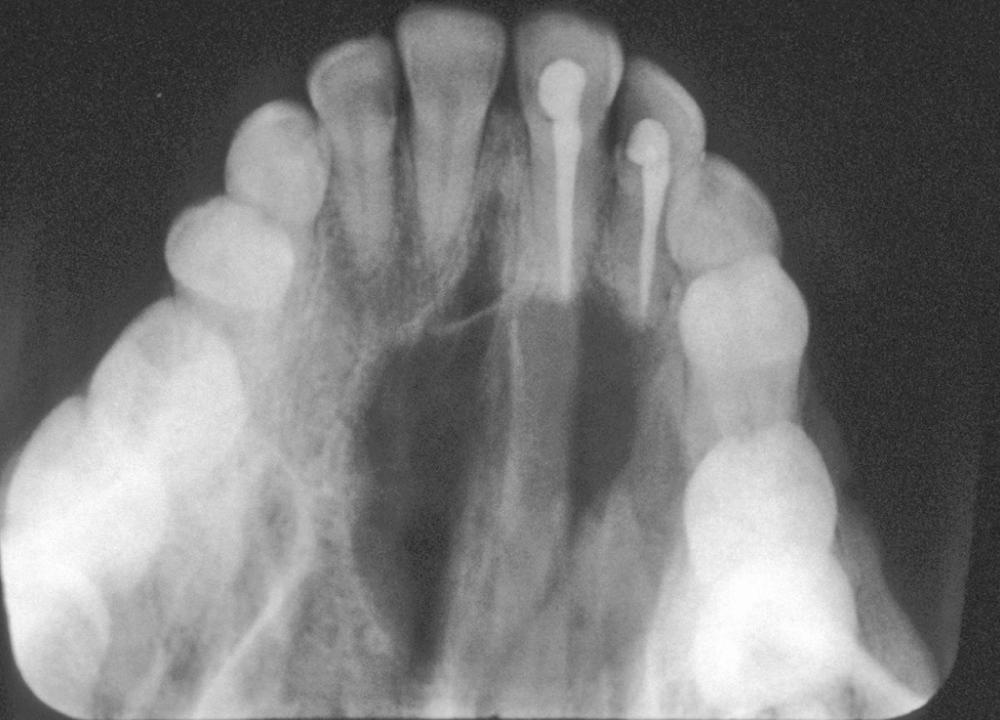
This is the classic inflamed “common odontogenic cyst” and as such the luminal lining will consist of nonkeratinized stratified squamous epithelium. This is an inflammatory cyst and inflammation is invariably present if sufficient sampling is performed ( Fig. 88.2 ). Rests of Malassez are possible in the connective tissue. However, odontogenic rests are rarely seen in the cyst wall, even though these rests are thought to be the source of the epithelial proliferation. Cholesterol slits, foreign body giant cells, and hemosiderin deposits are common findings. As in all “common odontogenic cysts,” squamous odontogenic tumor-like proliferations may be seen in long-standing lesions. These epithelial islands will be cytopathologically benign without evidence of dysplasia. If squamous odontogenic cyst-like proliferations are noted, they should essentially be ignored and are of no prognostic significance. In endodontically treated teeth foreign bodies secondary to endodontic therapy are common. Bacterial colonies may also be seen in these cysts. Although Actinomycetes colonies may portend a tendency for being slow to resolve, their presence should not result in a diagnosis of osteomyelitis. Such colonies are more commonly an incidental rather than a significant finding. Thus, owing to the multiple reasons earlier, the proper diagnosis of periapical cyst requires radiographic and/or clinical corroboration.
