Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Treatment decision making for arteriovenous malformations should take into account the lifetime risk associated with the aggressive natural history of these lesions. Whenever surgically feasible (specifically for grades I through III lesions), microsurgical resection with multimodality therapy (endovascular embolization) should be considered as the primary treatment modality in these patients.
Presentation of stroke in children will vary by age and etiology, with younger children more likely to present with nonfocal and nonspecific symptoms such as irritability, lethargy, and change in sleeping and feeding patterns.
Early diagnosis of moyamoya arteriopathy is of paramount importance to minimize the sequelae of strokes. The clinical status of the patient at the time of treatment is the most important predictor of long-term outcome. As such, any child with stroke should have moyamoya considered in the differential diagnosis.
Optimal treatment of moyamoya arteriopathy is operative. The surgical management of this condition is often successful in experienced centers and consists of cerebral revascularization through a variety of indirect means, such as pial synangiosis, or direct means in which an extracranial vessel is anastomosed to an intracranial vessel. Although direct methods afford immediate revascularization, it is not often technically possible in young children, and indirect methods also offer success with revascularization in months.
Stroke in children is uncommon relative to the adult population, but it can result in significant morbidity and mortality. Persistent neurologic deficits, seizures, or developmental compromise have been reported in up to 66% of patients, along with a 10% to 25% mortality rate. The World Health Organization's MONICA (monitoring trends and determinants in cardiovascular disease) Project defines stroke as “rapidly developing clinical signs of focal or global disturbance of cerebral function, with symptoms lasting 24 hours or longer or leading to death with no apparent cause other than of vascular origin.” This definition includes ischemic and hemorrhagic infarction as well as intracerebral and subarachnoid hemorrhage. The risk factors, presentations, outcomes, and impact of potential life-long impairment make stroke in the pediatric population a unique entity compared to adult stroke. In adults, 80% to 85% of stroke is ischemic, whereas 15% to 20% is hemorrhagic. In contrast, around 55% of stroke in children is ischemic, with the remainder presenting as hemorrhagic. This chapter reviews the major causes of stroke in children, with an emphasis on diseases with neurosurgical relevance, particularly moyamoya syndrome.
Limited data exist regarding pediatric stroke epidemiology. Estimated annual stroke incidence has been reported to be between 1.2 and 13 per 100,000 children, with the ischemic stroke incidence estimated to be between 0.63 and 7.8 and hemorrhagic stroke estimated to be between 1.1 and 2.9 per 100,000 children. Stroke risk is highest during the neonatal period, with an incidence of approximately 1 in 4000 live births. Incidence rates are likely underestimated given the frequency of undiagnosed or misdiagnosed cases. Boys carry a higher risk than girls for developing stroke, and even after accounting for the increased prevalence of sickle-cell disease, stroke is more common in black children than in children with white or Asian heritage. Approximately half of all children presenting with stroke have a previously identified risk factor, and recurrence is estimated at 20%.
The presentation of stroke in children varies by age and etiology, with acute ischemic stroke more commonly presenting with a focal neurologic deficit such as hemiparesis, and hemorrhagic stroke more commonly presenting with headache, emesis, and altered level of consciousness. Neurologic deficits in children may fluctuate in severity and frequency or present with recurrent manifestation. Seizure activity is common in both hemorrhagic and ischemic stroke, occurring in up to 50% of cases.
Age at time of stroke is significantly influential in the manner of presentation. Younger children are more likely to present with nonfocal and nonspecific symptoms. Neonates may present with focal seizures, irritability, lethargy, changes in sleep and feeding patterns, hypotonia, apnea, or developmental delay if the initial event is unrecognized. Focal deficits after stroke manifest in fewer than 25% of neonates. Toddlers may present with a wide variety of nonspecific symptoms, including irritability, developmental delay, failure to thrive, changes in sleep or feeding behaviors, emesis and crying, or sepsis-like symptoms. Older children, however, are more likely to present with specific or focal deficits, including hemiparesis, speech deficits, visual disturbance, and headache. Hemorrhagic stroke in older children will commonly present with acute-onset headache, emesis, and rapid neurologic deterioration.
