Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
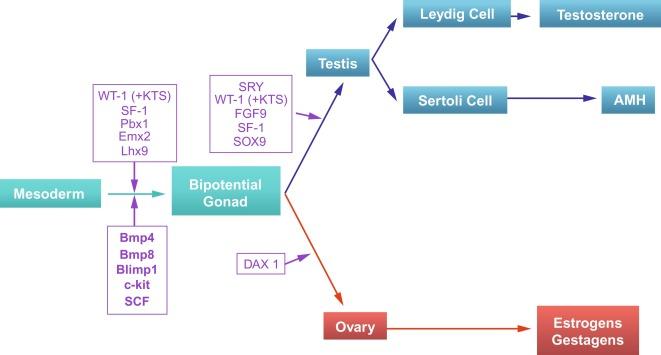
Sexual differentiation is the result of complex genetic and endocrine mechanisms that are closely associated with the development of both the genitourinary system and the adrenal glands. Formation of the bipotential gonad—and subsequently the testis or the ovary—depends on gene expression in both sex and autosomal chromosomes. Testes secrete steroid and peptide hormones, both of which are necessary for the development of internal and external genitalia. These hormonal actions are mediated by specific receptors that function as transcription regulators. Alteration of genetic events results in sexual dimorphisms involving the internal and external genitalia and may hinder development of other organs.
Determination of chromosomal gender takes place at the time of fertilization, with formation of an embryo of either 46,XY (male) or 46,XX (female) karyotype. The subsequent cascade of genetic events leads to development of either female (ovaries) or male (testes) gonads, referred to as gonadal gender. Hormonal secretions from the ovaries or testes are essential for development of external genitalia, thereby determining phenotypic gender. The relationship between the individual and the environment determines social gender.
Gonadal development comprises two phases. The first phase is characterized by the appearance of the bipotential gonad, or genital ridge, which is an indifferent gonad that is identical in males and females. Cells in the bipotential gonad may develop into either female or male gonads. The second phase is the development of a testis or an ovary.
In the fourth week of gestation the urogenital ridges appear as two parallel prominences along the posterior abdominal wall. This process is apparently driven by the expression of transcription factors Lim1 and Odd1. Each urogenital ridge gives rise to two important pairs of structures: the genital ridges arising from the medial prominences and the mesonephric ridges deriving from the lateral prominences. The genital ridges are the first primordium of the gonad, appearing as a pair of prominences about the midline. In 30- to 32-day embryos, each genital ridge is lateral to the aorta and medial to the mesonephric duct. The coelomic epithelium lining the genital ridges undergoes proliferation and thickening, protrudes into the coelomic cavity, and grows into cordlike structures giving rise to the gonadal ridges, the primary sex cords. Expression of steroidogenic factor 1 (SF1), triggered by the WT1 gene (Wilms tumor 1) (+ KTS isoform), the transcription factor Pbx1, and the homeobox proteins Emx2 and Lhx9, is essential for cell survival and proliferation during this period.
The coelomic epithelium also proliferates to invade the subjacent mesenchymal tissue. Cell proliferation in this phase also depends on Lhx9 expression. The basement membrane underlying the coelomic epithelium appears discontinuous and is rich in laminin content. As the coelomic cells proliferate, laminin production in the gonadal ridge increases, an apparently essential element for germ cell colonization. Immediately beneath the coelomic epithelium are several mesonephric tubules and glomeruli ( Figs. 12.1 and 12.2 ).
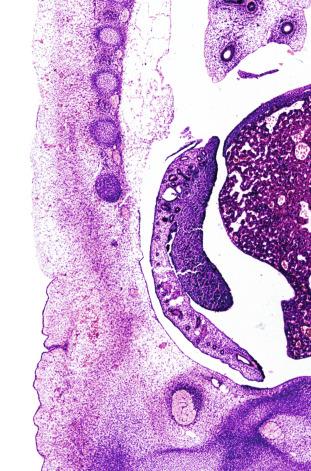
Initially the genital ridges are devoid of primordial germ cells, but they are detected in the third week of gestation in the extraembryonal mesoderm that lines the yolk sac posterior wall near the allantoic evagination. The germ cells are ovoid, 12 to 14 μm in diameter, and immunohistochemically express alkaline phosphatase, OCT3/4, NANOG, and LIN28. Nuclei are spherical and possess one or two large and prominent central nucleoli. The cytoplasm contains mitochondria with tubular cristae, lysosomes, microfilaments, lipid inclusions, numerous ribosomes, and abundant glycogen granules. Attracted by chemotactic factors, the primordial germ cells migrate along the mesenchyma of the mesentery and reach the genital ridge by 32 to 35 days. The appearance of these cells coincides with the expression of several proteins in the extraembryonal mesoderm, including Bmp4, Bmp8, and Blimp1.
Primordial germ cells begin to express two membrane proteins, fragilis and brachyurus, and the cells migrate through the primitive streak to settle into the developing endoderm (hindgut). The hindgut then invaginates into the future abdominal cavity and approaches the gonadal ridges. Primordial germ cells migrate by ameboid movements along the hindgut mesentery to reach the gonadal ridges. This emigration process occurs along autonomic nerve fibers that support them and requires the interaction of several factors: the integrin CXCR4-β1 (expressed by primordial germ cells), stromal cell–derived factor 1 (expressed by the body wall mesenchyma and gonadal ridges), and several extracellular matrix proteins. An essential mechanism for adequate primordial germ cell migration, survival, and chemoattraction is the interaction between CD117, expressed in the germ cell surface, and the stem cell factor present in the surrounding tissues. After entering the gonadal ridges, primordial germ cells colonize them; this process involves the expression of E-cadherin and germ cell interaction with a rich laminin network produced by organizing coelomic cells in the gonadal ridges. The association of coelomic-derived somatic cells, primordial germ cells, and a laminin-rich stroma in the gonadal ridge characterizes the gonadal blastema. Once inside the genital ridge, germ cells lose their motility and begin to aggregate.
Normal male determination depends on the expression of the SRY ( sex-determining region Y ) gene, located on the Y chromosome. In the absence of SRY , an ovary is formed. In the testicular blastema, SRY is exclusively expressed by the coelomic-derived somatic cells induced to differentiate into pre–Sertoli cells, which form the sex cords. These cells are believed to act as the organizing center of the male gonad, orchestrating differentiation of all other cell types. SRY expression is transient, ceases when sex cords form, and is activated by WT1 (+ KTS isoform), which is consistently expressed in the coelomic epithelium and the proliferating coelomic-derived somatic cells. Gonads lacking WT1 (+ KTS) show lower SRY levels per cell and also fewer SRY -positive cells. This observation led investigators to hypothesize that WT1 (+ KTS) contributes to SRY activation by increasing the number of pre–Sertoli cells. SRY expression is observed first in the anterior and central portions of the gonad and then in the poles. Expression of SRY requires proliferating gonadal somatic cells, and both SF1 and fibroblast growth factor 9 (FGF9) play a role in this proliferation. Immediately after SRY expression begins, FGF9 contributes to maintenance of cell proliferation necessary for sex cord formation. FGF9 regulates male-specific proliferation that produces pre–Sertoli cells.
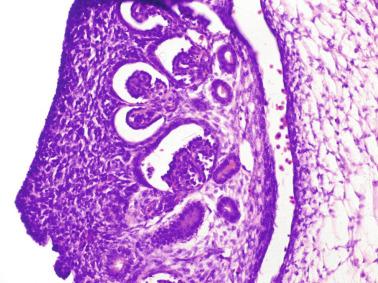
The origin of sex cord formation, and thus the first morphologic distinction between a testis and an ovary, also depends on the expression of SRY-box containing gene 9 ( SOX9 ). This expression occurs in the cytoplasm of somatic elements in the bipotential gonadal ridge. SOX9 is expressed in the pre–Sertoli cells in the same dynamic wave as SRY ; it originates in the center of the gonad and then continues to the rostral and caudal poles. Its transcription is activated by the synergistic action of SRY and SF1 . SOX9 also stimulates other factors that induce differentiation of Sertoli cells such as FGF9 and prostaglandin D 2 . SOX9 is also expressed in the female gonad, but there are important differences from the male gonad. Only males have an increase in SOX9 gene transcription and translocation of its protein product into the nucleus. This event occurs simultaneously with the initiation of sex cord formation. Therefore, like SRY , SOX9 is necessary and sufficient for both Sertoli cell differentiation and testis development. SOX9 expression in pre–Sertoli cells remains after sex cord formation, a finding indicating that SOX9 may have additional roles during proliferation and maturation of the testis, although it is dispensable for the development of embryonic and early postnatal testis. Contacts among pre–Sertoli cells during sex cord formation are regulated by neurotropic tyrosine receptor kinases ( Fig. 12.3 ).
Somatic coelomic epithelium-derived cells expressing nuclear SOX9 organize into clusters of pre–Sertoli cells as migrating primordial cells aggregate. These clusters are fused and transformed into tubular structures that form the primitive testis cords. The interaction of pre–Sertoli cells with peritubular myoid cells, which appear early, results in acquisition by pre–Sertoli cells of epithelial characteristics, polarization of organelles, and synthesis and deposition of collagen IV and small amounts of laminin. Myoid peritubular cells secrete fibronectin and collagens I and IV to form the basement membrane of the primitive testis cords. These pre–Sertoli cells express antimüllerian hormone under the synergistic action of SOX9 and SF1 . The primitive testis cords have a toroid structure, parallel to each other and aligned along the testicle. All have a point of contact in the dorsomedial part of the testicle, anastomosing to form a plexus that will be the future rete testis. The plexus has perforations through which vessels of the mesonephros penetrate.
For such development to occur, specific interactions are necessary between germ cells, Sertoli cells, endothelial cells, macrophages, and interstitial cells. Shortly after formation of the gonadal blastema in male gonads, cells of the adjacent mesonephros begin to migrate to the gonadal blastema. This migration depends on SRY expression by coelomic-derived cells and is controlled by the expression of FGF9.
The main emigration involves endothelial cells; they originate from the gonad-mesonephros border and migrate radially into the gonadal blastema parallel to the involution of the vascular mesonephric plexus from which they originate. When the endothelial cells reach the antimesonephric region, the celomic vessel forms under the coelomic epithelium, the male-specific main testicular artery. Branches of this vessel penetrate the gonad, delimiting about 10 avascular domains that form the cords and subsequently the seminiferous tubules. The process is mediated by platelet-derived growth factor receptor A (PDGFA) and vascular endothelial growth factor (VEGF). The action of VEGF and endothelial cells is a requirement for pre–Sertoli cells to organize into testis cords.
Macrophages play an important role at this stage of testicular differentiation. They arise from primitive yolk sac–derived progenitors and initially are in direct contact with pre–Sertoli cells and germ cells. Once the primitive cords form, macrophages remain extratubular with active involvement in regulation of vascularization, morphogenesis of cords, and phagocytosis of pre–Sertoli and germinal cells that remain to be incorporated into the cords ( Fig. 12.4 ).
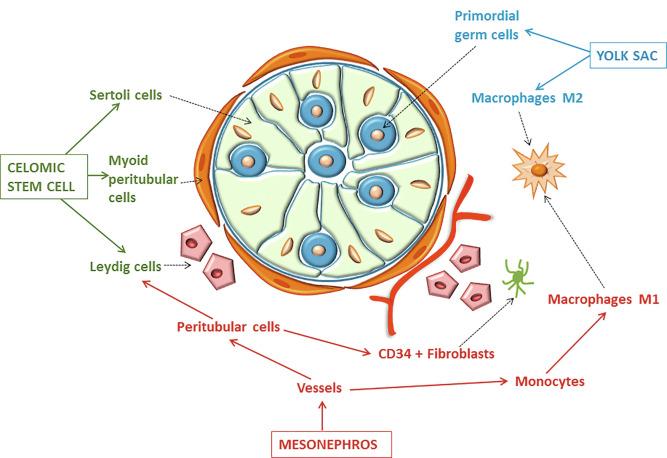
It is uncertain whether other cells are incorporated into the testicle from the mesonephros. Undifferentiated cells may penetrate the gonad following the endothelial cells and differentiate into a population of Leydig cells. The vessels not only contribute to Leydig cell progenitor migration but also affect their proliferation. Most stem Leydig cells arise directly from coelomic epithelium directly.
Leydig cell progenitors express LIM homeobox gene 9, but not gonadal somatic markers such as transcription factors GATA4 and SF1 . Fetal Leydig cell differentiation is regulated, at least in part, by three signaling molecules and pathways: desert hedgehog, PDGFA, and Notch signaling.
The same may apply to peritubular myoid cells that may differentiate either from the coelomic epithelium or from undifferentiated perivascular cells that migrate into the gonad from the mesonephric border, following the endothelial cells.
Primordial germ cells (that have proliferated in the seminiferous cords) that have undergone mitotic arrest in the G 1 and G 0 stages of the cell cycle are called gonocytes . Mitotic arrest depends on adequate cord formation and is probably mediated by inhibitory signals provided through gonocyte interactions with Sertoli cells. These cells remain in mitotic arrest until a few days after birth, when they resume proliferation.
Initially, gonocytes are in the central (“luminal”) portion of seminiferous cords. Later, during fetal and neonatal periods, gonocytes migrate toward the cord basement membrane because of gonocyte–Sertoli cell adhesion that is mediated by neural cell adhesion molecule. Gonocyte mitoses resume as soon as migration begins and may be identified at the basement membrane. These divisions result in the first generation of spermatogonia. Gonocytes that fail to migrate to the basement membrane undergo apoptosis. It has been suggested that Antimüllerian hormone (AMH) plays a role in gonocyte migration and the start of mitotic activity.
Near the end of the seventh week, pre–Sertoli cells differentiate from somatic cells in the sex cords, creating seminiferous cords. It was previously believed that interaction of peritubular myoid cells and pre–Sertoli cells was essential for seminiferous cord formation to promote basal lamina deposition and tubular organization. However, recent evidence indicates that peritubular myoid cells are not involved in the initial partitioning of the XY gonad into cord regions, which consist of clusters of both pre–Sertoli cells and germ cells.
As soon as seminiferous cords are formed by the interaction of peritubular myoid cells and pre–Sertoli cells, primordial germ cells become “entrapped” in tubules. This entrapment is mediated by interactions between primordial germ cells and pre–Sertoli cells through expression of E-cadherin and P-cadherin on the cell surfaces.
The differentiation of pre–Sertoli cells into Sertoli cells appears as polarization in which they form aggregates that assemble into seminiferous cords. Early events include the following: development of intercellular junctions between adjacent Sertoli cells; formation of a basal lamina that surrounds the external surface of seminiferous cords; and expression of AMH, sulfated glycoprotein-2, and clusterin by the Sertoli cells. Activin A, the major transforming growth factor-β protein, produced by fetal Leydig cells, acts directly on Sertoli cells to promote proliferation during late embryogenesis and plays an essential role in seminiferous cord morphogenesis in the murine testis ( Figs. 12.5 to 12.7 ).
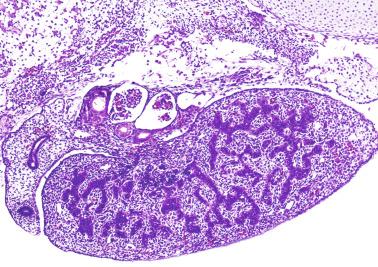
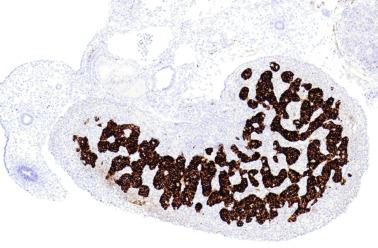
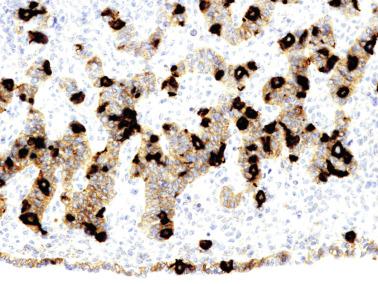
Peritubular myoid cells share expression of many genes with interstitial cells from early fetal development, so it has been hypothesized that they have an interstitial origin either from the mesenchymal cells that populate the initial genital ridge or from the somatic cells that proliferate from the coelomic epithelium.
Peritubular myoid cells form a single layer of flattened cells that surround the Sertoli cells and rim the seminiferous cords. Basal lamina formation by peritubular myoid cells is regulated through DHH homologue gene expression by the myoid cells themselves. Survival of peritubular myoid cells, and therefore seminiferous cord formation, depends on DAX1 (dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1) nuclear receptor expression, induced in turn by SF1 expression. DAX1 expression ceases in seminiferous cords after formation, whereas it is maintained in ovaries, a finding suggesting that dosage and stage-specific expression of this protein may be responsible for ovarian differentiation. Seminiferous cords lose their connection with the coelomic epithelium, whose height decreases to one or two cell layers.
Leydig stem cells proliferate actively and begin differentiation in the eighth week of gestation. Most originate from the same pool of NR5A1 + precursor cells from which Sertoli cells derive; others are perivascular NR5A1 − cells from the mesonephros. Differentiation is independent of hormonal stimulation, caused by two Sertoli cell–derived signaling molecules: DHH and PDGFA . Other factors involved in control of the development and functions of fetal human Leydig cells are GATA4 (transcription factor that recognizes the GATA consensus DNA sequence), insulin-like growth factor-1 (IGF1) (both are stimulatory factors), and the basic helix–loop–helix transcription factor POD1 (suppressive factor). Histochemical detection of 3β-hydroxysteroid dehydrogenase (3β-HSD) is the apparent first signal of differentiation and is completed with acquisition of ultrastructural characteristics of steroidogenesis.
As fetal development progresses, new cells differentiate from precursor Leydig cells located in the outer of the two peritubular layers ( Fig. 12.8 ). At 12 weeks of gestation, they begin to express LHCGR. Between weeks 14 and 18, Leydig cell number and testosterone level peak. After week 22, tubular walls are reduced to the internal layer. Differentiating Leydig cells are identified by characteristic expression of the androgen receptor (AR) at this stage. Fetal Leydig cells produce androstenedione, which in turn is converted into testosterone by HSD17B3 in Sertoli cells in a gonadotropin-independent process. Pituitary gonadotropins control Leydig cell function throughout the second and third trimesters, especially luteinizing hormone (LH).
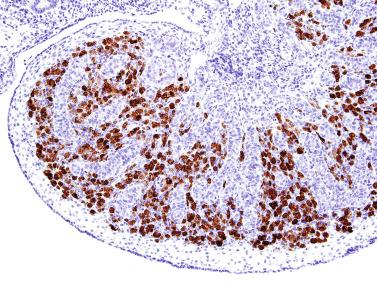
Rete testis develops from residual cords that persist from the mesonephros in continuity with seminiferous cords. The mesonephros and its testicular connection become progressively thinner and appear circular in cross sections. The testis remains between two ligaments: the cranial suspensory ligament and the caudal ligament. The caudal ligament gives rise to the gubernaculum ( Fig. 12.9 ).
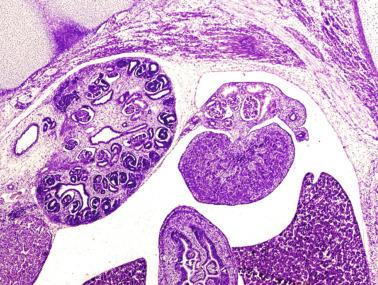
The development of the urogenital tract begins at the stage of the undifferentiated gonad, with the appearance of two different pairs of ducts: the wolffian ducts and the müllerian ducts ( Fig. 12.10 ).
Wolffian ducts arise inside the mesonephros, accounting for the close relationship between the reproductive and urinary systems. This pair of ducts originates in the third week of gestation, when the cranial region of the segmented intermediate mesoderm gives rise to 10 pairs of tubules, the nephric tubules, arranged with a segmental distribution. One end of each nephric tubule opens to the coelomic cavity, and the other end empties into an excretory duct. There are thus two excretory ducts, longitudinally placed at both sides of the embryonal axis, named pronephros. In the fourth week, the pronephros disappears and is replaced by another tubular excretory system, the mesonephros, derived from nonsegmented intermediate mesoderm. The medial ends of the mesonephric tubules are connected to glomeruli at one end and the wolffian ducts at the other end. The caudal ends of the wolffian ducts drain into the urogenital sinus. At the end of the second month, the mesonephros is replaced by the metanephros, the definitive kidney. In the male the most caudally located mesonephric tubules persist and give rise to the efferent ducts, whereas the wolffian ducts are the source of the epididymides, vas deferens, seminal vesicles, and ejaculatory ducts.
Both müllerian ducts originate from two longitudinal invaginations of the coelomic epithelium in the anterolateral aspects of the genital ridges. The cranial end of each duct is a funnel that opens into the coelomic cavity. The initial segments of both ducts run parallel and lateral to their respective wolffian ducts, and as they pass caudally, they cross over to lie medial to the wolffian ducts. Finally, in the distal portions, both müllerian ducts fuse into a single duct that serves as origin for the uterovaginalis duct. This duct elongates caudally to reach the posterior aspect of the urogenital sinus, forming a dilation named the Müller tubercle. Each wolffian duct drains at one side of this tubercle.
The remaining structures of the male genital system are derived from the urogenital sinus. Epithelium of this sinus with endodermal origin forms the prostate, the bulbourethral glands, the urethra, and the periurethral glands.
The primitive urogenital sinus derives from the cloaca, a structure that appears at the end of the first month and consists of a dilation of the final portion of the primitive posterior intestine. The cloaca is closed by the cloacal membrane.
During the third week, a crown of mesenchymal cells develops on the outer aspect of the cloacal membrane and gives rise to the cloacal folds. A knob in the middle of the cloacal fold is known as the cloacal eminence. In the sixth week the cloacal folds enlarge to form the genital folds, also known as the urethral folds. The cloacal eminence gives rise to the genital tubercle. External to the genital folds, two mesenchymal outgrowths develop to form the genital prominence or genital swellings.
In the fifth week the cloaca is divided by a septum into two cavities. The anterior cavity is the primitive urogenital sinus, which is covered by the urogenital membrane. The posterior cavity is the anorectal channel, which is covered by the anal membrane.
The primitive urogenital sinus divides further into two new compartments; the anterior compartment, the vesicourethral channel, becomes the urinary bladder and the urethra, whereas the posterior compartment, the definitive urogenital sinus, later differentiates according to gender.
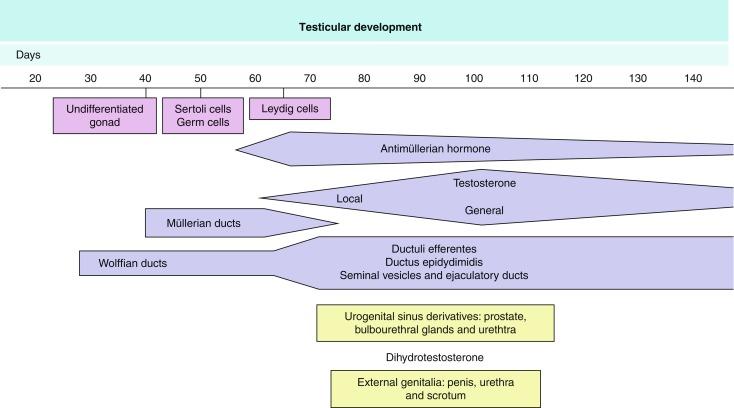
Subsequent development of the male genital system is under hormonal control. The mammalian fetal testis is initially independent of hormonal control, but then becomes LH (and possibly follicle-stimulating hormone [FSH]) dependent in the second half of gestation. At this point the most important hormones are AMH, testosterone, dihydrotestosterone (DHT), FSH, and LH.
AMH, also called müllerian-inhibiting substance, is secreted by Sertoli cells. It consists of a glycoprotein polymer with two identical subunits linked by a disulfide bridge. AMH is a member of the TGFB superfamily and is synthesized as a precursor peptide with proteolytic cleavage, which is required for hormone activation. AMH is encoded by a 2.75-kb gene, which comprises five exons and is located in the p13.3 region of chromosome 19.
AMH is secreted only by somatic gonadal cells that include male Sertoli cells and female granulosa cells. It is detected from the sixth week of development (eighth to ninth week of gestation), probably as soon as primordial germ cells come in contact with Sertoli cell precursors exactly 1 week before the müllerian ducts lose their responsiveness. AMH is at high concentration during the second trimester and drops markedly in the third trimester. Levels increase again during the first year of postnatal life and decrease during infancy and childhood. At the onset of puberty, AMH drops dramatically to low or undetectable levels, and this persists through adult life. The amount of hormone secreted by Sertoli cells is inversely proportional to their degree of maturation.
The regulation of AMH production is incompletely understood. Its expression is regulated by SOX9 ; SF1 (also called Ad4BP) also seems to be involved. SF1 is an orphan nuclear receptor that functions as a transcriptional regulator of all the steroidogenic genes within the P450 complex. It also has a regulatory effect on the SRY factor because SRY expression in Sertoli cells is detected shortly before AMH expression is detected. During puberty, AMH is negatively regulated by androgen levels.
AMH regulates the testis, genital tract, and extragenital structures, causing involution of the ipsilateral müllerian ducts that begins at the caudal end of the testis and progresses rapidly. In adulthood, remnants of this duct may be observed near the cranial (testicular hydatid) and caudal (prostatic utricle or verumontanum) ends of the testis. AMH is also responsible for formation of tunica albuginea, with accumulation of mesenchyma between the coelomic epithelium and the sex cords. The mesenchyma gives rise to collagenized connective tissue that contains several layers of fibers arranged parallel to the testicular surface. AMH also hinders spermatogonial proliferation into meiotic spermatocytes and has a paracrine role regulating fetal androgen production. The most important extragenital function of AMH involves maturation of the fetal lungs.
Testosterone synthesis by Leydig cells is regulated by human chorionic gonadotropin (hCG) and LH. hCG secretion reaches a peak between 11 and 14 weeks, whereas testosterone peaks between 11 and 17 weeks. From the 18th week forward, hCG declines markedly. hCG-dependent testosterone production plays an important role in genital differentiation. Wolffian duct differentiation occurs only in response to testosterone secretion by the ipsilateral testis, and this differentiation gives rise to the ipsilateral epididymis, vas deferens, and seminal vesicle. Anomalies of androgen synthesis during embryogenesis lead to incomplete masculinization and cryptorchidism.
DHT is formed from testosterone by the action of the enzyme 5α-reductase and causes differentiation of the prostate and development of the external genitalia, including the male urethra, penis, and scrotum. The scrotum is formed by the fusion of the labioscrotal folds in the midline, the so-called scrotal raphe. The penile urethra, initially a urethral groove, is formed by the fusion of urethral folds. The genital tubercle enlarges to form the glans penis. The terminal segment of the penile urethra is derived from an ectodermic invagination of the glans end. The urogenital sinus gives rise to the urinary bladder, the prostatic urethra, and the prostate. The first effects of DHT are observed on day 70; by about day 74, the urethral groove is closed; and between the 18th and 20th weeks, development of the external genitalia is complete.
The actions of testosterone and DHT on the male genital system must occur at precisely programmed times. Failure or delay of secretions or lack of responsiveness to these hormones are the main causes of genital malformations in disorders of sexual differentiation.
The fetal hypophyseal hormones FSH and LH play important roles in the last months of gestation. LH is first detected in the blood in the 10th week, peaking in the 18th week. Thereafter, levels decrease slowly until birth. LH controls androgen production in the second half of fetal life; fetal Leydig cells are devoid of luteinizing hormone receptors (LHRs) in the first half of gestation. LH does not exert negative control over LHRs and androgen production by fetal Leydig cells, whereas the converse occurs in the adult; also, the steroidogenic ability of fetal Leydig cells is higher than that in the adult. Fetal Leydig cells are insensitive to the inhibitory effects of estrogen.
FSH is an essential mitogen for Sertoli cells, which undergo maximal mitotic activity at the end of fetal life. This hormone appears to activate transcription factors such as GATA4 , which shows intense Sertoli cell expression from the nineteenth to the 22nd week following an increase in serum FSH. GATA transcription factors are structurally related zinc finger proteins that recognize a consensus DNA sequence (A/T)GATA(A/G), known as GATA motif, which is an essential cis -acting element in promoters and enhancers of multiple genes.
The structure of the fetal testis evolves under the influence of placental hormones and the hypophysis. Changes include modifications in external morphology (from elongate to ovoid) and development and differentiation of the cell types. The degree of development is uniform in both testes, and growth varies with gestational age.
The testicular covering, the tunica albuginea, increases in thickness 10-fold from the 10th to the 41st week of gestation. From the 29th week onward, two layers may be distinguished: an outer fibrous layer and an inner loose layer. Interlobular septa begin to appear between the 17th and 21st weeks and are completely formed between the 25th and 28th weeks. These septa support blood vessels. Nerve fibers are seen for the first time in the 16th week within the loose connective tissue of the albuginea (tunica vasculosa) and in the 20th week in the septa.
These irregular compact structures gradually acquire a cylindrical shape as they elongate and become convoluted. The diameter increases slowly up to the 16th week and stabilizes until birth. During fetal life the seminiferous cords consist of Sertoli cells and germ cells, surrounded by a tunica propria. The seminiferous cords are solid structures devoid of lumina. Between the cords the connective tissue forms the testicular interstitium, which contains numerous Leydig cells ( Fig. 12.11 ).
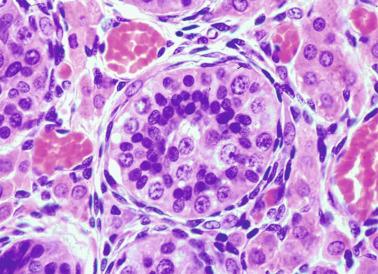
In contrast with other species, germ cells in the human fetal testis are not homogeneous, with several cell types that form the basis of different classifications. Three cell types are identified by immunohistochemistry: gonocytes, intermediate cells, and fetal spermatogonia (prespermatogonia) ( Table 12.1 ).
| Marker | Germ Cell Type | First Trimester | Second Trimester | Third Trimester | First Year of Life |
|---|---|---|---|---|---|
| PLAP | Gonocyte | +++ | ++ | + | + |
| Prespermatogonia | ++ | + | − | − | |
| Kit | Gonocyte | +++ | +++ | ++ | + |
| Prespermatogonia | +++ | +++ | ++ | + | |
| OCT3/4 | Gonocyte | +++ | +++ | + | + |
| Prespermatogonia | − | − | − | − | |
| TSPY | Gonocyte | − | − | − | − |
| Prespermatogonia | ++ | ++ | ++ | ++ | |
| Ki67 | Gonocyte | +++ | +++ | + | + |
| Prespermatogonia | ++ | ++ | + | − |
Gonocytes refers to the primordial germ cells once they reside in the gonadal ridge. They are prominent for the large size (twice that of the surrounding cells) and location in the center of the seminiferous cords during most of fetal life. Nuclei are spherical and possess prominent central nucleoli. The cytoplasm contains well-developed Golgi complex, lipid droplets, short rough endoplasmic reticulum cisternae, and microfilaments. Gonocytes connect with Sertoli cells by gap junctions and desmosome-like junctions. Adhesion molecules are present, including neural cell adhesion molecule (NCAM), PB-cadherin, and connexin 43. Immunoreactivity includes octamer-binding transcription factor 4 (OCT4), KIT, placental alkaline phosphatase (PLAP), serine/threonine-protein kinase 2 (CHK2), and proliferating cell nuclear antigen (PCNA), with absence of melanoma-associated antigen 4 (MAGE-A4).
Intermediate cells are morphologically similar to gonocytes, although the cytoplasm-to-nucleus ratio is lower, the number of cytoplasmic processes is higher, and rough endoplasmic reticulum cisternae are more numerous. Intermediate cells are connected by cytoplasmic bridges. They express PCNA, weakly express OCT4, but are negative for KIT and MAGE-A4.
Fetal spermatogonia are also joined by intercellular bridges, are grouped at the periphery of seminiferous cords, and differ from gonocytes by exhibiting more condensed nuclear chromatin and a higher cytoplasm-to-nucleus ratio. The cytoplasm is pale, and mitochondria are adjacent to one side of the nucleus and joined by electron-dense bars. Rough endoplasmic reticulum and lipid droplets are scant. Immunohistochemically, these cells are MAGE-A4 + and are negative for KIT and PCNA, indicating a quiescent phenotype.
The three germ cell types are rich in glycogen granules, polysomes, and chromatoid bodies. Chromatoid bodies consist of finely granular material intermingled with other larger granules, which are similar in size to ribosomes; their mission is to accumulate regulatory RNAs to be used during transcription.
The germ cell number per cross-sectional cord reaches a peak between the 12th and 22nd weeks. In the 10th week, most germ cells are gonocytes; at approximately the 15th week, many intermediate cells are present together with gonocytes, and fetal spermatogonia may be observed for the first time. From the 16th to the 20th week, germ cell degeneration occurs with Sertoli cell phagocytosis. From the 22nd week onward, most germ cells are fetal spermatogonia. Mitotic activity is high in the last trimester of gestation. Approximately 22% of testes between 14 and 33 weeks contain ectopic germ cells located beneath the coelomic epithelium, in the connective tissue that separates the testis from the epididymis, or in the rete testis. Some gonocytes persist after birth. The majority will be transformed into Ad spermatogonia during the 30th to 90th postnatal days (minipuberty).
Fetal Sertoli cells are the most numerous cells in the seminiferous cords, where they form pseudostratified epithelium that rests on the basal lamina. At approximately the 13th week of gestation, Sertoli cells exhibit an indented outline and long cytoplasmic processes, and are connected by desmosomes. Nuclei are spherical and contain small nucleoli. The cytoplasm is electron dense and contains numerous lysosomes, actin microfilaments, and intermediate filaments. Other organelles include microtubules, mitochondria, and well-developed Golgi complexes. In the apical region are numerous, parallel rough endoplasmic reticulum cisternae. The Sertoli cells progressively elongate, their cytoplasm becomes less electron-dense, and filaments predominate in the basal region. They express vimentin filaments throughout life, whereas low-molecular-weight cytokeratins (8, 18, and 19) are present until the 20th week. Desmin filaments may be observed from the 11th to the 14th week. Fetal Sertoli cells express inhibin and Stem cell factor (SCF) to secure a niche for gonocytes.
During gestation, the number of Sertoli cells increases even though mitoses are only occasionally observed. The number of Sertoli cells per cross-sectioned cord does not increase during this period, but their proliferation contributes to increased length and tortuosity of the cords. Sertoli cell proliferation and testicular cord expansion take place in response to activin A, which is secreted by Sertoli cells. At the end of gestation, there are approximately 260 million Sertoli cells per pair of testes. In the fetal and early postnatal period, the absence of AR expression in Sertoli cells characterizes androgen insensitivity within the male gonad during this period. Fetal Sertoli cell functions include AMH secretion, fetal Leydig cell differentiation induction, and prevention of entry of germ cells into meiosis.