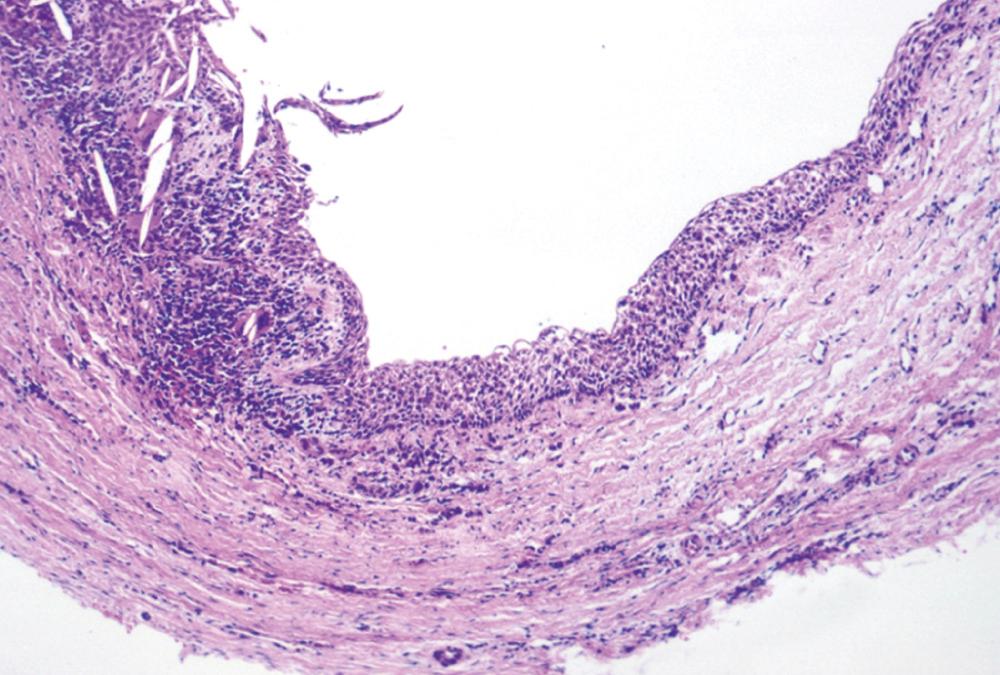
This cyst is treated with simple enucleation ( Fig. 88.3 ). Enucleation is often accomplished at time of tooth extraction. Uncounted numbers of these cysts are probably adequately resolved with endodontic therapy. If a radiolucency persists greater than 6 months following endodontic therapy, enucleation and histopathologic review are necessary.
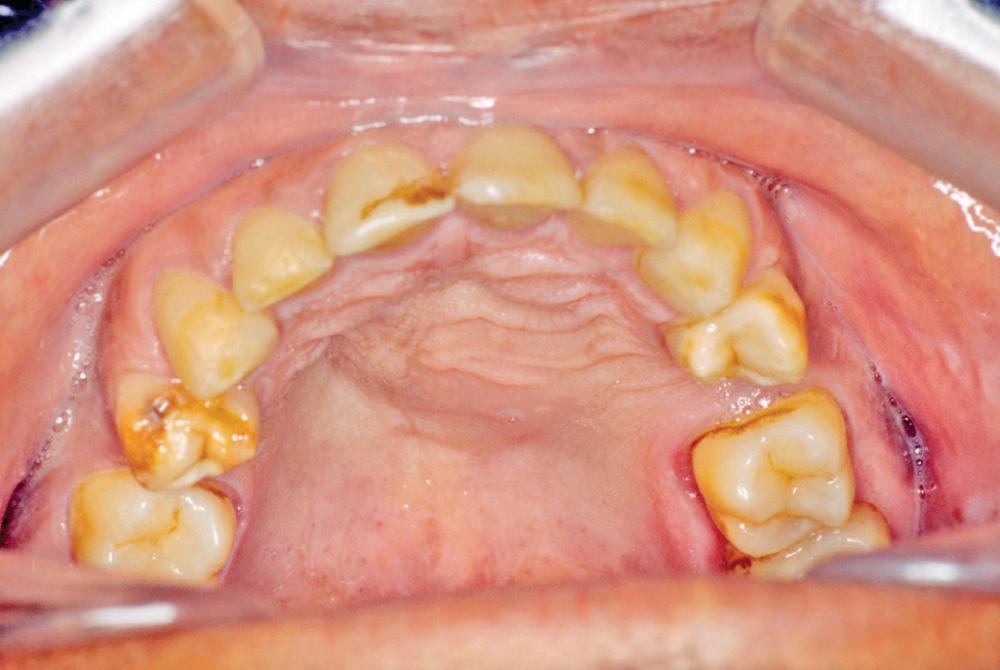
This cyst is simply a variant of the periapical cyst. It is associated with a nonvital tooth but instead of being at the apex of the tooth, the cyst is located lateral to the tooth root(s). This happens because the root canal system of teeth sometimes has exits on the lateral aspect of the root. Not just at the apex. Therefore if the path of least resistance is through one of these lateral canals, the lesion will be present laterally. Otherwise the clinical, microscopic, radiographic, and histologic features are identical to the periapical cyst.
The majority of these cysts will be the result of leaving a periapical cyst “behind” following tooth extraction. All of these cysts are inflammatory cysts. Occasionally an inflamed dentigerous cyst is incompletely removed and could also be the source of a residual cyst. The clinical, microscopic, radiographic, and histologic features are identical to the periapical cyst.