Computed tomography (CT) should be obtained emergently in the setting of acute onset of focal or deteriorating neurologic symptoms in order to exclude a hemorrhagic etiology of stroke. Magnetic resonance imaging (MRI) may be equally accurate for detecting hyperacute hemorrhage, and if this modality is available emergently, consideration should be made to substitute MRI for CT in order to minimize radiation dose.
CT may be normal within the first 12 hours after symptom onset in the setting of acute ischemic stroke, and MRI is a more sensitive modality for early detection. If an acute hemorrhage is identified, magnetic resonance venography (MRV) will establish the presence of cerebral venous sinus thrombosis (CVST), and magnetic resonance angiography (MRA) will establish patency of vascular anatomy.
Digital subtraction angiography (DSA) provides the most detailed and accurate information regarding vascular anatomy and is frequently necessary to identify and elucidate vasculitides, arterial dissection, smaller arterial abnormalities, or structural etiologies of stroke such as vascular malformations and moyamoya arteriopathy.
The procedural safety of cerebral DSA has been established in children at institutions that treat a high number of patients with pediatric vascular disease; however, the necessary use of fluoroscopy exposes these children to ionizing radiation. Following the 2012 publication of a reported increased incidence of brain tumors after CT imaging in children within a large national United Kingdom registry, Orbach and colleagues sought to calculate the predicted risk of secondary tumors in a large cohort of pediatric neurointerventional patients. The predicted lifetime risk of tumor development was found to be significantly increased in children undergoing neurointerventional procedures, with younger age at the time of the procedure significantly correlating to increased ionizing radiation dose.
The vascular pathologies requiring neurointerventional evaluation and treatment carry the potential for profound morbidity and mortality, therefore the risk-benefit ratio favors the use of these procedures for appropriate vascular pathologies. Regardless, improvements in angiographic technology and optimized fluoroscopic parameters for pediatric patients can substantially lower brain-absorbed radiation doses and limit associated long-term adverse effects, and they should therefore be implemented whenever possible. The lifetime risk for deleterious effects from radiation dose should be considered when weighing treatment options for these children.
Cerebral arteriovenous malformations (AVMs) are congenital lesions characterized by direct connections between arteries and veins without intervening capillary beds. These lesions are classically high flow and may increase in size or vascular caliber over time while recruiting new arterial feeders via angiogenesis and regulation by a variety of metalloproteinases and growth factors, such as vascular endothelial growth factor (VEGF).
A subpopulation of patients with AVM has known associated genetic mutations. These are most commonly found in the setting of a diagnosis of hereditary hemorrhagic telangiectasia (HHT), a condition associated with 3.4% of pediatric AVM. Two genes identified within this population, ENG and ACVRL1 , have been linked with causation of AVM in 85% to 95% of patients. Familial symptomatic AVMs have been connected to the RASA1 mutation in 35% of pediatric AVMs associated with HHT.
The annual incidence of symptomatic AVM has been estimated at 1.1 per 100,000, though it may be as high as 1.4% overall with 12% to 18% occurring in the pediatric population. AVMs are most commonly scaled on the Spetzler-Martin grading system, with the size, eloquence of surrounding cortex, and venous drainage characteristics considered in order to estimate the risk of lesional morbidity.
The rupture rate in children with AVMs has been estimated to be between 2.2% and 4% per year, with 52% of pediatric AVMs presenting with spontaneous hemorrhage. Mortality after AVM rupture has been reported as high as 21% in children. Headache (20%), seizures (12%), and incidental discovery (16%) are other common clinical presentations, along with progressive neurologic deficits due to cerebral steal phenomena.
MRA or computerized tomography angiography may definitely diagnose AVM; however, DSA is the most sensitive diagnostic study for this pathology and should be obtained prior to treatment in order to establish the presence of intranidal aneurysms as well as characterize the details of vascular feeding and draining channels ( Fig. 14.1 ). An acute hematoma may compress a small AVM and lead to a falsely negative DSA. It is therefore appropriate to consider repeating the angiogram after resolution of a hematoma in the setting of acute intracranial hemorrhage and an initial negative study.
