Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Miscarriage, fetal growth restriction, and preeclampsia are pathologies generally arising from events that go awry in the early developmental period. Most fetal malformations arise in early embryogenesis. Thus to accurately diagnose and design effective treatments for these conditions, an understanding of early human development is essential. Normal early placental and embryonic development requires a complex sequence and array of signaling pathways, cell-cell communication, and decidua-embryo and maternal immune cell–embryo crosstalk. This chapter reviews the earliest developmental events of the embryo and placenta.
After fertilization, the zygote moves through the fallopian tube to the endometrial cavity for implantation, a process that takes ∼7 days (0–7 days postfertilization). During this time, many events occur in both the zygote and the endometrium to permit successful continuation of the pregnancy. In the zygote, the earliest events occur under relative transcriptional silence, utilizing maternally-derived “stored” proteins and mRNAs (maternal messenger RNAs), which mediate the first two cleavage divisions. In the human embryo, zygotic control of cell divisions commences with the third cleavage division, at the four- to eight-cell transition, and is termed embryonic genome activation (EGA). Although the zygotic genome is biparental, the contributions to the early zygote from each parent are not equal: a phenomenon called imprinting silences transcription from one or the other parent’s allele in select genes, a process with significant implications for early human development, discussed in more detail later in this chapter.
Following the third cleavage division and EGA, at 3 days postfertilization, the zygote begins to undergo compaction to form the morula, taking the first morphological steps toward loss of radial symmetry. At this stage, cell junctions form, and positional cell polarity begins to develop, with inner and outer cells becoming distinct. Further changes in morphology and gene expression lead to the outer cells becoming trophectoderm (TE) and the inner cells forming the inner cell mass (ICM). Recently, keratins have been identified as one of the earliest asymmetrically inherited fate determinants that play a key role in the specification of outer cells as TE. While TE cells are the progenitor of all trophoblast cells (epithelial component) of the placenta, the ICM gives rise, not just to the embryo proper, but also to extraembryonic/primitive endoderm (yolk sac) and extraembryonic mesoderm, which forms the mesenchyme and fetal vascular components of the placenta.
An early factor expressed in the zygotic genome is POU homeodomain class 5 transcription factor 1 (POU5F1, also known as octamer-binding transcription factor 4 [OCT4]) ( Box 3.1 ), a critical protein involved in maintenance of pluripotency. In the early human zygote, all cells express POU5F1 (OCT4) up to, and including, the early blastocyst stage. The first lineage specification factor expressed in the outer cells of the compacted morula is caudal-type homeobox transcription factor 2 (CDX2), which is coexpressed with POU5F1 (OCT4) in cells destined to become TE. ,
CDX: Caudal-type homeobox transcription factors, important in trophoblast fate determination and later in intestinal epithelial cell differentiation.
HOX: Homeobox-containing transcription factors highly conserved evolutionarily and across species. The HOX gene complex provides specific regional distinction of the anterior-posterior axis of vertebrate embryos. HOX genes are also important in specific organogenesis (e.g., the gynecologic tract ) as well as limb, hindbrain, and gut patterning. Mutations, misexpressions, or deletions of HOX genes are often lethal but can also result in viability with often severe malformations (for review, see Goodman and Scambler ).
GATA: Family of zinc finger transcription factors that bind GATA motifs. GATA proteins are important in formation of mesendoderm and endoderm, hematopoiesis, heart and lung development, gonadal development, and T-cell differentiation.
NANOG: A homeodomain transcription factor key in maintaining inner cell mass pluripotency.
POU/OCT: Homeodomain and octamer domain transcription factors with pivotal roles in embryogenesis, neurogenesis, metabolism, and immunity.
SOX: Sex determining region Y (SRY)–related high-mobility group (HMG)-box transcription factors conserved throughout eukaryotic species. Important in many developmental regulatory events, including sex determination, neuronal development, and lung and gut patterning.
TBX: T-box containing transcription factors. The genes (about 17) in the TBX family produce proteins that are essential for the embryonic development of mesodermal derivatives, including the extremities and the fetal heart. TBX genes have been implicated in human malformation syndromes and defects, including DiGeorge, Holt-Oram, and some forms of cleft palate (for review, see Packham and Brook ).
BMP: A family of growth factors/cytokines that work as morphogens regulating patterning throughout development. Their importance during development is exemplified by the fact that mutations in bone morphogenetic proteins (BMPs) either are embryonic lethal or cause major malformations. ,
FGF: A conserved family of secreted growth factors (heparin-binding proteins) with 22 family members in humans. Fibroblastic growth factors (FGFs) have multiple roles in embryonic development, including cell fate determination, angiogenesis, branching morphogenesis, and limb patterning.
SHH: A critical evolutionarily conserved signaling molecule of the hedgehog family. Sonic hedgehog (SHH) is important in many aspects of development as a morphogen, including left-right asymmetry, anterior-posterior axis formation and mesodermal morphogenesis, axial segmentation and limb, gut, neural, and lung development. , Alterations of SHH, or its pathway, are the cause of many cases of holoprosencephaly and its associated cyclopia.
WNT: A family of signal transduction proteins important in body axis patterning, cell fate determination, cell migration and proliferation, and regeneration. , Wingless-type MMTV integration site family (WNT) proteins play an important role in gynecologic tract development, the endometrial cycle, uterine receptivity, blastocyst attachment and implantation, and trophoblast invasion (for review, see Nayeem and colleagues ). WNT proteins function through canonical (via nuclear β-catenin) or noncanonical pathways. ,
The developing embryo enters the uterine cavity on or about 4 days postfertilization and, shortly before it enters the uterus, undergoes cavitation to form the blastocyst. Although cell fate determination of the TE lineage likely begins at the compacted morula stage, morphologic differentiation of a human ovum into embryonic and TE cells first occurs in a 58-cell blastocyst, around 5 days postfertilization, as first described by Hertig and most recently confirmed by single-cell transcriptome analysis. In the early blastocyst (5–6 days postfertilization), one can identify the outer shell of TE and the ICM ( Fig. 3.1 ). By the late blastocyst stages (7–8 days postfertilization), the ICM further differentiates into the primitive endoderm/hypoblast (PrE, located nearest the blastocyst cavity) and the epiblast (see Fig. 3.1 ). The PrE goes on to form the yolk sac, while epiblast is the precursor to both the embryo proper and extraembryonic mesoderm.