This may or may not be considered by some to be a true cyst. However, because it is an occasionally used diagnosis, a quick summary is included here. This lesion is associated with periodontal disease of a vital tooth. Uncommonly, a deep intrabony periodontal pocket may be sufficiently isolated to allow for hydraulic expansion of the bone ( Fig. 88.4 ). As such, radiographically there will be a radiolucent periradicular lesion ( Fig. 88.5 ). There will also be a periodontal pocket associated with that radiolucency. This diagnosis should be limited to those cases where the clinician indicates the diagnosis as the most likely choice. Otherwise the clinical, microscopic, radiographic, and histologic features are identical to the periapical cyst.
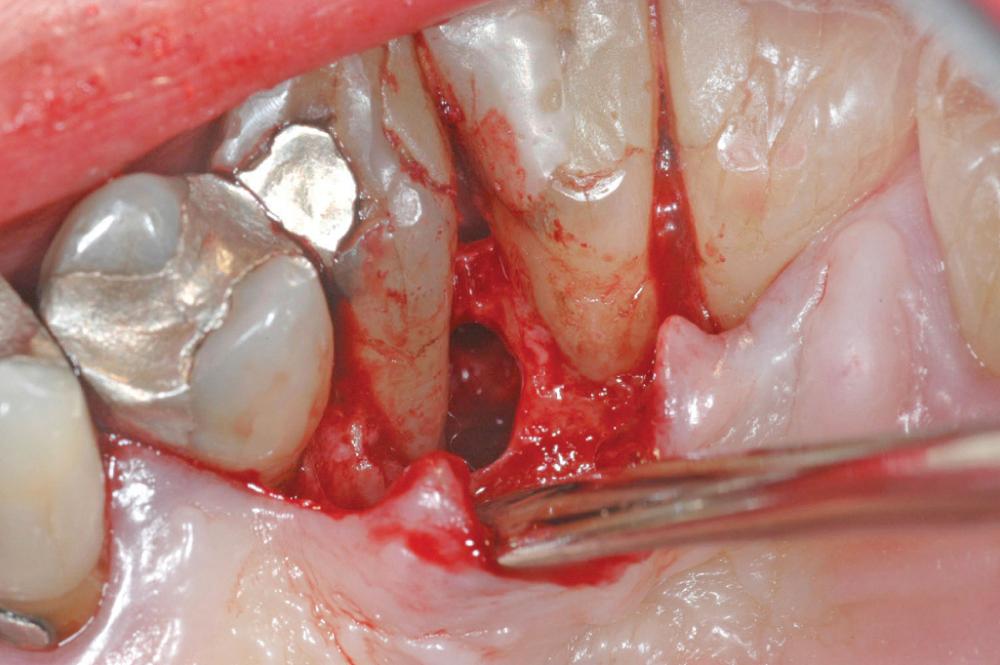
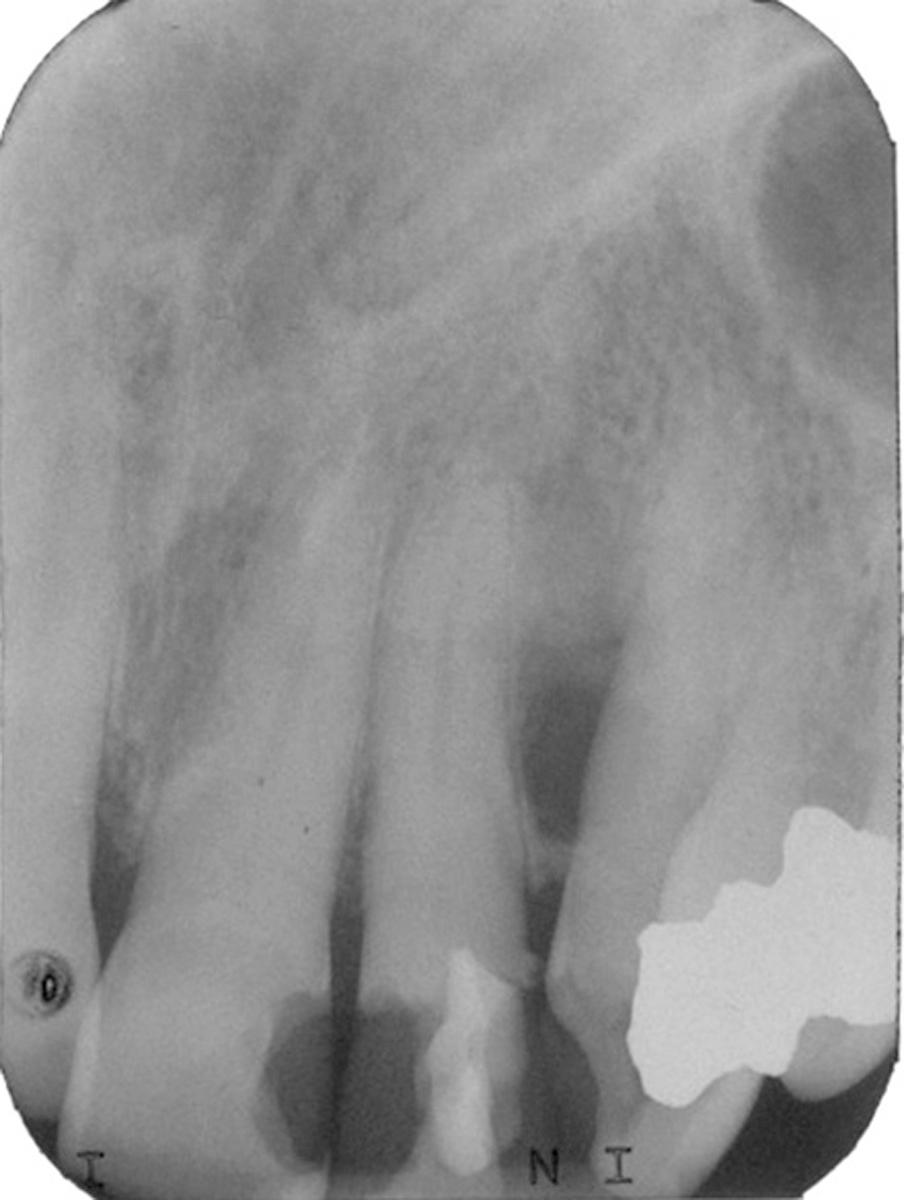
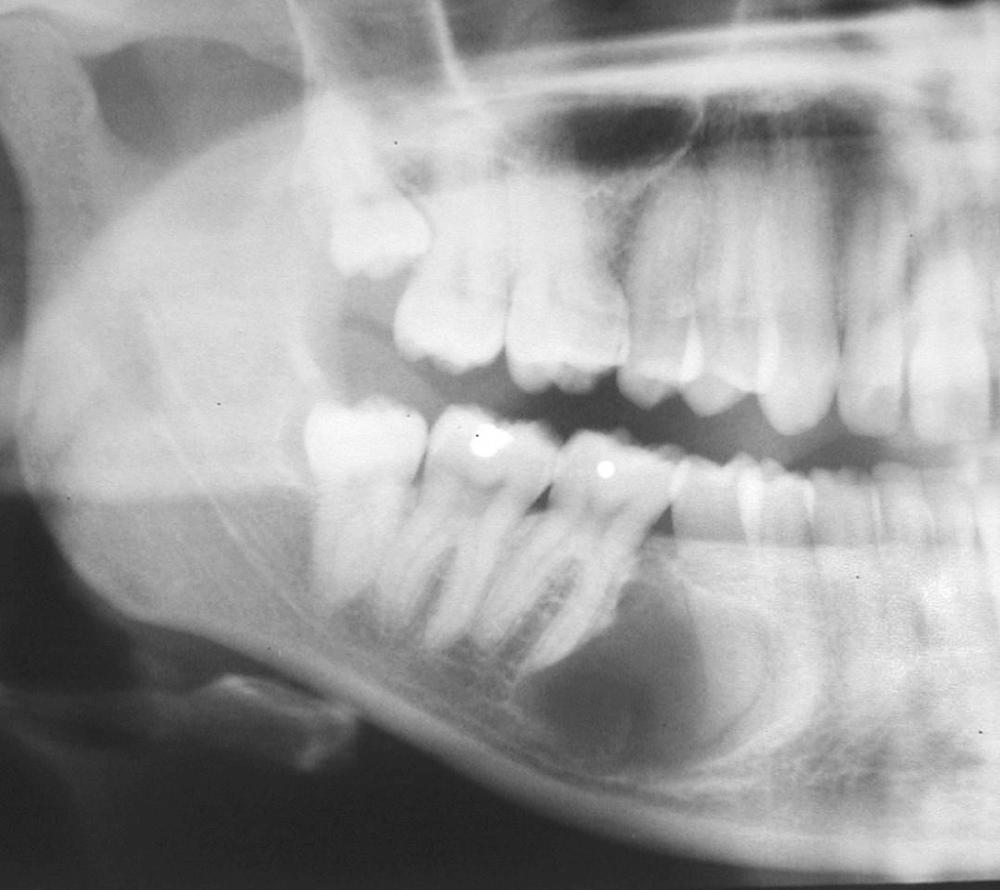
The dentigerous cyst by definition must be associated with the crown of an unerupted tooth, developing tooth, or odontoma. The eruption cyst is essentially a subtype of dentigerous cyst that is confined only by the overlying alveolar mucosa. Dentigerous cysts form when fluid accumulates between reduced enamel epithelium and tooth crown. As alluded to earlier, the accumulation of fluid may be partially or largely surrounded by connective tissue as well as epithelium. Because the third molars and maxillary canines are the teeth most frequently impacted, they are also the most likely to be associated with dentigerous cysts. However, any impacted tooth has an increased risk. There also may be inherent differences in impacted tooth development and how the reduced enamel epithelium is transformed/resorbed. They are generally found in the teenage years and early adulthood. However, the longer a tooth is impacted, the greater the chance of developing a dentigerous cyst.