From the 14th week, two types of peritubular cells may be observed: inner myoid cells and fibroblast-like cells. Fibroblast-like cells occupy the outermost layers. In total, there are four to five layers of peritubular cells. At this time the number of myoid cells is low, but are predominate by the 34th week. The probable precursors of myoid cells are the fibroblast-like cells because both coexpress Ki67. In the final weeks of gestation the number of peritubular cell layers decreases to only two, perhaps because of intense lengthening of seminiferous cords and Leydig cell differentiation from peritubular cell precursors. The presence of the AR in peritubular myoid cells suggests an important role in Sertoli cells control.
Leydig cells first appear among the seminiferous cords in the eighth week of gestation, and increase in number to 48 million per pair of testes (~ 50% of testicular volume at this moment) between the 13th and 16th weeks, coinciding with the testosterone peak ( Fig. 12.12 ). Leydig cell number is maintained up to the 24th week, although the testicular volume occupied by Leydig cells is lower at this time because the seminiferous cords have grown markedly during this period. From the 24th week to birth, the number of Leydig cells progressively decreases to 18 million.
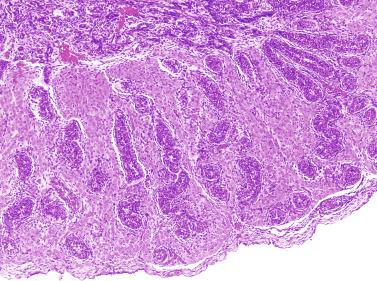
Leydig cells are polyhedral and measure between 30 and 37 μm in diameter. They have eccentric and pale nuclei, with voluminous nucleoli, and eosinophilic cytoplasm. There is an abundance of smooth endoplasmic reticulum, numerous mitochondria with tubular cristae, and a variable number of lysosomes and lipid droplets. The rough endoplasmic reticulum consists of some groups with a few short, parallel cisternae. These cells differ from adult Leydig cells by the absence of Reinke crystals and paracrystalline structures, and by the lesser amount of lipid droplets. Histochemical expression included acid phosphatase, glucose-6-phosphatase, and 3β-HSD. In addition to testosterone, these cells secrete several peptides that play important roles in endocrine and paracrine control of testicular function. One of these peptides, insulin-like factor 3 (INSL3), is important in testicular descent.
Macrophages and hematopoietic cells are usually observed in the testicular interstitium of the fetal testis. They derive from yolk sac hematopoietic progenitors and migrate to colonize the testis and other organs.
Macrophages are more numerous at the end of the fetal period, probably because of involution of Leydig cells. These cells are likely involved in Leydig cell paracrine regulation.
Hematopoietic cells appear in isolated clusters at 17 to 20 weeks in the testis, chiefly located beneath the tunica albuginea or near the testicular mediastinum. In the final weeks of gestation, more than two-thirds of testes show hematopoietic foci.
Most fetal testes (72%) receive blood through three arteries: the testicular (inner spermatic) artery, which originates from the abdominal aorta; the deferential (vassal) artery, which originates from the inferior vesical artery; and the cremasteric (outer spermatic) artery, which is a branch of the inferior spermatic artery. In 23% of fetal testes, only two arteries (testicular and deferential) are present, whereas 5% of testes have four arteries.
The testis and epididymis form an anatomic and functional complex, but the anatomic relationships vary widely. The most frequent finding (almost 90%) is connection of the testis limited to the caput and cauda of the epididymis. In other cases (about 8%) the testis is intimately attached to all parts of the epididymis (caput, corpus, and cauda), and uncommon cases (3%) have deficiencies in the testis-epididymis junction in the caput or the cauda. These variations are not related to the position of the testis or to the side of the body (right or left). During fetal life, androgenic receptors are observed in epithelial cells of both efferent ducts, the epididymal duct, and the peritubular stroma.
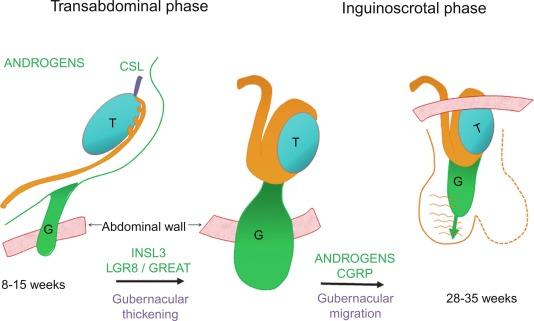
Testicular descent results from hormonal and mechanical influences that mediate migration through the abdominal wall and the inguinal canal to the scrotum. The process of descent begins between weeks 8 to 15 of gestation, accelerating from weeks 24 to 26. At week 23, most testes (90%) are still in the abdomen, and from weeks 26 to 28 they pass through the deep inguinal ring. Testicular displacement through the inguinal canal lasts a few days. At approximately week 28, they pass through the superficial inguinal ring and reach the scrotum, a process completed within 4 weeks. After week 35, descent is normally complete.
Three phases are classically recognized in testicular descent: nephric, transabdominal, and inguinal. In the nephric phase the gonad detaches from the metanephros (primitive kidney) by week 7. Transabdominal descent consists of the displacement from the posterior abdominal wall to the future inguinal region (inner inguinal ring, also called the deep ring) by week 15. This displacement is associated with regression of the cranial suspensory ligament and enlargement of the caudal suspensory ligament (gubernaculum). At the same time, marked growth of the lumbar backbone takes place, and as a result the testis moves away from kidneys. Inguinal descent refers to the entry into the inguinal canal and complete descent into the scrotal pouch, occurring between week 28 of gestation and birth.
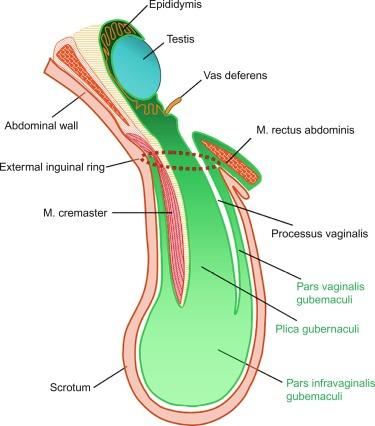
Testicular descent is directed by the gubernaculum testis, a structure that appears at approximately week 6 of gestation as an elongate condensation of mesenchymal cells (the caudal ligament) extending from the genital ridge to the presumptive inguinal region. This dynamic formation undergoes multiple morphologic changes. At this level of the abdominal wall, the gubernacular cells persist as simple mesenchyma, whereas the remaining abdominal wall cells differentiate into muscle. The mesenchymal cells give rise to the inguinal canal. Thus the testis lies on a continuous column of mesenchyma (plica gubernaculum) limited by the cranial testicular ligament in the upper pole and the plica gubernaculum joining the testis to the future scrotal region in the inferior pole. The periphery of this mesenchyma is invaded by the vaginal process, which develops from a blind peritoneal pouch that opens cranially into the abdominal cavity. The pouch partially encircles the gubernaculum except for its dorsal aspect, and divides the gubernaculum into two portions: central (plica gubernaculum) and peripheral (pars vaginalis gubernaculum).
Once the inguinal canal and the plica gubernaculum are formed, development slows. In the seventh month the processus vaginalis undergoes active growth, the cremasteric muscle develops from the mesenchyma outside the processus vaginalis, and the distal end of the gubernaculum enlarges markedly.
Gubernacular thickening occurs from weeks 16 to 24 of gestation, produced by an increase in number of cells and quantity of glycosaminoglycans and hyaluronic acid. This tissue later absorbs water to create the final volume of the gubernaculum. The tissue is reminiscent of Wharton jelly of the umbilical cord. By this time the testis-epididymis complex is pear shaped, and its largest component is the gubernaculum. The inguinal descent of the testis behind the gubernaculum begins in week 25. The testis and epididymis slide through the inguinal canal behind the gubernaculum. Simultaneously, development of the processus vaginalis concludes, and the gubernaculum begins to shorten and fibrose, located caudal to the testis and epididymis (gubernacular regression); the epididymis develops further, with lengthening of testicular blood vessels and vas deferens ( Figs. 12.13 and 12.14 ).
Testicular descent is a complex process integrating several essential factors that probably act sequentially and synergistically. The main prerequisites are normal hormonal stimulation, intraabdominal pressure, development of epididymis and spermatic vessels, development of the gubernaculum, and harmonic development of the processus vaginalis.
The critical role of hormonal function in testicular descent is exerted through placental gonadotropins, the hypothalamic–pituitary-testicular axis function, and successful synthesis and action of testosterone produced by the testis. In animal models, destruction of the pituitary blocks testicular descent. Anencephalic fetuses and patients with familial hypogonadotropic hypogonadism usually have undescended testes. Many cryptorchid patients have transient neonatal hypogonadotropic hypogonadism. Some cryptorchid testes descend after treatment with hCG or gonadotropin-releasing hormone (GnRH). Defective Leydig cell function caused by absence of LH, defective testosterone synthesis, or defective ARs interferes with testicular maldescent.
Another important prerequisite for the testicular descent is adequate abdominal pressure. In prune belly syndrome, bilateral abdominal cryptorchidism is associated with urologic malformations and lack of abdominal wall musculature. In a variant termed pseudo–prune belly syndrome, a positive correlation is seen between the development of abdominal wall musculature and testicular descent. The more developed the abdominal wall musculature is, the further the testes descend.
Development of the processus vaginalis also plays a critical role in testicular descent. Growth of the processus vaginalis into the gubernaculum takes place harmoniously. If this structure is invaded, even partially, by fibrous tissue, the testis will descend in an abnormal direction, thus giving rise to ectopia. If fibrous tissue completely replaces the gubernaculum, the processus vaginalis and cremasteric muscle fail to develop fully, and as a result the testis is mechanically blocked in its route of descent.
There is a close relationship between the development of the processus vaginalis and descent. If the processus does not extend far from the abdominal wall, then the testis remains intraabdominal. The processus protrudes throughout the outer inguinal ring only when testicular descent is initiated, and descends into the scrotum only after the testis has entered the inguinal canal.
Given that nephric displacement consists only of detachment of the testis from the mesonephros, descent may also be classified as occurring in two phases, each regulated by different factors. The most important factor for transabdominal displacement is androgen-independent peptide INSL3 (also called INSF3 or IGF3), a member of the relaxin-insulin family that is produced by fetal Leydig cells. This peptide reaches high levels in the first half of gestation, stimulating gubernacular swelling by the production of hyaluronic acid and glycosaminoglycan that trap large amounts of water. In animal models, mutations in the genes that encode INSL3 or its receptor LGR8 (leucine-rich repeat-containing G protein–coupled receptor 8) or another receptor called RXFP2 (relaxin/insulin-like family receptor 2) cause cryptorchidism by disrupting transabdominal descent.
In humans, however, mutations in the genes that encode INSL3 or its receptors have been found in only 1% of cryptorchid patients, even in studies of familial cryptorchidism. The low frequency of such mutations in human cryptorchid patients may account for the infrequent disruption of the first phase of descent in humans, but the inguinoscrotal phase is usually impaired. Analyses of other potential candidate genes for human cryptorchidism, such as homeobox genes HOXA10 and HOXA11 , and the estrogen receptor ESR1, also fail to elucidate mechanisms underlying cryptorchidism. Androgens facilitate regression of the cranial suspensory ligament, which also seems to contribute to positioning of the gonad.
In contrast, the inguinoscrotal phase of testicular descent depends on androgenic action, as explained by the genitofemoral nerve (GFN) hypothesis. The nucleus of the GFN is located in the spinal cord. The nerve courses along the anteromedial surface of the psoas muscle, and the genital branch crosses the inguinal canal to innervate the cremaster muscle, whose rhythmic contractions are likely transmitted to the gubernaculum, orienting it in a scrotal direction. Based on this hypothesis, androgens act on the GFN nuclei rather than directly on the gubernaculum. Under androgenic action, GFN neurons then undergo masculinization. Male mice have a greater number of neurons than females, and the neurons secrete calcitonin gene–related peptide (CGRP), which is a GFN neurotransmitter. The gubernaculum tip may contain an area of primitive mesenchymal cells. Growth of the gubernaculum apparently results from CGRP-induced cell proliferation and prevention of apoptosis. The range of GFN-mediated androgenic effects is broad and may include obliteration of the processus vaginalis, inguinal canal differentiation, cremaster muscle myocyte differentiation, and initiation of transabdominal descent through involution of the testicular cranial suspensory ligament.
Other factors that influence testicular descent include epidermal growth factor (EGF) and estrogens. EGF has a positive effect on descent through stimulation of the placental-gonadal axis. Maternal EGF levels increase just before fetal masculinization. The placenta has an elevated concentration of EGF receptors, and placental stimulation by EGF may stimulate hCG production, which in turn may stimulate Leydig cells to produce androgens that, alone or combined with other factors, may stimulate descent. In contrast, estrogens play a competing role in descent by preventing regression of the cranial gonadal ligament, gubernaculum growth, and Leydig cell proliferation, resulting in a decrease in androgen and INSL3 secretion. Exposure to environmental endocrine disrupters such as estrogen in utero has a negative effect on male genital tract development. During the first trimester of gestation, mothers of cryptorchid infants have free estradiol serum concentrations that are significantly higher than those of controls. Experimental studies have shown that estradiol diminishes gubernaculum swelling and stabilizes müllerian ducts; therefore estradiol may inhibit the cell proliferation that causes swelling through a reduction of INSL3 secretion by Leydig cell damage ( Fig. 12.15 ).
After birth the gubernaculum and processus vaginalis involute. The gubernaculum is replaced by fibrous tissue that forms the scrotal ligament. Once the testis has descended, the processus vaginalis undergoes atrophy and reabsorption, mainly in its cephalic portion. Failure of the processus vaginalis to regress may be a common cause of acquired cryptorchidism. In some patients a noticeable and wide processus vaginalis is associated with inguinal hernia and cryptorchidism, whereas a narrow processus vaginalis appears associated with hydrocele; if there is partial obliteration of the lumen with persistence of the processus vaginalis, the testis could be retractile.
From birth to puberty the testis is a dynamic structure, an important consideration when interpreting biopsy results in children. All testicular components undergo waves of proliferation and differentiation before puberty. Morphometric analyses and endocrinologic studies in infants and children revealed that the number of Sertoli cells and germ cells increases during this period, accompanied by significant production of AMH and inhibin.
During the prepubertal period, three waves of germ cell proliferation occur: during the neonatal period, in infancy, and at puberty. Germ cell proliferation at puberty gives rise to the adult testis with complete spermatogenesis. Leydig cell proliferation also has three waves (fetal, neonatal, and pubertal), the last of which corresponds to the pubertal wave of germ cell proliferation.
The newborn testis has a volume of approximately 0.6 mL, and it is covered by a thin tunica albuginea from which the intratesticular septa arise. These septa divide the testis into approximately 250 lobules containing the seminiferous tubules and testicular interstitium ( Fig. 12.16 ). The seminiferous tubules measure 60 to 65 μm in diameter, form solid cords with no apparent lumina filled with Sertoli cells and germ cells, and are surrounded by a thin basement membrane and isolated myoid cells and fibroblasts.
Sertoli cells are the most abundant cells, with 26 to 28 per tubular cross section ( Fig. 12.17 ). They form a pseudostratified cellular layer and have elongated to oval nuclei with darker chromatin than that of mature Sertoli cells, as well as one or two small peripheral nucleoli. The apical cytoplasm contains abundant rough endoplasmic reticulum, several Golgi complexes, and numerous vimentin filaments, with inhibin B expression ( Fig. 12.18 ). Interdigitations and small junctions of the occludens and adherens types join adjacent Sertoli cells, and desmosome-like junctions are present between Sertoli cells and germ cells. Mitotic figures are occasionally seen. These cells express AMH and vimentin, as well as weak staining for M2A oncofetal antigen. Also, in the apical pole, spherical or ovoid bodies show intense immunostaining with inhibin ( Fig. 12.19 ).
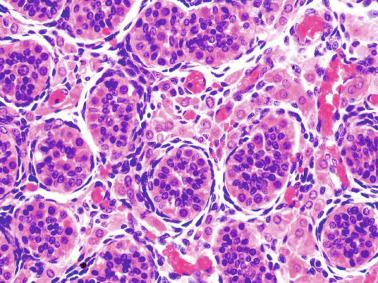
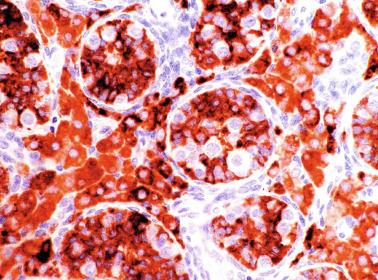
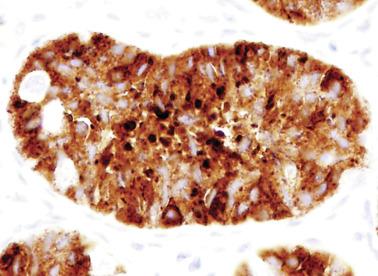
Germ cells comprise fetal spermatogonia, spermatogonia A dark (Ad), and gonocytes. Spermatogonia are present chiefly on the basal lamina in a discontinuous pattern, possessing smaller nuclei and less cytoplasm than gonocytes; nucleoli are peripheral and small. At birth, most spermatogonia correspond to the adult type A (Germ cell-Spermatogonia) ( Fig. 12.20 ). Spermatogonia Ad have smaller nuclei, and barely visible nucleoli are peripherally distributed. Gonocytes are usually located near the center of the tubules, with spherical and voluminous nuclei and large central nucleoli. Most gonocytes are immunoreactive with PLAP and KIT.
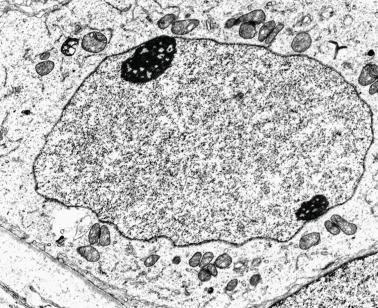
Seminiferous tubules are surrounded by the tunica propria , which comprises a basal lamina, myoid cells, fibroblasts, collagen fibers, and extracellular matrix. The peritubular myoid cells express intense nuclear immunostaining for AR, with expression similar to interstitial cells, easily distinguished from the negative staining in Sertoli cell nuclei.
The testicular interstitium is a loose connective tissue that contains fetal Leydig cells that resemble adult Leydig cells but lack Reinke crystalloids ( Fig. 12.21 ). These cells have well-developed smooth and rough endoplasmic reticulum, filament bundles, and lipid droplets. In addition, mast cells, macrophages, and hematopoietic cells are present.
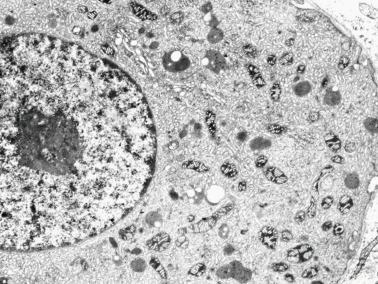
Minipuberty is first important postnatal development. It involves changes in germ cells, Sertoli cells, and Leydig cells caused by a transient increase in secretion of FSH and LH during the third postnatal month.
Testicular weight and volume increase twofold from birth to 5 months of age. FSH induces Sertoli cell proliferation, increasing fivefold to sixfold during the first year of life. Under the influence of LH, resident Leydig stem cells undergo stimulation, with new ones differentiating from peritubular myoid cells, and the number increases, peaking between 2 and 4 months after birth. In the following months, this cellular population declines rapidly so that by the end of the first year, Leydig cells are rare. As a consequence of the changes in Sertoli and Leydig cells, serum levels of inhibin B, testosterone, and INSL3 increase. Inhibin B, a Sertoli cell marker, remains elevated even when FSH and LH levels have decreased.
The total number of germ cells per testis increases up to threefold in the first months of life to the end of the neonatal period, and drops later. Gonocytes move from the center of the seminiferous tubule toward the basal lamina. This migration is probably facilitated by cell adhesion molecules on the immature Sertoli cell surface, including P-cadherin. Transformation of gonocytes into spermatogonia Ad is enhanced by testosterone and probably also by AMH, which is found at high levels between the 4th and 12th months of life ( Fig. 12.22 ). This transformation is complete by age 6 months and coincides with total loss of fetal germ cell markers PLAP and KIT by the end of the year one.
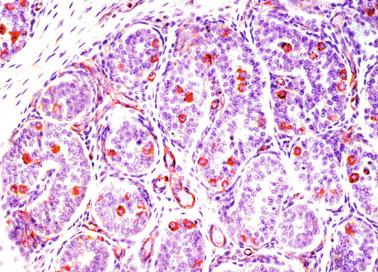
Paraganglia are often observed in epididymides and spermatic cords in newborns. This finding is not surprising because paraganglia are the main source of catecholamine before birth ( Fig. 12.23 ).
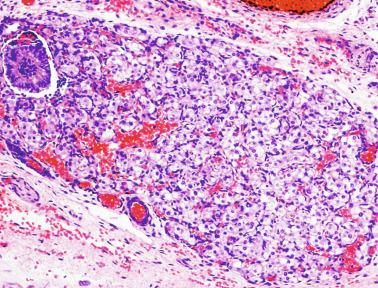
From the sixth month to approximately the second half of the third year of life, the testis is in a resting period . Tubular diameters decrease (from 80 to 60 μm), and spermatogonial proliferation is rarely observed. Leydig cells involute so that by the end of this period, only a few of these cells persist and are not easily detected in routine specimens. The thickness of the albuginea diminishes to 250 μm. Despite these findings, which permit investigators to define a resting period of the testis, Sertoli cells maintain active hormone synthesis. During these years Sertoli cells produce high levels of AMH and inhibin. AMH modulates the number and function of Leydig cells by hindering the differentiation of these cells from their mesenchymal precursors and diminishing synthesis of steroidogenic enzymes. Inhibin B plays a role in the inhibition of FSH during infancy. Immunohistochemically, its expression is observed throughout the cytoplasm and in a granular pattern in the apical pole.
This quiescence is broken at the end of the third year by the second wave of germ cell proliferation, the so-called growth period. The number of Ap spermatogonia increases, and B spermatogonia (derived from Ap spermatogonia) appear. In some normal testes from children who are older than 4 years, meiotic primary spermatocytes and round spermatids (Sa + Sb types) are observed ( Fig. 12.24 ). This second spermatogenic attempt fails, and many degenerate germ cells may be present but are phagocytosed by Sertoli cells. The testis continues to produce AMH (by Sertoli cells) and inhibin B. AMH modulates the number and function of Leydig cells by regulating differentiation of the mesenchymal precursors and expression of steroidogenic enzymes. Inhibin B plays a role in FSH inactivation during infancy.
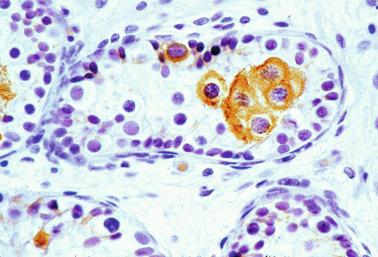
The cause of this second wave of germ cell proliferation is unknown; no elevation of FSH or LH serum concentrations occurs between 6 months and 10 years of life. After the sixth year, there is a slight increase in adrenal androgens, but testicular testosterone levels increase only after the tenth year. By the third year, most Leydig cells have degenerated: from a peak of approximately 18 million at birth, only 60,000 remain by the age of 6 years. At this age, testosterone levels are similar to those of girls, and most androgens are of adrenal origin. Testosterone levels during infancy are much higher in the tunica vaginalis than in plasma. It also could be important that Sertoli cells begin to express the AR in their nuclei at this age ( Fig. 12.25 ). Expression is probably related to development of this wave of proliferation and differentiation of germ cells.
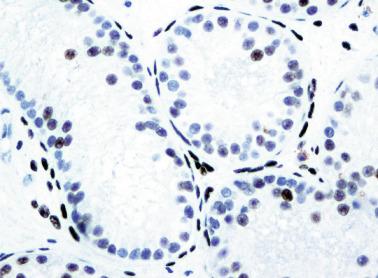
From the fourth to the ninth year of life, the seminiferous tubules and testicular interstitium undergo active growth and development. The seminiferous tubules increase in length, width, and diameter, and the epithelium changes from pseudostratified to columnar. Sertoli cell nuclei remain ovoid, but the outlines become increasingly irregular. The number of cells decreases gradually while the seminiferous tubules lengthen, and the result is that the total number of Sertoli cells per testis increases. At the same time, all spermatogonia types (Ad, Ap, and B) increase in number. The lamina propria now contains one to four fibroblasts embedded in collagen fibers. Cholinergic and adrenergic nerves are observed, with cholinergic nerves ending in the tubular basal lamina.
The testicular interstitium apparently lacks classic morphologic Leydig cells. Isolated Leydig cells persist that are fetal in origin or else developed during minipuberty, with pronounced signs of dedifferentiation, alongside a large number of fibroblast-like cells corresponding to adult Leydig stem cells. The tunica albuginea becomes progressively thicker and more collagenized.
At the end of the growth period, between the fourth and ninth years of life, moderate degeneration of spermatogonia occurs. During this period the control of gonadotropic secretion is likely mediated by inhibiting neuroendocrine secretions, whereas testicular hormone levels are low.
There is autonomic innervation of Leydig cells, with three different types of nerve endings. Type I contains many small agranular vesicles (30 to 60 nm) and occasionally large granular vesicles (100 nm); they are probably cholinergic fibers. Type II nerve endings, with many small granular vesicles (30 to 60 nm) and occasionally large granular vesicles (100 nm), are probably adrenergic fibers. Type III contains large granular vesicles of the mixed type. Most of these nerve fibers are “boutons en passant,” characterized by fibers are separated from Leydig cells by at least 150 nm, but true contact (20 nm) has also been reported.
At approximately 9 years of age, the maturation period begins. The third and definitive wave of spermatogenesis occurs, coinciding with a significant elevation of LH. This is followed by additional increases in the level of this hormone between 13 and 15 years of age. LH induces fibroblast-like Leydig cell precursors to differentiate into mature Leydig cells in the seminiferous tubule walls and in the interstitium. By the end of puberty, the number of Leydig cells per testis is estimated to be 786 million. Leydig cells secrete androgens that, together with the rise in FSH between 11 and 14 years of age, cause Sertoli cell maturation, germ cell development, and appearance of tubular lumina ( Fig. 12.26 ), thus increasing the size of the testes between the ages of 11.5 and 12.5 years. At 10 years of age, the testicular volume is 1.5 mL (three times that of the first year of life). This enlargement is assumed to be the first clinical manifestation of puberty. The spermarche, defined as the first spermaturia, occurs early, and may precede other androgen effects such as the development of secondary sex characteristics and the pubertal growth spurt. Spermaturia is a constant finding when testicular volume is greater than 4 mL (or even lower).
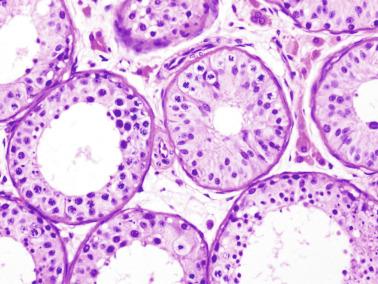
Morphologic changes occurring at puberty involve all testicular structures. Sertoli cells undergo active proliferation in the prepubertal period, a prerequisite to ensure normal spermatogenesis, beginning at about 11 years of age, but is not completed until 13 years of age. Sertoli cell nuclei become enlarged and irregular with indentations; the chromatin becomes looser, and nucleoli acquire a tripartite structure. Prominent cytoplasmic changes include development of endoplasmic reticulum (smooth and rough), elongation of mitochondria with longitudinal cristae, increase in the amount of lysosomes and lipid droplets, appearance of annulate lamellae and Charcot-Böttcher crystals, and development of inter-Sertoli junctional specializations that form the blood-testis barrier. The degree of Sertoli cell maturation may be deduced from AMH levels: high levels when Sertoli cells are immature, with marked decrease after puberty with the advent of meiotic spermatocytes and rise of testosterone.
Proliferation of Sertoli cells is accompanied by an increase in the number of peritubular myoid cells induced by PDGF ligands. The myoid cells in turn contribute to elongation of the seminiferous tubes.
Germ cell proliferation finally achieves efficient spermatogenesis, although morphologic anomalies of spermatozoa are frequent up to the end of puberty. The mean age for appearance of spermatozoa is 13.4 years.
Leydig cell differentiation is rapid, and many interstitial Leydig cell clusters are seen before seminiferous epithelium development is complete.
Collagenization of the tunica albuginea progresses up to the end of puberty, when thickness reaches 400 to 450 μm. Final testicular volume is approximately 20 mL.
From the first month of postnatal life to the 18th year of age, the most common testis-epididymis configuration is connection by the caput and cauda epididymidis (84% of cases), resulting in a digital fossa present between testis and epididymis. A less frequent configuration (12%) is complete testis-epididymis union. Other configurations are pathologic.
Testicular biopsy in children is necessary to determine the nature of the gonads in those with ambiguous genitalia, a history of leukemia or lymphoma whose testes underwent rapid enlargement, or precocious testicular maturation of unknown cause. Testicular biopsy has been replaced by fine needle aspiration in the study of enlargement in patients with leukemia or lymphoma. In other situations the value of biopsy is less clearly established. For example, biopsy of cryptorchid testes during orchidopexy is controversial, although routine performance of such biopsies provided information on precocious development of lesions in cryptorchidism, including explanations of the causes of cryptorchid lesions such as testicular dysgenesis or transient hypogonadotropic hypogonadism, and to abandon the disproven hypothesis of temperature-induced lesions.
Evaluation of biopsy samples of the prepubertal testis should involve assessment of tunica albuginea thickness, mean tubular diameter (MTD), and the number of germ cells, Sertoli cells, and Leydig cells.
The most frequent anomaly of the tunica albuginea is the presence of thin, poorly collagenized, altered tissue layers arranged parallel to the surface resembling ovarian stroma. There may be irregular seminiferous tubules protruding from the testicular surface, a configuration classically known as testicular dysgenesis , including mixed gonadal dysgenesis, dysgenetic male pseudohermaphroditism, and persistent müllerian duct syndrome (PDMS). This alteration results from insufficiency or defective action of AMH. This anomaly may affect all or part of the tunica albuginea.
This lesion should not be misinterpreted as simple seminiferous tubule ectopy, such as that seen in an otherwise normal, well-collagenized tunica albuginea and an orderly arrangement of layers. Focal ectopy of seminiferous tubules is a frequent finding in both normal and cryptorchid testes. In these testes, ectopic seminiferous tubules after puberty may undergo normal germ cell development or become hyalinized. Occasionally, ectopic tubules have cystic dilation that forms a bulbous zone that may be macroscopically visible ( Table 12.2 ).
Thin, poorly collagenized albuginea, ovarian-like stroma
Focal ectopy of testicular parenchyma
Presence of ovocytes in an ovarian-like stroma
In patients with disorders of sex differentiation, groups of ovocytes may replace the tunica albuginea, the characteristic structure of ovotestis.
Evaluation of seminiferous tubules includes qualitative study of the morphology of the epithelial cells and quantitative estimates of MTD and number of germ cells and Sertoli cells.
The MTD is an excellent indicator of development of the seminiferous epithelium. In the prepubertal testis, tubular diameter depends principally on the number and trophism of Sertoli cells, thus indicating whether there is adequate stimulation by FSH. Tubular diameter varies throughout, being smallest at the end of the third year of life, slowly enlarging up to 9 years of age, and rapidly enlarging thereafter up to 15 years, when the tubule reaches its definitive diameter (160 to 190 μm) ( Fig. 12.27 ).
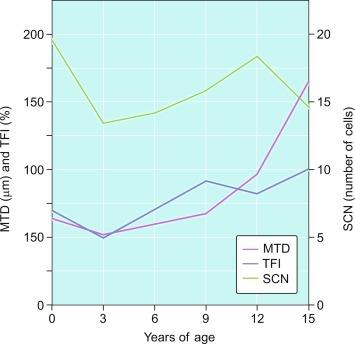
The most frequent abnormality in the prepubertal testis is a low MTD. This is seen in undescended testes and hypogonadotropic or hypergonadotropic hypogonadism ( Table 12.3 ). In the latter condition the lesion results from anomalous Sertoli cell responsiveness to FSH.
Hypogonadotropic hypogonadism
Hypergonadotropic hypogonadism
Undescended testis
Compensatory hypertrophy
Precocious puberty
Benign idiopathic macroorchidism
Macroorchidism associated with fragile X chromosome
Familial testotoxicosis
Macroorchidism associated with hypothyroidism
Megatubules, ring-shaped tubules, tubules with eosinophilic bodies with microliths
Sertoli cell intratubular neoplasia
The three levels of severity of low tubular diameter are slight tubular hypoplasia (≤ 10% reduction in relation to the diameter normal for the age), marked tubular hypoplasia (from 10% to 30% reduction), and severe tubular hypoplasia (> 30% reduction).
High MTD is observed in precocious puberty. There is a focal increase in diameter (precocious tubular maturation) of tubules at the periphery of Leydig cell tumors. This enlargement seems to be produced by elevated androgen concentration, which would also be responsible for precocious tubular maturation. The same occurs with some Sertoli cell tumors.
Diffuse increase in MTD may be unilateral or bilateral ( Table 12.3 ). Unilateral increase is found in monorchidism (compensatory testicular hypertrophy), as well as in some testes that are contralateral to cryptorchid testes. Most frequently, diffuse enlargement occurs in benign idiopathic macroorchidism, macroorchidism associated with fragile X chromosome, familial testotoxicosis, hypothyroidism, and other forms of precocious puberty. Focal increase in MTD is usually associated with precocious maturation of the seminiferous epithelium in tubules at the periphery of Sertoli cell tumors and Leydig cell tumors.
Frequently, infantile testicular biopsies of undescended testes contain several types of malformations such as nearly straight tubules or ramified tubules. Other findings are large tubules (megatubules or ring-shaped tubules), which are malformed tubules displaying a tight spiral course, or bell-shaped deformities (bell-shaped tubules). Megatubules may surround and isolate connective tissue that often develop eosinophilic bodies or microliths. These tubules are likely an indicator of poor prognosis in those with infertility.
The presence of one or more groups of enlarged tubules with marked thickening of the basal lamina in infant testes suggests intratubular Sertoli cell neoplasia. This pattern is frequently seen in infants with Peutz-Jeghers syndrome.
Some infantile testicular biopsies show enlarged tubules with prominent lumina or cystically dilated tubules. Testicular fluid is not produced before puberty, so normal prepubertal tubules do not contain lumina. Therefore the observation of such findings suggests cystic dysplasia of the rete testis . This disorder may include absence or dysplasia of the ipsilateral kidney and urinary excretory ducts.