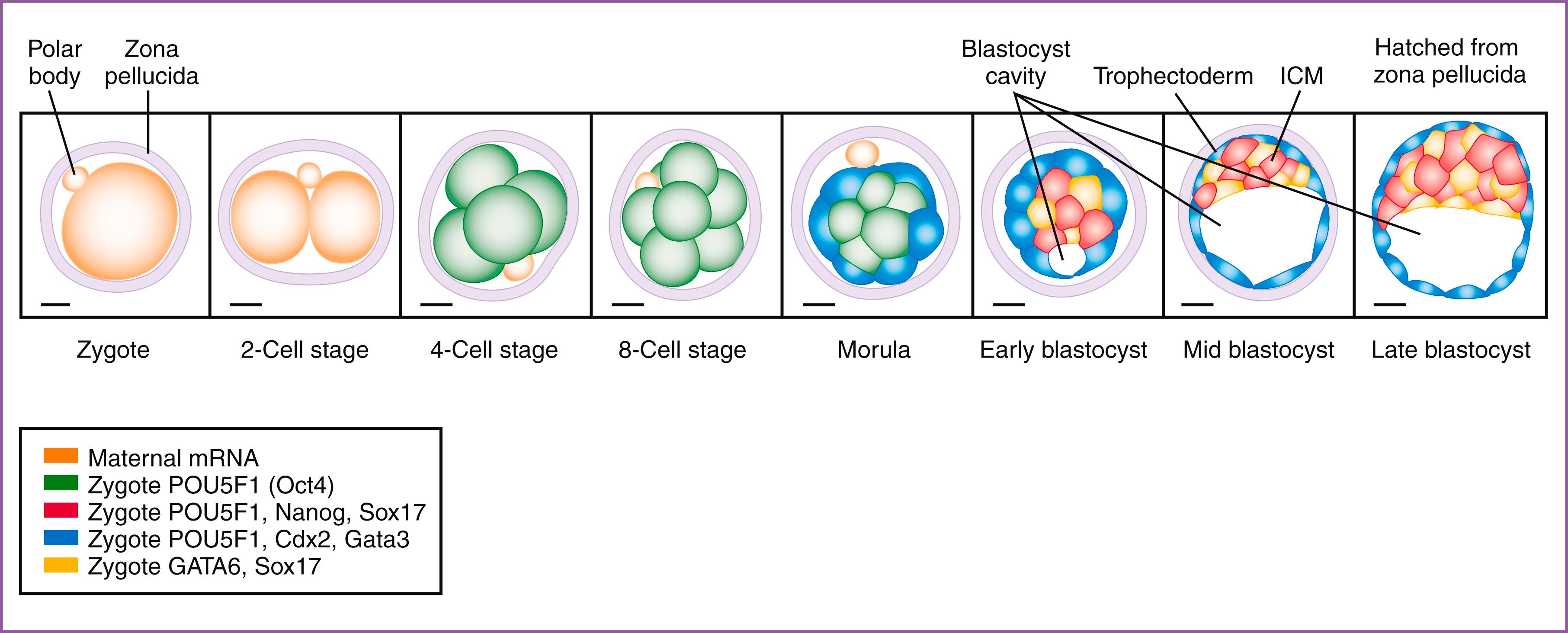
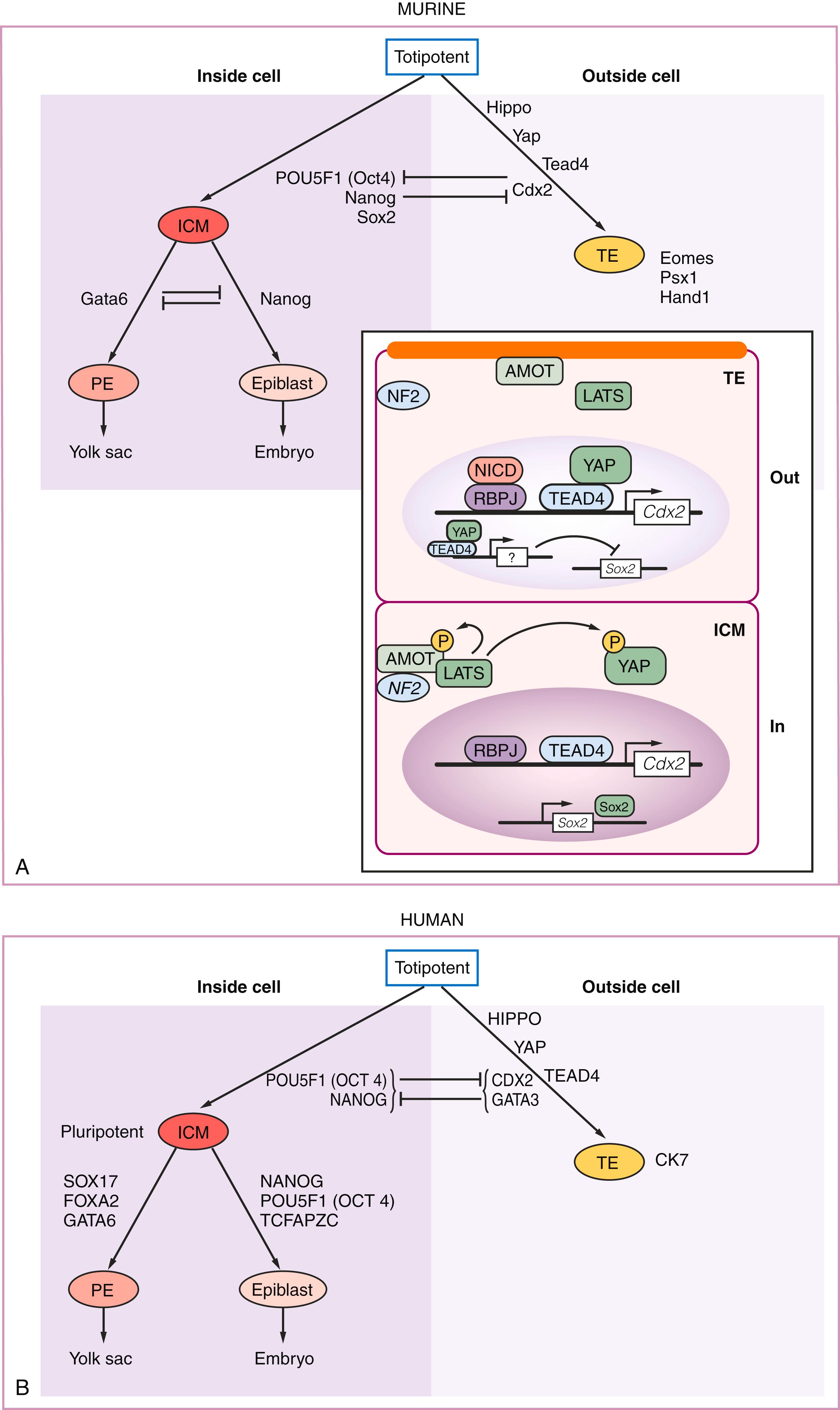
The molecular controls of these preimplantation events have been best described in the mouse, but with the advent of single-cell analysis , and recent advances in long-term culture of human embryos, , significant differences between murine and human embryogenesis have been identified, which deserve mention here. , For example, in the murine system, the totipotent blastomeres differentiate to TE and ICM by restricted expression of Cdx2 and Pou5f1 (Oct4) transcription factors, respectively. Along with Pou5f1, Nanog (a homeodomain transcription factor) and SRY- box 2 (Sox2, an SRY-related HMG-box transcription factor) (see Box 3.1 ) are coexpressed in the ICM, all of which are necessary to maintain pluripotency. The inner cell mass then differentiates to the epiblast and primitive endoderm by restricted expression of the transcription factor GATA binding protein 6 (Gata6) to the primitive endoderm ( Fig. 3.2 ). In the human embryo, however, while some of these same factors are expressed, significant differences have been identified. For example, POU5F1 (OCT4) is expressed in all cells of the human blastocyst, including those of the TE, through 6 days postfertilization, , , where it may play a role in establishment of this lineage. Compared to mouse TE, human TE is defined by low levels of CDX2 expression, but significantly higher levels of another transcription factor, GATA3. , In addition to POU5F1 coexpression with low CDX2 levels, the human TE also lacks expression of ELF5 and EOMES, two transcription factors involved in TE lineage identity and maintenance in the mouse. Perhaps as a result of all these differences, human TE has been shown to remain non–lineage-restricted with the ability to contribute to NANOG + inner embryonic cells in a reaggregation assay. At 7–8 days postfertilization, the human PrE expresses GATA6, as in the mouse, but also strongly expresses SOX17.
Recent studies have shown that all the above events, which occur through 10 days postfertilization in vivo, can progress relatively normally, in the absence of maternal cues, in vitro. , This is an important finding, particularly since in vitro fertilization practice now favors later stage embryo transfers. , It appears that implantation rates are superior with transfer of blastocyst-stage embryos, compared to earlier cleavage- and morula-stage embryos. ,
Development to the blastocyst stage occurs within the zona pellucida, a semiporous specialized “shell” that functions to hold the blastomeres of the early cleavage embryo together. Nevertheless, the maternal environment can still affect preimplantation development via secretions from the epithelia of the fallopian tube and endometrium. These histotrophic agents are important for the early zygote and must penetrate the zona pellucida.
Following arrival within the uterine cavity, at approximately 6–7 days postfertilization, the late-stage blastocyst hatches from the zona pellucida, orients itself with polar TE (TE cells closest to the ICM) apposed to the luminal endometrial epithelium, and adheres to and then implants into the endometrium ( Fig. 3.3 ). This process involves coordinated endometrial and trophoblastic differentiation and signaling among TE, endometrial epithelium, maternal immune cells, and specialized endometrial stromal cells—the decidua.