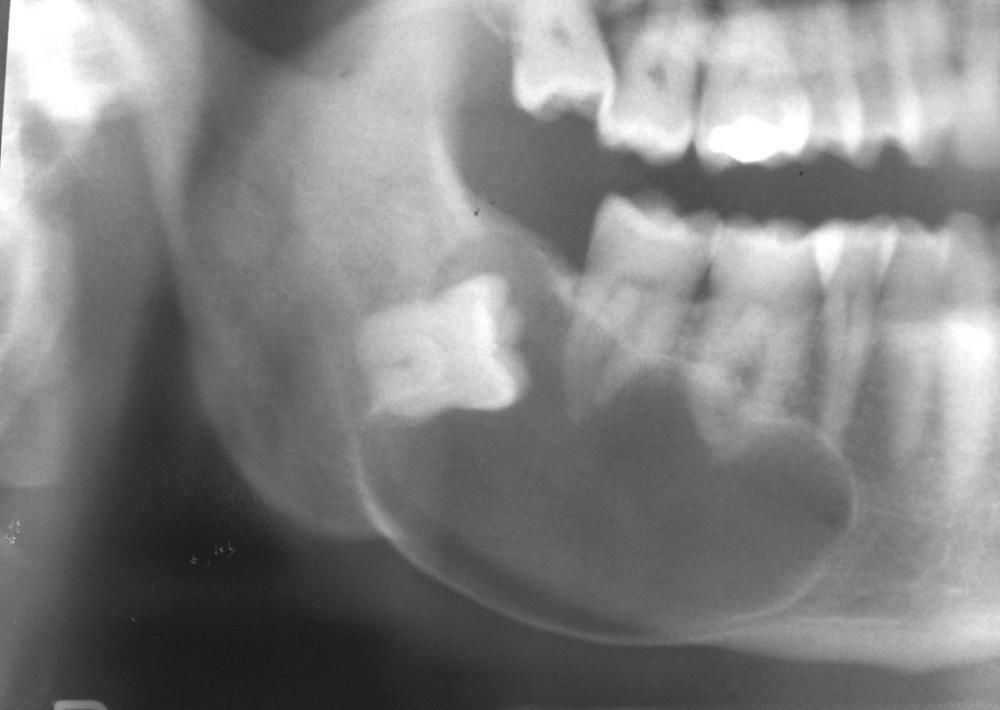
A dentigerous cyst presents as a unilocular radiolucency, which is associated with an unerupted tooth ( Fig. 88.6 ). Dentigerous cysts may also involve odontomas, which by their nature also have “tooth crowns.” The radiolucency is generally well demarcated and well corticated. The border may become sclerotic or display rarifying osteitis if secondary infection is present. Even large cysts that have pushed the associated tooth considerable distances will display evidence of origin from the cementoenamel junction if the film angle is adequate. In large lesions the origin from the cementoenamel junction is best visualized as an area of cortication at the cementoenamel junction. There is considerable overlap between the appearance of small dentigerous cysts and hyperplastic follicles.
The specimen will present primarily as variably dense fibrocollagenous connective tissue with some areas being loose and myxomatous. Odontogenic epithelial rests are usually scattered within the connective tissue and are most common near the epithelial lining. The luminal lining consists of nonkeratinized stratified squamous epithelium. The presence of mucous prosoplasia within the lumen is not uncommon. Care should be taken to not overinterpret the mucus prosoplasia. Cholesterol slits and their associated multinucleated giant cells may be present in inflamed cysts and when seen are generally associated with the connective tissue wall. As mentioned earlier, the lumen may by partially or mostly lined by connective tissue. If present in the specimen, crevicular epithelium may make microscopic separation of an inflamed dentigerous cyst from pericoronitis impossible. Thus proper diagnosis requires radiographic or clinical corroboration.
Dentigerous cysts appear to retain the ability to transform into true neoplasms. One study reported that 17% of ameloblastomas were associated with an existing dentigerous cyst. However, this figure varies by study. Both squamous cell carcinomas and mucoepidermoid carcinomas have been reported in association with dentigerous cysts.