Germ cells may be counted in two ways: calculation of the number of cells per tubular cross section or determination of the tubular fertility index (TFI). The first method counts the number of germ cells in a light microscopic field and divides this by the number of cross-sectioned tubules. In the first 6 months of postnatal life, the normal testis has two germ cells per cross-sectioned tubule, dropping to 1.5 at the end of the first year and to 0.5 at the end of the third year. The number then increases to 1.8 cells at the age of 3 to 4 years, which coincides with the appearance of spermatocytes in some tubules. This number then increases slowly up to 8 years of age, decreases again up to 9 to 10 years, and increases once more, rising markedly from 12 years of age to the end of puberty. Separation of spermatogonia and gonocyte counts reveals time of last transformation of gonocytes into Ad spermatogonia.
TFI reflects the percentage of tubular sections containing germ cells. In newborns, 68% of tubular sections contain at least one germ cell. From birth to 3 years, this decreases to 50%, followed by a progressive increase to 100% at puberty. The most accurate measure is calculation of total germ cell number per testis. This is more difficult because it requires morphometric assessment of intratubular volume and careful clinical measurement of the three axes of the testis.
Three levels of severity of germinal hypoplasia are recognized: slight (TFI > 50), marked (TFI between 50 and 30), and severe (TFI < 30). Marked and severe germinal hypoplasia is usually associated with marked or severe tubular hypoplasia, in most cases resulting from tubular dysgenesis. It may also be useful to determine whether the seminiferous tubules devoid of germ cells are randomly distributed. If grouped, they probably belong to the same lobule or group of lobules that will never develop normally.
Congenital decrease of germ cells occurs in numerous conditions, including trisomies 13, 18, and 21; some forms of primary hypogonadism such as Klinefelter syndrome; anencephaly; cryptorchid testes and posterior urethral valves; and severe obstruction of urinary ducts ( Table 12.4 ). Congenital germ cell decrease may result from deficient colonization of genital ridges by primordial germ cells, reduced germ cell proliferation, or increased germ cell loss. In Klinefelter syndrome, defective germ cell colonization has been suggested as the cause because of the high incidence of extragonadal germ cell tumors in such patients.
13, 18, 21 trisomies.
Klinefelter syndrome.
Anencephalia.
Cryptorchidism.
Patients with posterior urethral valves.
Treatments with antitumoral chemotherapy.
Treatments with immunosuppression.
Therapies in transplantation.
Parenchyma close to either germ cell tumors or gonadal-stroma tumors.
During infancy and childhood a significant reduction in the number of spermatogonia occurs in children undergoing chemotherapy or immunosuppression. An increased number of germ cells may be seen at the periphery of germ cell tumors, gonadal-stromal tumors, and paratesticular sarcomas.
Other altered germ cells include multinucleate or hypertrophied spermatogonia and gonocyte-like cells. Multinucleate spermatogonia have two to four nuclei, and Ap and Ad nuclei may coexist within the same cell, representing a failure of cytokinesis. Hypertrophic spermatogonia are located over the basal lamina and exhibit large, usually hyperchromatic nuclei and abundant cytoplasm, findings indicating polyploid cells that are unable to complete cellular division. Most of the hypertrophic spermatogonia degenerate rapidly. Gonocyte-like cells are located among Sertoli cells in the center of tubules, appearing as large cells with ovoid nuclei, large central nucleoli, and small heterochromatin granules. These cells should not be misinterpreted as cells from germ cell neoplasia in situ (GCNIS). Tumoral cells of GCNIS, which share with gonocytes immunoreactivity for PLAP, KIT, and OCT3/4, also show immunoreactivity for SCF. The presence of gonocytes is common in gonads of patients with disorders of sexual differentiation, and this finding often indicates delay in germ cell maturation.
Complete maturation of the seminiferous epithelium at early ages may occur in patients with precocious puberty, as well as in the testicular parenchyma at the periphery of Leydig cell tumors.
The number of Sertoli cells per tubular cross section varies during childhood as a result of slow proliferation from 4 to 12 years and redistribution as seminiferous tubules become longer and broader. An apparent decrease in Sertoli cell number results from cellular displacement as a consequence of slow growth in length and tortuosity of tubules and not from degeneration. The pseudostratified cellular pattern characteristic of Sertoli cells at birth and the first months of life changes slowly to a columnar pattern during later infancy. During puberty, three phenomena occur: proliferation of Sertoli cells with emergence of pseudostratified seminiferous epithelium that ensures growth of tubules; Sertoli cell maturation; and subsequent transformation of pseudostratified epithelium into the columnar epithelium that is characteristic of the adult testicle. Proliferation is under the control of FSH and testosterone, ensuring optimum sperm production.
Testicular biopsies may reveal hypoplasia or hyperplasia of Sertoli cells. Hypoplasia may indicate congenital hypogonadotropic hypogonadism, Kallmann syndrome, Prader Willi syndrome, DAX1 mutation, or multiple pituitary hormone deficiency. Hyperplasia may be observed in several pathologic states and manifests itself at different moments of development. In childhood, hyperplasia is observed in most cases of macroorchidism, because most of the volume of the testis at this time depends on the number of Sertoli cells. This also applies for patients with the syndrome of fragility of the X chromosome and in those with peripheral precocious pseudopuberty, a component of McCune-Albright syndrome (MAS). Sertoli cell hyperplasia seen at the beginning of puberty is characteristic of cryptorchid testicles and reflects the inability of the growth in length and tortuosity of the seminiferous tubules, and, to a lesser extent, an absolute increase in number of Sertoli cells. This is considered a sign of tubular dysgenesis. Biopsies may reveal one or several tubular sections containing Sertoli cells with eosinophilic and granular cytoplasm that is positive for CD68 and α 1 -antitrypsin, oncocytic changes that result from lysosomal accumulation; this is considered a primary anomaly.
Calculation of Leydig cell number during childhood is difficult because of the low numbers. Use of semithin sections or immunohistochemistry to detect cells containing testosterone or calretinin may be helpful. Selection of the appropriate denominator to express the Leydig cell population is another problem. The most frequent measures are Leydig cell number per tubular section or per unit area, or total Leydig cell number per testis.
Low numbers of Leydig cells are observed in undescended testes, hypogonadotropic hypogonadism, disorders of sexual differentiation caused by an anomaly in LHRs, and anencephaly. High numbers occur in congenital Leydig cell hyperplasia, triploidy, variants of precocious puberty, and several syndromes such as leprechaunism and Beckwith-Wiedemann syndrome ( Table 12.5 ). Focal accumulation of Leydig cells with broad and microvacuolated cytoplasm caused by the presence of lipids is characteristic of patients with sexual developmental disorders secondary to mutations of the NR5A1 ( SF1 ) gene. They may also be seen in patients with defects in androgen synthesis.
Hypogonadotropic hypogonadism.
Undescended testes.
Defects in luteinizing hormone receptors.
Anencephalic fetuses.
Congenital hyperplasia of Leydig cells (maternal diabetes mellitus).
Malformative syndromes (Beckwith-Wiedemann syndrome, leprechaunism).
Precocious puberty.
The seminiferous tubules are normally closely packed, separated only by a small amount of loose connective tissue that maintains cohesion among the tubules and contains scant Leydig cells, macrophages, mast cells, blood vessels, and nerves. This intertubular connective tissue may be altered, including increased amount, increased cellularity, abnormal development of lymphatic vessels, and the presence of cell types that are unusual in this location.
An apparent increase in loose connective tissue is found in patients with marked tubular hypoplasia. The cellular basis for increased connective tissue is uncertain. Some testes have thick fusiform cell bundles that separate groups of closely packed seminiferous tubules. These cells are reminiscent of the cells that form ovarian stroma and are the most characteristic histologic finding in Botella-Nogales-Morris syndrome (a sex differentiation disorder secondary to androgen insensitivity).
Other alterations include the presence of overly developed lymphatic vessels (congenital testicular lymphangiectasis), focal hematopoiesis, leukemic infiltrate, and the presence of cells reminiscent of the adrenal cortex (tumors of the adrenogenital syndrome).
The adult testis is an egg-shaped organ suspended in the scrotum from the spermatic cord, the retroepididymal surface, and the scrotal ligament. Mean weight in white men is 21.6 ± 0.4 g for the right testis and 20 ± 0.4 g for the left. Mean testicular diameter is 4.6 cm (range, 3.6 to 5.5 cm) for the longest axis and 2.6 cm (range, 2.1 to 3.2 cm) for the shortest. Testicular volume varies from 15 to 25 mL. Testicular volume correlates with height, weight, body mass index, and body surface area, and it decreases in adulthood.
The tunica albuginea and interlobular septa make up the connective tissue framework of the testis. The tunica albuginea consists of three connective tissue layers: an outer layer of mesothelium apposed to the basal lamina ( tunica vaginalis ), a middle layer of dense fibrous tissue, and an inner layer of loose connective tissue ( tunica vascularis ) with nerve fibers and abundant blood and lymphatic vessels. From the outer to the inner layers, the amount of collagen fibers decreases, whereas the number of cells increases. The fibers and cells in the two outermost layers form planes parallel to the testicular surface; cell types include fibroblasts, myofibroblasts, mast cells, and nervous fibers. Myofibroblasts are more numerous in the posterior portion of the testis ( Fig. 12.28 ). The thickness of the tunica albuginea increases with age from 400 to 445 μm in young men to more than 900 μm in older men. The tunica albuginea acts as a semipermeable membrane that produces the fluid of the vaginalis cavity. The presence of many contractile cells showing high concentrations of guanosine monophosphate suggests that the tunica albuginea undergoes cycles of contraction and relaxation. The cells may regulate testicular size and favor the transport of spermatozoa into the epididymis. The interlobular septa consist of fibrous connective tissue with blood vessels supplying the testicular parenchyma. The interlobular septa divide the testis into approximately 250 pyramidal lobules, with the bases at the tunica albuginea and vertices at the mediastinum testis. Each lobule contains two to four seminiferous tubules and numerous Leydig cells.
Adult seminiferous tubules are 180 to 200 μm in diameter and 30 to 80 cm long. The total combined length of the seminiferous tubules is approximately 540 m (range, 299 to 981 m). These tubules are highly convoluted and tightly packed within the lobules. The seminiferous tubules comprise approximately 80% of testicular volume. The tubular lining of germ cells and Sertoli cells is surrounded by a lamina propria (tunica propria) ( Fig. 12.29 ).
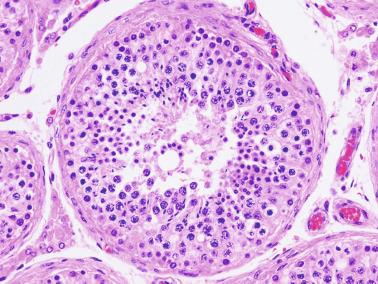
Sertoli cells are columnar cells that extend from the basal lamina to the tubular lumina, with 10 to 12 cells per cross-sectioned tubule. They are easily identified by their nuclear characteristics. Nuclei are located near the basal lamina and appear triangular with an indented outline. They are euchromatic, as expected of cells that express a great proportion of the genome to synthesize a large variety of substances required for its multiple functions. Nucleoli are central and voluminous ( Fig. 12.30 ). The cytoplasm has lateral processes that spread out and surround adjacent germ cells and touch other Sertoli cells, facilitating direct contact with 40 to 50 germ cells and 6 to 7 adjacent Sertoli cells. Charcot-Böttcher crystals and lipid droplets often are visible in the cytoplasm.
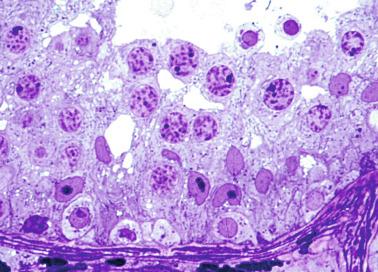
The number of Sertoli cells per testis decreases from approximately 250 million in young men to 125 million in men who are older than 50 years of age. A positive correlation exists between the number of Sertoli cells and daily sperm production. Sertoli cells are the target of FSH and androgen action. In adulthood these cells produce testicular fluid through an active transport mechanism, creating and maintaining the lumen of the seminiferous tubule. Testicular fluid is isosmotic, with a high content of potassium that exposes various membrane and water transporters. The fluid provides an optimal milieu for developing spermatozoa and a vehicle to transport them from the testis.
Ultrastructurally, Sertoli cells have characteristic nucleoli, plasma membranes, and cytoplasmic components. Nucleoli have a tripartite structure with round fibrillar centers, compact granular portions, and three-dimensional nets composed of intermingled fibrillar and granular portions. The plasma membrane has two types of intercellular junctions that develop at puberty: junctions between adjacent Sertoli cells and junctions between Sertoli cells and germ cells. The ectoplasmic junction is a unique and specific junction that consists of Sertoli-Sertoli cell junctions (basal ectoplasmic specializations) and Sertoli-germ cell junctions (apical specializations). Sertoli-Sertoli junction specializations consist of adherens, tight junctions, and gap junctions that are dynamically remodeled to allow the movement of germ cells across the seminiferous epithelium and the timely release of spermatids into the tubular lumens. This dynamic remodeling of cell junctions is mediated by several mechanisms at transcription and posttranslation. In addition, ectoplasmic specializations also confer cell orientation and polarity in the seminiferous epithelium. Within the plasma membrane are adhesion molecules such as connexin 43 that may regulate other proteins of intercellular junctional complexes that occur between adjacent cells. The presence of connexin depends on the seminiferous epithelium stage, absent at stages II and III when primary spermatocytes cross from the basal to the adluminal compartment.
Three different types of membrane protein have been identified in the testis: occludin, claudins, and adherens junction molecules. In addition, beneath the Sertoli cell plasma membrane and cisterns of the endoplasmic reticulum, several molecules are recognized: actin filaments, anchorage molecules, vinculin, zonula occludens 1 (ZO-1) and ZO-2, plakoglobin, radixin, and nectins.
The inter-Sertoli cell junctions are the morphologic basis for the blood-testis barrier and divide the seminiferous epithelium into two compartments; the basal compartment contains spermatogonia and newly formed primary spermatocytes, whereas the adluminal compartment contains meiotic primary spermatocytes, secondary spermatocytes, and spermatids. These junctions permit each compartment to have its own microenvironment for spermatogenic development. The Sertoli cells, the only cell types present in both compartments, together with primary spermatocytes, become the key to control the passage of information from one compartment to another. There is a transient compartment between the basal and adluminal compartments, where preleptotene and leptotene spermatocytes linger before entering the adluminal compartment. The blood-testis barrier is always secured by the existence of tight junctions both apically and basally to the cells. Definitive establishment of the blood-testis barrier is complete when Sertoli cell proliferation and maturation ceases, features coinciding with the initiation of meiosis. Terminal differentiation of Sertoli cells is regulated to a great extent by retinoblastoma protein.
Sertoli cell-germ cell junctions (desmosomes and tight junctions) persist from the primary spermatocyte stage through spermatozoon release. Adhesion of Sertoli cells and germ cells is mediated by N-cadherin. These glycoproteins are involved in maintaining the seminiferous epithelium architecture and germ cell migration from the basal to the adluminal compartment. These junctions may also be present between spermatogonia.
Sertoli cell basal cytoplasm contains abundant smooth endoplasmic reticulum (involved in steroid synthesis), scant rough endoplasmic reticulum (involved in protein synthesis), annulate lamellae, Golgi complex, lysosomes, residual bodies, glycogen granules, lipid droplets in amounts that vary with the seminiferous tubule cycle, Charcot-Böttcher crystals (structures several micrometers long, formed of multiple parallel laminae of protein), and ribosomes. The apical cytoplasm contains elongate mitochondria, numerous microtubules, and large numbers of vesicles with degraded cytoplasmic fragments derived from phagocytosis of residual bodies of spermatids.
Sertoli cells are immunoreactive for inhibin, WT1 ( Fig. 12.31 ), GATA4, SOX9, follicle-stimulating hormone receptor (FSHR), vimentin, nuclear AR ( Fig. 12.32 ), F-actin filaments, and α-tubulin in the cytoplasm. Actin filaments appear in both inter-Sertoli cell junctions and ectoplasmic specializations that surround germ cells. F-actin bundles contribute to formation of Sertoli-Sertoli cell junctions arranged at regular intervals beneath the plasma membrane and cistern of the endoplasmic reticulum connected to microtubules. Actin distribution varies during the cycle of the seminiferous epithelium. It is more abundant next to the heads of elongate spermatids (ectoplasmic specializations) and in the Sertoli cell cytoplasm that surrounds spermatids. Actin filaments, intermediate filaments (vimentin), and microtubules make up the cellular cytoskeleton, one of the more developed somatic cell cytoskeletons ( Fig. 12.33 ).
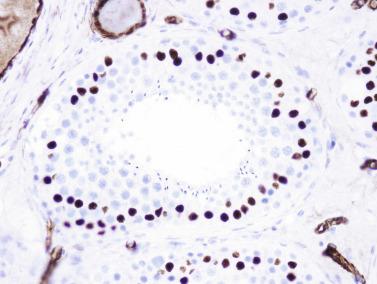
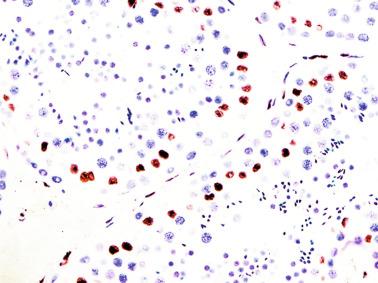
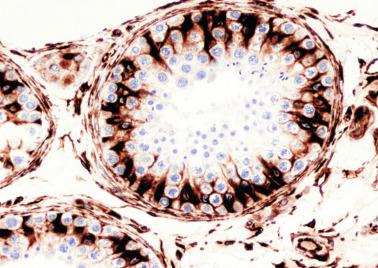
Sertoli cells synthesize multiple products to ensure the nutrition, proliferation, and maturation of germ cells; they also stimulate other cells such as Leydig cells and peritubular cells, and contribute to hormonal regulation (inhibin secretion) ( Table 12.6 ). Transport of small molecules (< 600 to 700 Da) such as pyruvate, lactate, and probably choline from the Sertoli cell to germ cells occurs through gap junctions. Large or small soluble molecules are transported by proteins that are synthesized by Sertoli cell and include androgen-binding protein (ABP), transferrin, ceruloplasmin, sulfated glycoproteins, α 2 -macroglobulin, and γ-glutamyl transpeptidase. Activin and inhibin are Sertoli cell–secreted proteins that induce proliferation and differentiation of germ cells. Activin stimulates FSH production and resultant spermatogonial proliferation, and controls the seminiferous epithelium cycle, whereas inhibin B inhibits FSH secretion and is an important marker of spermatogenesis ( Fig. 12.34 ). Other Sertoli cell secretions include interleukins, mainly IL1, and growth factors such as TGFB, IGF1 and IGF2, and seminiferous growth factor. Some of these growth factors, such as TGFA, TGFB, and IGF1, are involved in regulation of Leydig cell function. Other secreted substances include clusterin, the steroid 3-α-4-pregnen-20-one, and prostaglandin D synthase (PGDS) ( Table 12.7 ). Sertoli cells are also involved in migration of differentiating germ cells toward the tubular lumens. This movement leads to continuous remodeling of the plasma membrane and requires synthesis of several proteases, including urokinase, tissue-type plasminogen activator, cyclic protein 2, collagenase IV, other metalloproteins, and several antiproteases, such as cystatin C, tissue inhibitor of metalloproteinase type 2, and α 2 -macroglobulin. Differentiation of germ cells requires different molecules such as SCF, bone morphogenetic protein 4, and retinoic acid. Sertoli cells also regulate germ cell apoptosis. Approximately one-half of spermatogonia in differentiation undergo apoptosis. Dead cells are degraded by Sertoli cells and then recycled. Regulation of apoptosis occurs by production of Fas-ligand, which binds to its receptor (APO-1, CD95) in germ cell plasma membranes. In addition, Sertoli cells possess receptors for several factors such as the nerve growth factor produced by spermatocytes and young spermatids, underscoring the complexity of the Sertoli cell–germ cell relationship. Sertoli cells also produce steroid hormones (estradiol and testosterone) and several components of the seminiferous tubule wall, including laminin, type IV collagen, and heparin sulfate–rich proteoglycans.
| Paracrine Factor | Origin | Receptor | Action |
|---|---|---|---|
| Androgens | Leydig cell | Sertoli cell | Regulate/maintain function and differentiation |
| Proopiomelanocortin peptides | Leydig cell | Sertoli cell | Decrease FSH actions |
| β-Endorphin | Leydig cell | Sertoli cell | Decrease steroidogenesis |
| GnRH-like factor | Sertoli cell | Leydig cell | Decrease steroidogenesis |
| Estrogens | Sertoli cell | Leydig cell | Decrease steroidogenesis |
| TGFA | Sertoli cell | Leydig cell | Decrease steroidogenesis |
| Interleukin-1 | Sertoli cell | Leydig cell | Decrease steroidogenesis |
| IGF1 | Sertoli cell | Leydig cell | Increase steroidogenesis |
| Inhibin | Sertoli cell | Leydig cell | Increase steroidogenesis |
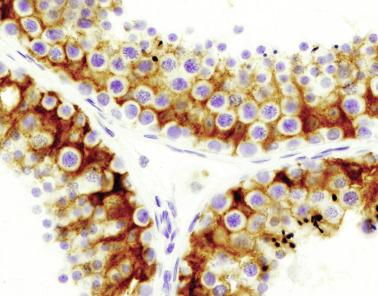
| Products | Functions/Characteristics |
|---|---|
| Transport-Binding Proteins | |
| Androgen-binding protein (ABP) | Androgen transport |
| Transferrin | Iron transport |
| Ceruloplasmin | Copper transport |
| Sulfated glycoprotein-1 | Sphingolipid binding |
| Regulatory Proteins | |
| Inhibin | Endocrine-paracrine agent |
| Müllerian duct inhibitory agent | Development |
| Sulfated glycoprotein-2 | Sperm coating, immunosuppressant |
| Growth Factors | |
| GDNF | Glial cell–derived neurotrophic factor |
| FGF2 | Fibroblast growth factor 2 |
| TGFA | Growth stimulation |
| TGFB | Growth inhibition |
| IGF1 | Maintain growth/differentiation |
| Interleukin-1 | Growth regulation |
| Metabolites | |
| Lactate-pyruvate | Energy metabolites |
| Estrogens | Steroid hormone, endocrine-paracrine agents |
| Proteases/Inhibitors | |
| Plasminogen activator | Plasminogen activation |
| Cyclic protein-2 | Cathepsin activity |
| α 2 -Macroglobulin | Protease inhibitor |
| Extracellular Matrix Components | |
| Laminin | |
| Collagens I and IV | |
| Proteoglycans | |
Germ cells of the adult testis include spermatogonia, primary and secondary spermatocytes, and spermatids ( Fig. 12.29 ).
There are two types of spermatogonia: A and B. Type A spermatogonia are approximately 12 μm in diameter, rest on the basal lamina, and are surrounded by the cytoplasm of adjacent Sertoli cells. Nuclei are spherical, contain several peripheral nucleoli, and have four different patterns according to their shape, size, and nuclear staining features: Ad (dark), Ap (pale), Al (long), and Ac (cloudy). The cytoplasm of Type A spermatogonia contains a moderate number of ribosomes, small ovoid mitochondria joined to each other by electron-dense bars, and Lubarsch crystals. These are several micrometers long and are composed of numerous 8- to 15-nm parallel filaments intermingled with ribosome-like granules. Ad spermatogonia are thought to be stem cells in spermatogenesis, and under normal conditions do not divide. Some Ad spermatogonia replicate their DNA and acquire an elongate shape (Al spermatogonia). They later divide to make another Ad (maintaining the stem cell reservoir) and an Ap spermatogonia. During replication, Ap spermatogonia become Ac and then divide by mitosis to form two type B spermatogonia.
Type B spermatogonia are the most numerous, and their contact with the basal lamina is less extensive than that of type A. Nuclei usually are more distant from the basal lamina than are those of type A spermatogonia and contain one or two large central nucleoli. The cytoplasm contains more ribosomes than type A spermatogonia, and intermitochondrial bars are usually not observed. Type B spermatogonia divide to form primary spermatocytes.
Although all type A spermatogonia are located on the basal membrane, their distribution varies in the seminiferous tubules from one area to another. Spermatogonial stem cells (SSCs) are in specific areas of the basal membrane known as the SSC niche. The niche may be defined as a space limited by somatic cells (Sertoli cells) and basal membrane. The number of SSCs and niches is determined by the Sertoli cells. The niches are preferably located in those segments of seminiferous tubules that line the small intertubular spaces between three or four seminiferous tubules that frequently contain small blood vessels and Leydig cells. In contrast, the spermatogonia in differentiation reside along the basal membrane, where seminiferous tubules are in contact with each other ( Fig. 12.35 ).
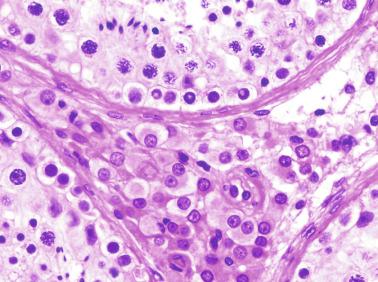
Sertoli cells, Leydig cells, myoid cells, macrophages, and endothelial cells with several growth and transcription factors participate, either directly or indirectly, in regulation of self-renewal or differentiation of spermatogonia. The best-known growth factors are glial cell line–derived neurotropic factor (GDNF), FGF2, SCF, activin A, bone morphogenic protein 4 (BMP4), and colony-stimulating factor 1 (CSF1). GDNF and FGF2 are secreted by Sertoli cells, and CSF1 is secreted by Leydig cells, myoid cells, macrophages, and endothelial cells. Different factors regulate proliferation, self-renewal, and expansion of SSCs. Among transcription factors, the following have similar actions: Bcl6c, Etv5, and Lhx1 dependent on GDNF, Taf4b (TATA box-binding protein-associated factor 4b), and Plzf. Activin A, SCF, and BMP4, produced by Sertoli cells, seem to be involved in stem cell differentiation ( Fig. 12.36 ). Each step in differentiation of spermatogonia, including formation of spermatocytes and spermatids, is regulated by the microenvironment.
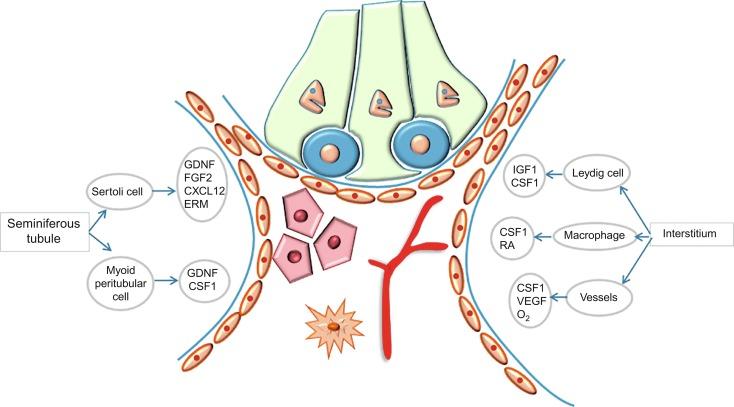
Primary spermatocytes at interphase of the cell cycle lose contact with the basal lamina and inhabit cavities formed by the Sertoli cell cytoplasm. Their cytoplasm contains more rough endoplasmic reticulum than that of spermatogonia, and the Golgi complexes are more developed. Meiotic primary spermatocytes are tetraploid and are readily identified by their chromatin pattern. The leptotene spermatocyte, with filamentous chromatin, leaves the basal compartment and migrates first to an intermediate compartment and then to the adluminal compartment. In the zygotene spermatocyte, chromosomes are shorter, and pairing of homologous chromosomes begins. Ultrastructural studies show coarse chromatin masses in which synaptonemal complexes and sex pairs may be present. Nucleoli acquire a peculiar pattern of segregation of the fibrillar and granular portions. Associated with nucleoli are the round bodies that contain proteins but no nucleic acids. In pachytene spermatocytes, homologous chromosomes are completely paired, and ultrastructurally show chromatin masses that are larger and less numerous than in zygotene spermatocytes. In diplotene spermatocytes, the larger spermatocytes, paired homologous chromosomes begin to separate and remain joined by the points of interchange (chiasmata); neither synaptonemal complexes nor sex pairs are observed. Diakinesis spermatocytes show maximal chromosome shortening, and the chiasmata begin to resolve by displacement toward the chromosomal ends. Nuclear envelopes and nucleoli disintegrate. The spermatocytes complete the other phases of the first meiotic division (metaphase, anaphase, and telophase), thus forming two secondary spermatocytes; the first meiotic division lasts 24 days. Prophase of the first division usually lasts from 1 to 3 weeks, whereas the remaining phases of the first meiotic division occur in less than 2 days.
Secondary spermatocytes are haploid cells, smaller than primary spermatocytes, and contain coarse chromatin granules and abundant rough endoplasmic reticulum cisternae. These cells rapidly undergo second meiotic division and within 8 hours give rise to two spermatids. The newly formed spermatids have smaller nuclei with homogeneously distributed chromatin, unlike secondary spermatocytes.
Transformation of spermatids into spermatozoa is called spermiogenesis. During this process pronounced changes occur in the nuclei and the cytoplasm. Nuclei become progressively darker and elongate, and chromatin is rearranged before it becomes completely condensed. The cytoplasm develops the acrosome and flagellum, mitochondria cluster around the first portion of the spermatozoon tail, and the remaining cytoplasm is phagocytized by Sertoli cells. By electron microscopy, four transient stages of spermatid development are seen: Golgi, cap, acrosome, and maturation. These stages correspond to those defined by light microscopy of nuclear morphology: Sa, Sb, Sb 1 , Sb 2 , Sc, Sd 1 , and Sd 2 . These phases may be grouped as early (or round) spermatids that comprise the stages with round nuclei (Sa + Sb) and as late (or elongate) spermatids that comprise the stages with elongate nuclei (Sc + Sd). Mature spermatids (Sd 2 ) are the spermatozoa released into the tubular lumens (spermiation). The presence of spermatids and phagocytosis of their residual bodies stimulates the Sertoli cells to initiate a new cycle of the seminiferous epithelium and to produce tubular fluid, inhibin, ABP, IL1, and IL6. All germ cells derived from the same stem cell remain interconnected by cytoplasmic bridges to ensure synchronous maturation during the spermatogenic process.
At first glance the germ cells in the seminiferous tubules appears disordered. However, closer study reveals that these cells are grouped into six successive steps, designated I to VI. In contrast with other mammals, in humans the volume occupied by each step is small, so several steps may be observed in the same tubular cross section. Stereologic studies have shown that the successive steps are organized helically along the length of the seminiferous tubule. Each association persists for a specific number of days (I, 4.8 days; II, 3.1 days; III, 1 day; IV, 1.2 days; V, 5 days; and VI, 0.8 days), and each successively transforms into the following association. Finally, at the end of step VI, the cycle is repeated; the spermatogenic process requires several cycles. The time from initiation of spermatogenesis to the appearance of sperm in the ejaculate is only 64 days, instead of 74 days, as was formerly believed ( Fig. 12.37 ).
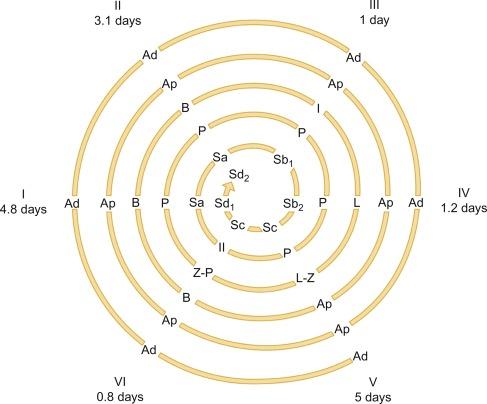
The succession of different steps probably depends on cyclic Sertoli cell activity. Cyclic changes in the mitochondria, rough endoplasmic reticulum, Golgi complexes, lysosomes, and lipid droplets have been reported. This cyclic activity is probably regulated by numerous factors, both intrinsic (Sertoli and germ cells) and extrinsic (androgens and retinoic acids).
The efficiency of spermatogenesis (daily sperm production per gram of parenchyma) in humans is only 25% to 35% of that found in most species, including other primates, and is no more than 3 to 7 million per gram of testis per 24 hours. Germ cell degeneration at the end of meiosis is important. The ratio of spermatids to primary spermatocytes is 2.45:1 instead of the expected 4:1 because of a high degree of spontaneous apoptosis, which mainly affects primary spermatocytes but also involves spermatogonia and spermatids. When spermatogenesis is defective, the number of stages that may be observed in the same cross-sectioned tubule decreases.
The seminiferous tubules are surrounded by a 6-μm-thick lamina propria (tunica propria) consisting of a basement membrane, myofibroblasts (peritubular myoid cells), fibroblasts, collagen and elastic fibers, and extracellular matrix. The basement membrane measures 100 to 200 nm in thickness and has three layers: the lamina lucida (beneath the Sertoli cells), lamina densa (basal lamina), and lamina reticularis (a discontinuous layer containing fibers). The basal lamina contains laminin, type IV collagen, entactin (nidogen), and heparan sulfate. External to the basal lamina are five to seven discontinuous layers of flat elongate peritubular cells. The two outer layers are formed by fibroblasts and the remainder by peritubular myoid cells.
Peritubular myoid cells , also known as myofibroblasts, form three or five innermost layers of the tubular walls. Ultrastructurally these cells have numerous actin and myosin-immunoreactive filaments, dense plaques, an abundance of free ribosomes, small mitochondria, and poorly developed rough endoplasmic reticulum and Golgi complexes. Peripheral borders of myofibroblasts are divided into laminar prolongations arranged in two planes. Myofibroblasts express ARs, smooth muscle cell antigens (smooth muscle actin, α-actin, desmin, GB-42, and myosin) ( Fig. 12.38 ), and fibroblast antigens (vimentin, fibroblast surface protein). The cells are contractile and secretory, facilitating rhythmic tubular contractions that propel spermatozoa toward the rete testis. They also synthesize numerous products, including those involved in paracrine regulation of Sertoli cells such as PModS (peritubular modifies Sertoli) that modulates the secretion of ABP, transferrin, inhibin, IGF1, bFGF, and many interleukins. Others such as GDNF and CSF1 are involved in regulation of SSC renewal. Finally, myoid cells contribute to formation of tubular walls, secreting fibronectin, collagens, proteoglycans and entactin, and angiogenic factors such as vascular endothelial growth factor-C and antiangiogenic pigment epithelium-derived factor involved in the avascularity of the seminiferous tubules ( Table 12.8 ).