Once the blastocyst is formed and hatches from the zona pellucida, adhesion molecules and their receptors from endometrial epithelial projections (pinopodes), and from the microvilli or atypical podosomes of the TE, interact to facilitate attachment. The process of attachment requires the endometrium to be “receptive.” Receptivity is a process by which the proliferating endometrium undergoes a marked transformation to a nonproliferative tissue rich in secretory factors necessary for blastocyst survival. In menstruating humans, endometrial receptivity is a function of a cyclic response to the ovarian hormones progesterone (P4) and estradiol (E2) in the midluteal phase of the menstrual cycle. The endometrial response to the major surge in P4 and minor surge in E2 results in decreased proliferation of the endometrial stromal and epithelial cells, the marked transformation of the stromal cells to large specialized secretory cells called decidua, and an influx of specialized immune cells. This unique cellular milieu depends on autocrine and paracrine signaling among the cells and interaction with the hatched and activated blastocyst. This receptive endometrium is short lived, and the “window of implantation” is only a couple of days in the human.
Studies have shown that epithelial-to-mesenchymal signaling is critical for development of the receptive state of the endometrium. P4 hormone exposure induces several specific epithelial and stromal events leading to decidualization and endometrial receptivity ( Table 3.1 ). The specialized decidualized stromal cells express many factors important in conferring receptivity, including prolactin and insulin-like growth factor–binding protein 1 (IGFBP-1). One important P4-regulated transcription factor is cyclooxygenase-2 (COX2) which is also influenced by human chorionic gonadotropin (hCG). The COX2 protein is an enzyme important in synthesis of prostaglandins. COX2 is expressed in the endometrial epithelium and subepithelial stroma. COX2 is also important in inducing angiogenesis in receptive endometrium required for normal implantation [reviewed in Staun-Ram and Shalev ]. Another critical factor in inducing endometrial receptivity is a member of the hedgehog family of secreted proteins, Indian hedgehog (IHH), expressed in the endometrial epithelium. Epithelial IHH induces expression of chicken ovalbumin upstream promoter transcription factor 2 (COUP-TFII) in the stromal cells. This signal transduction pathway leads to stromal cell expression of bone morphogenetic protein 2 (BMP2) and wingless-type MMTV integration site family, member 4 (WNT4), both secretory factors necessary for receptivity (reviewed in Large and DeMayo ). Also essential is the expression of two members of the homeobox (HOX) family of transcription factors, HOXA10 and HOXA11. These HOX proteins are required for decidualization, and their expression is regulated by E2 and P4. Interestingly, HOXA10 and HOXA11 are embryologically important in uterine formation as well (reviewed in Taylor and coworkers ).