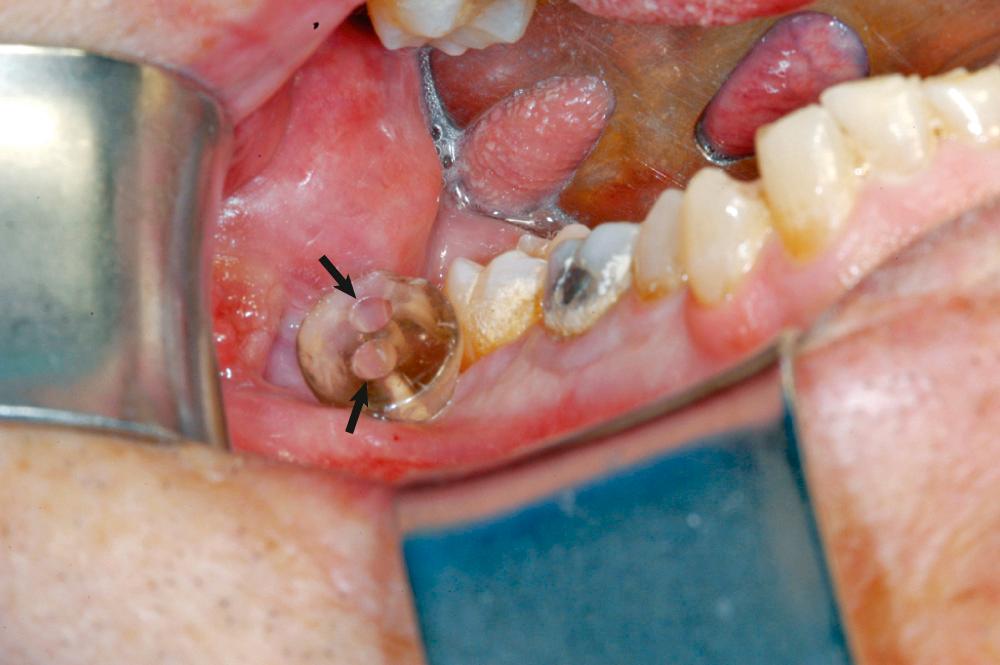
Dentigerous cysts are usually easily enucleated at the time of tooth extraction. In large lesions, decompression with subsequent enucleation may be appropriate ( Fig. 88.7 ).
The eruption cyst is a form of dentigerous cyst that is found in the soft tissue overlying an erupting tooth. Because by definition it must be associated with an erupting tooth, eruption cysts occur only during the ages of tooth development. They may be seen with erupting deciduous or permanent teeth, but the majority of lesions are seen in the first decade. The lesion will present as a soft tissue swelling of the alveolar ridge overlying an area of age-appropriate tooth development. Some eruption cysts will have a slightly blue hue color, although the normal coral pink color of the surrounding mucosa is common ( Fig. 88.8 ). Unlike dentigerous cysts, it is not uncommon for an eruption cyst to be associated with deciduous teeth. Eruption cysts may be seen in newborns with an incidence of 2 per 1000 births reported. Clinical follow-up may also serve to confirm the diagnosis as the tooth will erupt within several weeks to months through the cystic expansion.
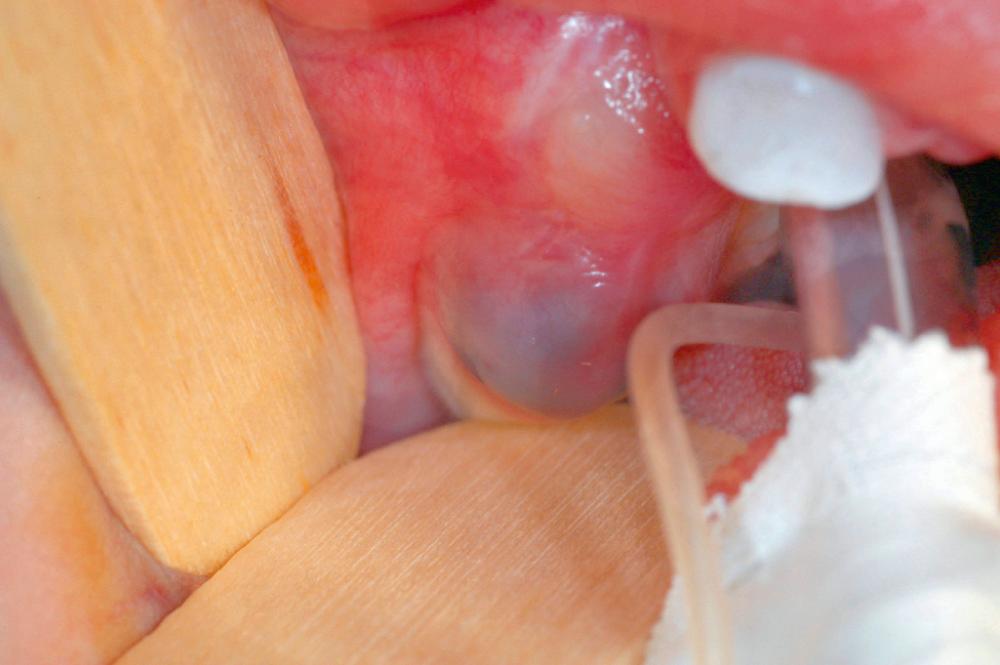
The eruption cyst almost always resolves without intervention. In uncommon cases eruption of the underlying tooth may be impeded or delayed. In such cases a “deroofing” will allow eruption.