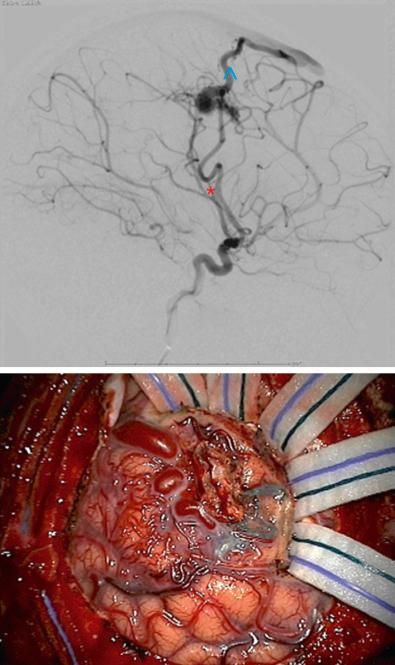
The presence of an AVM in eloquent cortex may lead to relocation of the associated neurologic function to adjacent or contralateral cortex. Establishing the location of cortical function therefore has significant implications for surgical resection, and functional MRI (fMRI) may be especially helpful in this determination.
Treatment options for cerebral AVM include surgical resection, endovascular embolization, stereotactic radiosurgery (SRS), and observation. Treatment decision making must consider the lifetime risk associated with the natural history of an AVM balanced against the risk of treatment. Due to the dangerous natural history of pediatric AVMs, available evidence generally supports treating these anomalies.
A multimodal treatment plan is frequently applied, with microsurgical resection considered to be the most definitive treatment option and the optimal modality when surgically feasible. Embolization is an excellent adjunct to surgical treatment, as it may reduce flow through the AVM and therefore reduce surgical hemorrhage risk.
Microsurgical resection is associated with high rates (86%–100%) of angiographic-confirmed obliteration with low rates of significant complications. The higher rates of obliteration were found in settings where perioperative angiography was applied. Recurrence rates have been reported at 0.9% after surgical resection, with 5-year postresection hemorrhage rates at 0.3%. Children have been reported to have better outcomes than adults after microsurgical resection of AVMs, and given the increased cumulative life-year risk in the pediatric population, surgical resection with multimodality therapy (endovascular embolization) should be carefully considered as the primary treatment modality in patients with Spetzler-Martin lesions of grades I through III.
For surgically inaccessible AVMs, especially selected Spetzler Martin grades IV and V AVMs, stereotactic radiosurgery with or without endovascular treatment should be considered as the primary treatment. Radiographic obliteration of AVM after SRS has been reported to be between 68% and 88% after 3 to 5 years of follow-up.
No clear guidelines exist for the duration or interval of posttreatment follow-up, though many reported series utilized a paradigm of postoperative DSA at 1 year, followed by annual MRI/MRA for 5 years.
Pediatric arteriovenous fistulae (AVF) are rare CNS vascular anomalies classified as pial AVF or dural AVF. Pial AVF are characterized by a direct arterial connection to a pial vein with no intervening nidus. These lesions may present with cardiac insufficiency, epilepsy, macrocrania, mass effect from enlarged venous varices, or intracranial hemorrhage. Pial AVF has been shown to be associated with hereditary hemorrhagic telangiectasia (0%–25% association reported), and cavernous malformation–AVM syndrome associated with RASA1 gene mutation. Treatment with endovascular and microsurgical techniques to close the fistulous connection has been shown to be effective for obliterating these lesions. Patients should be monitored closely after treatment for the development of hydrocephalus, which has been reported as high as 19%.
Dural AVF are associated with significant potential morbidity and mortality. They may present with intracranial hemorrhage, high output cardiac failure, macrocrania, developmental delay, cognitive impairment, seizures, and venous infarctions. Dural AVF have been associated with prior head trauma or neurosurgical procedures, venous sinus thrombosis and hypercoagulable states, and venous sinus hypertension. Endovascular obliteration of these connections is the most common therapeutic approach, though microsurgical ligation or a combination of both modalities may be necessary in some cases.
Cavernous malformations (CMs) are characterized by a compact mass of dilated, sinusoidal vessels lined by flattened endothelium and without elastin, smooth muscle, or surrounding adventitia. These lesions have no intervening neural parenchyma, and 10% to 20% are associated with a developmental venous anomaly (DVA).
CMs are angiographically occult but typically appear on CT as a well-defined collection of multiple compact densities, often with calcification. MRI will demonstrate a classic “popcorn” appearance with surrounding bloom on susceptibility imaging due to low-pressure hemorrhages that lead to hemosiderin deposition ( Fig. 14.2 ).