| Factor a | Expression Location | Effect on Endometrial Receptivity |
|---|---|---|
| Estrogen- And/Or Progesterone-Regulated Factors | ||
| IHH | Epithelium | Paracrine signal for epithelial-stromal interaction, stromal cell proliferation |
| HAND2 | Stroma | Important for decidualization; downregulates epithelial differentiation |
| LIF | Epithelium/lumina | Signals to the blastocyst and endometrial epithelium for uterine receptivity and implantation |
| Non–Estrogen/Progesterone-Responsive Factors | ||
| MSX1 | Epithelium | Increased expression during window of implantation; upstream activator of stromal BMP2 expression and inhibitor of WNTs |
| BMP2 | Stroma | Required for decidualization; also plays a role in embryo spacing (may be a clue to placenta previa) |
| LKF5 | Epithelium to stroma | Downregulated in epithelium at implantation and upregulated in stroma with decidualization; role in implantation |
| HOXA10, HOXA11 | Stroma | Crucial for decidualization |
a BMP2, Bone morphogenetic protein 2; HAND2, heart- and neural crest derivatives–expressed protein 2; HOXA, homeobox protein HOXA; IHH, Indian hedgehog; KLF5, Kruppel-like factor 5; LIF, leukemia inhibitory factor; MSX1, muscle segment homeobox 1.
Receptivity is also marked by expression of integrins. These transmembrane proteins, important in cell-cell communication, are expressed in response to the E2-responsive leukemia inhibitory factor from the endometrial epithelium. Leukemia inhibitory factor (LIF) is a critically important factor in the process involved in generating endometrial receptivity as well as inducing factors necessary for attachment and implantation. Other factors important in endometrial receptivity are highlighted in Table 3.1 .
One of the important processes in uterine receptivity is the interaction of the endometrium and trophectoderm with specialized immune cells in the decidua (for reviews, see Sharma and Robertson and Moldenhauer ). The normal decidua contains multiple groups of beneficial immune cells, including decidual natural killer (dNK) cells and T-regulatory (Treg) cells. The dNK cells are unique cells that are either recruited from peripheral blood and converted to decidual NK cells by decidualized stromal cell secretion of transforming growth factor-β1 (TGF-β1) and interleukin-15, 30 or are differentiated from tissue-resident (immature) NK cells in situ. The dNK cells provide important controls for trophoblast invasion and remodeling of the uterine spiral arteries (reviewed in Lash and colleagues ) as well as aiding in preventing maternal immune rejection of the hemiallograft zygote (reviewed in Warning and associates ). Recent single-cell analysis of early gestation decidua indicate heterogeneity of dNK cells, suggesting that distinct subpopulations carry out the different functions in vivo. Treg cells are specialized antiinflammatory and immunosuppressive T cells that modulate the immune response. They are increased at the implantation site and mediate tolerance of the semiallogeneic fetus. Deficiencies in both dNK cells and Tregs are thought to play a role in early pregnancy loss. ,