The paradental cyst is considered by some to be a variant of the dentigerous cyst. This is because the various forms of paradental cyst all originate from the cementoenamel junction just like the dentigerous cyst. However, the paradental cysts are almost uniformly inflamed so they are generally classified as inflammatory cysts rather than as developmental cysts. Paradental cysts occur on the buccal or distal aspect of an erupted mandibular molar. Although the mesial aspect of a mandibular tooth may rarely be involved, there have been no reported occurrences to the lingual. Craig reported the occasional presence of developmental enamel projections near the furcation of some teeth. This is particularly true of the subcomponent of paradental cysts known as infected buccal bifurcation cysts. How big a role these projections play in pathogenesis remains debatable. In one series the paradental cyst accounted for 3% of all odontogenic cysts.
The lesion will present as a well-defined radiolucency associated with but not surrounding the coronal area of a partially erupted tooth ( Fig. 88.9 ).
It is with great regret that another diagnosis is added to the list of odontogenic cysts but there appears to be no good alternative. On occasion the clinician and pathologist may come upon a lesion that is not classifiable by histologic, radiographic, or clinical features. As mentioned earlier, there are several odontogenic cysts that have identical microscopic features. Odontogenic cysts with identical or potentially identical histologic features are referred to as the “common odontogenic cysts.” These “common” cysts are separated on the basis of clinical features alone.
An odontogenic cyst of undetermined origin is a unilocular radiolucent cyst of the jaws with histologic features of common odontogenic cysts but lacking the clinical, histologic, and radiographic features of any defined common odontogenic cyst.
The problem in diagnosis comes when a cyst is not associated with the crown of a tooth, residually, or with the root of a nonvital tooth. Additionally, the cyst is not consistent with a fissural cyst but yet the cyst is at least partially located in the alveolar process. Historically, any cyst that occurred in an area where a tooth should have developed, or where supernumerary teeth could occur, was called a primordial cyst. This term was used to allude to development from the tooth primordium. Unfortunately this was proposed in 1945 before delineation of the features defining the KCOT and a large percentage of these primordial cysts had features of what would now be diagnosed as a KCOT. When the odontogenic keratocyst (as it was then known) was defined in the 1950s some pathologists had already recognized those histologic features in what they termed primordial cysts and thus they began to use the term interchangeably with odontogenic keratocysts too. International journals especially came to use the term “primordial cyst” as a synonym of the odontogenic keratocyst. Americans often avoided the term primordial, or left the moniker of primordial cyst for those lesions without features of a keratocyst.
The term primordial cyst is avoided to eliminate any confusion between the histopathologist and the clinician. It is hoped that this will ensure the more aggressive treatment of a KCOT is NOT performed for this innocuous lesion.
These cysts are thought to be derived from the dental lamina and are thus thought to retain some limited neoplastic growth potential. This limited neoplastic potential is best displayed by the histologically similar lesion known as a botryoid odontogenic cyst. Lateral periodontal cysts are located on the lateral surface of a vital tooth. This assumes that the tooth has not been rendered nonvital by dental caries or trauma unrelated to cyst formation. The most common location is the mandibular premolar/canine area. If present in the maxilla, the lateral incisor area is the most common location. However, the lateral periodontal cyst may be seen in any area of the alveolar processes. This cyst is seen in the interproximal area between tooth roots and is usually an incidental radiographic finding. Demographically the cyst is most common in males by a 2:1 ratio with a peak incidence in the fifth and sixth decades. The gingival cyst of the adult and the botryoid odontogenic cysts are essentially subtypes of lateral periodontal cysts. Other cysts also present interproximally, especially the KCOT, odontogenic cyst of undetermined origin, and the lateralized periapical cyst. Histopathologic features and tooth vitality are important diagnostic considerations to separate these lesions. All of these lesions can be separated histologically and the lateralized periradicular cyst will be associated with a nonvital tooth.
The lesion presents as a unilocular radiolucency of the alveolus that is usually well corticated. Larger lesions may result in diverged roots. Multilocular lesions are a special subset and are classified as botryoid odontogenic cysts (see section on botryoid odontogenic cysts ).