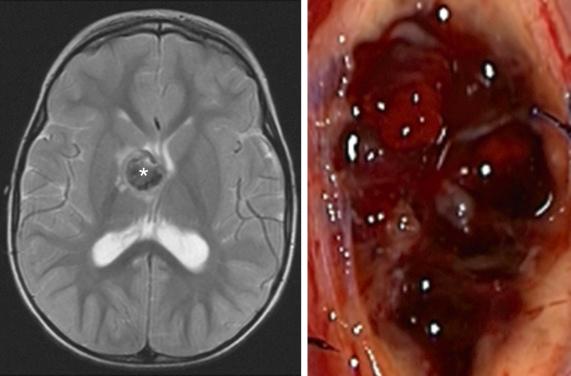
Prevalence of CM in the pediatric population has been reported at 0.6% in a large cohort study, with lower prevalence found in infants (0.2%). The minority (10%) of CM cases are reported to be familial, though this association is significantly increased in the setting of multiple lesions. About 9% are associated with prior radiation exposure.
A clear understanding of the natural history of these malformations in children is limited by the paucity of medical literature on the topic. Pediatric CMs may be incidentally found or present with hemorrhage, seizure, headache, or focal neurologic deficits. Patients presenting with symptomatic CM have a significantly worse natural history than those presenting with incidentally found lesions. Available literature for pediatric CM reports annual hemorrhage rates at 0.2% to 0.5% for incidentally found lesions and 8% to 11.3% for symptomatic or hemorrhagic lesions.
Risk factors that increase the likelihood of hemorrhage include a prior hemorrhage of the CM, brainstem location, or an associated DVA. Size does not appear to be associated with hemorrhage risk. A recently hemorrhaged lesion carries a significantly higher risk for subsequent hemorrhage, reported at up to 24% per lesion per year. Most children do not recover completely to baseline after hemorrhage, and with each subsequent hemorrhage the level of function may decline. Due to these reports, many advocate early treatment in children given their potential future life span. Brainstem location of a CM is generally associated with a more aggressive natural history in the pediatric population. Approximately 79% to 82% of brainstem CMs have been reported to present with hemorrhage and a subsequent annual bleed rate of 11.7% to 16.7%. It is important to note that the literature is consistent with reporting an early clustering of hemorrhage risk after the initial bleed, with risk apparently decreasing after 3 years of the initial bleed.
Treatment options include observation or surgical resection. SRS is a more controversial and poorly accepted treatment modality for CM. Surgical resection is associated with excellent outcomes, with most series reporting 0% mortality and a 4% to 5% risk of new neurologic deficit. Higher risk locations such as the brainstem are associated with a 12% to 25% risk of new postoperative deficit and should be approached with increased caution. Resection of asymptomatic lesions is controversial. Decision making must take into account the location and surgical risk along with the available information on natural history.
Treatment with radiosurgery has been reported but is controversial. Available literature reports a reduction in the frequency of hemorrhage from 17.3% to 4.5% per year, though it is unclear how much of this effect is due to the known natural history of early clustering of hemorrhagic risk. SRS is associated with increased complications, including a 16% incidence of new permanent neurologic deficit and a 3% mortality rate. Given the expected life-years of a pediatric patient with CM, as well as the increased short-term risk, unknown long-term risk, and possible secondary injury from radiation dose, the use of SRS as a treatment modality must be carefully balanced against known natural history. Surgical resection should be considered as a primary treatment whenever feasible. Genetic counseling should be considered for patients with multiple lesions.
Vein of Galen malformations (VOGMs) are characterized by fistulous connections between the choroidal arteries and the residual dilated median prosencephalic vein, with choroidal and mural subtypes. VOGMs may present with macrocrania, hydrocephalus, high-output cardiac failure, cerebral atrophy, and ischemia due to cerebral steal phenomena or intracranial hemorrhage. Endovascular embolization at a high-volume center is the treatment of choice. Outcome without intervention is poor, with mortality reported up to 100%. Advances in endovascular technology have improved outcomes for treated patients significantly, with one meta-analysis reporting good outcome in 68% of patients and a 10% mortality rate.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here