Implantation begins with attachment of the activated blastocyst to the receptive luminal endometrial epithelium (for reviews, see Cha and coworkers and Tu and associates ). This involves the specialized polar TE, cells that border the ICM and have a distinct phenotype ( Fig. 3.3 ), adhering to and then invading into the receptive endometrium. Both E2 and P4 are necessary for the expression of arguably the most important factors for blastocyst attachment, heparin-binding epidermal growth factor–like growth factor (HB-EGF) and its receptors ErbB1 and ErbB4. HB-EGF is expressed in both a soluble and a membrane-bound form by the endometrial epithelium in the pinopodia. Its receptors are expressed on the polar TE of the blastocyst associated with their microvilli/podosomes. HB-EGF–associated binding of the TE to the endometrium is the critical event in blastocyst attachment. HB-EGF plays other roles in implantation, and defects in its regulation are associated with abnormal implantation and preeclampsia. Other factors that also play key roles in the attachment process include leukemia inhibitory factor, , endometrial epithelial HOXA10 and HOXA11, 43 and canonical WNT signaling in the endometrial epithelium. , Blastocyst-to-endometrium signals and epithelial-stromal crosstalk , also function in the initial steps of implantation.
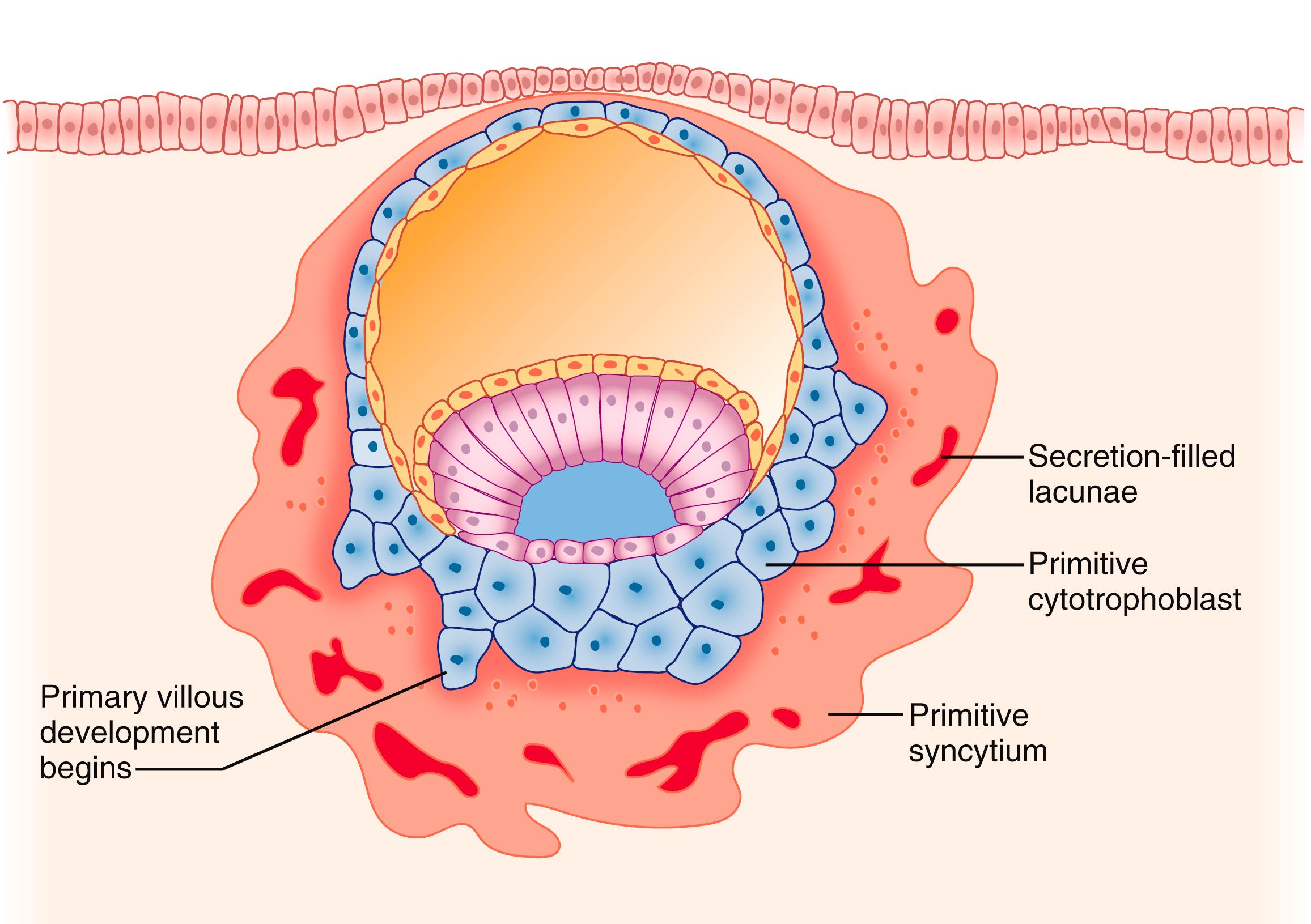
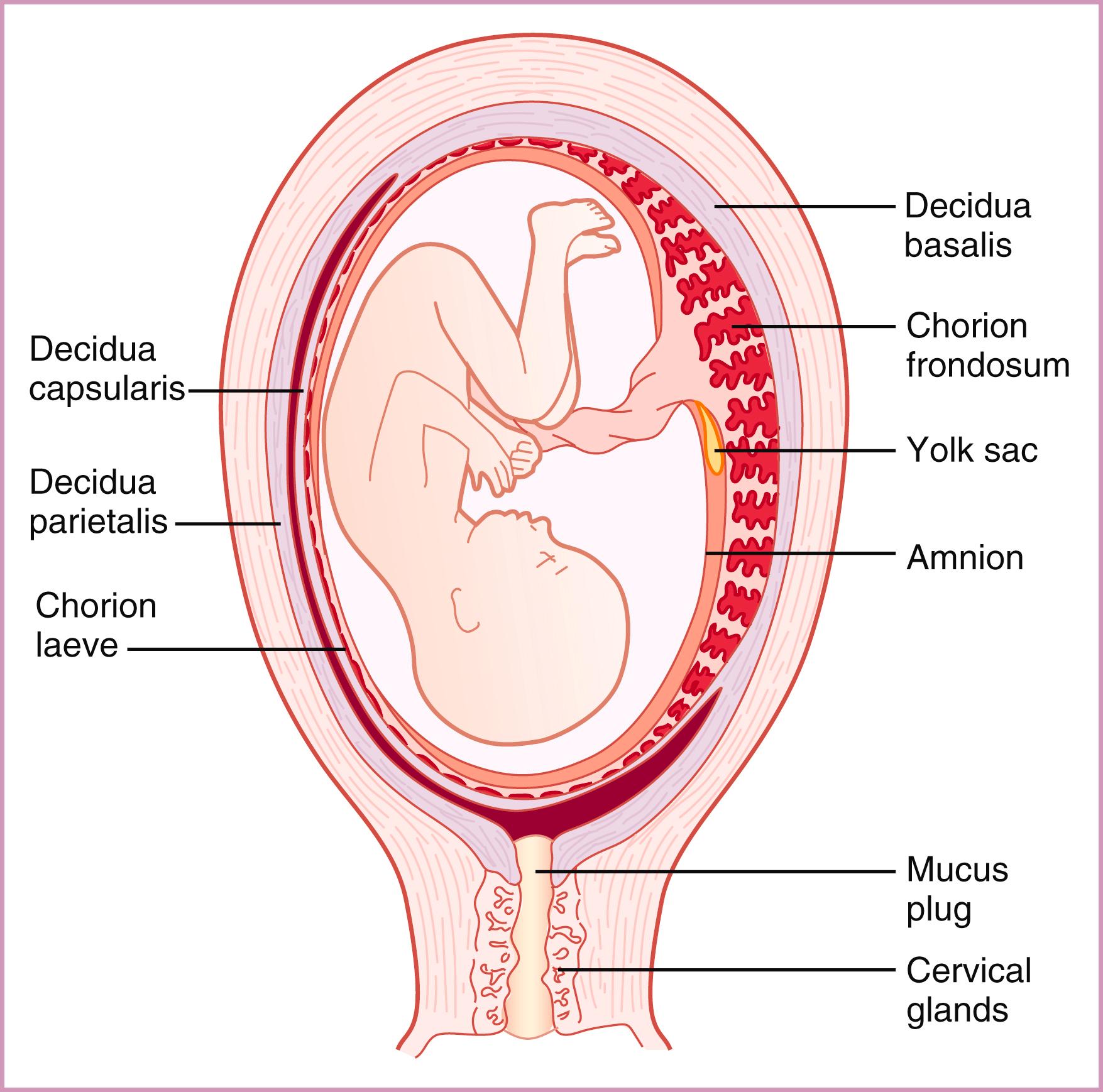
Once attachment has occurred, the cells of the polar TE break through the endometrial epithelium, burrowing underneath and forming a shell of cells, called cytotrophoblast, around the embryo ( Fig. 3.3 ). The cells from the cytotrophoblastic shell subsequently differentiate into primitive syncytium, a unique trophoblast cell type with both invasive and secretory functions (see Fig. 3.3 , and more detail later). The blastocyst continues to invade the endometrium via the primitive syncytium, and a completely interstitial implantation of the blastocyst is usually accomplished by the ninth day of gestation. The entire blastocyst thus comes to assume an interstitial position (i.e., it sinks entirely into the endometrium at the site of attachment). The process may be aided by the collapse of the blastocyst cavity that occurs at this time. The implanted cytotrophoblastic shell encases the blastocyst and comes to be surrounded by endometrium (decidua) on all sides. The portion of decidua lying between blastocyst and myometrium is the decidua basalis; the other portion is the decidua capsularis that eventually fuses with the decidua vera on the opposite side of the uterine cavity to form the decidua parietalis, which in turn becomes part of the chorion laeve ( Fig. 3.4 ) and comes to lie on the outside of the placental membranes.