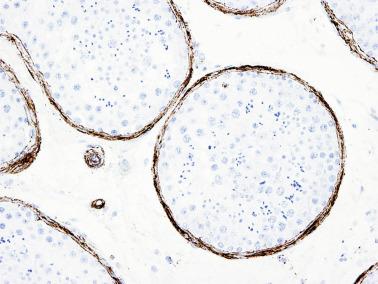
| Products | Functions/Characteristics |
|---|---|
| Paracrine Regulation of Sertoli Cells | |
| P-mod-S | Modulate ABP, transferrin, and inhibin secretion |
| IGF1, βFGF, several interleukins | Regulate multiple functions |
| Plasminogen activator inhibitor | Inhibition of plasminogen activator activity |
| Niche | |
| CDNF and CDF1 | Self-renewal of spermatogonia stem cells |
| Tubular Wall Formation | |
| Fibronectin, collagen I, proteoglycans, and entactin | Extracellular matrix component |
| Angiogenic (VEGF-C) and antiangiogenic (PEDF) factors | Prevent seminiferous tubule vascularization |
Most functions of peritubular cells are under androgenic control. Contractile function is regulated by angiotensin II activin via type angiotensin receptor, oxytocin, PDGF, neurotransmitters, and prostaglandins. Tumor necrosis factor-α strongly upregulates production of proinflammatory interleukins.
Fibroblasts in the two outermost layers lack desmin filaments and have less actin and myosin than the myofibroblasts. Two types of fiber may be found: collagen and elastic fibers. Collagen fibers are present among the peritubular cells and are abundant between the basal lamina and the peritubular cells. Elastic fibers are located mainly at the periphery of peritubular cells. Elastic fibers appear at puberty, so their absence in adults is a sign of tubular immaturity or dysgenesis. In addition, the tubular walls may contain capillaries and Leydig cells. These cells are similar to interstitial Leydig cells and are referred to as peritubular Leydig cells.
The interstitium between the seminiferous tubules contains Leydig cells, macrophages, neuron-like cells, mast cells, blood vessels, lymphatic vessels, and nerves, accounting for 12% to 20% of testicular volume.
The most numerous connective tissue cells are fibroblasts and myofibroblasts. Fibroblasts are also known as interstitial dendritic cells or CD34+ stromal cells. They display a network around the seminiferous tubules and Leydig cells, and form the outermost layers of the tubular wall ( Fig. 12.39 ). This distribution begins in fetal life. Some of these cells are in contact with typical macrophages, so it has been suggested that they may be involved in immune surveillance. Myofibroblasts, in addition to their presence in the inner layer of the tubular wall, are numerous in the tunica albuginea.
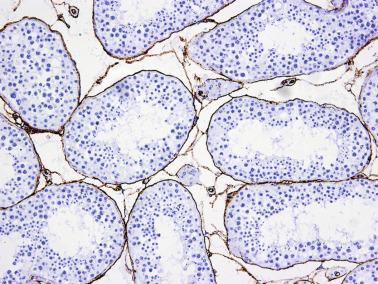
Leydig cells are distributed singly or in clusters, comprising approximately 3.8% of testicular volume. Most are in the interstitium, although they may also be found in ectopic locations such as inside the tubular tunica propria, mediastinum testis, tunica albuginea, epididymis, and spermatic cord. Extratesticular Leydig cells are usually seen within or near nerve trunks.
Leydig cells have spherical eccentric nuclei with one or two eccentric nucleoli and prominent nuclear lamina. The cytoplasm is abundant and eosinophilic, containing lipid droplets and lipofuscin granules (residual bodies) ( Fig. 12.40 ). Reinke crystalloids are found only in adults. Although it was formerly believed that these crystals were present exclusively in humans, they have also been observed in the wild bush rat. Reinke crystalloids dissolve completely in formalin and partially in alcohol. They stain with anti–3β-HSD antibodies. Reinke crystalloids are rodlike, up to 20 μm long and 2 to 3 μm wide, consisting of a complicated meshwork of 5-nm filaments with a trigonal lattice arrangement. Depending on the plane of section, three basic aspects of this lattice may be discerned. Frequently the crystalloids display pale lines, considered to be potential planes of cleavage. The filaments are grouped into 19-nm-wide hexagons visible on cross section. Some areas have aggregates of electron-dense, rod-shaped structures. Leydig cells may harbor other types of paracrystalline inclusions, the most common of which are multiple parallel-folded laminae.
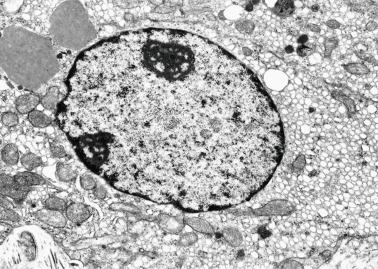
Leydig cells contain abundant, well-developed smooth endoplasmic reticulum, pleomorphic mitochondria with tubular cristae, numerous lysosomes, and peroxisomes. Marked morphologic changes in Leydig cells occur during the six stages of the seminiferous epithelium cycle.
Leydig cells in the adult originate at puberty from fibroblast-like precursor cells under LH stimulation. Studies in adult rats reveal that Leydig cells originate from peritubular cells, vascular smooth muscle cells, and blood capillary pericytes. Leydig cell precursors resemble nervous system stem cells because of their expression of nestin and some neuron and glial cells features. Adult Leydig cells infrequently undergo mitosis. The human testis contains approximately 200 million Leydig cells. This number decreases with age; the testes of 60-year-old men contain approximately one-half as many Leydig cells as do those of 20-year-old men. Leydig cells in aging men often show cytoplasmic hypertrophy to balance the loss in number, and some of these cells are giant and multinucleate. Lipids and lipofuscin increase progressively. Testosterone production is maintained at normal levels up to the end of the fifth decade because of the high number of persistent Leydig cells. Most elderly men have increased LH levels, but only 22% have low testosterone levels.
Leydig cells comprise a specialized population of cells with endocrine, neuroendocrine, and paracrine functions. They are immunoreactive for LHRs, 3β-HSD, relaxin-like factor, inhibin, and ghrelin. Relaxin-like factor, more commonly known as INSL3, is a peptide involved in testicular descent that may be found in serum. Its concentration is a marker of Leydig cell functional status. As occurs with testosterone, INSL3 production is associated with LH. Leydig cells are immunoreactive for calretinin, a 29-kDa calcium-binding protein that has a buffering effect to avoid abnormal increase in intracellular calcium. Calretinin is a more sensitive, albeit less specific, marker than inhibin ( Fig. 12.41 ). Leydig cells also contain VEGF and its two receptors (Flt-1 and KDR) and endothelin and its two receptors (α and β). VEGF and endothelin are involved in paracrine and autocrine control of Leydig cells. These cells also contain certain substances that suggest neuroendocrine function, including oxytocin, proopiomelanocortin, substance P, endorphin, NCAM, and microtubule-associated protein-2 (MAP2). Immunohistochemical studies have demonstrated synaptophysin, chromogranins A and B, neurofilament proteins, neuron-specific enolase, S100 protein, and gliofibrillary acidic protein expression in Leydig cells adjacent to nerves, justifying inclusion in the diffuse endocrine system or paraneuron family. Leydig cells are the targets of LH and several paracrine and growth factors produced by Sertoli cells and other cells, including IGF1 (secreted by Sertoli cells and by the Leydig cell itself) that improves Leydig cell response to hCG administration; inhibin and activin, which enhance Leydig cell function; and IL1 (synthetized by Sertoli cells, germ cells, Leydig cells, and macrophages), a stimulant of DNA synthesis in immature Leydig cells. TGFB (produced by Leydig cells and Sertoli cells) is a potent Leydig cell inhibitor. In response to LH stimulation, Leydig cells produce testosterone and other androgens necessary for maintenance of spermatogenesis and many structures of the male genital tract and other tissues of the body (bone, muscle, and skin). Mean daily testosterone production by Leydig cells is 6 to 7 mg, representing more than 95% of the circulating testosterone, reaching levels of intratesticular concentration more than 100 times serum levels. These cells are also the main source of estrogen in adult males. Testosterone influences Sertoli cells either directly or indirectly through the P-mod-S (protein modulating Sertoli cell) factor secreted by peritubular myofibroblasts in the tunica propria. Nuclear testosterone receptors are found in Sertoli cells, Leydig cells, and peritubular myofibroblasts.
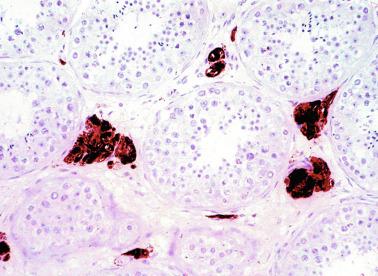
Leydig cells also secrete numerous nonsteroidal factors, including oxytocin, which acts on myofibroblasts and stimulates seminiferous tubule contraction; β endorphin, which inhibits Sertoli cell proliferation and function; and other factors with less certain actions such as angiotensin, proopiomelanocortin-derived peptide, which inhibits Sertoli cell proliferation and function, some other proopiomelanocortin peptides, and α-melanotropic–stimulating hormone ( Table 12.9 ). EGF is secreted in the testes mainly by Leydig cells, modulating spermatogenesis by stimulating germ cell differentiation and reducing spermatogonial proliferation. EGF is also an autocrine regulator of Leydig cells. Together with Sertoli cells, peritubular cells, and endothelial cells, Leydig cells produce Nitrous oxide (NO), which has a relaxing effect on smooth muscle of seminiferous tubules and blood vessels, thus regulating spermatozoon transport and testicular blood flow, respectively.
| Products | Functions/Characteristics |
|---|---|
| Androgens | Steroid hormone/endocrine-paracrine agent |
| Estrogens | Steroid hormone/endocrine-paracrine agent |
| Insulin-like growth factor 3 (INSL3) | Maintenance growth/differentiation Self-renewal of spermatogonia stem cells |
| Colony-stimulating factor 1 (CSF1) | Self-renewal of spermatogonia stem cells |
| Proopiomelanocortin peptides | Opiates/proopiomelanocortin regulatory agents |
Leydig cells are spatially related to adrenergic and cholinergic nerve fibers. Varicosities containing synaptic vesicles have been found near Leydig cells, and nerves that end on Leydig cells have been identified. The functional meaning of this innervation is unknown.
Macrophages are a normal component of the testis. Young adult men have one macrophage per 10 to 50 Leydig cells, and this number increases with age. They are classified by phenotype into two groups: resident (M2 macrophages), which are the most numerous, and activated (M1 macrophages). Both express CD68, but only resident macrophages express CD163. Resident macrophages appear in the testis early in development, present in the urogenital ridges, and are believed to be derived from fetal yolk sac progenitors. Activated macrophages are likely derived from circulating monocytes.
Macrophages help maintain normal testicular function and homeostasis, including interaction with Leydig cells. Slender Leydig cell cytoplasmic expansions penetrate deep into the cytoplasm of the macrophages. One of the factors that favorably influence steroidogenesis in Leydig cells is 25-hydroxycholesterol. Macrophages regulate spermatogenesis through CSF1 and enzymes involved in the synthesis of retinoic acid, and participate in proliferation and differentiation of Leydig cell fibroblastic precursors and in the proliferative activity of newly formed cells. Activated macrophages secrete abundant antiinflammatory cytokine IL10, and produce IL1 and IL6, tumor necrosis factor-α, and TGFA, which is important in the control of bacterial infections.
Immunohistochemistry has revealed the presence of fusiform or star-shaped cells known as neuron-like cells in the interstitium. Their number is low in adult testes. These cells express neuron-specific intermediate filament NF-200, voltage-activated sodium (Na) channel, and intratesticular catecholamines, which appear to be increased in some disorders such as Sertoli cell–only syndrome and hypospermatogenesis.
Mast cells are a normal finding in the interstitium, in peritubular and perivascular locations, among Leydig cells, and inside interlobular septa and the tunica vasculosa. The number decreases with age, and this change seems not to be influenced by Sertoli Growth Factor (SGF) produced by Sertoli cells. The main product of mast cells is tryptase. Among mast cell functions, participation in intercellular matrix formation may be fundamental. The number of mast cells increases in several diseases, and it is greatly increased in patients with nonobstructive azoospermia.
The testis is supplied by the testicular artery, which arises from the abdominal aorta. In the spermatic cord the testicular artery gives rise to two or three branches that obliquely penetrate the tunica albuginea, spawning multiple branches that run along the intralobular septa of the testis. These centripetal arteries lead to the mediastinum testis. Along their course the centripetal arteries give off branches that abruptly reverse direction, the so-called centrifugal arteries. At puberty, both the centripetal and centrifugal arteries develop pronounced spiral architecture. The centrifugal arteries develop additional branches in the interstitium that give rise to arterioles and capillaries that form intertubular plexuses, some of which are apposed to the tunica propria. Capillaries are of the continuous type, except for the seminiferous tubule capillaries, which are partially fenestrated, and their endothelial cells are similar to those of brain capillaries, with scant pinocytosis, intercellular junctions of the fascia adherens type, and low permeability. The mediastinum testis is poorly vascularized.
The inner two-thirds of the testicular parenchyma is drained by veins that follow the interlobular septa to the mediastinum testis (centripetal veins). The outer one-third is drained by veins that lead to the tunica albuginea (centrifugal veins). Both centripetal and centrifugal veins join and anastomose, exiting the testis by the veins of the pampiniform plexus, which drains the testis via the spermatic cord.
Lymphatic vessels are poorly developed in the testis and are limited to the tunica vasculosa, interlobular septa, and mediastinum, where they accompany arterioles and venules. Prelymphatic vessels have been reported in the interstitium and probably drain interstitial fluid into the true interlobular lymphatic vessels.
Efferent innervation of the testis is mainly supplied by neurons of the pelvic ganglia, where contralateral and bilateral neural connections occur. Postganglionic nerve fibers enter the testis via the pelvic nerves, extend throughout the tunica vasculosa, and follow the interlobular septa to reach the interstitium. These nerve fibers end in the walls of arterioles, the walls of seminiferous tubules, and the Leydig cells. Adrenergic nerve fibers innervate the tunica albuginea and blood vessels of the tunica vasculosa, but do not enter the testicular parenchyma. Most nerve fibers are peptidergic. Afferent nerve endings form corpuscles like those of Meissner and Pacini in the tunica albuginea.
In summary, testicular histology may be understood only by considering the different autocrine, endocrine, and paracrine relationships that occur among the different cells of the testicular parenchyma. Only in that way may we understand the complex processes of cellular interrelation that take place in spermatogenesis and that culminate in spermatozoon production with adequate morphology, vitality, and fertilizing capacity.
The rete testis is a network of channels and cavities that connects the seminiferous tubules with the ductuli efferentes. Differences in the configuration and size of channels and cavities distinguish three portions of the rete testis: septal (intralobular), composed of the tubuli recti; mediastinal, composed of a network of interconnected channels; and extratesticular, composed of dilated cavities (≤ 3 mm in diameter) termed the bullae retis .
The tubuli recti are short tubules (0.5 to 1 mm long) that connect the seminiferous tubules to the mediastinal rete, although some seminiferous tubules, principally those in the central region of the testis, may connect directly to the mediastinal rete. The tubuli recti are lined by cuboidal epithelium. There are approximately 1500 tubuli recti (or their analogous seminiferous tubule segments). The tubuli recti in the cranial, central, and anterior testis are perpendicular to the mediastinal rete testis channel into which they drain, and those in the caudal testicular region are parallel to their respective channels. The transitional segments between the seminiferous tubules and the tubuli recti are formed by modified Sertoli cells.
The epithelium of the mediastinal rete testis consists of flattened cells interspersed with small areas of columnar cells ( Fig. 12.42 ). Both cell types have a single centrally located cilium and numerous microvilli on their free surfaces and contain keratin and vimentin filaments. Interdigitations are present between adjacent cells. The epithelium rests on a basal lamina surrounded by a layer of myofibroblasts and a rim of fibroblasts and collagen and elastic fibers.
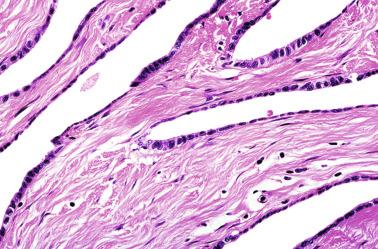
The rete channels and cavities are traversed by the chordae rete, columns 15 to 100 μm long and 5 to 40 μm wide, arranged obliquely to the long axis of the cavity. The chordae rete consists of fibrous connective tissue and fibroblasts covered by flattened epithelium. The rete testis probably has the following functions: damping of differences in pressure between the seminiferous tubules and the ductuli efferentes; reabsorption of protein and potassium from tubular fluid; and, occasionally, phagocytosis of spermatozoa.
Anorchidism refers to absence of one (monorchidism) or both testes (testicular regression syndrome). Unilateral anorchidism may present as true congenital testicular absence or vanishing testis. Monorchidism is estimated to occur in approximately 4.5% of cryptorchid testes, in 40% of the testes that are impalpable on physical examination, and in 1 in 5000 males. Bilateral anorchidism occurs in approximately 1 in 20,000 males. Evaluation of a child with a solitary palpable testis begins with scrotal palpation. Laparoscopy is required for patients in whom no apparent testicular nubbin tissue is found in the scrotum or for those with a patent vaginal processus.
The hormonal pattern in prepubertal patients with monorchidism is similar to that of normal children, whereas children lacking both testes have undetectable levels of AMH and elevated levels of gonadotropins that fail to respond to stimulation with hCG even in the first months of postnatal life. Although hCG stimulation challenge is often positive in children with bilateral cryptorchidism, it is negative in some children with bilateral intraabdominal cryptorchidism, further complicating the differential diagnostic separation of anorchidism and cryptorchidism. Exceptionally, anorchidism may be associated with hypogonadotropic hypogonadism.
For unknown reasons the left testis is more frequently absent (69%) than the right. In such cases the contralateral scrotal testis undergoes compensatory hypertrophy, and its volume increases to more than 2 mL. Compensatory hypertrophy has also been reported in association with abdominal cryptorchid testis.
The absence of testicular parenchyma should be confirmed before diagnosing monorchidism. Laparoscopy has been proposed as the usual procedure to localize a nonpalpable or absent testis. At exploration the finding of a vas deferens ending near or in a hypoplastic epididymis is not sufficient for the diagnosis of monorchidism. The only acceptable finding is blind-ending spermatic vessels. If inguinoscrotal exploration fails to identify these vessels, intraabdominal exploration is required to exclude undescended testis and avoid development of a testicular tumor. All remnants found at exploration should be removed.
Testicular regression syndrome refers to a variety of conditions, including agonadism, anorchidism, testicular agenesis, rudimentary testes, hypoplastic testes, and embryonal testicular dysgenesis. Each syndrome shares complete absence or involution of both testes, but they differ in the time of testicular disappearance during development. The most frequently observed are Swyer syndrome (see discussion of Disorders of sex development (Gonadal dysgenesis)), true agonadism, rudimentary testes, bilateral anorchidism, vanishing testes syndrome, and Leydig cell–only syndrome ( Table 12.10 ).
| Embryonal Period | Fetal Period | ||||
|---|---|---|---|---|---|
| Early | Late | Early | Middle | Late | |
| Müllerian structures | Vestigial | Differentiated | Differentiated/vestigial | Vestigial | Vestigial |
| Wolffian structures | Vestigial | Vestigial | Vestigial/differentiated | Differentiated | Differentiated |
| External genitalia | Female | Female | Ambiguous | Ambiguous-male | Male |
Patients are phenotypically girls, and the male gender may be discovered only at the time of referral for other symptoms such as primary amenorrhea. External genitalia are female with or without clitoromegaly, labioscrotal fusion, and short vagina. Examination of the internal genitalia demonstrates the absence of müllerian and wolffian derivatives, although the presence of uterine and uterine fallopian hypoplasia may occur. No gonads (not even in an ectopic location) are found. Early testicular regression occurs between the seventh and eighth weeks of embryonal development, just after the onset of AMH secretion.
Sporadic and familial cases are both with associated extragenital anomalies. In some cases the cause is heterozygous mutation of WT1 . In most familial cases, inheritance is either recessive autonomic or X-linked, and the cause seems to be either unknown anomalies in the WT1 gene or known anomalies in other genes involved in development. SRY molecular defect has never been observed. Agonadism may be associated with several syndromes, including PAGOD (hypoplasia of lungs and pulmonary artery, agonadism, omphalocele/diaphragmatic defect, dextrocardia), Seckel, and CHARGE (ocular coloboma [C], heart disease [H], choanal atresia [A], retarded growth or development [R], genitourinary defects or hypogonadism [G], and ear anomalies or deafness [E]).
Patients with rudimentary testes have a normal male phenotype. Müllerian remnants are absent, and wolffian derivatives are usually present. The testes are cryptorchid and small, less than 0.5 cm long. Seminiferous tubules are few in number ( Fig. 12.43 ). Testicular regression occurs between the weeks 14 and 20 of gestation. This syndrome has been reported in several members of the same family, a finding suggesting genetic transmission, but this is not a constant feature.
Congenital bilateral anorchidism is defined as complete absence of testicular tissue in a patient with a normal male karyotype and phenotype. Congenital bilateral anorchidism occurs in 1 in 20,000 newborns. Patients have male external genitalia, but the internal genitalia consists only of normal wolffian derivatives without müllerian derivatives, a finding suggesting that the testes were present and functionally active up to approximately the 20th week of gestation, thus producing sufficient amounts of AMH and androgens. Patients have male external genitalia with hypoplasia of both the scrotum and penis. The disorder may be associated with other malformations, such as anal atresia, rectourethral and rectovaginal fistula, and urinary exstrophy. Patients diagnosed in adulthood have male phenotype, androgen insufficiency symptoms, and elevated levels of both FSH and LH. The cause of congenital bilateral anorchia is uncertain, with numerous hypotheses, including intrauterine torsion of both testes, endocrinologic or immunologic disfunction, or genetic abnormality. The possibility of genetic anomaly was suggested by the occurrence of several familial cases. However, isolated mutations in the SF1 gene have been identified in only one case to date, and no mutations have been found in the SRY gene, the INSL3 gene (necessary for correct testicular descent), or in the gene of its receptor.
The basal plasma concentration of AMH, inhibin B, and testosterone is undetectable in anorchic patients. The increase in testosterone levels after GnRH or hCG administration is low or undetectable. FSH and LH levels are abnormally high during the first months of life and then progressively decrease. LH decreases more rapidly to normal levels than does FSH in 70% of anorchic patients until pubertal age, when gonadotropins increase to high levels. A case of bilateral anorchidism was reported in association with hypogonadotropic hypogonadism in a pubertal patient with Kallmann syndrome.
Vanishing testes syndrome applies to disappearance of one or both testes between the last months of intrauterine life and the beginning of puberty. Two criteria are required for diagnosis: (1) absence of a palpable testis on examination with the patient under anesthesia; and (2) blind-ending spermatic vessels visualized within the retroperitoneum, or spermatic vessels and vas deferens seen exiting a closed internal inguinal ring. Testicular regression occurs after the seventh month of embryonal life, so exploration finds the vas deferens in the inguinal canal or high in the scrotum; it may be accompanied by the epididymis and less frequently by testicular remnants consisting of small groups of seminiferous tubules ( Figs. 12.44 and 12.45 ). Patients who lack both testes experience hypergonadotropic hypogonadism after puberty, with gynecomastia, infantile phallus, hypoplastic scrotum, and impalpable prostate. The condition is usually secondary to perinatal scrotal torsion, although rarely it has a genetic cause.
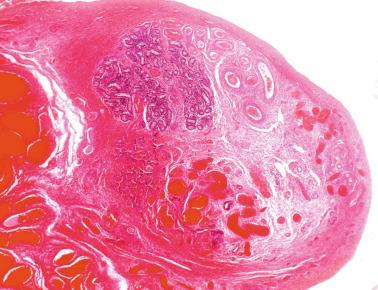
Patients with Leydig cell–only syndrome have agonadism without eunuchoidism and normal male phenotype, although meticulous surgical exploration fails to find testicular remnants. Study of serial sections from the spermatic cord reveals clusters of Leydig cells. Detection of testosterone in spermatic vein blood indicates that these ectopic Leydig cells are functionally active and synthesize testosterone in amounts sufficient to induce a rudimentary male phenotype but are insufficient to support complete development of secondary sex characteristics.
Macroscopic and histologic findings in patients with testicular absence differ according to the cause of anorchidism (true congenital absence or disappearance of a former testis). In true congenital absence the lack of testis is associated with absence of both the ductus epididymis and ductus deferens. In acquired testicular absence, the morphology of spermatic cord remnants is similar to monorchidism and testicular regression syndrome occurring after the 20th week of gestation. The ductus deferens, testicular artery, and a venous plexus may usually be identified in spermatic cord sections. Grossly, a small, firm mass is found at the end of the spermatic cord ( Fig. 12.44 ). Histologic examination reveals vas deferens, epididymis, or small groups of seminiferous tubules in 69% to 83% of cases. Vas deferens is the most constant finding (79%), followed by epididymis (36%) and seminiferous tubules (0% to 20%). These tubules may or may not contain germ cells. Small vessel groups are present in 83% of patients, but in only 24% is the number of vessels sufficient to identify them as spermatic vessels. Blind-ending cord structures have been found in the abdomen (21% of cases), the inguinal canal (59%), the superficial inguinal ring (18%), and the scrotum (2%).
Areas of dystrophic calcification, hemosiderin deposition ( Fig. 12.46 ), and giant cell reaction may be found within the mass in place of the testis. Other reported findings include arterial and venous vessels (88%), fat (44%), and nerves that may resemble traumatic neuroma (56%).
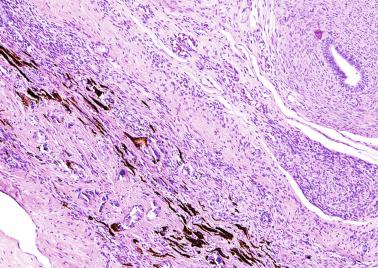
The minimum requirement for diagnosis is identification of either a vascularized fibrous nodule with calcification or hemosiderin, or a fibrous nodule with cord elements.
There are few histologic studies of evanescent testes removed in adulthood that refer to residual testicular parenchyma. In three cases from our files, we have not observed areas of calcification or macrophages with hemosiderin. All contained tubules with complete spermatogenesis mixed with Sertoli cell–only tubules with dysgenetic characteristics (mixed atrophy [MAT]); the interstitium had significant Leydig cell hyperplasia ( Figs. 12.47 and 12.48 ). No GCNIS cells were observed.
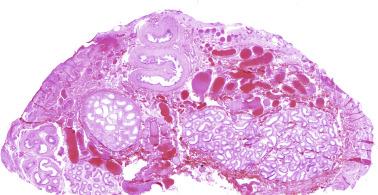
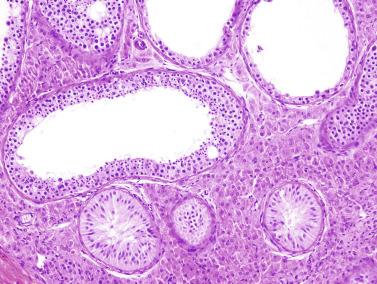
Optimal management of the testicular remnant associated with the vanishing testis syndrome is controversial. Some urologists advocate surgical exploration, either laparoscopic or through an inguinal scrotal approach, whereas others believe these procedures are unnecessary based on the low percentage (21%) of seminiferous tubules found in the removed testicular nubbins; only 14% contain seminiferous tubules with germ cells, and thus the probability of a tumor is minimal. Only one case of GCNIS has been reported. Thus some authors defend conservative management, whereas others believe that these remnants should be removed, given the potential for malignant degeneration.
Most cases of unilateral and bilateral testicular loss apparently occur during the fetal period after the testis has inhibited the müllerian ducts and induced differentiation of wolffian duct derivatives, or else postnatally. Two hypotheses account for the disappearance of the testes. The first involves atrophy secondary to a vascular lesion such as thrombosis or intrauterine torsion, trauma, or neonatal scrotal hematoma. The presence of macrophages with hemosiderin and dystrophic calcification suggests a vascular event. In addition, the morphology of the contralateral testis is normal. If the disappearance resulted from hormonal influences, the contralateral testis would show abnormalities, as is typical in the contralateral testis in cryptorchidism. The second hypothesis of disappearance invokes a primary anomaly of the gonad such as a true congenital testicular absence, which would be responsible for only 27% of nonpalpable testes. This theory is supported by multiple factors: histologic presence of dysgenetic lesions in the residual testicular parenchyma; absence of evidence of old hemorrhage or ischemia; evidence of disturbance in endothelial development leading to a reduction in vascular formation; and the occasional presence of malformations of the urogenital system, such as absence of the kidney, cystic seminal vesicles, or ipsilateral renal dysgenesis.
The clinical term microorchidism refers to diverse conditions characterized by small testicular size, including Klinefelter syndrome, hypogonadotropic hypogonadism, and bilateral cryptorchidism. In adulthood, microorchidism refers to testes with a volume ≤ 12 mL. Patients with microorchidism are candidates for genetic or hormonal studies to identify the underlying disease or syndrome. For example, some patients with Kenny-Caffey syndrome exhibit short stature, cortical thickening and medullary stenosis of long bones, delayed closure of anterior fontanelles, hypoparathyroidism, and ocular abnormalities. FSH serum level is elevated in some cases, whereas LH and testosterone levels are normal. Adult testes are small, with seminiferous tubules showing complete but diminished spermatogenesis. Leydig cells are hyperplastic. Unlike patients with the rudimentary testes syndrome, a patient with microorchidism has a normal-sized penis and no epididymal or prostatic atrophy.
The most frequent causes of adult microorchidism are Klinefelter syndrome, testicular maldescent, varicocele, secondary atrophy, and other idiopathic clinical disorders. Most patients have azoospermia or oligoasthenoteratozoospermia.
Polyorchidism refers to congenital presence of more than two testes. It is a rare condition, with slightly more than 200 reported cases. The first histologic description appeared in 1880, and the first case treated surgically and confirmed histologically was reported in 1895. Although the existence of three testes is the most common presentation, four testes have been reported in 10 patients, and five testes in one case report that lacked histologic confirmation. The age at diagnosis varies from newborn to 74 years, with a mean of 17 years. Most patients are phenotypically similar to persons of their age. With the exception of isolated cases with 46,XX karyotype with XY mosaicism and deletion of the long arm of chromosome 21, patients typically do not have chromosomal abnormalities. Testicular duplication is usually an incidental finding during surgery for inguinal hernia, cryptorchidism, or testicular torsion, but has also been detected in patients with infertility or unexplained fertility after bilateral vasectomy. The extra testis is often intrascrotal (75%) and less frequently inguinal (20%), abdominal, or retroperitoneal (5%). Duplication is three times more frequent on the left side than on the right. Testicular maldescent (40%), inguinal hernia (30%), hydrocele (9%), varicocele, and contralateral cryptorchidism are the most frequently associated anomalies. Testicular torsion (13%) and testicular cancer (6.4%) are occasional complications. In isolated cases, imperforate anus, idiopathic infertility, and contralateral anorchidism have been observed. High-resolution sonography is indicated, followed by magnetic resonance imaging (MRI) when sonographic findings are inconclusive.
The extra testis may be histologically normal, but usually is not, containing Sertoli cell–only tubules, hypospermatogenesis, maturation arrest, or microlithiasis. Lack of spermatogenesis has been attributed to the anomalous location of the testis and the absence of communication between the testis and excretory ducts, although in some cases the lesions are probably primary.
Embryologic origin of polyorchidism remains uncertain, and the following mechanisms have been proposed to account for the variety of findings in different cases ( Fig. 12.49 ):
Duplication of the genital ridge. All structures of the genital ridge and mesonephric ducts are duplicated. Each of the two testes resulting from duplication has an excretory duct and develops active spermatogenesis.
Longitudinal division of the genital ridge . Of the two resulting testes, the medial loses its connection with the mesonephric ducts and undergoes atrophy.
High transverse division of the genital ridge . The two resulting portions are in continuity with the mesonephric ducts that give rise to the ductuli efferentes. Each testis may have its own ductus epididymidis or shares a common one, but there is a separate vas deferens for each.
Low transverse division of the genital ridge . The more caudal testis has no excretory ducts.
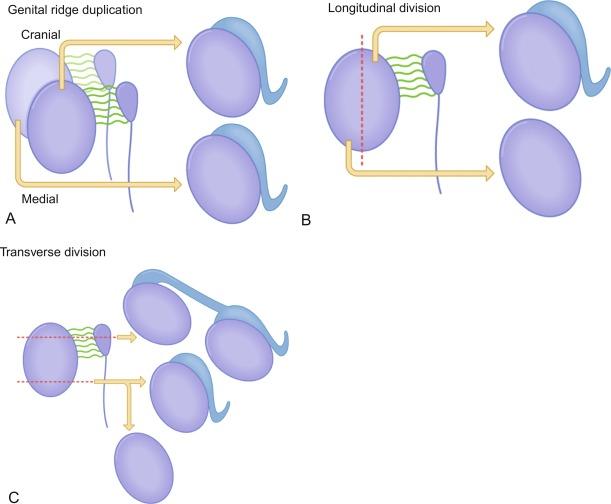
Several classifications of polyorchidism have been proposed, including one based on embryology and another on reproductive potential. In embryologic classification, type II is the most frequent variation ( Table 12.11 ), and types II and III together comprise more than 90% of cases. This classification does not consider certain isolated cases such as the occurrence of double testes on one side, each connected to their own epididymis and vas deferens. This finding may be explained embryologically by complete splitting of the genital ridge and entire mesonephros with wolffian duct elements along the dorsoventral line of cleavage. Embryologic classification also fails to consider double testes on one side that are not connected with an epididymis or vas deferens; this anomaly is explained by horizontal splitting of the genital ridge to form the gonad.
Type I: A supernumerary testis lacks an epididymis or vas deferens and has no attachment to the usual testes (division of the genital ridge only).
Type II: The supernumerary testis drains into the epididymis of the usual testis, and they share a common vas deferens (division of genital ridge occurs in the region where the primordial gonads are attached to the metanephric ducts, although the mesonephros and metanephric ducts are not divided; i.e., incomplete division).
Type III: The supernumerary testis has its own epididymis, and both epididymides (that of the supernumerary testis and that of the ipsilateral testis) drain into one vas deferens (complete transverse division of mesonephros, as well as the genital ridge).
Type IV: Complete duplication of the testes, epididymis, and vas deferens (vertical division of the genital ridge and mesonephros).
In the reproductive potential classification ( Table 12.12 ), type I includes types II, III, and IV of the embryologic classification, but excludes type II and all ectopic testes (types IB and IIB). Supernumerary testis type IA may be excluded if the patient has at least one intrascrotal testis with normal drainage, if the testicular biopsy of the supernumerary testis shows a Sertoli cell–only pattern or malignancy, or if difficulties in patient follow-up are expected. Proposed modification of the reproductive potential classification is shown in Table 12.13 .
Type I: The accessory testis (supernumerary testis) is attached to the draining epididymis and vas deferens with reproductive potential (30% of polyorchidism).
Type IA: The accessory testis is intrascrotal.
Type IB: The accessory testis is in an ectopic location.
Type II: The testis lacks such an attachment and has no reproductive potential.
Type IIA: The accessory testis is intrascrotal.
Type IIB: The accessory testis is in an ectopic location.