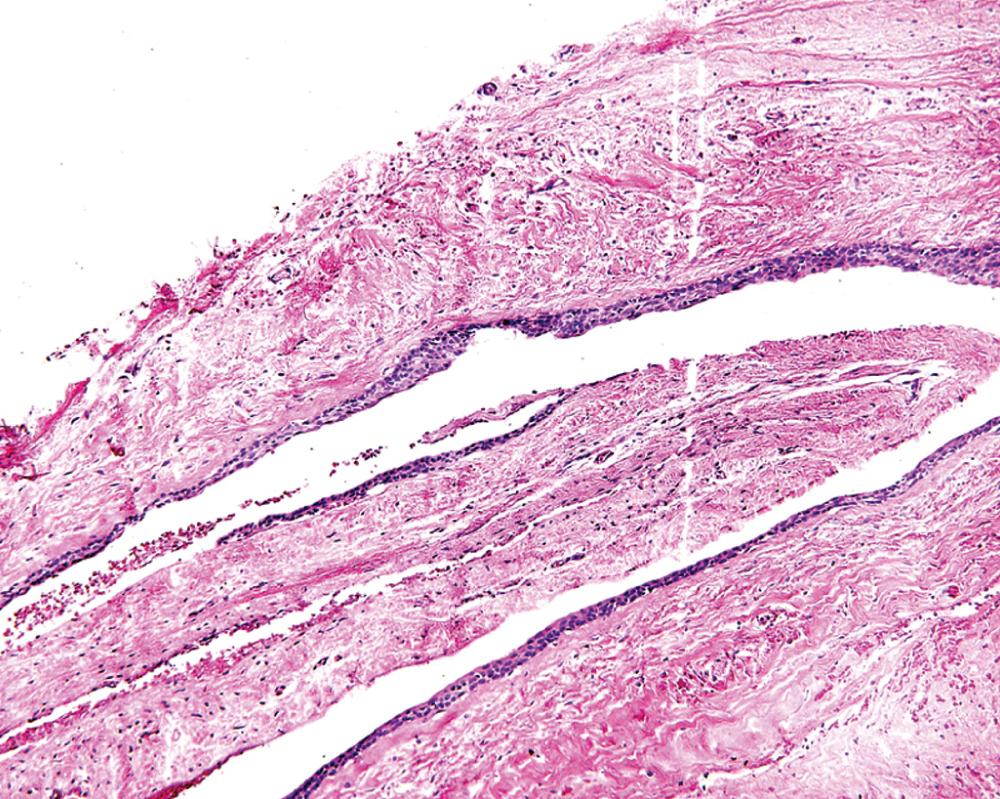
The cyst lining is composed of nonkeratinized simple to stratified squamous epithelium. The lining is most notable for being only a few cells in thickness ( Fig. 88.10 ). Intermixed within this otherwise thin epithelial lining are nodular epithelial thickenings or plaques. The plaques may display somewhat whorled epithelial cell aggregates. The central cells in the aggregate may classically display cytoplasmic clearing. The clear cells contain glycogen, which can be demonstrated by staining with periodic acid–Schiff with and without diastase digestion. Cells containing glycogen will not retain Schiff positivity after diastase digestion, thus confirming that they contained glycogen. The diagnosis of lateral periodontal cyst must be reserved for lesions displaying the thin epithelium described earlier. The plaque-like thickenings are helpful microscopic features but are not always found. The connective tissue wall may contain dental lamina rests but these are not required for diagnosis.
These cysts are treated with simple enucleation, although the surgeon is reminded to look for intraoperative multilocularity, which may not have been evident radiographically. If multilocularity is present, the lesion is probably a botryoid odontogenic cyst. In such cases light curettage of the surrounding bone wall is advised. Recurrence of the simple lateral periodontal cyst is not a problem and even the recurrences of botryoid cysts are usually easy to manage.
The botryoid odontogenic cyst will usually present radiographically as a multilocular lesion and is a special variant of the lateral periodontal cyst. Botryoid refers to the grape-like cluster these lesions may resemble both histologically and radiographically.
Radiographically they present in the same preferred alveolar process locations as the lateral periodontal cyst. Small locules may not be seen on radiographs and will need to be detected intraoperatively or histologically.
Like the lateral periodontal cyst, treatment consists of enucleation with bony curettage. However, the multilocular nature may make this difficult; this is especially true of large lesions and if teeth are to be spared. With full enucleation and bone curettage/peripheral ostectomy recurrence should be uncommon. The slow growth of these lesions suggests that a 10-year radiographic follow-up is reasonable, although the small sample size makes this a suggestion at best.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here