Once within the endometrium, the blastocyst begins gastrulation (further differentiation and development of the embryo proper). Simultaneously, placental growth proceeds in an accelerated manner (in comparison to embryonic growth), which is required for gestation to proceed. It has been estimated that most pregnancy losses that occur in the first trimester are failures of periimplantation events, resulting from problems either with uterine receptivity or with early placentation itself ; therefore successful completion of these events is necessary for embryonic survival. For this reason, we will first focus on events related to placental development, before discussing gastrulation and embryonic development.
Once the polar TE has attached and the embryo has invaded into the decidua, marked changes begin to occur, starting with formation of the cytotrophoblastic shell. The transition from polar TE to cytotrophoblast involves loss of CDX2 expression, retention of GATA3, and gain of expression of p63 and VGLL1, among other transcription factors ( Fig. 3.5 ). Differentiation of TE cells into cytotrophoblast heralds the establishment of a true trophoblast progenitor cell, which will persist throughout gestation and gives rise to subsequent terminally differentiated and specialized progeny, the syncytiotrophoblast and the extravillous cytotrophoblast (described further later). Although a unique molecular signature for human trophoblast stem cells (TSC) has yet to be defined, Okae and colleagues have developed culture conditions for derivation of such cells from both human blastocyst-stage embryos and first-trimester cytotrophoblast, which can maintain self-renewal long-term in vitro.
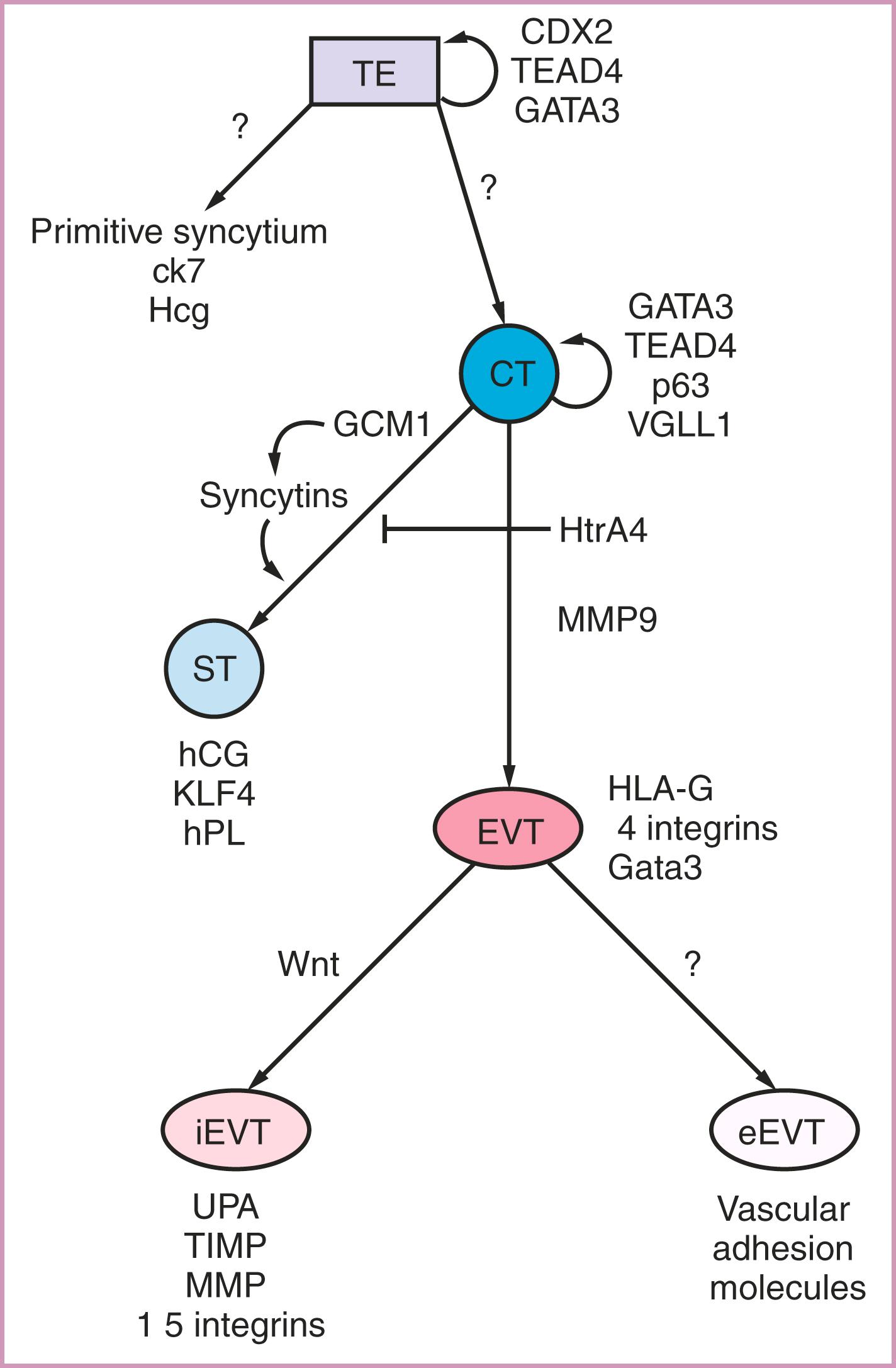
As noted, the cells of the cytotrophoblastic shell also differentiate into a specialized multinucleated, invasive cell type, called the primitive syncytium ( Fig. 3.3 ). These cells secrete enzymes that break down surrounding decidual cells and matrix, leading to development of clefts (lacunae), which later coalesce to form the intervillous space. These lacunae are filled with endometrial glandular secretions, into which the primitive syncytium taps, to provide nutritional support for the recently-implanted embryo, a process referred to as histiotrophic nutrition. Otherwise, very little is known about the formation or function of primitive syncytium, though it is important to distinguish these cells from true syncytiotrophoblast, which, though multinucleated, are noninvasive cells (see later). The primitive syncytium express the trophoblastic hormone hCG and the pantrophoblast marker cytokeratin 7, but not the characteristic marker of invasive extravillous cytotrophoblast, human leukocyte antigen G (HLA-G) (see later; M. Parast, unpublished observations). It is not clear what becomes of the primitive syncytium. Both villous and extravillous trophoblastic differentiation proceeds soon after implantation, as described in the next section.