Type A1: The drained supernumerary testis has its own epididymis and vas.
Type A2: The drained supernumerary testis may have its own epididymis but shares a common deferens duct with its neighbor.
Type A3: The drained supernumerary testis may share a common epididymis (and duct) with its neighbor.
Type B1: The undrained supernumerary testis does have its own epididymis.
Type B2: The undrained supernumerary testis does not have its own epididymis and thus consists of testicular tissue only.
The clinical differential diagnosis of polyorchidism includes other conditions that enlarge the scrotum and spermatic cords, including spermatocele, hydrocele, cyst and tumor of the spermatic cord, crossed testicular ectopia, adrenal cortical ectopia, and splenogonadal fusion. Orchidectomy has been replaced as treatment of choice for atrophic and nonscrotal testes by fixation of the testis to the scrotal pouch and re-creation of a “simple testis” when permitted by the anatomic condition and after exclusion of malignancy. This treatment may allow spermatogenesis, as well as psychological and cosmetic benefits. Intrascrotal rhabdomyosarcoma, testicular teratoma, and seminoma have been reported in patients with polyorchidism.
Macroorchidism may be unilateral or bilateral, and is caused by excessive development of seminiferous tubules, Leydig cells, or both. It may be associated with chromosomal anomalies, tumors, or endocrine alterations. An increase in the testicular parenchyma occurs in several conditions, including congenital Leydig cell hyperplasia, compensatory hypertrophy, benign idiopathic macroorchidism, bilateral megalotestes with low gonadotropins, fragile X chromosome, and testicular hypertrophy observed in juvenile hypothyroidism.
Congenital Leydig cell hyperplasia is uncommon and may be diffuse or nodular. Diagnosis of diffuse Leydig cell hyperplasia requires quantification of Leydig cells by morphometry using normal newborn testes as controls ( Fig. 12.50 ). Nodular Leydig cell hyperplasia is characterized by the presence of nonencapsulated cellular aggregates in the mediastinum testis, adjacent testicular parenchyma, and connective tissue among the ductuli efferentes ( Figs. 12.51 and 12.52 ).
The differential diagnosis of nodular Leydig cell hyperplasia includes intratesticular adrenal rests and bilateral Leydig cell tumor. Excluding adrenogenital syndrome, intratesticular adrenal rests are rare. Rests are encapsulated, except with adrenogenital tumors, and consist of radially arranged cells with vesicular nuclei and small nucleoli displacing the rete testis or seminiferous tubules. Leydig cell tumors may be bilateral, poorly circumscribed, and surrounded by testicular parenchyma, features making it difficult to distinguish from Leydig cell hyperplasia. However, Leydig cell tumors are rarely congenital, whereas those occurring during infancy often induce precocious maturation of the adjacent seminiferous tubules and early macrogenitosomia.
Congenital Leydig cell hyperplasia is caused by large quantities of hCG entering the fetal circulation. Mothers with diabetes, particularly those with hypertension, may experience development of hyperplacentosis; the resulting edema in the placental villi alters vascular permeability and allows passage of hCG to the fetus. Congenital Leydig cell hyperplasia decreases rapidly during the first months of postnatal life after maternal hCG is gone. Combined diffuse and nodular Leydig cell hyperplasia occurs in several malformative syndromes, including Beckwith-Wiedemann syndrome, leprechaunism, triploid fetuses, and fetuses with Rh isoimmunization, as well as in several complications of pregnancy.
Compensatory hypertrophy may occur in monorchidism, cryptorchidism ( Fig. 12.53 ), and varicocele, as well as after testicular injury. The disorder is characterized by increased volume of the descended testis, defined as more than two standard deviations of the corresponding size in normal children. A volume greater than 2 mL or even lower is considered to be predictive of monorchidism. Compensatory hypertrophy develops between birth and 3 years of age, and the testis may reach a volume twice normal when the other testis is absent. Hypertrophy persists and may increase during childhood and puberty, but ceases thereafter; the hypertrophied testis then becomes normal or remains slightly enlarged. The degree of compensatory hypertrophy is determined by three factors: (1) volume of the remaining testicular parenchyma, (2) age at which the underlying pathologic event occurred, and (3) functional ability of the descended testis. Compensatory hypertrophy results from alteration of hypophyseal hormonal feedback, followed by an increase in secretion of FSH, evidence that the hypertrophied testis is normal. In monorchidism, it is likely that the absent testis was initially of normal size during fetal development, but later underwent progressive shrinkage.
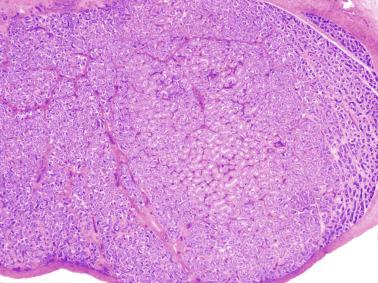
When 50% reduction of testicular mass occurs (probably before birth), endocrine feedback changes, and the resulting secretion of FSH (before or immediately after birth) causes accelerated growth of the contralateral testis. The hypertrophied testis contains an increased number of germ cells, explaining why patients with solitary testis may not necessarily be at additional risk for infertility. In cryptorchidism the reduction in overall testicular mass is less pronounced than in monorchidism, and the scrotal testis may also be abnormal, resulting in less marked compensatory hypertrophy.
Some prepubertal and pubertal patients have pronounced unilateral or bilateral testicular hypertrophy in the absence of other pathologic findings. This condition probably results from hormonal receptivity in the testicular parenchyma. Clinical manifestations are usually related to the onset of the puberty.
Several disorders, such as bilateral testicular tumor (germ cell, stromal tumor, leukemia, or lymphoma), adrenal rest tumor, X-linked mental retardation, hypothyroidism, and idiopathic or cerebral precocious puberty, should be excluded before testicular enlargement is diagnosed as idiopathic benign macroorchidism.
Morphometric studies indicate that testicular enlargement results from an increase in length of the seminiferous tubules, although increases in tubular diameter and Sertoli cell number have also been observed. Elevated FSH serum level, reported in some cases, or hyperactive FSH receptors (FSHR) could cause excessive Sertoli cell proliferation and lengthening and thickening of seminiferous tubules. In addition, Leydig cell hyperplasia and deficient spermatogenesis are frequent findings in adult life. In some cases the underlying mechanism is unclear because the sequential analysis of 10 exons of the FSHR was normal.
The development of the two testes may be asynchronous during puberty, so some cases of unilateral macroorchidism may reveal differences that are unusually exaggerated. This situation is also known as transitory unilateral testis enlargement of puberty. The enlarged testis, usually on the right (75% of cases), may reach 20 mL in volume at the onset of puberty. The contralateral testis grows during puberty until it reaches the same volume as the hypertrophic testis, whereas growth of the hypertrophic testis slows. After puberty neither testis is larger than 25 mL in volume.
Approximately 2% of adults with fertility problems have enlarged testes, with volumes greater than 25 mL, and low levels of FSH, LH, testosterone, prolactin, and estradiol. Despite these important hormonal changes, sperm concentration and total number of spermatozoa are higher than normal. Low FSH levels may be attributable to increased inhibin secretion because the number of Sertoli cells is elevated, but reduction in other hormone levels is of unknown cause.
Fragile X chromosome is the best-known form of inherited mental retardation, with an incidence of 1 in 4000 males and 1 in 6000 females. Inheritance is dominant and X-chromosome linked, with a low penetrance in females and variable expression in males. In addition to facial dysmorphia (large ears, prognathism, high forehead, and arched palate), macroorchidism (Martin-Bell syndrome) is often an associated finding first described in 1943.
The impaired gene, the fragility mental retardation 1 gene ( FMR1 gene), is mapped to Xq27.3, which is genetically fragile in these patients. The gene alteration is caused by lengthening of trinucleotide CGG repeat that results in FMR1 gene silencing. The repeat is present in the 5′-untranslated region of the FMR1 gene and shows 5 to 50 CGC units in the normal population. If the CGG sequence is repeated fewer than 200 times, the disorder is considered a premutation, and males show no symptoms; if the number of repetitions exceeds 200, mutation is complete, and all affected persons show the disorder. Full mutations involve hypermethylation of the gene promoter and lead to transcriptional silencing of the gene, with resulting total or partial loss of the FMR1 protein. This protein is present in many cells and regulates the translation of numerous proteins with a central role for cerebral maturation and function. Genotype and phenotype are associated in patients with fragile X chromosome. Great CGC unit repeats correlate with the most severe forms of the phenotype. Conversely, nonmethylated CGC repeats show little or no semiologic repercussion. Some syndromes result from intragenic FMR1 variants.
In affected men, mean testicular volume is more than 70 mL (four times greater than normal). The penis is larger than normal, and both anomalies are apparent in infancy. Testicular enlargement probably begins during fetal life. The scrotum is also enlarged and prematurely pigmented. This precocious genital development is difficult to explain because the hypothalamopituitary-gonadal axis is normal, but the condition may be caused by increased sensitivity to stimulation by FSH. Testicular biopsies from adults may be normal or show interstitial edema and hypospermatogenesis ( Fig. 12.54 ). Usually, one sees normal testicular parenchyma with focally reduced spermatogenesis and Sertoli cell hyperplasia ( Fig. 12.55 ) or tubules containing only immature Sertoli cells. In other cases, severe pathologic changes have been reported, including marked tubular ectasia and atrophy of the seminiferous epithelium, granular changes in Sertoli cells ( Fig. 12.56 ), MAT with or without Sertoli cell hyperplasia, and Sertoli cell nodules. Testicular enlargement is chiefly the result of lengthening and coiling of seminiferous tubules as a consequence of Sertoli cell proliferation. The low number of spermatids is attributed to atrophy caused by compression of the seminiferous epithelium by marked increase in intratubular fluid. Meiotic anomalies have been excluded. The fragile X syndrome is second in frequency only to Down syndrome as a cause of mental retardation. However, this chromosomal anomaly is not always associated with mental retardation or macroorchidism, and some men with fragile X syndrome are otherwise normal.
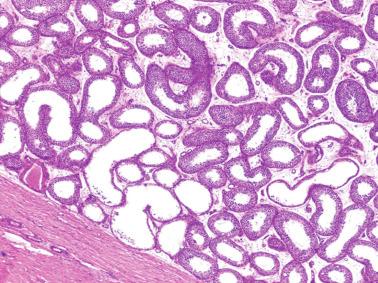
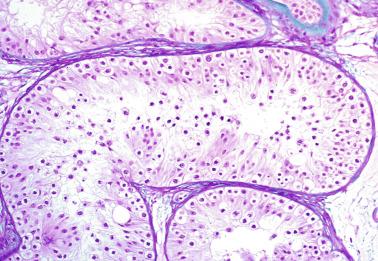
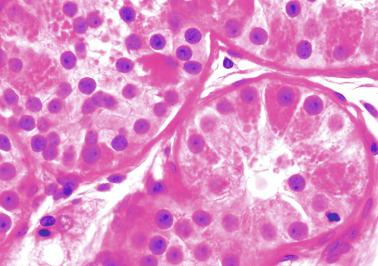
The terms fragile X–negative Martin-Bell syndrome and mental retardation–macroorchidism refer to patients with X-linked mental retardation–macroorchidism or X-linked mental retardation and macroorchidism who have the Martin-Bell syndrome phenotype, but not the fragile X site. The gene responsible for this disorder is mapped to Xq12-q21. Isolated patients with fragile X chromosome have experienced testicular torsion or neoplasms (testicular or extratesticular).
Testicular hypertrophy is associated with several glandular disorders such as FSH-secreting pituitary adenoma, hyperprolactinemia, hypoprolactinemia, and hypothyroidism. The most frequent association of testicular hypertrophy is with hypothyroidism.
Children with hypothyroidism often show testicular enlargement without virilization. Approximately 80% of these patients have macroorchidism, most have elevated FSH levels, and one-half have increased LH levels. Testosterone levels are normal during infancy. The response of FSH and LH to GnRH is altered, and no pulsatile LH release occurs ( Fig. 12.57 ). Testicular biopsies before puberty show accelerated development with pubertal maturation of seminiferous tubules, but not Leydig cells. Testicular biopsies in untreated adults show tubular and interstitial hyalinization with few Leydig cells. Testicular size in this type of macroorchidism diminishes as soon as substitution therapy starts.
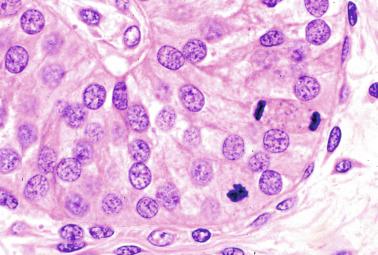
The etiopathogenesis of macroorchidism associated with primary hypothyroidism may be explained by several hypotheses: increase in gonadotropin secretion caused by thyrotropin-releasing hormone (TRH) stimulation of gonadotropic cells ; direct thyroid-stimulating hormone (TSH) effect on the testis resulting from structural similarity between TSH receptors and FSHRs in the testis ; and lack of steroid hormones that are required for testicular maturation (in their absence, Sertoli cell proliferation is excessive, giving rise to testicular enlargement). Macroorchidism associated with secondary hypothyroidism is related to loss-of-function mutations in IGSF1, hyperprolactinemia, and alterations in steroid metabolism of testicular cells.
Most adenomas that are considered nonfunctioning secrete variable amounts of FSH, although only exceptionally do they have any clinical manifestations. Functioning pituitary adenoma, with FSH secretion during infancy and puberty, gives rise to variable clinical symptoms in relation to size and accelerated development of pubic hair and genitalia. In adults, macroorchidism secondary to length growth of the seminiferous tubules is accompanied by normal spermatogenesis. Once the most frequent causes of macroorchidism have been ruled out, diagnosis is suggested by a discrepancy between normal or elevated FSH and decreased LH, as well as by the detection of elevated inhibin B. Atypical cases of FSH-secreting pituitary adenoma are accompanied by hypogonadism with erectile dysfunction, loss of libido, and absence of macroorchidism.
Precocious puberty is defined by the onset of secondary sex characteristics at a chronologic age that is younger than the mean middle age for the population, 2.5 standard deviations lower than the mean of a defined population. For practical purposes, this is before 8 years of age in girls and 9 years in boys. The incidence is estimated at between 1 in 5000 and 1 in 10,000, with a female-to-male ratio higher than 20:1. In boys the first symptom is rapid testicular enlargement followed by growth of pubic and axillary hair, enlargement of the penis, and acceleration of skeletal growth. According to hypothalamopituitary-gonadal axis function, precocious puberty may be classified into three groups: central or gonadotropin dependent, which results from activation of this axis; peripheral or gonadotropin independent, mediated by sex steroid hormones secreted by the testis or adrenal glands; and a mixed group that first appears as peripheral precocious puberty and, thereafter, because of the secondary response of the hypothalamus, becomes gonadotropin dependent.
Central precocious puberty (CPP), also known as true precocious puberty , is isosexual. CPP is caused by premature activation of the hypothalamic-hypophyseal-gonadal axis. The first manifestation in boys is an increase in volume (> 4 mL) or length (> 2.5 cm) of the testes. It is the most common form of precocious puberty in girls and accounts for more than 50% of cases in boys. Age of presentation is between 4 and 10 years.
The cause is known in only 60% of cases; most are related to lesions in the central nervous system, whereas others are usually idiopathic. Increasingly, cases previously considered idiopathic are found to have an underlying genetic basis.
Lesions in the central nervous system that cause CPP share alterations of specific areas, including the posterior hypothalamus (median eminence and tuber cinereum), mammillary bodies, the bottom of the third ventricle, or the pineal gland. The most frequent causes are as follows:
Tumor of the hypothalamus (astrocytoma, ganglioneuroma, ganglioglioma, craniopharyngioma, cyst of the third ventricle, and suprasellar cyst of the arachnoid space), pineal cyst, hamartoma (gangliocytoma) of the tuber cinereum and mammillary body, tumor of the pineal gland (teratoma and pinealoma), tumor of the optic nerve (glioma), cerebral and cerebellar astrocytoma, and granular cell tumor of the neurohypophysis
Cerebral trauma (including postpartum and accidental trauma) that stimulates extrahypothalamic areas responsible for hypothalamic activation
Infections such as meningitis, encephalitis, toxoplasmosis, and syphilis
Cerebral malformations, including hydrocephaly, microcephaly, and craniosynostosis, pituitary duplication, and midline defects
Hereditary diseases such as neurofibromatosis and tuberous sclerosis; children with type I neurofibromatosis often also have optic pathway tumors
Cerebral irradiation, as occurs in hypothalamopituitary selective irradiation, prophylactic irradiation in children with acute lymphoblastic leukemia, and irradiation of cerebral tumor that is far from the hypothalamopituitary region
Knowledge of the etiology in male patients has expanded with the use of computed tomography (CT) and MRI. One of the most important contributions of these techniques is the finding of high numbers of hamartomas in children with precocious puberty. These lesions, also known as gangliocytomas, consist of abnormally located neurons and glial cells. Lesions are usually multiple, small, and located on the hypothalamus between the anterior part of the mammillary body and the posterior part of the tuber cinereum. These neurons contain LH-releasing hormone–positive neurosecretory granules, a finding suggesting that this hormone may be released into the blood draining the hypophyseal portal system and reach the gonadotropic cells. Other hamartomas associated with CPP do not show immunohistochemical reactivity to GnRH, but they do show reactivity to TGFA protein and its messenger RNA (mRNA), as well as to the receptors for TGFA and EGF, which could also be involved in precocious pubertal development.
Precocious puberty resulting from cerebral tumors usually occurs at an advanced stage of the tumor, preceded by symptoms such as hydrocephaly, papillary edema, or psychic alterations. The same occurs when precocious puberty results from cerebral inflammation or malformation.
Although pineal gland tumor is rare in children, 30% of these produce precocious puberty, principally in boys. This tumor is usually a teratoma or nonparenchymatous tumor that destroys the pineal gland, thus hindering its antigonadotropic action and initiating puberty. In contrast, pinealocyte-derived tumor secretes great amounts of melatonin, which delays the onset of puberty.
Idiopathic precocious puberty . The cause of CPP cannot be determined in approximately 80% of girls and 40% of boys. This difference between the sexes may be attributed to the higher sensitivity of female gonadotropic cells to GnRH stimulation. The presentation of idiopathic precocious puberty is familial in nearly 50% of cases, and puberty starts after 7 years in most of these boys. Inheritance may be autosomal recessive or sex linked with variable penetrance. Genetic causes may play an important role in the development of some cases. Activating mutation (P745) in the KISS1 gene, encoding GPR54s ligand (Kisspeptin), may occur in boys with CPP. Mutations resulting in MKRN3 gene deficiency induce pulsatile secretion of GnRH, and that triggers precocious puberty.
The diagnosis of CPP is easy when there are elevated gonadotropins (both basal values and in response to GnRH) associated with high testosterone levels and an increase in either the LH/FSH ratio or in LH and FSH values after stimulation with GnRH agonists. However, in some cases it is necessary to measure nocturnal LH secretion to identify secretion pulses before a dynamic test may reveal the pubertal pattern. The treatment of choice is GnRH agonists.
Associated disorders that have been reported are intrauterine growth retardation, Silver-Russell syndrome, bilateral retinal degeneration, epilepsy, cryptorchidism, and inguinal hernia.
Peripheral precocious puberty is also known as precocious pseudopuberty. It may be caused by a primary testicular disorder, lesion in other endocrine glands, or hormonal treatment. Primary testicular disorders causing precocious pseudopuberty include familial testotoxicosis, functioning testicular tumor, excessive aromatase activity, and Leydig cell hyperplasia with focal spermatogenesis. The principal secondary anomalies include adrenal cortical anomaly (congenital adrenal hyperplasia, virilizing tumor of the adrenal, and Nelson syndrome) and hCG-secreting tumor, which accounts for one-half of cases of precocious pseudopuberty; testicular germ cell tumor and tumors of the retroperitoneum, mediastinum, and pineal gland are responsible for the other one-half ( Table 12.14 ). The best-known treatment that may cause precocious puberty is long-term administration of hCG.
Gonadotropin-independent precocious puberty
Familial male-limited precocious puberty
Leydig cell tumor
Intratubular hyalinizing large cell Sertoli cell tumor
Large cell calcifying Sertoli cell tumor
Adrenal cortex virilizing tumors
Hepatoblastoma
Extratesticular human chorionic gonadotropin–secreting germ cell tumors
Teratoma
Choriocarcinoma
Seminoma.
Precocious pseudopuberty secondary to disorders of aromatase activity.
Precocious pseudopuberty secondary to Leydig cell hyperplasia with focal spermatogenesis.
Familial testotoxicosis is a form of male sexual precocity characterized by early differentiation of Leydig cells and initiation of spermatogenesis in the absence of stimulation by pituitary gonadotropin. This condition is assumed to be a primary testicular abnormality with autosomal dominant inheritance that is limited to male patients. Some cases occur sporadically. The patients, usually 1 to 4 years of age, have signs of pubertal development, including rapid virilization, acceleration of growth with eventual closure of epiphyses, and short adult stature. Although the testes are enlarged, testicular size does not correlate with the degree of virilization. Histologic and ultrastructural studies confirm adult Leydig cell pattern and complete spermatogenesis, although many spermatids are abnormal ( Fig. 12.58 ).
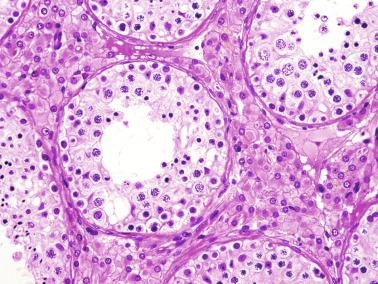
The cause of familial testotoxicosis is a constitutive activating mutation of the LH/CGR gene. This gene comprises 11 exons and has been mapped to 2p21, and approximately two dozen LH/CGR gene mutations have been reported.
Hormonal measurements show elevated serum levels of testosterone and low levels of dehydroepiandrosterone sulfate, androstenedione, 17-hydroxyprogesterone, GnRH, and LH, as well as absence of a pulsatile pattern. In addition, serum level of inhibin B appears elevated before the normal age of onset of puberty. In some patients a mutation in the LHR may induce Leydig cell hyperplasia.
Patients do not respond to treatment with GnRH analogues, which are used for treatment of CPP. Therapy with the antifungal drug ketoconazole is effective, but patients may experience significant hepatotoxicity and, in some cases, suffer escape phenomenon (secondary CPP), which requires additional therapy with GnRH agonists. A proposed alternative to GnRH analogue therapy is administration of cyproterone acetate or the aromatase inhibitor anastrozole, as well as bicalutamide, a nonsteroidal antiandrogen. Inactivating mutations of GnRH cause male undermasculinization as a result of the absence or hypoplasia of Leydig cells.
Precocious pseudopuberty may be caused by Leydig cell tumor, Sertoli tumor, adrenal cortex virilizing carcinoma, and extratesticular hCG-secreting germ cell tumor.
With Leydig cell tumor , the involved testis is enlarged in response to tumor growth and maturation of the seminiferous tubules adjacent to the tumor; such maturation results from androgen secretion by tumor cells ( Figs. 12.59 and 12.60 ). An activating mutation of the LHR gene, Asp578His has been detected in some patients with Leydig cell tumors. In most cases the contralateral testis is not enlarged. Occasionally, tumors cause only precocious tubular maturation, and symptoms of precocious pseudopuberty are absent, probably because of early diagnosis.
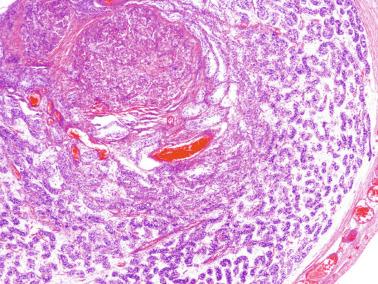
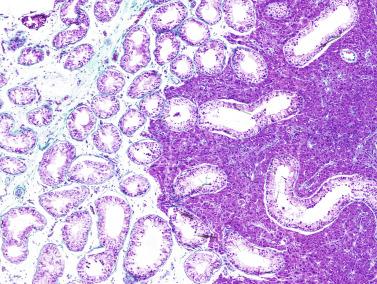
Intratubular large cell hyalinizing Sertoli cell neoplasia and large cell calcifying Sertoli cell tumor may give rise to precocious pseudopuberty that is isosexual (development of musculature and axillary and pubic hair) and heterosexual (gynecomastia). This precocious testicular maturation and the development of the tumor itself cause testicular enlargement ( Fig. 12.61 ). Tumor cells may stimulate Leydig cells to produce androgens that are aromatized to estrogens by the Sertoli tumor cells themselves, thus accounting for the clinical symptoms. These tumors are frequently observed in Peutz-Jeghers syndrome and Carney complex.
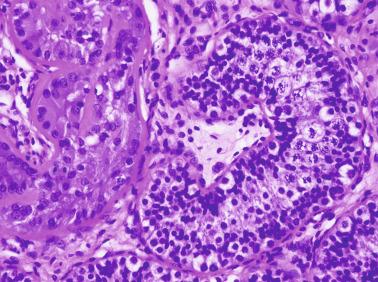
Most infants with adrenal cortex virilizing tumor have small testes, but hypertrophy has also been observed. Testicular development in these cases is attributed to adrenal androgenic action on seminiferous tubules. In untreated (or maltreated) congenital adrenal hyperplasia, both testes may be enlarged because of growing masses of cells resembling adrenal cortex. Patients with Nelson syndrome may have a similar condition. Surgical removal of virilizing adrenocortical carcinoma may induce CPP that requires treatment.
Testicular enlargement is modest in paraneoplastic precocious pseudopuberty secondary to hepatoblastoma or extratesticular hCG-secreting germ cell tumor , although nodular or diffuse precocious maturation has been occasionally reported. These nodules consist of hyperplastic Leydig cells and seminiferous tubules that may show complete spermatogenesis. Outside the nodule the seminiferous tubules maintain their prepubertal pattern. In some cases, only diffuse Leydig cell hyperplasia is observed.
Biosynthesis of C18 estrogens from C19 androgens occurs by three consecutive oxidative reactions that are catalyzed by an enzymatic complex known as estrogen synthetase or aromatase. This complex has two components: P450 arom (a product from the CYP19 gene located on 15p21.1), which joins C19 substrate and catalyzes the insertion of oxygen in C19 to form C18 estrogens; and reduced nicotinamide-adenine dinucleotide phosphate–cytochrome P450 reductase, a ubiquitous flavoprotein that conveys reducing equivalents to any form of cytochrome P450 it meets. Aromatase is in the endoplasmic reticulum of estrogen-synthesizing cells and is expressed in placenta, ovarian granulosa, Sertoli cells, Leydig cells, adipose tissue, and several central nervous system regions, including the hypothalamus, amygdala, and hippocampus.
Excessive aromatase causes massive conversion of androgens to estrogen. It is a genetic disorder with autosomal dominant inheritance caused by gain-of-function mutation in the aromatase ( CYP19A1 ) gene that results in heterosexual precocious pseudopuberty with gynecomastia in boys and isosexual precocity and macromastia in girls. Ultimately, patient stature is short because of the potent ability of androgens to accelerate epiphyseal closure. FSH, LH, and serum testosterone are decreased. Although many patients have mild hypogonadotropic hypogonadism, testicular size in adults is normal, and most are fertile and have normal libido. Generally the inhibitory estrogenic effect on testicular function is less than that observed with estrogen-producing tumors or in patients treated with exogenous estrogens. Patients with aromatase excess syndrome are effectively treated with aromatase inhibitors.
Aromatase deficiency syndrome, a rare recessive autosomal disorder, was first reported in 1991. It is the result of several mutations in the coding region of the CYP19A1 gene that lead to decrease or loss of enzymatic function with subsequent estrogenic deficit. Most patients are homozygous for inactivating mutations because they are sons of consanguineous parents. Other patients are compound heterozygous from nonrelated patients.
In both sexes the first symptom appears in the mothers during pregnancy; these women become progressively virilized because of the placental incapacity to aromatize androgens. In a female fetus, excessive exposure to androgens in utero leads to ambiguous external genitalia. In puberty, normal adrenarche is present; however, these patients have primary amenorrhea, absence of mammary development, and progressive hypergonadotropic hypogonadism with hyperandrogenism.
In children, FSH and LH levels and the gonadotropin response to GnRH are normal findings suggesting that the role of estrogens in pituitary regulation is weak during infancy. Most cases are diagnosed at puberty. The most significant symptom is continuous linear growth in adulthood. In both genders, epiphyseal closure is delayed because the absence of estrogen results in failure of growth plate fusion despite high levels of androgens, and patients develop a eunuchoid habitus. Men have small testes, severe oligozoospermia, and complete asthenozoospermia; FSH and LH levels are high, testosterone levels are normal, and serum estrogen levels are low. All patients with aromatase deficit have tall stature, with continuing linear growth into adulthood, unfused epiphyses, osteoporosis, bilateral genu valgum, and eunuchoid proportions. Many male patients are obese with dyslipidemia, hyperinsulinemia, acanthosis nigricans, and diabetes mellitus. Estrogens play an important role in pituitary regulation in male adults, so men with aromatase deficit have low libido and infertility. Hormonal analysis reveals undetectable estradiol and estrone levels and elevated gonadotropins, findings suggesting a lack of negative regulation of both FSH and LH by estrogens. Androstenedione and testosterone may be normal or increased. There is macroorchidism with normal consistency in some cases, and the testes are small with severe oligozoospermia and 100% immotile spermatozoa in other cases.
A syndrome like that in men with aromatase deficit is found in patients with estrogen resistance caused by disruptive mutations of the ER gene . These patients have macroorchidism, increased testosterone level, and increased levels of FSH, LH, estradiol, and estrone. The spermiogram reveals a low number of spermatozoa (25 million/mL) with decreased viability (18%).
Estrogen therapy in patients with aromatase deficit is effective in achieving epiphysial closure, but this treatment does not improve spermatogenesis. The role of estrogens in male infertility has yet to be discussed.
This entity may manifest with clinical symptoms like those of functioning Leydig cell tumor; this is a form of precocious pseudopuberty with ipsilateral testicular enlargement. The testis contains hypertrophic Leydig cell nests associated with normal spermatogenesis. No tumor mass is present. Leydig cells do not contain Reinke crystalloids and do not compress seminiferous tubules. Tubules with spermatogenesis and infantile immature tubules are clear delimited. The differential diagnosis between this entity and Leydig cell tumor with precocious pseudopuberty is based on histologic pattern. Open excisional testicular biopsy is recommended; if the patient has a Leydig cell tumor, or if the diagnosis by frozen section is not conclusive, testicular removal is advisable. There is no evidence to suggest that this hyperplasia may develop into Leydig cell tumor.
The best-known form of mixed precocious puberty is the MAS, characterized by the association of “coffee and milk” pigmentary lesions in the skin, bone lesions (polyostotic fibrous dysplasia), enlarged testes, prepubertal size of the penis, and the absence of pubic and axillary hair. Although testicular enlargement is usually bilateral, unilateral macroorchidism may be the first symptom. The diagnosis may be delayed until adulthood. This syndrome is caused by mutations that activate the guanine nucleotide–binding protein α-subunit gene ( GNAS1 ), which encodes the α subunit of the trimeric G protein (20q13.32). Because these mutations are lethal in utero, male patients with MAS bear mosaicism chromosomal constitution for this deficiency. The anomaly is followed by inactivation of both LHR and FSHR.
An interesting finding is that the onset of testicular maturation is induced by the testis itself, which produces steroid secretion secondary to autonomous hyperfunction of Sertoli cells without evidence of Leydig cell involvement. This secretion causes early maturation of the hypothalamopituitary-testicular axis and subsequently true precocious puberty. The testicular parenchyma shows maturation of both seminiferous tubules and Leydig cells. Testicular microlithiasis is a frequent finding (62%).
The previously described developmental pattern is the rule. However, some cases with a different hormonal or clinical pattern have been reported. Some patients have isolated Sertoli cell hyperfunction, whereas others have only Leydig cell hyperfunction. The macroorchidism resulting from autonomous Sertoli cell hyperfunction shows abnormally elevated serum levels of inhibin B and AMH in correlation with decreased FSH and testosterone. Therefore pubertal inhibin B serum level suggests Sertoli cell hyperfunction that would exert negative feedback on FSH secretion. These testes show Sertoli cell hyperplasia and absence of maturation of both germ cells and Leydig cells, with subsequent lack of steroidogenesis ( Figs. 12.62 and 12.63 ). This peculiar condition has been related to the presence of a somatic mutation in the GNAS1 gene in Sertoli cells, but not in Leydig cells. The different early embryologic origin of precursor cells that contribute to Sertoli cell and Leydig cell lineages may underlie the differential occurrence of the mutated GNAS1 gene. In other cases, and preferentially in late childhood and adult patients, clinical, biochemical, and histologic data suggest isolated Leydig cell hyperfunction with Leydig cell hyperplasia but without precocious activation of Sertoli cells; this condition leads to complete spermatogenesis in some tubules. In these cases, autonomous testicular function and the gonadotropin suppression may persist for a long time.
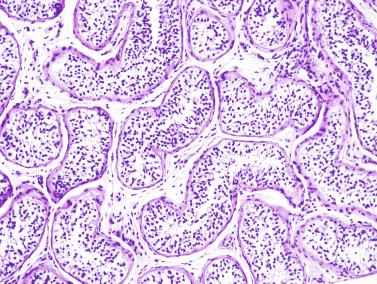
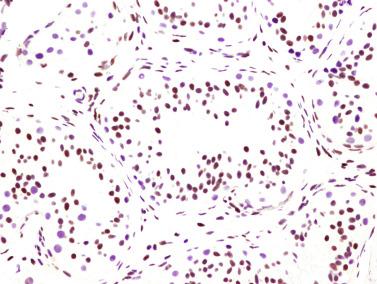
Therapy in MAS is symptomatic. Precocious puberty is treated with aromatase inhibitors, which block the conversion of testosterone into estradiol; hypophyseal surgery in some cases of fibrous dysplasia; and bromocriptine, cabergoline, and long-acting somatostatin analogues to stop growth hormone (GH) secretion.
A testis is ectopic when it is located outside the normal path of descent. Unlike cryptorchidism, ectopic testes are nearly normal in size and accompanied by spermatic cord that is normal or even longer than normal, as well as by a normal scrotum. Testicular ectopia is estimated to account for about 5% of all undescended testes. Testicular ectopia is classified according to location ( Fig. 12.64 ). In decreasing order of frequency, major sites are:
Interstitial or inguinal superficial ectopia . This is the most frequent form and may be confused with inguinal cryptorchidism. After passing through the external inguinal ring, the testis ascends to the anterosuperior iliac spine and remains on the aponeurosis of the major oblique muscle. These testes are more likely to be normal histologically than cryptorchid testes.
Femoral or crural ectopia. After passing through the inguinal canal, the testis lodges in the high crural cone in the Scarpa triangle.
Perineal ectopia. The testis is located between the raphe and the genitocrural fold in a subcutaneous location. Bilateral cases have been reported.
Transverse or crossed ectopia. This condition is also referred to as pseudoduplication, unilateral double testis, and transverse aberrant maldescent, first reported in 1886. Both testes descend through the same inguinal canal and lodge in the same scrotal pouch. The ectopic testis may be located in the internal inguinal ring, the inguinal canal, or the contralateral hemiscrotum. Each testis possesses its own vascular supply, epididymis, and vas deferens. In addition, ipsilateral hernia is present. More than 150 cases have been reported, mostly in adults. External genitalia ranges from normal to severe undermasculinization. Most cases are diagnosed during herniotomy surgery; others are identified by sonography, CT, or MRI. Isolated cases have been identified through treatment of incarcerated inguinal hernia or testicular torsion. Transverse ectopia must be suspected in cases of inguinal hernia associated with the absent contralateral testis. Treatment consists of transeptal orchiopexy or extraperitoneal transposition of the testis. Between 20% and 40% of patients have PMDS. Müllerian remnants must be removed with caution, if at all. It is better to leave them in place than to perform extensive dissection that may harm spermatic cord structures. High incidence of germ cell tumor (18%) has been reported.
Pubopenile ectopia. The ectopic testis is on the back of the penis near the symphysis pubis. Penile ectopy associated with groin testicular ectopy and scrotal absence was reported in a patient with popliteal pterygium syndrome. Approximately 60% of patients show genital anomalies.
Pelvic ectopia. The testis is in the pelvis, usually deep in the Douglas cul-de-sac.
Other unusual testicular ectopias include retroumbilical, craniolateral to the inner inguinal opening between the outer and inner oblique muscles, subumbilical, and anterior abdominal wall.
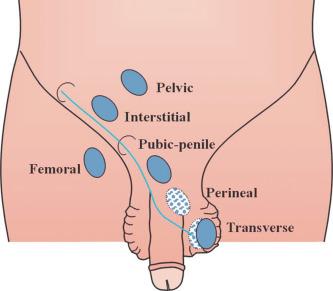
Ectopic testes in locations other than the superficial inguinal region typically exhibit histologic changes similar to cryptorchid testes. Findings include low germ cell number, low testicular volume, persistence of the vaginal process, and epididymal anomalies. Microlithiasis may occur in a patient with transverse testicular ectopy. Approximately 8% of patients with ectopic testes have contralateral cryptorchid testis.
There are multiple hypotheses explaining the different types of ectopia. Accepting that testicular descent occurs in two phases, some ectopias may be explained by failure in the first phase and others by a failure in the second phase. The first phase requires the gubernaculum to be attached to the abdominal wall at the site of the future inguinal canal. Attachment to the wrong side or disruption of that attachment would result in the following ectopias: attachment to femoral canal would result in femoral ectopia; attachment to the Spigelian triangle would result in Spigelian ectopia; absence or loss of attachment to the inguinal canal would result in transverse ectopia or pelvic ectopy. In the second phase of descent the gubernaculum must emigrate from the inguinal canal to the scrotum. This event is under the control of CGRP from the GFN. The GFN has two main branches, the genital branch supplying the scrotum and the femoral branch supplying the inner side of the thigh. Erroneous distribution of GFN sensory fibers or altered secretion of CGRP occurring in the wrong branch would disrupt the usual path of emigration of the gubernaculum. If the femoral branch of the GFN directs migration, femoral ectopia occurs; if the genital branch reaches the perineum instead of the scrotum, perineal ectopia occurs; and if the genital branch supplies the penis instead of the scrotum, pubopenile ectopia occurs.
Finally, there are ectopias that occur despite normal attachment of an abnormally developed gubernaculum. This occurs in patients with defects in AMH in whom an excessively long gubernaculum allows ample displacement that may lead the testicle even to the contralateral vaginal process.
In testicular exstrophy the testis and the spermatic cord are prolapsed externally through a defect in the scrotal wall. The lesion may be unilateral or bilateral. The gubernaculum is normal, and testicular descent is not impaired. Testicular exstrophy may result from mesodermal failure, similar to what causes the defect of the anterior abdominal wall in newborns with gastroschisis, scrotal wall necrosis secondary to meconium peritonitis, and iatrogenic trauma during cesarean section.
Testicular fusion is a rare anomaly characterized by fusion of the testes to form a single structure, usually in the midline. Each testis has its own epididymis and vas deferens. This anomaly is often associated with other malformations such as fusion of the adrenal glands or horseshoe kidney. Testicular fusion was also observed in a patient with transverse testicular ectopia and PMDS. The fused testes had a single vas deferens.
Bilobed testicle is a rare malformation, with only four cases reported. Patients have enlarged hemiscrotum related to testicular enlargement and hydrocele formation. The contralateral testis is normal. Sonography may exclude a tumor and thus avoid surgical exploration and testicular biopsy. This condition is considered an incomplete expression of polyorchidism. As in polyorchidism, follow-up with ultrasound imaging is recommended because of the higher risk for testicular tumor development.
Hamartoma is a term used to refer to abnormal and excessive development of structures that usually form part of the testis, epididymis, or spermatic cord. Hamartomatous lesions of the testis and sperm excretory ducts include cystic dysplasia of the rete testis, hamartoma of the rete testis, fetal gonadoblastoid testicular dysplasia (FGTD), Sertoli cell nodule, tubular hamartoma, congenital testicular and epididymal lymphangiectasis, and muscular hyperplasia of paratesticular structures. The main concern with hamartoma is not the biologic behavior, which is always benign, but their significance, because most are associated with specific disorders or are markers of complex syndromes.
Cystic dysplasia of the rete testis is a congenital lesion characterized by cystic transformation of an excessively developed rete testis that may extend to the tunica albuginea of the opposite pole. It was first reported in 1973 as “cystic dysplasia of the testis,” arising in a 4-year-old with right renal agenesis. To date, approximately 50 cases have been reported, mostly in children, with median age at presentation of 6 years (range, 0 to 23 years). The most remarkable clinical symptom is scrotal swelling, followed by scrotal pain suggesting testicular tumor in some cases. Approximately 35% have abdominal cryptorchidism. Ultrasound images are characteristic: small cystic formations of variable size, located in the testicular mediastinum, extend to the proximal testicular parenchyma and cause its compression. In addition, small hyperechoic foci, originating in the interphase between the walls of distal cystic and adjacent parenchyma, are observed. The observation of ipsilateral renal anomalies in ultrasound exploration may be of great importance for diagnosis.
Macroscopically the testis in adult patients has a cut surface reminiscent of the thyroid, with cysts of varying size filled with colloid-like material ( Fig. 12.65 ). The extent of the cystic transformation is widely variable. Small groups of cysts may be limited to the region of the mediastinum testis, or cysts may extend throughout the entire testis. In extensive cases, residual seminiferous tubules occupy only a small crescent beneath the tunica albuginea, and the testis is grossly spongy.
Cysts, which may be of variable size (from microscopic to several millimeters), arise in the septal and mediastinal rete testis ( Fig. 12.66 ). They are interconnected and contain acellular, eosinophilic, periodic acid–Schiff + (PAS + ) material. The cavities are lined by cuboidal cells that resemble those of normal rete testis both immunohistochemically (expressing both cytokeratin and vimentin) and ultrastructurally. Immunoreactivity to epithelial membrane antigen, which is usually not expressed in the normal rete testis, has been reported in some cases. Connective tissue between the cysts is scant and histologically similar to interstitial connective tissue. In some cases this tissue is so abundant that it partially collapses the cavities, and psammoma bodies and small inflammatory infiltrates may be present beneath the epithelium.
In cystic dysplasia, during infancy and childhood the residual testicular parenchyma may mature normally, and cryptorchid testes with cystic dysplasia have a marked decrease in spermatogonial number ( Fig. 12.67 ). However, in all cases the proximal end (the opening to the rete testis) of the seminiferous tubules shows a small dilatation. The seminiferous tubules may be dilated and atrophic; this is more evident after puberty.
Cystic dysplasia occurs in normally descended and cryptorchid testes, both in children and adults, and may affect one or both testes. In adults, residual parenchyma often shows morphologic characteristics of obstructive lesions: complete tubular sclerosis or hypospermatogenesis with intratubular accumulation of spermatozoa and Leydig cell pseudohyperplasia. In most cases the epididymis is altered. The head of the epididymis is small and contains few ductuli efferentes with irregular, usually dilated lumina, and abundant loose stroma. The ductus epididymidis is dilated, with atrophic epithelium consisting of cuboidal cells lacking stereocilia. Thick connective tissue replaces the muscular layer ( Fig. 12.68 ). Marked epididymal hypoplasia has been described. The ductus deferens may also be dilated. Ipsilateral absence of the ductus deferens occurs infrequently.
Cystic dysplasia is frequently associated with severe anomalies of the kidney or the urinary system. These problems greatly overshadow the significance of the testicular changes. Renal agenesis (51%), renal dysplasia (21%), hydronephrosis (8%), hydroureter, dilated or cystic epididymis (8%), absence of ipsilateral ureteric orifice (6%), ureteral duplication (4%), urethral stenosis, ectopic ureter draining to the seminal vesicle, ipsilateral renal agenesis and contralateral crossed ectopia, and genitourinary manifestations of VATER (vertebra/anus/cardiac/trachea/esophagus/radius/renal anomalies) syndrome have been reported ipsilateral to testicular cystic dysplasia.
The clinical differential diagnosis consists of all cystic lesions involving the prepubertal testes, including epidermoid cyst, cystic teratoma, juvenile granulosa cell tumor, testicular lymphangiectasis, simple cyst of the testis, and posttorsion cystic degeneration of the testis. Previously, total or partial orchiectomy was the treatment of choice. Currently, conservative therapy is recommended if the ultrasound diagnosis is highly suggestive, and results of α-fetoprotein and hCGβ subunit assays are negative. Spontaneous regression may occur. However, it is uncertain whether attempts to save the testis preserve spermatogenesis.
The etiology and pathogenesis of cystic dysplasia are uncertain. Given that the rete testis is a mesonephric derivative and most of the associated renal malformations are apparently caused by failure of induction of renal blastema by the mesonephros, cystic dysplasia is the result of abnormal mesonephros. During childhood the normal rete testis has no lumina because these form during puberty. The adult rete testis is a conduit for the passage of tubular fluid and spermatozoa, and actively reabsorbs part of this fluid while adding ions, proteins, and steroids. Primary lesion of mesonephric cells that form the rete testis could result in abnormal function of the rete testis epithelium, which could produce fluid with abnormal composition at an inappropriate time.
Lesions similar to cystic dysplasia of the rete testis have been experimentally induced by sodium intoxication after administration of salt-retaining hormones or deoxycorticosterone acetate in fowls, as well as by estradiol administration in newborn rats.
Cystic dysplasia of the epididymis (CDE) is a recently described anomaly characterized by the presence of irregular, segmental cystic dilatation of the epididymal ducts with aberrant forms and immature appearance ( Fig. 12.69 ). There is a significant decrease in the number of sections of efferent ductules without lesions in the lining epithelium. The irregular cystic dilatations cause loss of epididymal head architecture (loss of typical hemispherical shape). It may affect one segment of the epididymal duct in association with lesions in the head of the epididymis or as an isolated lesion.
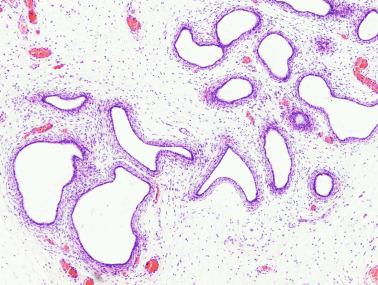
CDE was observed in 19 fetal and neonatal autopsies of males from 27 weeks of gestation to 10 days of life, and in one surgical specimen from a 4-year-old. The lesion was bilateral in all evaluable cases. Eighteen of 20 cases had renal or urinary tract anomalies, including renal dysplasia (8 cases), renal agenesis (4 cases), autosomal recessive polycystic renal disease (1 case), and renal hypoplasia (1 case). In eight cases, testes were cryptorchid. One patient had associated ipsilateral testicular cystic dysplasia.
CDE is a novel congenital anomaly of mesonephric differentiation that should be added to the spectrum of male excretory system disorders associated with renal and urinary malformations.
Only one case of rete testis hamartoma has been reported. It was in a 3-year-old who presented with a testicular mass. The lesion consisted of a disordered tubular proliferation lined by epithelium like that of the rete testis within a loose connective tissue stroma. A peculiar hamartoma that consisted of three separate components (cystic dilatation of the rete testis, diffuse interstitial smooth muscle proliferation, and extensive stroma with myxoid areas) was reported in a 26-year-old.
FGTD refers to abnormally differentiated testicular parenchyma. This disorder was first described in 1986 in a 28-week premature newborn who died 1 hour after delivery. The only remarkable antecedent during pregnancy was polyhydramnios. The karyotype was 46,XY. The most relevant data from autopsy were hypertelorism, low-set ears, fenestrated secundum-type atrial septal defect, idiopathic bilateral hydronephrosis without apparent urinary duct obstruction, unilateral double renal collecting system, and pleural effusion. Genitalia were characteristic of a normal male infant. Three other cases (18-week-old fetus, 22-week-old fetus, and a 3-year-old) were later reported, all in association with Walker-Warburg syndrome (WWS; https://www.omim.org/entry/236670 ), including left ureter malformations and ipsilateral renal cystic dysplasia. Two additional cases of FTGD were observed. The most interesting autopsy findings were hydrops, muscular lesions suggesting mitochondrial myopathy that did not fit the diagnostic criteria of WWS, and undescended testes lodged near the inferior pole of both kidneys. Weinberg reported a 10-month-old with WWS whose gonads were located in the inguinal canal and consisted of epididymis, vas deferens, and a rete testis that ended blindly in fibrous streaks; “follicle-like structures” were noted microscopically. This observation lends support to the notion of an association between WWS and FGTD. Incidentally, Weinberg’s case appears to describe the oldest patient in whom FGTD has been identified. In contrast, Whitley and colleagues reported a patient with WWS in whom testicular abnormalities included small size attributed to shortening of seminiferous tubules and low numbers of Leydig cells. No features resembling FGTD were present. WWS represents the most severe of the dystroglycanopathies, in which defective interaction between membrane receptors and extracellular matrix components results in muscle, brain, and nerve derangements. Two new cases of GTD have been reported in association with Noonan syndrome.
In all cases of FGTD, the testes were grossly unremarkable, with normal tunica albuginea, and the histologic testicular pattern was similar. Seminiferous tubules were well developed (number of germ cells and Sertoli cells per cross section within normal limits) only in the central zone of the testicular parenchyma, although with reduced numbers of seminiferous cords. Beneath the tunica albuginea, the peripheral testicular parenchyma showed a crescent-like zone with large malformed tubules, cords, and solid nodules of spherical or irregular shape embedded in fibrous connective tissue reminiscent of ovarian stroma organized into several concentric layers. Each of these solid nodules was surrounded by a delimiting basement membrane ( Fig. 12.70 ). Each structure was composed of three cell types: cells with vesicular nuclei and vacuolated cytoplasm, cells with hyperchromatic nuclei, and germ cell–like cells. The former two cell types were arranged at the periphery, forming a pseudostratified epithelium with the long axes perpendicular to the nodule periphery. The third cell type resembled fetal spermatogonia and was fewer in number, although number varied from one tubular formation to another. These structures contained eosinophilic, PAS + material, similar to Call-Exner bodies ( Fig. 12.71 ). There may be continuity between these structures and normal seminiferous tubules.
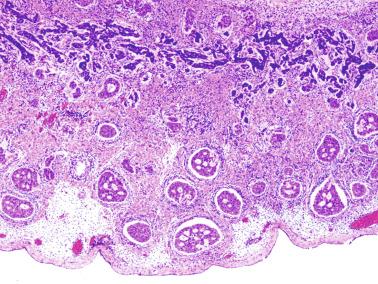
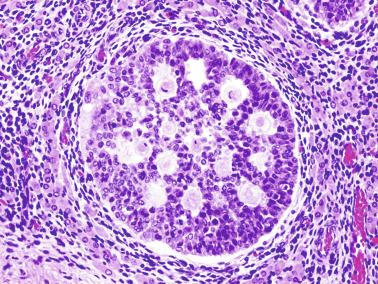
Immunohistochemically, cells within the nodular lesions revealed a gradient in the expression of inhibin, most strongly at the periphery and negative in the center. The surrounding spindle stromal cells expressed vimentin and muscle-specific actin. Laminin and collagen type IV were expressed in the basement membrane and revealed deep invaginations of basement membrane material toward the center of the nodules, resembling Call-Exner bodies ( Fig. 12.72 ). Only isolated cells morphologically resembling germ cells expressed PLAP, but most were negative for all markers. Cytokeratin (AE1/AE3) expression was only focally positive. The remaining parenchyma showed normal development according to age and revealed expression of vimentin in Sertoli cells. Inhibin was intensely and diffusely expressed in Sertoli and Leydig cells.
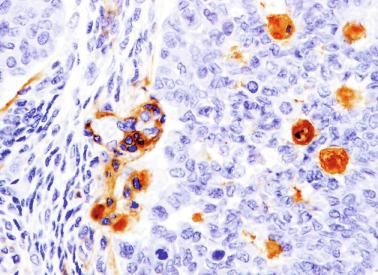
The differential diagnosis includes conditions with anomalous seminiferous tubules at the gonadal periphery, including dysgenetic testis and gonadoblastoma. Dysgenetic testis also exhibits tubular or cordlike structures, but these are differentiated (some form true seminiferous tubules) and may also be present within a poorly collagenized tunica albuginea. Both contain intertubular stroma consisting of fibrous connective tissue similar to the ovarian-like stroma characteristic of dysgenetic gonads. However, patients with dysgenetic testis are 46,XY disorder of sex development (DSD) with müllerian remnants, a condition that is absent in FGTD.
Distinguishing FGTD from gonadoblastoma is more difficult. Gonadoblastoma is usually found in an ovarian streak gonad or dysgenetic gonad and contains granulosa–Sertoli cells and germ cells that are like those of dysgerminoma or seminoma; these cells are absent in FGTD.
FGTD has been reported in patients with WWS, so one could hypothesize that the genetic deficiency causing this syndrome may also be responsible for normal testicular development. Other hypotheses suggest a defect in segmentation of primitive cords secondary to poor fetal development of the vascular system or mutations in the PTPN11 gene because two patients were carriers of Noonan syndrome.
One may also speculate about the potential progression pathway of FGTD. Given that no patient has survived beyond a few months of life, it is not possible to ascertain how the lesion would have progressed. Incidentally, it is puzzling to recognize the vague resemblance between FGTD and granulosa cell tumor of the juvenile type, another congenital testicular tumor. The determination whether FGTD is a precursor lesion of full-blown gonadoblastoma, juvenile granulosa cell tumor, or another neoplasm, or would rather undergo atrophy, as may be surmised by the observations of Weinberg, awaits identification of FGTD in patients with longer survival.
Sertoli cell nodule consists of the presence in the adult testis of one or several foci of infantile (immature) seminiferous tubules. The term “Sertoli cell nodule” was first reported in 1973, although Pick described a lesion in 1905 known as Pick adenoma that has similar characteristics. This lesion has also been reported as Sertoli cell hyperplasia, tubular dysgenesis, or hypoplastic zones.
Each group of tubules appears well delimited but unencapsulated. Nodule size usually varies from microscopic to 5 mm; however, in at least 10 reported cases, size has reached up to 10 mm, termed macroscopic Sertoli cell nodule . These patients may have palpable nodules at presentation. The nodules may be single or multiple. On section, each nodule is distinguished by its whitish color. Ultrasonography may suggest testicular tumor.
Sertoli cell nodule is found in about 60% of adult cryptorchid testes, regardless of when the testes descended. It is also present in 22% of normal scrotal testes in some series and is an occasional finding in men with idiopathic infertility and in the parenchyma surrounding germ cell tumor. The seminiferous tubules have a prepubertal diameter and may be anastomotic. The epithelium is columnar or pseudostratified, devoid of lumina, and usually consists only of Sertoli cells ( Fig. 12.73 ). The cells have elongate hyperchromatic nuclei with one or several peripherally placed small nucleoli.
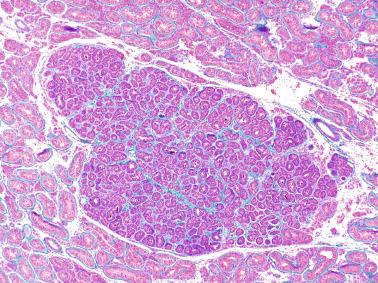
The interstitium varies from scant to well collagenized. Leydig cells are usually absent in these areas, and, if present, their numbers are low; exceptionally, they are abundant ( Fig. 12.74 ). In some nodules, isolated germ cells may be observed ( Fig. 12.75 ). Study of serial sections sometimes reveals continuity between some tubules and normal tubules. Sertoli cell nodules change with advancing age. These changes are not related to a significant enlargement of the size because that remains unaltered, but rather reflect development of structures inside the lesions. The Sertoli cells produce large amounts of basal lamina that become intensely PAS + and protrude inside the hypoplastic tubules. In transverse and oblique sections, these protrusions may be misinterpreted as intratubular accumulations of basal lamina material ( Fig. 12.76 ). This material may undergo calcification to form microliths. Immunohistochemical study reveals two components of the basal lamina (collagen IV and laminin), thus confirming extracellular origin; the protrusions consist mainly of laminin, whereas collagen IV delimits the outer profile of the seminiferous tubules. So, although the amount of collagen IV is uniform around the tubules, the depth of laminin varies within the same tubule and from one to another tubule. Sertoli cells in this lesion also show immunohistochemical markers of immature cells, including expression of D2–40, AMH, calretinin, and inhibin bodies. The diameter of hypoplastic tubules is usually smaller than that of the adjacent tubules, but in some cases the inverse occurs because of hypertrophy of Sertoli cells, which show some evidence of pubertal maturation without reaching full maturation of adult cells.
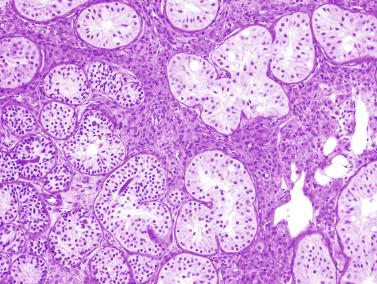
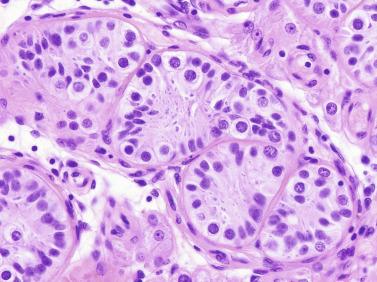
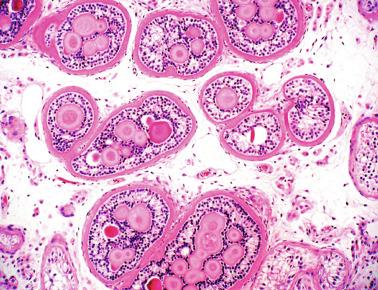
Sertoli cell nodules are assumed to be primary testicular lesions and are included in the spectrum of testicular dysgenesis syndrome. They represent seminiferous tubules that are unable to undergo pubertal development despite the same hormonal stimuli as adjacent normal tubules. This dysgenesis includes immature Sertoli cell pattern showing D2–40 immunoexpression, low inhibin secretion, absence of ARs, and lack of maturation of peritubular myoid cells that fail to synthesize elastic fibers ( Fig. 12.77 ). The presence of hypoplastic zones (Sertoli cells nodules) in a testicular biopsy is an adverse prognostic sign for fertility, as is the presence of isolated dysgenetic tubules or hypoplastic tubules in testicular parenchyma. Attempts have been made to identify the precursor zones of these hypoplastic zones in prepubertal biopsies to obtain an additional predictor of fertility in cryptorchidism. Infantile precursor hypoplastic zones are not as well delimited as definitive hypoplastic zones of adults. Differences in diameter between hypoplastic tubules and the other tubules of the testicular parenchyma are not as clearly marked in infants as in adults. Although small tubules predominate in the hypoplastic zone precursors of the infantile testis, the degree of maturation of these tubules may be similar to or more advanced than the outer tubules of the parenchyma ( Fig. 12.78 ). The most reliable histologic findings for identifying the precursor zones in infant testes are the cordlike arrangement of these tubules, occurrence of anastomoses among the cords, scant cellularity (Sertoli number is lower than in the remaining testicular parenchyma), and wide Sertoli cell cytoplasm, which often shows granular changes.
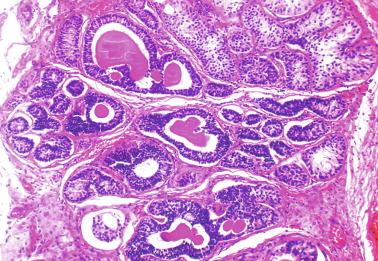
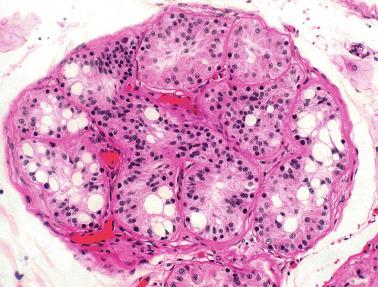
The differential diagnosis includes Sertoli cell adenoma, tubular hamartoma in androgen insensitivity syndrome (AIS), testis with focal Sertoli cell–only tubules (MAT of the testis), Sertoli cell neoplasm, and gonadoblastoma.
Sertoli cell adenoma and tubular hamartoma are characteristic findings in AIS. Like Sertoli cell nodule, adenoma and tubular hamartoma contain nodules composed of solid tubules of immature Sertoli cells without a capsule. The three lesions differ macroscopically in size; adenoma and tubular hamartoma are usually larger than Sertoli cell nodule. Histologically, both Sertoli cell adenoma and tubular hamartoma are formed by Sertoli cells with spherical, rather than elongate, nuclei that do not display pseudostratification. Although the tubular wall may be widened, it does not have nodular intratubular projections. In Sertoli cell adenoma, tubules are back to back, and the sparse interstitium does not usually contain Leydig cells. The interstitium may be densely cellular in tubular hamartoma, with fusiform cells. Tubular hamartoma always has many Leydig cells, whereas these cells are lacking or scant in Sertoli cell nodule. The parenchyma surrounding tubular hamartoma has the same grade of development as the lesion. In contrast, the testes in patients with Sertoli cell nodule are always delayed in development.
Testes with focal Sertoli only–cell tubules show two seminiferous tubule types: Sertoli cell–only tubules and tubules with germ cells, although the spermatogenetic degree may vary widely in the tubules with germ cells. Sertoli cell–only tubules do not form clusters such as those seen in the hypoplastic tubules of Sertoli cell nodules. In addition, the tubular size and the degree of Sertoli cell maturation are higher in Sertoli cell–only tubules to such an extent that even lumina may be observed in some tubules. Finally, the usual cell components of the testicular interstitium (Leydig cells, macrophages, and some mast cells) are found in these Sertoli cell–only tubules.
Sertoli cell tumor not otherwise specified consists of larger cells with vesicular nuclei and prominent nucleoli with isolated atypical nuclei and mitotic figures arranged in cords and tubules without significant widening of basal membrane that is characteristic of Sertoli cell nodule.
Intratubular large cell hyalinizing Sertoli cell neoplasia may resemble Sertoli cell nodule at low magnification because both may produce multiple nodules and show widened basal membranes and intratubular projections of basal membrane–derived material. However, the tubules in intratubular large cell hyalinizing Sertoli cell neoplasia are not anastomotic and are larger in diameter than those seen in Sertoli cell nodule. Sertoli cells have an apparent higher grade of maturation (vesicular nuclei with central nucleoli and large eosinophilic cytoplasm). Immunohistochemically the cells express cytokeratin that is usually absent in Sertoli cell nodule. Also, intratubular hyalinizing Sertoli cell neoplasia does not contain germ cells, usually develops in scrotal testis, and is associated with Peutz-Jeghers syndrome.
Sex cord tumor with annular tubules is exceptionally rare in the testis. The resemblance to Sertoli cell nodule is high, but features favoring this diagnosis include large size of the lesion and greater complexity of the tubular organization, with solid areas and hyalinized stroma.
When Sertoli cell nodule contains germ cells, the differential diagnosis of gonadoblastoma should be considered. Most germ cells in Sertoli cell nodules are spermatogonia; these cells are scant, do not show signs of proliferation, and are TSPY + (testis-specific protein Y-encoded + ). In rare cases, Sertoli cell nodule contains gonocyte-like cells in both the basal membrane and the center of the tubules; these cells express OCT3/4. These gonocyte-like cells thus represent GCNIS ( Fig. 12.79 ). In these cases the lesion itself is like gonadoblastoma. Criteria for ruling out gonadoblastoma are as follows: Sertoli cell nodule with GCNIS is surrounded by or near germ cell tumor, and gonadoblastoma characteristically arises in malformed gonads (gonadal dysgenesis, dysgenetic testis in DSDs), whereas Sertoli cell nodule is found in well-configured testes. The disposition of tumor cells is also important. In Sertoli cell nodule, tumor cells are preferentially disposed at the center of the lesion, whereas in gonadoblastoma, the distribution is more diffuse.
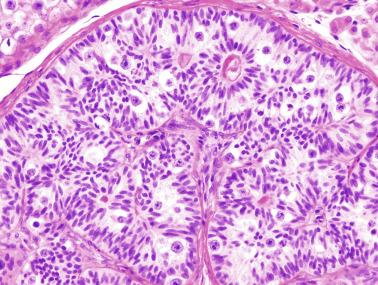
Another entity that should be included in the differential diagnosis is sex cord–stromal tumor of the testis with entrapped germ cells. These tumors measure several centimeters in diameter. The cells are arranged in cords and tubules and may show germ cells without atypia, mainly at the periphery.
Tubular hamartoma consists of unencapsulated whitish nodules that are well delimited from the parenchyma containing small seminiferous tubules and numerous Leydig cells. It is also known as Sertoli–Leydig cell hamartoma (see later discussion of Androgen Insensitivity Syndromes).
Congenital testicular lymphangiectasis is characterized by abnormal and excessive development of lymphatic vessels in the tunica albuginea, mediastinum testis, interlobular septa, and testicular interstitium. Ultrastructurally these dilated vessels are similar to normal lymphatic capillaries, although some are markedly dilated, and the testicular interstitium is slightly edematous ( Fig. 12.80 ). Testicular lymphangiectasis occurs in both cryptorchid and scrotal testes. One patient had Noonan syndrome.
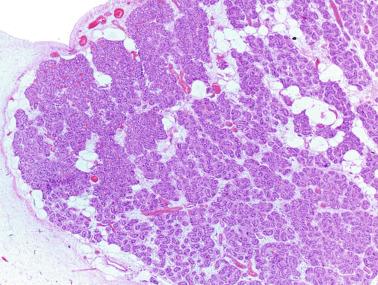
It does not seem to affect the seminiferous tubules, and low numbers of spermatogonia and reduced tubular diameters are observed only in cryptorchid testes. The epididymis and spermatic cord are not affected, and congenital testicular lymphangiectasis is not associated with pulmonary, intestinal, or systemic lymphangiectasis. During fetal life, lymphatic vessels are visible only immediately beneath the tunica albuginea and in the interlobular septa. During childhood the number and size of the septal lymphatic vessels decrease. By adulthood the vessels are inconspicuous, although ultrastructural and immunohistochemical evidence of their presence is visible in the interstitium, and they are easily identified by staining with monoclonal antibody D2–40.
In lymphangiectasis, septal lymphatic vessels are large and often massively dilated. It occurs only in childhood, a finding suggesting that these dilated vessels undergo involution at puberty or that pubertal development of the seminiferous tubules masks the lymphangiectasis.
Testicular lymphangiectasis may result from alterations in lymphatic drainage caused by surgical treatment of the inguinal region, radiation therapy of retroperitoneal lymph nodes, or chronic inflammation of the spermatic cord. Lymphatic dilatation involves vessels that were previously normal, resulting in development of small cysts chiefly in the tunica vasculosa and epididymis. Similar dilatations have been observed in cryptorchid testes and in patients with Morris syndrome.
Epididymal lymphangiectasis has been described in adults as “lymphangiectasis” and “lymphangioma,” terms referring to pseudotumoral lesions consisting of abnormal development of lymphatic vessels in the caput epididymis. Dilated vessels compress the ductuli efferentes and cause irregular dilation by compression, thereby distorting the normal architecture ( Fig. 12.81 ). In some cases, these malformative or hamartomatous lesions are likely primary, and the diagnosis is made by examination of orchiepididymectomy for suspected tumor or lesion. In other cases, it is considered secondary to previous herniorrhaphy, and only epididymectomy is performed.
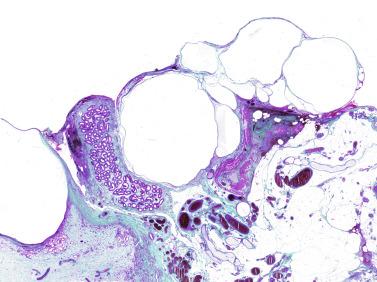
Smooth muscle is a normal component of sperm excretory ducts, as well as of two other specific structures: the tunica albuginea of the inferior pole and the interstitial tissues of the cauda epididymis among the numerous folds of the ductus epididymis. Muscular hyperplasia involving any of these structures has been reported in patients from puberty to 81 years of age. The minimum size required for diagnosis is not well defined, but previously reported cases indicate that the width of muscular proliferation was 0.6 to 7 cm.
In young men, smooth muscle hyperplasia is especially frequent in those with AIS. The lesion has been reported as leiomyoma, and it may be associated with multiple tubular hamartomas. This smooth muscle proliferation is located in the inferior pole, involves the tunica albuginea and the adjacent soft tissue (i.e., in the zone that should be occupied by the cauda epididymis), and may form nodules measuring more than 1 cm in diameter ( Fig. 12.82 ).
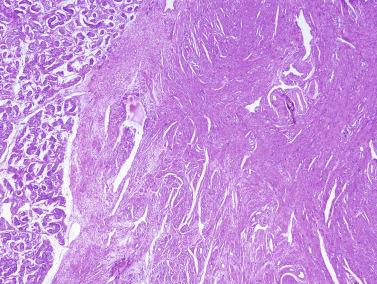
In other adult patients the reported distribution of smooth muscle hyperplasia has been related to the ductus deferens (periductal), the surrounding vessels (perivascular), the different structures of the cauda epididymis (interstitial), the seminiferous tubules (peritubular hamartoma) ( Fig. 12.83 ), or the tunica albuginea ( Fig. 12.84 ). In periductal hamartoma, ductus deferens thickness (normally 3 mm) can reach 10 mm. The cauda epididymis (normally 0.5 to 0.7 mm) may enlarge up to 2 mm. Smooth muscle cells create a concentric pattern around sperm excretory ducts and blood vessels. In other hamartomas such as those in the tunica albuginea and the interstitial tissue of the cauda epididymis, muscle bundles are irregularly arranged and intermingled with variable amounts of connective tissue ( Fig. 12.85 ).