The self-renewing cytotrophoblast (CTB) progenitor cell can differentiate down one of two pathways, forming either specialized invasive extravillous cytotrophoblast (EVT), or true syncytial cells, the syncytiotrophoblast (STB), which cover the placenta and serve transport and endocrine functions throughout pregnancy. Both of these cell types are discussed in this section; for additional details, see the review by Farah and colleagues.
Within anchoring villi, at the junction with the uterus, CTB progenitor cells give rise to extravillous cytotrophoblast (EVT). The role of EVT is to anchor the gestation and access oxygenated maternal blood for the growing embryo. To achieve these ends, EVT need to invade the maternal endometrium and remodel the endometrial vasculature from muscular vessels to high-capacitance slow-flow vessels. At this early time in gestation, the embryo is growing under low oxygen conditions (<30 mmHg or <3%); thus, the bulk of differentiation from CTB progenitor cells into EVT occurs under physiologic hypoxia. In fact, it has been shown that hypoxia-inducible factor (HIF), a transcription factor which is stabilized under low oxygen tension, plays a key role in specification of this lineage. , Many other pathways are involved in induction, maintenance, and function of EVT, some of which are also induced under physiologic hypoxia (for a review, see Chang and associates ). One such key transcription factor is ASCL2, a helix-loop-helix factor induced under low oxygen, which promotes EVT differentiation while simultaneously inhibiting STB differentiation of CTB progenitor cells. Another key pathway involved in EVT induction is canonical WNT signaling through T-cell factor 4/β-catenin system. Recent studies using first-trimester trophoblast organoids have shown that, while the initial transition from CTB to proximal column EVT (pcEVT) requires turning off WNT signaling, the subsequent maturation of these cells into functionally invasive EVT require this pathway to be turned on. The transition from CTB to pcEVT (immature EVT) to distal column (mature) EVT also involves changes in expression of integrins, which are receptors for various types of extracellular matrix components. Specifically, the integrin switch involves loss of integrins α6 and β4 (receptors for matrix components of the basement membrane), and gain of α5 and α1 (receptors for fibronectin, the primary matrix protein secreted by EVT and matrix component of cell columns and decidua). EVT also express factors important in facilitating invasion, including factors for degrading the extracellular matrix, such as matrix metalloproteinases (MMPs). Molecules such as MMP-9 and plasminogen activators and their inhibitors (tissue inhibitors of matrix metalloproteinases and plasminogen activator inhibitors, respectively) are some of the important factors expressed by these invasive EVT.
EVT can be further subdivided into two sublineages, the interstitial EVT (iEVT) which invade endometrial stroma and the superficial one-third of the myometrium, and endovascular EVT (eEVT) which invade maternal arterioles and replace the endothelium in a process known as vascular remodeling. The eEVT enter the opened lumens of maternal arterioles and penetrate deeply along their endothelial linings, herniating into open maternal vessels ( Fig 3.6A ). This endovascular invasion initially results in obstruction of maternal vessels, and maintains the very low oxygen concentrations in the developing embryo. Hustin and Schaaps offered evidence that these eEVT completely occlude the vessels in early pregnancy, thus allowing only filtrate of maternal blood to enter the intervillous space. This process is critical in human development and if it does not happen appropriately, pregnancy failure results. Significant blood flow to the conceptus does not occur until approximately 10–12 weeks’ gestation (for reviews, see Jaffe and associates and Burton and coworkers ). Thus, as noted earlier, nutritional support for the developing early embryo primarily comes from histiotrophic nutrition via direct continuity of the syncytium to glands. , When the eEVT plugs are dissolved (toward the end of first trimester), oxygenated maternal blood is free to flow into the intervillous space, increasing oxygen tension in the fetoplacental unit.
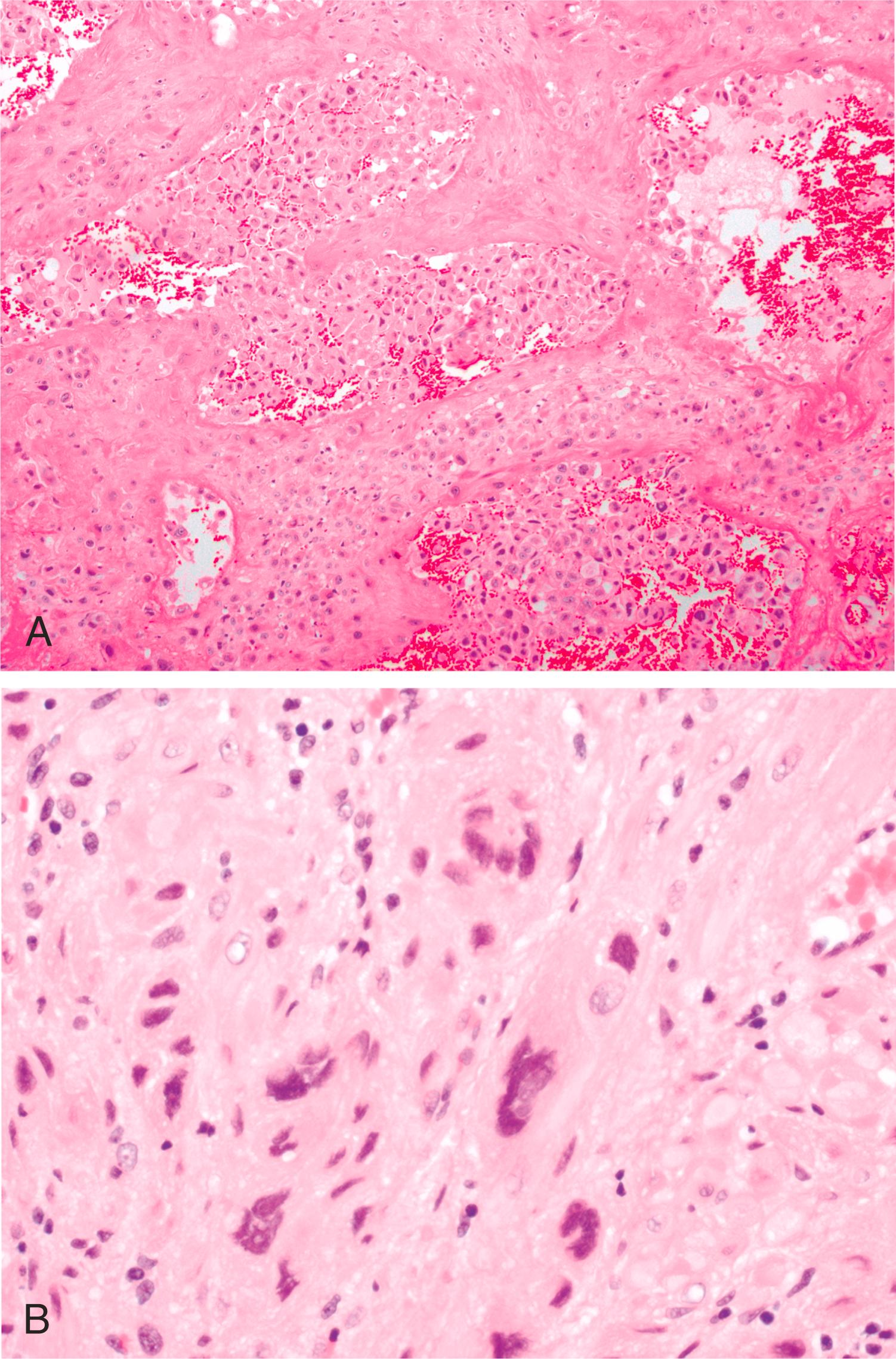
As the eEVT performs the above processes, the iEVT invade the decidua, as either isolated cells or columns of cells. Their primary role is anchoring the fetoplacental unit to the uterine wall. This process will continue through the superficial one-third of the myometrium up through about 20 weeks’ gestation (reviewed in Harris , ). One factor that has recently been identified as unique to iEVT is PLAC8, a protein involved in promoting migration and invasion that is also regulated by oxygen tension. As iEVT infiltrate the decidua and myometrium, they sometimes fuse to form multinucleated placental giant cells at the completion of their invasion ( Fig. 3.6B ).