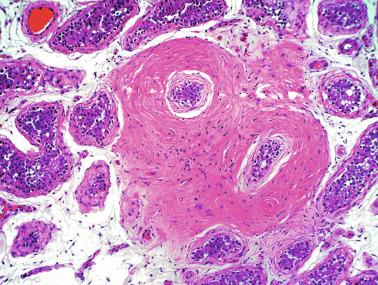
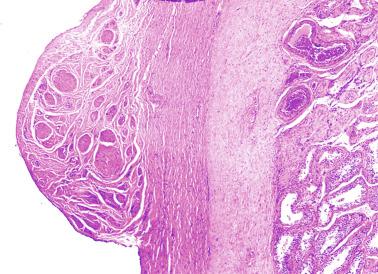
Most muscular hyperplasias are assumed to be hamartomas, similar to smooth muscle proliferations in other organs, including the gingiva, palate, esophagus, gastric pylorus, small intestine, large intestine, trachea, and breast. Differential diagnosis includes leiomyoma, but hyperplasia lacks characteristic features of leiomyoma such as the cohesive pattern of smooth muscle bundles, nodularity, and well-defined delimitation from the adjacent tissues.
The term persistence of testicular blastema refers to the presence of gonadal blastema in an otherwise normal testis for age. Testicular blastema includes immature sex cords, germ cells, and mesenchymal components. The primitive tissue gives rise to testicular parenchyma and is not expected to be present in completely developed testes.
This infrequent ectopia occurs in newborns as a rare autopsy finding. It was present in both testes of three fetuses from a total of more than 3000 consecutive autopsies: one fetus was spontaneously aborted as a result of chorioamnionitis; one was electively aborted because of a neural tube defect, omphalocele, and asymmetric arthrogryposis; and a third had trisomy 18 and classic phenotypic features of Edwards syndrome.
The blastema is near the upper testicular pole at the implantation site of the caput of the epididymis. It has a crescent shape and extends throughout the depth of the tunica albuginea and the adjacent parenchyma.
Blastema consists of epithelial cords of cells or solid masses in continuity with mesothelium ( Fig. 12.86 ). These cells are intermingled with others that are larger, with pale cytoplasm, vesicular nuclei, and prominent nucleoli. This second population, resembling germ cells, is less frequently seen, sparsely distributed among the cords. The remaining parenchyma, tunica albuginea, and epididymis are normal for age. Blastomatous epithelial cells display immunoreactivity for vimentin, laminin, type IV collagen, and cytokeratin; the expression of cytokeratin in the most superficial cells is like that of mesothelial cells and decreases in intensity in the deeper cells. This characteristic suggests that these may be pre–Sertoli cells. The cordlike structures are delimited by laminin and type IV collagen. The larger cell type is immunoreactive for PLAP on the surface, and lacks vimentin and cytokeratin expression, suggesting that it is related to the gonocyte. Leydig cells have not been observed among the cords of gonadal blastema.
The differential diagnosis of gonadal blastema ectopia includes ovotestes. The small size of the gonocytes distinguishes these cells from ovocytes, which are several times larger. In addition, no intersex condition is observed.
The most likely evolution of blastema is differentiation toward testicular parenchyma. This possibility is supported by two features: the disorder may occur only in newborns, and in the zone where blastema cells are found (superior testicular pole), ectopic seminiferous tubules or ectopic Leydig cells have also been observed.
The presence of seminiferous tubules within the tunica albuginea is a rare and usually incidental histologic finding. Ectopic tubules are present in approximately 0.8% of pediatric autopsies and 0.3% of adult autopsies. The lower incidence in adults may be explained by proportionally less sampling. The lesion ranges from microscopic to a few millimeters in diameter, and it may be visible in children as rounded macules on the surface of the testis as minute bulges in which multiple small vesicles protrude through a thin tunica albuginea. Histologically, there are groups of seminiferous tubules in the tunica albuginea, sometimes accompanied by Leydig cells. In children, ectopic tubules appear normal ( Fig. 12.87 ), whereas in adults they are usually slightly dilated ( Fig. 12.88 ), although some tubules may be hyalinized. Serial sections reveal continuity with the intraparenchymatous seminiferous tubules.
Ectopia of the seminiferous tubules is probably congenital, although it has been found in older men. It does not appear to be the result of trauma. The malformation probably arises in the sixth week of gestation, when the primordial sex cords have formed and are branching toward the gonadal surface, and the developing testes are covered by only one to three layers of coelomic epithelium. Later, the tunica albuginea forms around the sex cords and under the coelomic epithelium. Failure of insertion of the tunica albuginea between the sex cords and coelomic epithelium may entrap seminiferous tubules.
Ectopia differs from testicular dysgenesis, a distinctive form of 46,XY DSD with müllerian remnants. Numerous features characteristic of ectopic seminiferous tubules distinguish ectopia from other conditions, including normal thickness and collagenization of the tunica albuginea, absence of interstitial tissue resembling ovarian stroma (characteristic of testicular dysgenesis), and clear delimitation of the tunica albuginea and testicular parenchyma (see discussion of Disorders of Sex Development (Dysgenetic testis), below).
In a unique case, multiple clusters of seminiferous tubules and Leydig cells were present in the wall of a hernia sac that accompanied an undescended testis removed from an adult. The ectopic tubules were not surrounded by tunica albuginea and were like those in cryptorchid testicular parenchyma with only dysgenetic Sertoli cells ( Fig. 12.89 ). Sertoli cells in the tubules expressed inhibin and ARs ( Fig. 12.90 ). In another case, ectopic seminiferous tubules immunoreactive for inhibin were in the epididymis next to extratesticular rete testis ( Fig. 12.91 ). These extratesticular seminiferous tubules were surrounded by loose stroma and seemed to be connected to the extratesticular rete testis.
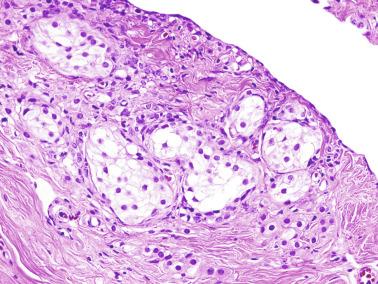
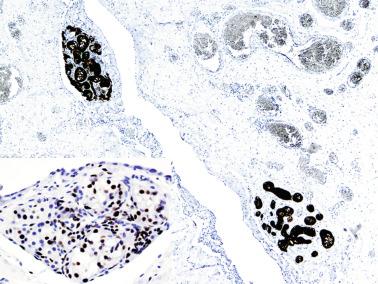
Leydig cells occur normally in the testicular interstitium (interstitial Leydig cells) and in the wall of the seminiferous tubules (peritubular Leydig cells). However, clusters of cells are often observed in other locations in the testis, in the epididymis, or in the spermatic cord. In men the incidence rate is 40% to 90%.
Inside the testis, ectopic Leydig cells may be found in the interlobular septa, rete testis, or tunica albuginea ( Figs. 12.92 to 12.94 ), as well as within hyalinized seminiferous tubules. Intratubular Leydig cells are found only in tubules with advanced atrophy and marked thickening of the tunica propria, including the tubules in adult cryptorchid testes, those of men with Klinefelter syndrome, and in some other primary hypogonadisms ( Fig. 12.95 ). Immunohistochemical studies suggest that the endocrine function of these Leydig cells is low. Several theories have been offered to account for these ectopic cells, including in situ differentiation, migration from the testicular interstitium, and trapping of peritubular Leydig cells in the tunica propria during its thickening and seminiferous tubule shortening in atrophy progression. The presence of small capillary vessels within the intratubular Leydig cell clusters would favor the last hypothesis.
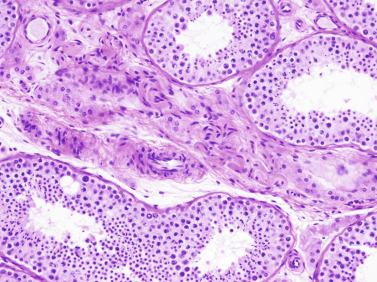
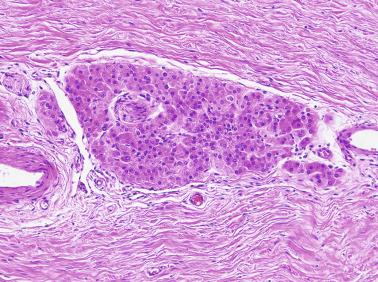
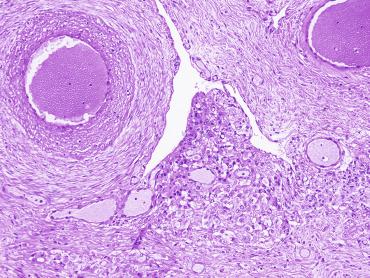
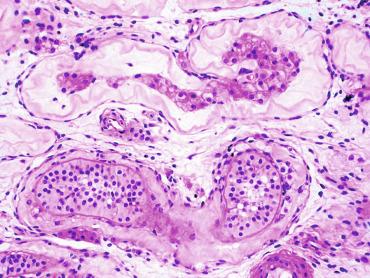
Leydig cells are commonly found in the epididymis, as well as in the spermatic cord, both in newborns and adults. Extratesticular Leydig cells usually form small groups within or adjacent to nerves ( Fig. 12.96 ), present in 41% of autopsies. Extraparenchymal Leydig cells arise in more than 90% of orchiectomy specimens, and study of tissue microarrays found an incidence rate of 35%. Extratesticular location of Leydig cells is apparently common, and thus finding them depends only on the number of sections obtained. In most cases, these cells are in proximity to small nerves or even inside the nerves and vegetative nervous system ganglia. These cells, probably originating from in situ differentiation of Leydig cell precursors, express testosterone. Inhibin and calretinin are other useful immunohistochemical markers to enhance detection of ectopic Leydig cells. Calretinin is more sensitive but less specific than inhibin.
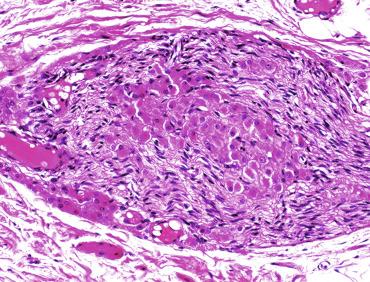
Ectopic Leydig cells, both intratesticular and extratesticular, suffer the same atrophic and hyperplasic alterations as their eutopic counterparts, including disappearance during childhood. In older men and in those with chronic alcoholism, ectopic Leydig cells may show atrophic features, whereas in patients with hCG-secreting germ cell tumors, they show hyperplasia.
The occurrence of ectopic Leydig cells in the tunica albuginea, epididymis, or spermatic cord may account for rare cases of Leydig cell tumor in these paratesticular structures. Ectopic Leydig cells should not be misinterpreted as tumor cells (infiltration or metastasis) when malignancy of a testicular Leydig cell tumor is suspected.
Adrenal cortical ectopia is a frequent finding outside of the testis, although it has also been observed inside. Ectopic adrenal cortex is the most frequent incidental finding in male urologic surgery. In a series of boys who underwent inguinoscrotal surgery the incidence rate of this finding varied from 3% to 3.8%. The frequency was higher (5.1%) in a series of children with cryptorchid testis who underwent more complete exploration of the spermatic cord. Most adrenal cortical ectopias are found in extratesticular locations such as the epididymis ( Figs. 12.97 and 12.98 ), tunica albuginea, and hernia sacs ( Fig.12.99 ). Macroscopically, ectopic adrenal tissue forms firm yellowish nodules, measuring 1 to 4 mm in diameter, spherical or ovoid, and sometimes umbilicated. The nodules are covered by a capsule, have three well-defined layers of adrenal cortex, and lack medulla. Adrenal ectopia represents aberrant adrenal tissue that has accompanied the testis in its descent.
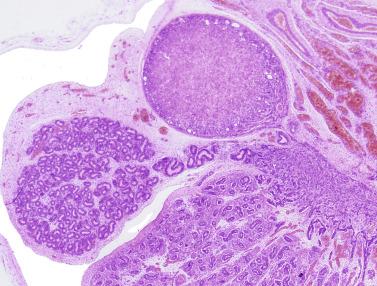
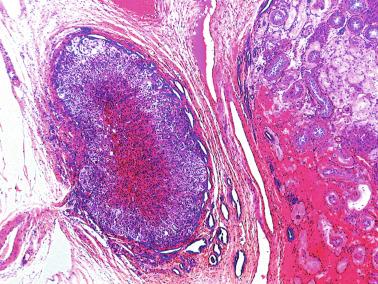
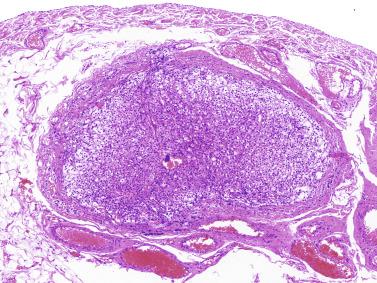
Ectopia of cells similar to adrenal cortex may also be found inside the testis. Adrenal ectopia has been observed between the rete testis and adjacent seminiferous tubules, as well as in the parenchyma near the tunica albuginea. Adrenal ectopic nodule may be solitary or multiple. Cytomegalic cells have been observed in newborns with adrenal cytomegaly, both in patients with Beckwith-Wiedemann syndrome and in isolated cases of adrenal cytomegaly ( Fig. 12.100 ). The origin of these intratesticular nodules may be pluripotential testicular hilus steroid cells.
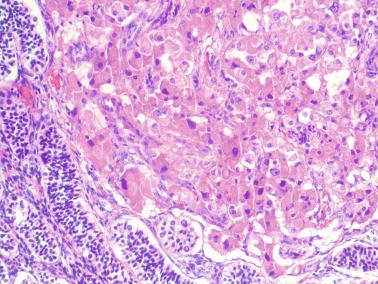
Intratesticular nodules may undergo hyperplasia, thus causing testicular enlargement that suggests a tumor in two disorders: adrenogenital syndrome and Nelson syndrome (see later).
Other rare forms of ectopia are found within and outside the testis. Osseous and adipose tissue and ectopic ductus epididymis may be formed within the testis. Extratesticular ectopia includes splenic ectopia (splenogonadal fusion), hepatic ectopia (hepatotesticular fusion), and renal blastema ectopia.
Cartilaginous heterotopia , which may appear as small immature cartilage nodules in the caput of the epididymis, has been attributed to metaplasia of metanephric rests.
Osseous heterotopia (testicular osteoma, stone in the testicle, testicular calculus) is a rare type of metaplasia that occurs in areas of the parenchyma with fibrosis. It is possible that some cases are secondary to prior tuberculosis, hematoma resorption, or traumatic injury. In some cases, such lesions cannot be distinguished from a tumor, prompting orchiectomy ( Fig. 12.101 ). Reported cases and our own observations reveal that osseous metaplasia manifests as hard intraparenchymatous nodules that vary from a few millimeters to several centimeters. These nodules consist of compact osseous tissue surrounded in all cases by cholesterol clefts and a fibrous pseudocapsule. They probably represent a metaplastic process in response to testicular trauma or damage.
Adipose metaplasia is frequent in undescended testes and older men. Adipose cells are preferably located in the proximity of the rete testis ( Fig. 12.102 ). Adipocytes are frequently found at the periphery of intratesticular adrenal cortical tumors and in some Leydig cell tumors. Testicular lipomatosis is associated with Cowden syndrome (usually bilateral), Proteus syndrome, and Bannayan-Riley-Ruvalcaba syndrome. These syndromes, together with adult Lhermitte-Duclos disease and autism spectrum disorders associated with macrocephaly, collectively form the PTEN hamartoma tumor syndrome (PHTS), which is related to PTEN gene mutations. Intratesticular lipomatosis associated with these syndromes is visualized by ultrasound as multiple bilateral hyperechoic lesions. Given the rarity of intratesticular lipoma, the presence of lipomatosis may be an indicator of underlying PTEN mutation.
Intratesticular and paratesticular mesonephric remnants. Most of the structures derived from embryonic remnants (testis and epididymis hydatids, aberrant upper and lower ducts, the Giraldes organ) have a specific location and are well-known wolffian or müllerian derivatives. However, in systematic study of surgical specimens of testis, epididymis, spermatic cord, and hernial sacs, glandular or tubular formations may frequently be found that are reminiscent of the epididymis but may be misinterpreted as either normal structures of the spermatic pathways or, more significantly, as primary or secondary neoplasm. These mesonephric remnants may be found in other parts of the urogenital tract, including the kidney, renal pelvis, prostate, prostatic urethra, and paratesticular and intratesticular regions.
Histologically, mesonephric remnants have variable patterns, ranging from small acini or tubules lined by low columnar epithelium with or without colloid type material inside. They resemble both histologically and immunohistochemically the efferent ducts, the main duct of the epididymis or the vas deferens, but differ by smaller size and absence of a well-structured muscular cell layer.
Mesonephric remnants in the spermatic cord are the most frequent and are observed in 28% of cords at autopsy. Most are incidental, but some present clinically as cystic structures with or without papillary formations. Most glandular inclusions in the wall of hernial sacs represent mesonephric remnants, with an incidence rate of 1.5% to 6%. Less frequent is the presence of mesonephric remnants in the testicular tunic, where these remnants, whose epithelium usually resembles that of efferent ducts, may produce cysts in the tunica albuginea. Even rarer is the appearance of remnants inside vestigial structures of müllerian origin, as is the case of aberrant epididymal tissue reported in a testicular hydatid.
Intratesticular mesonephric remnants are of special interest with respect to histogenesis and because they may be confused with neoplasm. Intratesticular structures reminiscent of efferent ducts or the main epididymal duct were found bilaterally in 5 of 1442 autopsies, and in 1 patient from a series of 271 orchiectomies. Patient age ranged from 69 to 75 years. In autopsy cases, both testes were shrunken. The lesion was found in an orchiectomy specimen from a 67-year-old with suspected testicular tumor. The testicles showed multiple whitish areas located in the central part, as well as near the rete testis and under the tunica albuginea. Most of the mesonephric remnants corresponded to epididymis-like or efferent ductile-like structures. The luminal size varied from that of seminiferous tubules to cystic formations. Interiorly, there was granular or fibrillar PAS + eosinophilic material with microcalcifications and Liesegang rings. A muscular layer surrounded most of the formations. Frequently the intratubular material was extravasated, triggering a minimal inflammatory reaction. The number and extent of these formations was so great in the reported orchiectomy specimen that in comparison with remnants observed in other organs, one could speak of florid hyperplasia of intratesticular mesonephric remnants. In most testes the adjacent parenchyma had large areas of tubular hyalinization, likely of ischemic origin ( Figs. 12.103 through 12.105 ).
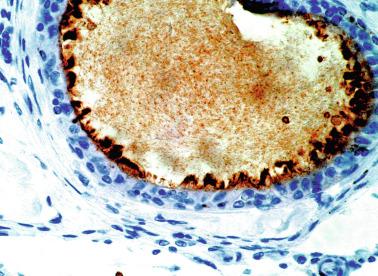
The differential diagnosis includes teratoma and burned-out germ cell tumor, but the distinction is not difficult. The location of these epididymal-like tubular formations within the parenchyma is difficult to explain from an embryologic point of view. Such displacement would have to occur at an early stage, before differentiation of the albuginea by AMH secreted by the Sertoli cells. Mesonephric cords would be trapped between nests consisting of pre–Sertoli and germ cells. An alternative hypothesis is metaplastic change in Sertoli cells.
Testicular descent is not always complete at birth, and approximately 3% of full-term newborns have incompletely descended testes. Most of these testes descend within 3 months, and only 1% of infants have incompletely descended testes 12 months after birth. Spontaneous testicular descent is exceptional after the first year. In recent decades there has been a significant increase in the incidence of cryptorchidism.
Only 5% of patients with impalpable testes are devoid of testes. Other causes include true cryptorchidism, testicular ectopia, and retractile testes. True cryptorchidism includes abdominal, inguinal, and high scrotal testes that cannot be moved to the scrotum on physical exploration. Ectopic testes are those located out of the normal path of testicular descent, most frequently in the superficial inguinal pouch. Other rare locations of ectopia include the abdominal wall, upper thigh, perineum, and base of the penis. Retractile testes may be moved to the scrotum at exploration and account for approximately one-third of clinically diagnosed undescended testes.
Patients with true cryptorchidism account for approximately 25% of cases of empty scrotum. These testes most frequently are found in the inguinal canal or upper scrotum; arrest within the abdomen is less frequent and accounts for only 5% to 10% of cases. Cryptorchidism is slightly more frequent on the right side than on the left (46% versus 31%, respectively), and in approximately 23% of cases is bilateral. Family history of cryptorchidism is present in 14%. Cryptorchid testis is usually smaller than the contralateral one, and this difference is often discernible at 6 months of age. One-third of cryptorchid testes are soft.
The causes of testicular maldescent are probably multiple and have not been fully elucidated. Several conditions are predictive of high risk for cryptorchidism, including increased maternal age, maternal obesity, pregnancy toxemia, bleeding during late pregnancy, exposure to phthalates, alcohol consumption and smoking, tall stature, paternal subfertility antecedents, cesarean birth, low birth weight, preterm birth, twin birth, hypospadias, other congenital malformations, and birth from September to November, as well as May to June. Of these associations, low birth weight seems to be the most important. Cryptorchidism may be congenital or acquired.
This type of cryptorchidism is mainly caused by anomalies of development or hormonal mechanisms involved in testicular descent (see earlier). Impalpable undescended testes are infrequent because the transabdominal phase follows the simple mechanism of relative movement of the testis. Conversely, palpable undescended testes are more frequent because the second phase of testicular descent is more complex. Unilateral cryptorchidism may be caused by androgen failure, which leads to either an ipsilateral lesion in the development of GFN neurons or defect in CGRP release that hinders normal migration of the gubernaculum.
Normally descended testis may become cryptorchid and may even settle in the abdominal cavity. Acquired cryptorchidism has a prevalence rate of 1% to 7% and peaks at approximately 8 years of age. Two categories of acquired undescended testis have been described: postoperative trapped testis and spontaneous ascent from unknown causes.
Postoperative trapped testis is a normally descended testis that leaves the scrotal pouch after surgery for inguinal hernia or hydrocele. This iatrogenic cryptorchidism occurs in 1% of children after herniotomy. Adherence of the testis or the cremasteric muscle to the surgical incision causes testicular ascent when the incision heals and undergoes retraction.
Various mechanisms have been proposed for spontaneous ascent from unknown causes , including inability of spermatic blood vessels to grow adequately, anomalous insertion of the gubernaculum and reabsorption of the vaginal process, failure in postnatal elongation of the spermatic cord, and prenatal and postnatal androgen disruption because boys with severe hypospadias are at increased risk for acquired and retractile testes. The spermatic cord measures 4 to 5 cm at birth and reaches 8 to 10 cm by 10 years of age. This growth does not occur if the peritoneal-vaginal duct becomes a fibrous remnant. The cause may be a defect in postnatal CGRP release by the GFN. Acquired undescended testis spontaneously descends at puberty in 78% of cases.
Testicular maldescent has multiple causes, including anatomic anomalies of the gubernaculum testis, hormonal dysfunction (hypogonadotropic hypogonadism), mechanical impairment (insufficient intraabdominal pressure, short spermatic cord, underdeveloped processus vaginalis), dysgenesis (primary anomaly of the testis), neuromuscular conditions (CGRP and cremaster nucleus), genetic factors (mutations in INLS3 or its receptor), and other hereditary or acquired conditions.
Most cases of cryptorchidism likely appear to be caused by a deficit of fetal androgens or an excess of maternal estrogens. Androgen insufficiency seems to be slight and transient because anomalies other than hypoplasia of the epididymis are not seen. It may result from deficient gonadotropic pituitary stimulation or low production of placental gonadotropins. Elevated maternal estrogens could cause diminution of FSH secretion by the fetal pituitary, thus leading to low Sertoli cell proliferation, and could create decreased testosterone production because of the inhibitory effect of estrogens on Leydig cells. Three mechanisms seem to be involved in this process:
Primary testicular anomaly. Cryptorchid testes may bear an anomalous germ cell population. More than 40% of cryptorchid patients have a marked decrease in the TFI, even with nearly normal numbers of spermatogonia; these cells also have abnormal DNA content.
Lesions secondary to transient perinatal hypogonadotropic hypogonadism. Cryptorchid patients do not have gonadotropin elevation, which normally occurs between 60 and 90 days after birth, and this deficiency of LH could cause Leydig cell involution. Subsequent androgen deficiency could account for failure of gonocytes to differentiate into spermatogonia.
Injury caused by increased temperature. This was suggested in the past based on animal studies. In follow-up biopsies from testes that were descended surgically or with hormonal treatment, the sole factor that improved during childhood was tubular diameter. Diameter depends on Sertoli cells, so temperature may be more important for Sertoli cells than for spermatogonia.
The most frequent findings in congenital and acquired cryptorchidism in infancy are decreased germ cell numbers and diminished tubular diameter. In the normal testis, transient formation of spermatocytes occurs at 4 to 5 years of age. This meiotic attempt is probably an androgenic event that does not occur in cryptorchid testes and corresponds to the characteristic low numbers of spermatogonia in the prepubertal age.
Undescended testis is usually smaller than contralateral, descended testis, a difference that is already significant at 6 months of age. However, no functional studies have revealed the existence and severity of congenital lesions in cryptorchid testes. Numerous biopsy studies of cryptorchid testes in the first years of life have been conducted, but no agreement exists about the severity of damage or time of onset. The presence of lesions in the first year of life in most suggests that they are primary rather than acquired as a consequence of long-standing cryptorchidism. One should not forget that the testis is a dynamic structure, with waves of proliferation and differentiation from birth to puberty. Moreover, the parameters usually applied to studies of the adult parenchyma should not be used for the prepubertal testis.
The pathologist should evaluate both semiquantitative and qualitative parameters to obtain as much information as possible from histologic study of biopsies from undescended testes. Semiquantitative morphometric parameters include MTD, TFI, number of germ cells per cross-sectioned tubule, Sertoli cell index (SCI), and number of Leydig cells in the interstitium. Qualitative findings include the pattern of germ cell distribution in the parenchyma (regular or irregular), abnormal spermatogonia (multinucleated, hypertrophic, or atypical), focal granular changes in Sertoli cells, abnormal tubules (megatubules, ring-shaped tubules), microliths, and ARs in children who are older than 4 years. Based on the evaluation of four parameters (TFI, MTD, SCI, and the spermatogonial distribution pattern), most testicular biopsies from cryptorchid testes of children may be classified into one of three groups ( Table 12.15 ).
Type I (testes with slight alterations) (about 31% of cases). TFI is higher than 50, and MTD is normal or slightly (< 10%) decreased ( Fig. 12.106 ).
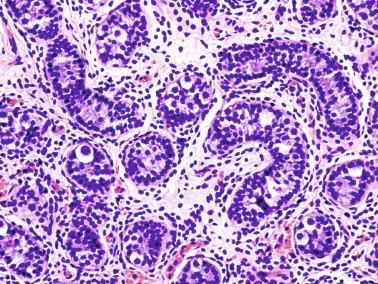
Type II (testes with marked germinal hypoplasia) (about 29% of cases). TFI is between 30 and 50, and MTD is 10% to 30% lower than normal. The spermatogonia are distributed irregularly, and most are in tubular sections that are grouped in the same testicular lobule ( Fig. 12.107 ).
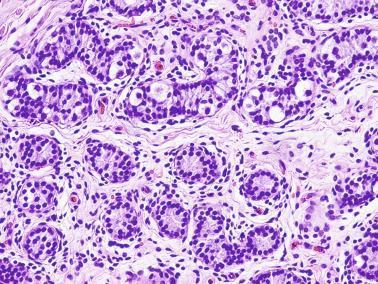
Type III (testes with severe germinal hypoplasia) (about 40% of cases). TFI is less than 30, and MTD is less than 30% of normal. Many spermatogonia are giant with dark nuclei ( Fig. 12.108 ). The testicular interstitium is wide and edematous.
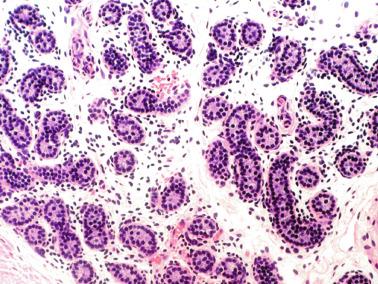
| Type of Lesion (Incidence Rate) | MTD | TFI | SCN | Spermatogonia Distribution |
|---|---|---|---|---|
| Type I (31%) | Slightly decreased (90% normal values) | > 50% | Normal | Regular |
| Type II (29%) | Markedly decreased (60%–90% normal values) | 30%–50% | Decreased | Irregular |
| Type III (40%) | Severely decreased (< 60% normal values) | 0%–30% | Low | Irregular |
The seminiferous tubules of testes with type II or III lesions have a thickened lamina propria during childhood and, at puberty, Sertoli cell hyperplasia, and more than 30% of these testes have microliths ( Fig. 12.109 ), ring-shaped tubules, and granular changes in Sertoli cells ( Fig. 12.110 ). Intense immunoexpression for inhibin is observed in Sertoli cells in all types of cryptorchid testes ( Fig. 12.111 ). Patients with bilateral cryptorchidism have a higher incidence of type II and III lesions than those with unilateral cryptorchidism. Approximately 8% of testes with type I lesions contain many multinucleated spermatogonia (with three or more nuclei; Fig. 12.112 ), and both KIT expression and PLAP expression persist in approximately 5% of immature germ cells.
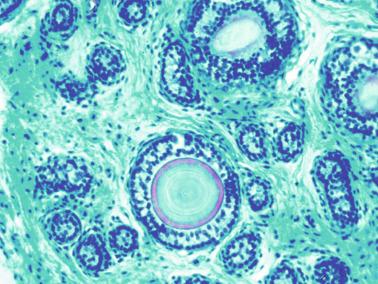
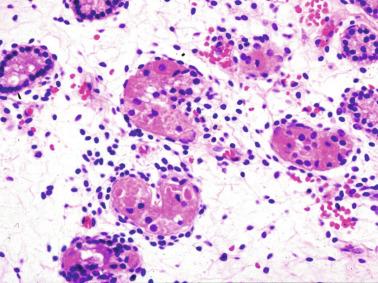
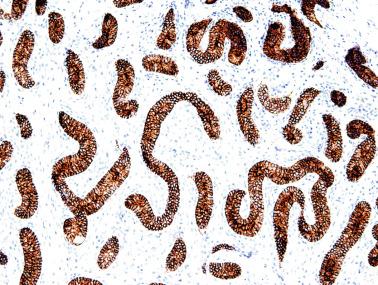
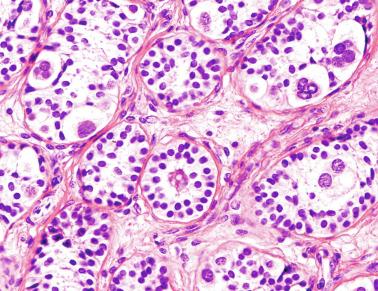
Type I lesions are comparable with those seen in experimental cryptorchidism: normal testes in which lesions are induced by increased temperature. Testes with type II or III lesions bear variable degrees of dysgenesis that, in addition to germ cells, involves Sertoli cells, peritubular myofibroblasts, and Leydig cells. The dysgenesis of these other cell types is evident only after puberty and is more severe in intraabdominal testes. In approximately 25% of cases the contralateral scrotal testis also has histologic lesions of variable severity. This finding supports the hypothesis of bilateral defect in many cases of unilateral cryptorchidism. Microdeletions in the long arm of Y-chromosome genes (DAZ, RBM, [azoospermic factor] AZFa, b, and c) are not increased in cryptorchidism.
Unilateral cryptorchidism with normal contralateral testis may result from end-organ failure. In cryptorchidism secondary to spontaneous ascent, lesions are like those of congenital cryptorchidism, whereas in cryptorchidism secondary to herniotomy, germ cell depletion is slight and becomes important only after 5 years of age.
Most pubertal and adult cryptorchid testes have anomalies in all testicular structures. During puberty, undescended testes show severe maturation delay in both tubular and interstitial components ( Fig. 12.113 ). Seminiferous tubules display apparent Sertoli cell hyperplasia as a result of failure in lengthening and coiling of the seminiferous tubule during pubertal development, and tubules also have decreased diameter and delayed germ cell proliferation when these cells are present. Frequently, as puberty progresses, testicular development is irregular, varying from one lobule to another. Seminiferous tubules that have initiated spermatogenesis are adjacent to other tubules showing prepubertal maturative pattern. Careful study reveals a delay in Sertoli cell maturation, estimated by nuclear morphology. Irregular expression of ARs is found in these Sertoli cells ( Fig. 12.114 ). Tubules with a prepubertal pattern also show delayed maturation in mural myofibroblasts, which are unable to express muscle-specific actin.
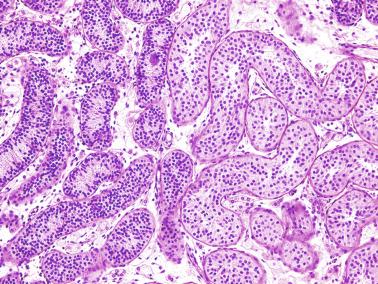
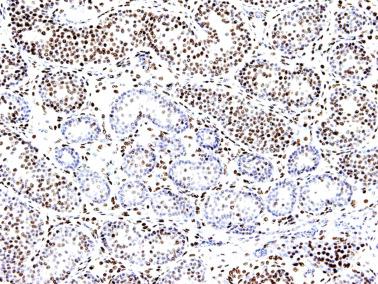
Surgically removed cryptorchid testes from adults reveal that most have histologic lesions in all structural components and the rete testis and epididymis. Seminiferous tubules have decreased diameters and deficient spermatogenesis. In decreasing order of frequency, the most common germ cell lesions are tubules with a Sertoli cell–only and spermatogonia-only pattern, tubules with Sertoli cells (dysgenetic) only, tubular hyalinization, and MAT. The lamina propria has scant elastic fibers, as well as irregular deposits of collagen IV and laminin in the basal lamina. Peritubular myoid cells in experimental cryptorchidism show disorganization of actin filaments. Sertoli cells are present in greater number and do not mature normally except in tubules with germ cells ( Fig. 12.115 ). In tubules with advanced spermatogenesis, the morphology of Sertoli cell nuclei is normal. In addition to being immunoreactive for vimentin, similar to normal adult Sertoli cells, the dysgenetic Sertoli cells in Sertoli cell–only tubules immunoexpress cytokeratins and desmin in the basal cytoplasm. These immunoreactions are also positive in intraluminal accumulations of sloughed Sertoli cells. Often, groups of tubules containing only Sertoli cells with a prepubertal pattern (small diameter and total absence of maturation) may be found and are considered hypoplastic (showing a small lumen and a thick wall lined by a tall columnar epithelium, formed only by Sertoli cells of eosinophilic cytoplasm) ( Fig. 12.116 ), dysgenetic, or hamartomatous; seminiferous tubules with granular changes in Sertoli cells may also be present. Areas of apparent Leydig cell hyperplasia are frequent ( Fig. 12.117 ), and many of these cells contain vacuolated lipid-laden cytoplasm ( Fig. 12.118 ).
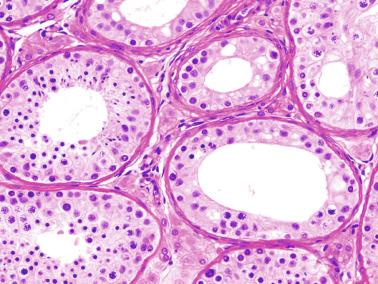
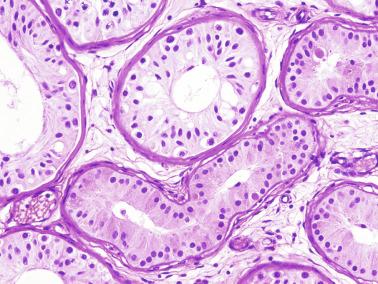
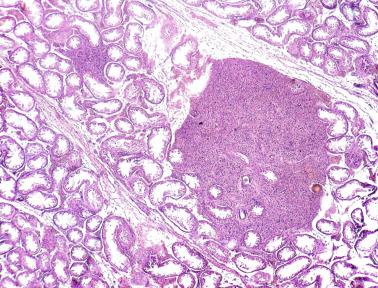
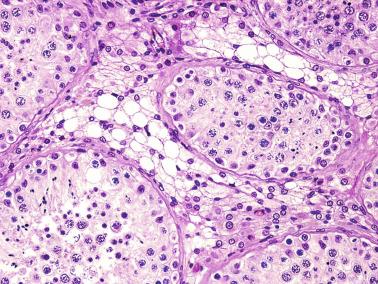
The rete testis is hypoplastic in most cases, lined by columnar epithelium with rare areas of flattened cells. Cystic dilation is common, and adenomatous hyperplasia may be present. Most cryptorchid testes, and especially those with Sertoli cell–only tubules, contain underdeveloped rete testis whose epithelium has not differentiated between squamous and columnar cells (conversely to what normally occurs during puberty), retaining an infantile pattern referred to as “dysgenetic” ( Fig. 12.119 ). Near the rete testis, the parenchyma frequently contains metaplastic fat. In some cryptorchid testes, several tubular segments are destroyed by inflammation that probably has an autoimmune cause (focal orchitis). Epididymal tubules are poorly developed, and peritubular tissue is immature.
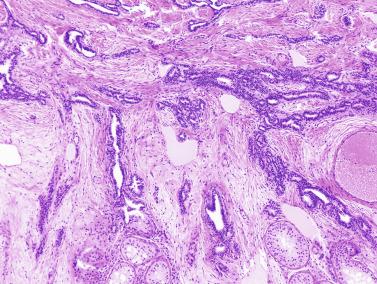
Needle aspiration biopsy of the normally descended contralateral testis shows a variety of histologic patterns, varying from normal to with alterations like those of cryptorchid testes. This variability is probably related to the cause of cryptorchidism. Bilateral lesions suggest congenital or genetic cause, whereas normal contralateral testis suggests local anatomic anomalies.
Study of serial sections of bilateral testicular biopsies allow comparison of histologic findings in boys with cryptorchidism at the time of orchidopexy and in adulthood (performed for evaluation of infertility). One report described diffuse and complete spermatogenesis in such testes with higher TFI (normal testes and those with type I lesions) in more than two-thirds of cases, accompanied by a quantitatively abnormal number of germ cells; MAT developed in the other one-third of cases. MAT is defined as synchronous occurrence of tubules containing germ cells (with variable degrees of spermatogenesis) and Sertoli cell–only tubules ( Fig. 12.120 ). All testes with type II lesions and 85% of those with type III lesions experience development of MAT, indicating that MAT is the most frequent lesion (68%) in adult ex-cryptorchid patients presenting with infertility ( Table 12.16 ). In most patients with unilateral cryptorchidism, MAT is observed in both the undescended testis and contralateral descended testis, even if the surgical correction occurred at an early age. There is an inverse correlation between severity of the lesions in childhood and amount of spermatogenesis in adulthood. Approximately 85% of adult testes with type III lesions in infancy experience MAT, with spermatogenesis in less than 50% of tubules; another 5% develop hypospermatogenesis and spermatocyte I sloughing; and the remaining 10% have Sertoli cell–only tubules. Patients whose prepubertal testes had clustering of germ cell–containing tubules usually had incomplete spermatogenesis in adulthood, and when spermatogenesis was complete, it occurred in less than 50% of tubules.
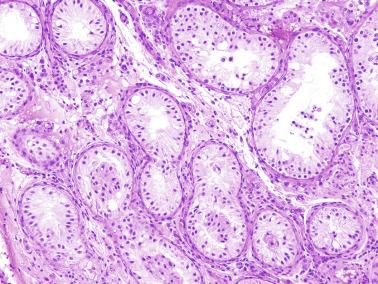
| Type of Lesion | Postpubertal Testicular Pathology |
|---|---|
| Type I | Adluminal compartment lesions (57%) |
| Mixed testicular atrophy (29%) | |
| Basal and adluminal compartment lesions (14%) | |
| Type II | Mixed testicular atrophy (100%) |
| Type III | Mixed testicular atrophy with < 50% tubules with spermatogenesis (85%) |
| Sertoli cell–only tubules (10%) | |
| Hypospermatogenesis + spermatocyte I sloughing (5%) |
Sperm excretory duct anomalies occur in 9% to 36% of cryptorchid patients. These anomalies may cause obstruction of the excretory ducts, so biopsies may also reveal an obstructive pattern superimposed on the seminiferous tubule lesions.
In summary, the classification of testicular lesions in childhood into three types (I, II, and III) correlates with the amount of spermatogenesis found in postpubertal biopsies. The most important parameter is TFI. Spermatogenesis may also be impaired by an obstructive process that is complete or incomplete, and functional or organic. Biopsy allows adequate evaluation of congenital or obstructive lesions, and detects testes with focal spermatogenesis to establish the possibility of treatment with assisted reproductive techniques.
Optimal treatment of undescended testis is controversial. The European Association of Urology recommends mandatory surgical treatment in infancy; patients with palpable inguinal cryptorchid testes should undergo orchidopexy within 1 year and no later than 2 years of age. The Nordic Consensus statements recommend anticipating orchidopexy as early as the first 6 to 12 months of life. Surgical treatment benefits all patients with primary cryptorchidism and retractile testis, even those whose testes are surgically descended at an older age.
The efficacy of hormonal treatment after orchidopexy remains controversial. Results are contradictory because great variations in the effects of hormonal treatment have been reported. Whereas use of hCG and GnRH in the treatment of cryptorchidism was found effective in some cases, it was useless in others. Metaanalysis showed that approximately 15% to 20% of retained testes descend during hormonal treatment, although one-fifth subsequently reascend. Moreover, treatment with hCG increases germ cell apoptosis, with subsequent damage to future spermatogenesis. Testicular biopsy is the only sure method for identification of cryptorchid boys who need hormonal treatment with luteinizing hormone-releasing hormone (LHRH) after successful surgery; consequently, some advocate mandatory biopsy during orchidopexy. Neonatal gonocytes transform into type A spermatogonia at 3 to 12 months, and this process is disrupted in undescended testes, although there is a chance of reversal by orchidopexy at an early age. The presence of Ad spermatogonia at surgery is an excellent prognostic parameter for future fertility. Cryptorchid boys who lack these cells experience infertility despite successful orchidopexy at an early age. In those whose testes contain a low number of Ad spermatogonia and suboptimal response to hCG stimulation, hormonal treatment is advised to increase the number of germ cells. It is uncertain whether different modalities and periods of hormonal therapy in patients with impaired Leydig cell response can improve histologic features prognosis for future fertility.
In childhood the chance of detecting occult cancer or precancer by biopsy is low because intratubular germ cell neoplasia is not diffusely distributed throughout the testis. Nonetheless, testicular biopsy should be performed in all patients with intraabdominal testes, abnormal external genitalia, or abnormal karyotype.
Autotransplantation has been used in some patients with high intraabdominal testes as an alternative to the Fowler-Stephens technique. These testes represent approximately 5% of all undescended testes. Histologic studies performed after autotransplantation reveal evidence of Leydig cell development, tubular diameter increase, and spermatogenetic development, although paternity has not been reported.
Most cryptorchid patients have a patent processus vaginalis, and 65% to 75% have a hernia sac, although most hernias are not clinically visible. Urologic anomalies are present in 11%, the most frequent being hypospadias, complete duplication of the urinary tract, nonobstructive ureteral dilatation, kidney malrotation, and posterior urethral valves. Cryptorchidism is more frequent in those with microcephaly, myelomeningocele, bifid spine, omphalocele, gastroschisis, micropenis, imperforate anus, and cloacal exstrophy.
The incidence of sperm excretory duct anomalies is high in patients with undescended testes, and these anomalies involve both the intratesticular and extratesticular ducts. Dysgenesis of the rete testis is observed in more than 80% of adult undescended testes. The incidence of extratesticular spermatic duct anomalies varies from 9% to 79%. Sperm excretory duct anomalies are classified into three types :
Ductal fusion anomalies (25% of cases). These consist of anomalous fusion of the caput of the epididymis to the testis or segmental atresia of the epididymis and vas deferens. This category is chiefly associated with intraabdominal or high scrotal cryptorchid testes.
Ductal suspension anomalies (59% of cases). The caput of the epididymis is attached to the testis, whereas the corpus and the cauda of the epididymis are separated from the testis by a mesentery. A variant consists of excessively long cauda of the epididymis that descends along the inguinal duct to the scrotum ( Fig. 12.121 ).
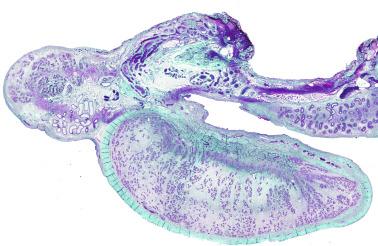
Anomalies associated with absent or vanishing testes (16% of cases).
The ductuli efferentes and the ductus epididymidis of cryptorchid patients show evidence of abnormal development from infancy. Remarkably, epithelial cell height of both types of ducts is low, and the muscular wall is poorly developed. In adults, ductuli efferentes show an inner circular outline instead of the wavy outline that is characteristic of normal caput of epididymidis, reflecting different epithelial cell heights. Incomplete development of the muscular wall suggests that propulsion of testicular fluid is compromised, resulting in stasis of ductuli efferentes, rete testis, and even seminiferous tubules.
Patent vaginal process is associated with epididymal anomalies, regardless of the location of the cryptorchid testis, with an incidence rate as high as 80%, compared with only 5% in those with descended testes with a closed vaginal process.
Anomalies of the gubernaculum are also frequent in cryptorchidism. Comparative studies between normal fetuses and cryptorchid infants reveal differences in the union of the gubernaculum to the testis and epididymis (72% vs 99% incidence rate, respectively). The gubernaculum is joined exclusively to the testis (the cauda epididymis is free) in 22% of cryptorchid testes and in 1% of normal fetal testes. The gubernaculum is joined exclusively to the cauda epididymis in 6% of cryptorchid testes, whereas this condition is not observed in normal fetuses.
Another anomaly is the occurrence of short spermatic vessels that hinder testicular descent. This is a frequent finding in cryptorchid testes with type III lesions and, to a lesser extent, in those with type II lesions. This observation further supports the dysgenetic nature of these testes.
Many unfavorable results obtained after surgical descent of these testes may be explained by ultrasonographic images that reveal anomalies associated with undescended testes.
Cryptorchidism may be isolated, or is often be associated with congenital, endocrine, or chromosomal disorders, as well as with disorders in sexual differentiation. Cryptorchidism may occur in patients with GnRH deficit; Kallmann, Prader-Willi (PWS), Klinefelter, Noonan, Smith-Lemli-Opitz (SLOS), Aarskog-Scott, Rubinstein-Taybi, prune belly ( Fig. 12.122 ), Cornelia de Lange, and caudal regression syndromes; testicular feminization syndromes caused by AR anomalies such as 5α-reductase deficiency (an enzyme required for conversion of testosterone to DHT); several types of undermasculinization caused by AMH absence; DiGeorge syndrome; Beckwith-Wiedemann syndrome; CHARGE association; and trisomies 13, 18, and 21. The association of impalpable testes with hypospadias suggests a disorder of sexual differentiation (this suggestion was confirmed in 27% to 30% of cases).
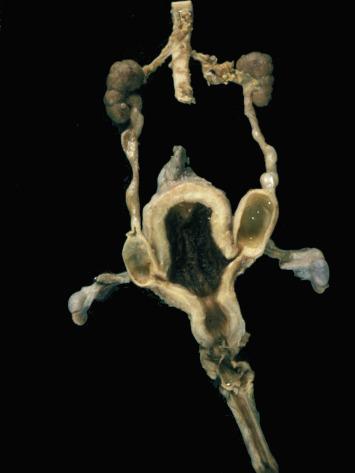
Cryptorchidism is part of the testicular dysgenesis syndrome , first described in 2001. This syndrome consists of a spectrum of male reproductive disorders with a range of clinical presentations, including abnormal testicular development that predisposes to cryptorchidism, hypospadias, spermatogenetic alterations, and testicular cancer. The association of these disorders with cryptorchidism has been corroborated by numerous clinical, epidemiologic, and genetic studies. The least severe form of this syndrome is defect in spermatogenesis; the most severe is testicular cancer.
A constellation of histologic lesions is common in the testes of men with testicular dysgenesis, including Sertoli cell–only pattern, MAT, hypoplastic tubules (Sertoli cell nodules), testicular microlithiasis, malformed tubules, granular changes in Sertoli cells, nodular Leydig cell hyperplasia, and GCNIS. It is assumed that the lesions develop prenatally as a result of several genetic, environmental, or endocrine disruptor factors that would interfere with the estrogen-to-androgen ratio and possibly lead to disruption of Sertoli or Leydig cell function. The initial disorder is probably an imbalance between estrogens and androgens during fetal life related to increased estrogen exposure in utero. Exposure to environmental toxins, acting as endocrine disruptors, could disrupt fetal sexual differentiation by an estrogenic or antiandrogenic effect. Various environmental chemicals may alter endogenous levels of androgens (certain phthalates) and estrogens (polychlorinated biphenyls, polyhalogenated hydrocarbons). Estrogens may induce cryptorchidism and hypospadias by suppressing androgen production or action, or by suppressing INLS3. Although testicular dysgenesis syndrome still raises many questions of epidemiology and pathogenesis, it is accepted as a single unifying hypothesis, particularly when so little is known of etiology.
The main complications of cryptorchidism are testicular cancer, infertility, testicular torsion, and psychological problems derived from an empty scrotum.
Approximately 1% of 1-year-olds have cryptorchidism, and approximately 10% of patients with testicular cancer had cryptorchidism. Cancer risk of undescended testis is 6.3 versus 1.7 of the contralateral testis by metaanalysis. The risk for testicular cancer in cryptorchid boys and men is 4 to 10 times higher than that of the general population. Cancer risk in abdominal testes is six times higher than that of other cryptorchid testes in an inferior location. Testes with an elevated number of multinucleated spermatogonia have a higher risk for cancer during adulthood. The risk increases when cryptorchidism is bilateral and associated with external genitalia anomalies or chromosomal anomalies such as 45,X/46,XY. Approximately 5% of biopsies in children contain cells similar to those seen in GCNIS, although possible evolution to invasive malignancy remains a subject of controversy ( Fig. 12.123 ). The presence of GCNIS may be missed in infancy because in the prepubertal testis atypical gonocytes may not be located directly on the basal lamina; instead, these gonocytes may be present in the center of the tubules, and their number may be low. Approximately 2% to 3% of adult cryptorchid testes have evidence of GCNIS, and the most frequent tumor in these patients is seminoma. Regardless of timing, orchidopexy does not reduce the risk for cancer, although it facilitates early detection because the intrascrotal testis is palpable. Twenty percent of testicular tumors arise in properly descended testes contralateral to cryptorchid testes; this finding suggests a primary bilateral testicular anomaly in cryptorchidism. Intraabdominal testes also have a higher incidence of tumors, with a similar prognosis.
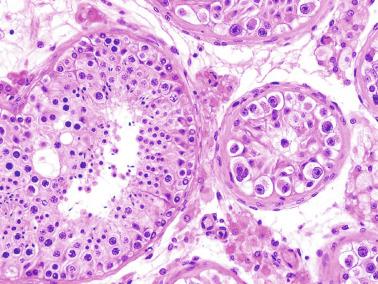
Infertility is the most frequent problem caused by cryptorchidism. In a series of patients with infertility, almost 9% had cryptorchidism ( Fig. 12.124 ). Hormonal serum levels in ex-cryptorchid patients show high levels of FSH, normal LH, and low testosterone.
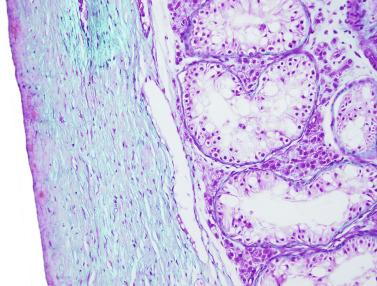
Infertility is influenced by several factors, including bilaterality, number of germ cells, location and size of the testis, germ cell distribution, anomalous DNA content in germ cells, Leydig cell dysfunction, congenital anomalies of sperm excretory ducts, and possible iatrogenic injury to the testes, epididymis, ductus deferens, or spermatic cord during orchidopexy.
The most important risk factors are bilaterality and germ cell number; 16% to 25% of men with bilateral cryptorchidism have normal sperm counts (≥ 20 million/mL). Highest sperm counts occur with testes in the superficial inguinal pouch. Patients with bilaterally impalpable testes are usually azoospermic. Fertility rates in unilateral cryptorchidism vary from 25% to 81%. Many patients with repaired bilateral cryptorchidism require assisted reproductive techniques such as intracytoplasmic sperm injection to obtain successful fertilization.
The number of germ cells per cross-sectioned tubule is the most important prognostic factor. Patients with no increase in inhibin B during the postoperative period usually have a low number of spermatogonia and low TFI. Usually a cryptorchid boy with less than 0.2 spermatogonia per cross-sectioned tubule will have a deficient spermiogram in adulthood, and fertility will be decreased. In unilateral cryptorchidism, fertility depends on the number of spermatogonia in the contralateral testis. However, if the number of germ cells per cross-sectioned tubule in the cryptorchid testis is less than 1% of normal, the risk for infertility is 33%, increasing to 75% to 100% in bilateral cryptorchidism. Spermatogonial number correlates with sperm number in the spermiogram, volume of surgically repaired testis, inhibin B serum level, and volume of this testis in adulthood. In a series of 142 azoospermic men with history of cryptorchidism, testicular sperm extraction (TESE) was successful in 62% of cases overall and 63% of those with bilateral cryptorchidism, favorably altering the prospect of fertility. Those patients whose FSH level is normal or testicular volume is higher than 10 cm 3 have an even better prognosis because the rate of TESE with positive sperm retrieval is 75%.
Preoperative location of the unilaterally cryptorchid testis and the small size of the testis at the time of orchidopexy cannot predict likelihood of fertility. Patients with high scrotal testes or superficial inguinal pouch testes have the best spermiograms, whereas those with intraabdominal or canalicular testes are oligozoospermic or azoospermic. Testicular damage may result either from the presence of more severe histologic lesions or injury produced during orchidopexy because these testes require more mobilization those in lower locations. Patients whose testes are nearly normal in size also have better spermiograms. An important fertility factor is the permeability of sperm excretory ducts. Between birth and 4 years of age, patient age at orchidopexy may also influence fertility, although this has not been proven. Beyond 4 years, orchidopexy does not enhance fertility.
Undescended testes have a 10-fold higher risk for spermatic cord torsion than do normally located testes. This increased risk results from the abnormal suspension system. Fixation of the contralateral testis should be performed using orchidopexy if anomalies in testicular suspension are observed. The percentage of testes that may be preserved is low because of the delay in seeking consultation and making the diagnosis.
Tunica albuginea calcifications, coinciding with site of fixation, are found in adult testes that were fixed at orchidopexy. These calcifications appear mainly when chromic suture material was used, and tare not related to pathologic features present in the cryptorchid testis at the time of orchidopexy or will develop later. Intraabdominal testes may also undergo torsion.
The high number of children subjected to orchidopexy has led to knowledge of postsurgical atrophy. The frequency of this complication is estimated to be 1% to 5% for inguinal canal testes and 25% for intraabdominal testes ( Fig. 12.125 ).
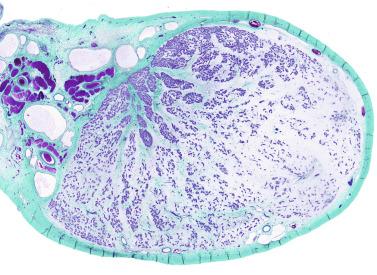
The importance of having two testes should be considered before orchidopexy because of potential damage to the male identity. Studies carried out in adults whose testes were surgically descended before puberty revealed normal male development and behavior, although these men seemed to be sexually less active than controls. This observation does not appear to be related to treatment method or age when treated. Patients should be offered testicular prosthesis and information on the risks involved (infection, migration).
Testicular biopsies of infantile testes at orchidopexy are useful for determining baseline germ cell status and whether surgery should be supplemented by hormonal treatment. However, even if biopsy supplies significant data, it is not considered a routine procedure.
When the number of spermatogonia is nearly normal, spermatogenesis may not occur because of deficient spermatogonium development during childhood or failure of spermatogenesis at puberty, even in the best of cases. If complete spermatogenesis occurs, this may be associated with obstruction of sperm excretory ducts.
In childhood, risk for occult cancer or precancer at biopsy is low because GCNIS is not diffusely distributed throughout the testis. Testicular biopsy is recommended in patients with intraabdominal testes, abnormal external genitalia, or abnormal karyotype.
The situation is different in adults because GCNIS is present in 2% to 3% of cases and is diffuse. When GCNIS is detected in a child, further examination of the testis and repeat biopsy after puberty are recommended. In adults, if GCNIS is unilateral, orchiectomy should be performed, but if bilateral, radiation therapy may be used to eradicate neoplasm while maintaining Leydig cell function.
Obstructed testes are in the superficial inguinal pouch (Denis-Browne pouch) and are considered ectopic by some authors and cryptorchid by others. Histologic studies reveal that most obstructed testes bear the same lesions as true cryptorchid testes. Type I lesions are observed in one-half, type II lesions are seen in more than one-third, and the remaining lesions are type III. The higher proportion of type I lesions suggests a better prognosis than in true cryptorchidism.
Some authors assume that retractile testes are normal and exclude them from studies of cryptorchidism. However, these testes may have significant lesions, and many consider them to be a form of cryptorchidism. Retractile testes may not always be movable to the lower scrotum (70 to 75 mm from the pubic tubercle), and in 50% of cases are smaller than scrotal testes. Approximately 50% of retractile testes remain high after age 6 years, when cremasteric activity declines. Retractile testes have a 32% risk for becoming ascending or acquired undescended testes. The risk is higher in boys younger than 7 years or when the spermatic cord is tight or inelastic. During childhood, MTD and TFI decrease. Adults with retractile testes that descended spontaneously but late may be fertile or infertile. Usually there is germ cell atrophy that varies in severity from lobule to lobule ( Fig. 12.126 ). Regular examination of retractile testes is advisable during childhood, and if complete testicular descent does not occur, orchidopexy is indicated. In 14% of patients with retractile testicles treated by orchiopexy, abnormalities of the epididymis are similar to those observed in cryptorchid testes.
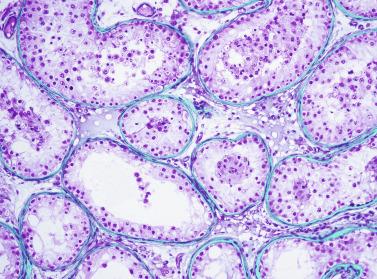
Calcifications may arise in the testis, but the high frequency of this finding has emerged with routine use of ultrasonography. Histologic studies combined with ultrasonography have elucidated the nature and significance of microliths, macroliths, clumps or calcified scars, phlebolites, stones, or calculus.
Testicular microlithiasis is characterized by the presence of numerous calcifications diffusely distributed throughout the parenchyma. Classically the term testicular microlithiasis was used to describe cases containing a large number of microliths. Microlithiasis may occur in infancy or adulthood, associated with tumoral and nontumoral pathologic features. In infancy, it may be seen in undescended testes, Klinefelter syndrome, Down syndrome, developmental delay and testicular asymmetry, undermasculinization XY testicular hydatid torsion, polyorchidism, congenital adrenal hyperplasia, and secondary hypogonadism, as well as in otherwise normal patients studied for other conditions. In adults, microliths are frequently observed in cryptorchid and ex-cryptorchid testes, in seminiferous tubules located at the periphery of germ cell tumors, in patients with GCNIS, in testicular dysgenesis, in testes with ischemic injury, in infertile men, and in some with orchialgia. Isolated cases associated with pulmonary microlithiasis and calcifications in the vegetative and central nervous system have been reported.
Ultrasonography is used to quantify calcifications, usually revealing multiple < 2-mm diameter hyperechoic foci without posterior acoustic shadow and diffusely distributed throughout the parenchyma (“snowstorm” pattern), or radiography is used, usually revealing multiple uniformly distributed microcalcifications. Ultrasonography identifies two types of testicular microlithiasis: classic type, in which the number of microliths is five or more; and limited type, with fewer than five microliths ( Fig. 12.127 ).
The incidence of testicular microlithiasis varies according to the diagnostic method used (light microscopy, radiography, or ultrasonography), whether the population is symptomatic or asymptomatic, and the nature of the disease process being studied (cryptorchidism, infertility, or testicular tumor).
In histologic studies, testicular microlithiasis is found in approximately less than 1% of testicular pediatric biopsies, in 1% of biopsies of infertile men, and in 4% of adult male autopsies. The incidence rate in two radiologic series varied from 1% to 74%.
The real incidence of testicular microlithiasis has been established by the routine use of ultrasonography in evaluating intrascrotal abnormalities. The prevalence rate of testicular microlithiasis in infants is less than 3%. Classic testicular microlithiasis is found in 2% and limited testicular microlithiasis in 2% of asymptomatic boys. The prevalence rate of classic testicular microlithiasis in children with disorders such as cryptorchidism, hydrocele, scrotal swelling, chromosomal anomalies, orchialgia, and testicular torsion who were evaluated ultrasonographically varied from 1% to 3%. Several cases of testicular microlithiasis have also been observed in infant testes with neoplasm. The prevalence seems to increase from birth to adolescence.
The prevalence of classic testicular microlithiasis in asymptomatic young men varies from 2% to 6% in the general population between 18 and 35 years of age, and is bilateral in 66% of affected patients. The incidence shows ethnic differences; it is low in whites (4%), high in blacks (14%), and intermediate in Hispanics (9%) or Asian or Pacific Island men (6%). Ultrasonographic studies performed in adults with several disorders showed a prevalence rate varying from 1% to 4%. In adults, testicular microlithiasis is most often identified during investigations for infertility, pain, or testicular asymmetry. In series of infertile patients, the incidence varied from 2% to 18%. The incidence ranged from 5% to 20% in subfertile patients, and it was 10% in ex-cryptorchid testes.
Testicular microlithiasis is observed in 30% to 50% of adult testes with germ cell tumor. It has also been observed in the contralateral, apparently normal testis, sometimes in association with GCNIS.
Pain is the most common clinical symptom in patients without a palpable testicular mass, attributed to dilation of seminiferous tubules secondary to obstruction by microliths.
In the prepubertal testis, microliths are surrounded by a double layer of Sertoli cells and measure up to 300 μm in diameter. When microliths are large, the seminiferous epithelium may be destroyed, and the microlith is surrounded by peritubular cells. Testes with microliths have subnormal MTD and TFI.
Adult testes with microliths have incomplete spermatogenesis ( Fig. 12.128 ). Microliths may appear in the tubular wall, in the seminiferous epithelium, or free in the tubular lumen. Some seminiferous tubules with microliths are cystically dilated and lined by markedly thin atrophic epithelium ( Fig. 12.129 ). The cause of this cystic transformation may be the obstruction caused by microliths that pass through the thin tubuli recti. Microliths are also numerous in the conglomerates of hypoplastic tubules of the so-called Sertoli cell nodule or hypoplastic zone ( Fig. 12.130 ), which are mainly observed in cryptorchid testes. However, microliths may also be observed in seminiferous tubules away from these areas.
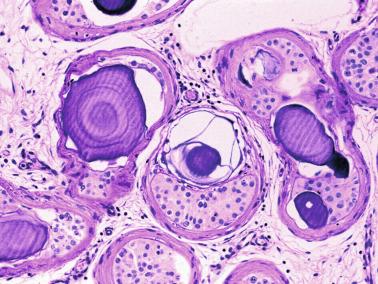
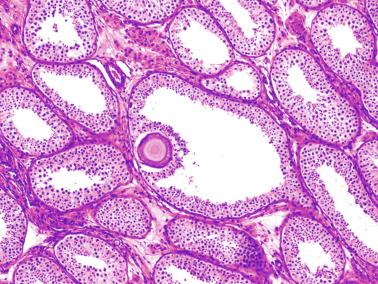
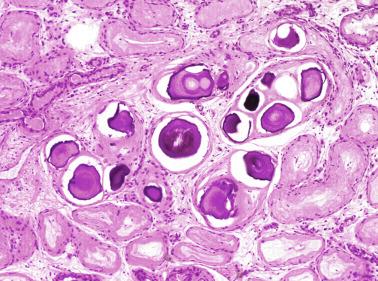
The origin of microliths is hypothesized to represent sloughed germ cells or glycoprotein secretions concentrated inside seminiferous tubules. Some have hypothesized that they arise from disordered tunica propria, forming extratubular eosinophilic bodies that mineralize and pass into tubular lumina. The material surrounding microliths expresses laminin and type IV collagen, both important components of basal lamina, which suggests an extratubular origin. This observation has led to the hypothesis that microliths originate from eosinophilic basal lamina material, giving rise initially to a spherical body, which advances toward the center of the tubule, displacing the basal lamina components and Sertoli cells that are in the path. Microliths thus become surrounded by these two elements. After internalization the nodular eosinophilic material becomes mineralized. In infantile testes, nonmineralized round eosinophilic bodies may be observed in normal and malformed seminiferous tubules. Many of the largest malformed tubules are known as ring-shaped tubules. Study of serial sections of these tubules reveals that they have a bell shape and contain eosinophilic bodies showing different degrees of mineralization, embedded in connective tissue. X-ray diffraction and Raman spectroscopy studies suggest that the mineralized material corresponds to hydroxyapatite crystals.
The possible association between microlithiasis and testicular cancer is controversial. Testicular microlithiasis is associated with both GCNIS and germ cell tumors ( Figs. 12.131 through 12.133 ). In infancy, it is associated with GCNIS and gonadal stromal tumors, but only in isolated cases. Several findings favor this association: (1) nearly one-half of testes with germ cell tumors also show testicular microlithiasis; (2) patients with testicular microlithiasis may later experience development of testicular tumor; (3) patients with testicular microlithiasis and extratesticular germ cell tumor, either retroperitoneal or mediastinal, have been reported; and (4) increased prevalence of testicular microlithiasis is noted in men with familial testicular cancer and in their relatives. The most important finding that mitigates against an association between testicular microlithiasis and cancer is the unquestionably high incidence of testicular microlithiasis compared with the low incidence of testicular germ cell tumors in adults.
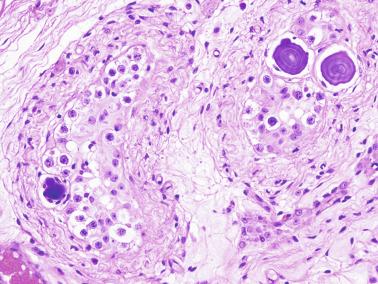
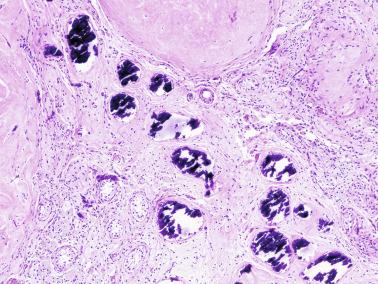
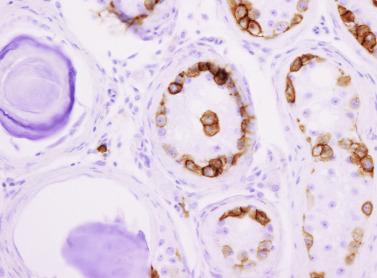
Isolated microlithiasis that is not associated with another disorder does not require follow-up. Three methods have been proposed for surveillance of patients with testicular microlithiasis when needed: abdominal and pelvic CT, ultrasound and testicular tumor marker study, or simple ultrasonography and physical examination every 6 months. The risk for malignancy is higher in classic than in limited testicular microlithiasis. The surveillance policy may be modified depending on the associated disorders. Patients found in infancy to have isolated testicular microlithiasis without other associated disorders warrant yearly physical examination, whereas follow-up sonography should be limited to the subgroup of patients with other associated risk factors. Yearly ultrasound examination is recommended in patients with testicular microlithiasis associated with cryptorchidism, infertility, atrophic testes, or contralateral testis with germ cell tumor. Testicular biopsy should be performed when clinically indicated.
When testicular microlithiasis is associated with infertility, the incidence of cancer varies according to the unilateralism or bilateralism of testicular microlithiasis. Subfertile patients with unilateral and bilateral microlithiasis have GCNIS in 0% and 20%, respectively. Therefore only patients with bilateral testicular microlithiasis should undergo rigorous follow-up.
The nexus between testicular microlithiasis and cancer does not seem to be the predisposition of one disorder toward the other, but rather the predisposition of both to develop in abnormal testes, accounting for frequent coincidence. This may also explain the association between testicular microlithiasis and infertility or subfertility, and these entities may be expressions of testicular dysgenesis syndrome.
In patients with germ cell tumor, other forms of calcification may be observed. The most frequent are conglomerates of microcalcifications around the tumor or amorphous intratubular calcifications in necrotic intratubular tumors such as embryonal carcinoma, calcifications in the scar of burned-out tumors, and calcifications associated with intratubular hyalinizing Sertoli cell tumor or calcifying large cell Sertoli cell tumor. Dystrophic calcifications and ossification may be observed in the testis and paratesticular structures in patients with a history of orchitis, epididymitis, trauma, or testicular infarction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here