To successfully carry out their functions, EVT, which are fetal-derived hemiallografts, must avoid immunologic rejection. While all trophoblast (including EVT) lack classical human leukocyte antigen (HLA) -A and -B proteins, EVT additionally achieve immunoprotection by expressing a specialized set of HLA proteins that modulate the maternal immunologic response. These “nonclassical” HLA factors include HLA-G, -C, and -E, with HLA-G the best characterized. , HLA-G facilitates crosstalk between EVT and maternal immune cells, including decidual NK cells and macrophages, helping to promote immunotolerance by increasing the Treg pool, among other functions. ,
What remains unknown is why and how EVT stop invading. While oxygen tension has been suggested as a regulator of invasion, the difficulty of studying this process separately from EVT formation has left some knowledge gaps. Nevertheless, it is known that EVT invasion is tightly regulated, with the decidual stromal and immune cells all playing important roles in directing and limiting this process. Decidualized stromal cells express tissue inhibitors of matrix metalloproteinases and plasminogen activator inhibitors to moderate the depth of invasion of the invasive extravillous trophoblast. Decidual natural killer (dNK) cells produce chemoattractants to direct migration to the spiral arteries (reviewed in Le Bouteiller ). Perhaps the best studied mechanism for regulation of EVT invasion is interferon-gamma, which is primarily secreted by dNK. Lack of proper decidualization, accompanied by lack of the associated immune cells and secreted factors, is the likely culprit in many complications of pregnancy. This includes pathologically deep placentation, as in placenta accreta spectrum (PAS) or gestational trophoblastic disease (GTD), as well as pathologically shallow implantation, as in preeclampsia and some cases of fetal growth restriction. , It is believed that invasive extravillous cytotrophoblast and vascular remodeling is abnormal in preterm preeclampsia and is the cause of the oxidative stress to the trophoblast and developing placenta. This results in release of factors, including antiangiogenic factors, that produce the systemic disease features (reviewed in Jauniaux and colleagues ). Better understanding of the implantation process should provide insights into these pathologies and (hopefully) better and directed treatment options.
Within floating villi, the cytotrophoblast progenitor cells fuse to give rise to the multinucleated STB. The controls of this lineage have been well studied and involve localized expression of the glial cells missing homolog 1 (GCM1) transcription factor, which may be regulated by Twist-related protein 1 (TWIST1), a basic helix-loop-helix transcription factor. GCM1 induces expression of syncytin proteins, necessary for the syncytialization. Syncytin proteins (syncytin-1 and -2) are captive retroviral envelope proteins whose main function is in placental STB formation. Once STB form they secrete high levels of hCG in a positive feedback loop to induce syncytins via GCM1 expression. GCM1 is also important later in instigating placental branching morphogenesis (discussed later).
Villous STB play a critical role in transport of factors between the maternal and fetal vascular spaces, help to maintain the underlying pluripotency of the cytotrophoblast, and are the major hormone-secreting cells of the placenta. STB line villi across the entire placenta, coming into direct contact with maternal blood after about 10–12 weeks of gestational age. STB do not express any HLA molecules (including nonclassical HLAs expressed by EVT), so they are fully “invisible” to the maternal immune system. They also have hemostatic and anticoagulant properties along with barrier and protective functions, as evidenced by clotting pathology sites of STB damage (intervillous thrombi, villous fibrinoid necrosis, or fibrinoid material). STB are also sources of growth factors, including the antiangiogenic factor soluble fms-like tyrosine kinase 1 (sFLT1). STB and STB “sprouts” are a significant source of fetal-derived cellular fragments and extracellular vesicles in maternal blood (see later). Recent technical advances in identification and measurement of small RNA and proteins have led to an explosion of knowledge in extracellular vesicle biology, including in pregnancy, where applications range from identification of fetal aneuploidy to development of diagnostic biomarkers for pregnancy complications. Maturation of STB across gestation is associated with loss of excess cytoplasm and condensation of nuclei, resulting in formation of “syncytial knots” near term (see later); this is accompanied by a switch from hCG and inhibin expression to placental lactogen (hPL) as the main secreted factor produced by STB.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here