Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Developmental (accessory tragi; first branchial cleft anomalies, others)
Infectious and inflammatory
Keloid
Epidermal and sebaceous cysts
Idiopathic cystic chondromalacia
Chondrodermatitis nodularis helicis chronicus
Autoimmune systemic diseases:
Relapsing polychondritis
Gout
Granulomatosis with polyangiitis (formerly Wegener granulomatosis)
Others
Exostosis
Synovial chondromatosis
Developmental and congenital anomalies
Infectious (otitis media) and inflammatory
Otic or aural polyp
Cholesteatoma
Langerhans cell histiocytosis
Heterotopias (central nervous system tissue; salivary gland)
Otosclerosis
Paget disease
Ménière disease
Others
The ear, including the external, middle, and internal ear, often is the target organ for congenital anomalies.
Congenital abnormalities occur as an isolated defect or in combination with other aural and extra-aural abnormalities and vary from cosmetic defects to complete hearing loss.
A complete discussion of the developmental defects of the ear is beyond the scope of this chapter; the interested reader is referred to other texts that detail this subject.
This section includes the more common developmental abnormalities that the surgical pathologist is likely to be confronted with in daily practice.
Synonyms : Supernumerary ears, accessory auricle, polyotia
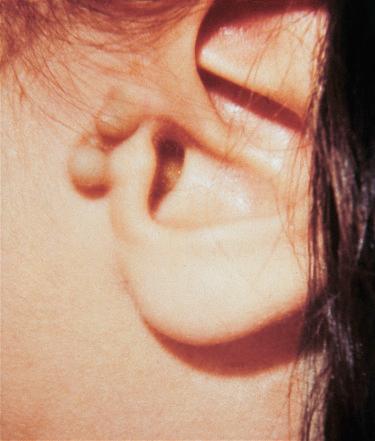
Appear at birth
Unilateral or bilateral, solitary or multiple, sessile or pedunculated, soft or cartilaginous, skin-covered nodules or papules.
Located on the skin surface often anterior to the auricle and may clinically be mistaken for a papilloma
Thought to be related to second branchial arch anomalies
May occur independent of other congenital anomalies but may occur in association with cleft palate or lip, mandibular hypoplasia, or in association with other anomalies such as Goldenhar syndrome (oculoauriculovertebral dysplasia)
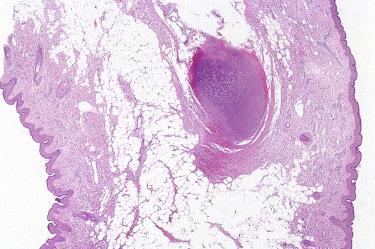
Histologically, accessory tragi recapitulate the normal external auricle and include skin, cutaneous adnexal structures, and a central core of cartilage.
Squamous papilloma:
The absence of cutaneous adnexal structures and cartilage differentiates squamous papilloma from accessory tragi.
Simple surgical excision is curative.
First branchial cleft anomalies typically occur in the area of the external ear and include cysts, sinuses, and fistulas.
See the Section on the Neck for more complete discussion.
Numerous developmental or congenital anomalies affect the middle ear and temporal bone; a complete discussion of the developmental defects of the ear is beyond the scope of this chapter. The interested reader is referred to other texts.
In brief, developmental or congenital anomalies of the middle ear and temporal bone include a variety of anatomic variations and anomalies, hereditary deafness (primarily conductive) occurring in syndromes, deafness caused by noxious prenatal influences, first and second branchial arch syndromes, dysplasias of the osseous and membranous cochlea and vestibular labyrinth, and hereditary/genetic sensorineural hearing loss.
Other dysplasias of the external, middle, and inner ear include a combination of hypertelorism, microtia, and clefting, and 18q syndrome.
Anatomic variations and anomalies may involve the facial nerve, jugular bulb, and intratemporal carotid artery:
Facial nerve anomalies include congenital bony dehiscence of the facial canal, anatomic variation, or anomalies in the course of the facial nerve, mastoid segment anomalies, anatomic variation of the chorda tympani nerve, and abnormal facial artery and vein.
Jugular bulb anomalies include superolateral extension of the bulb or superomedial enlargement of the bulb.
Abnormalities of the intratemporal carotid artery include aneurysm, aberrant location, abscess and inflammatory necrosis, traumatic lacerations, and atherosclerotic changes.
Hereditary deafness primarily conductive occurring in syndromes include:
Mandibulofacial dysostosis (Treacher Collins syndrome)
Acrofacial dysostosis (Nager syndrome)
Craniofacial dysostosis (Crouzon syndrome)
Klippel-Feil syndrome (fused vertebra, short neck, facial asymmetry, visceral abnormalities, deafness)
Marfan syndrome (arachnodactyly, ectopia lentis, deafness)
Pierre Robin syndrome (cleft palate, micrognathia, glossoptosis, low-set ears, deformed pinna, and hearing loss)
Other hereditary diseases causing conductive hearing loss, include osteoporosis (Albers-Schönberg disease or marble bone disease), otosclerosis, osteogenesis imperfecta
Deafness caused by noxious prenatal influences (embryopathic atresias) include:
Maternal rubella
Birth injuries
Hyperbilirubinemia (erythroblastosis fetalis)
Drugs, including thalidomide, quinine
Cretinism
First and second branchial arch syndromes include a variety of abnormalities with nonotologic and otologic manifestations
Otologic abnormalities include:
Malformed or absent external ears
Atretic external auditory canal and impaired hearing
Nonotologic features include:
Asymmetric facies
Temporomandibular joint
Neuromuscular abnormalities
Associated abnormalities of the cardiovascular, renal, and central nervous systems; Goldenhar syndrome, also known as oculoauriculovertebral dysplasia, is a first and second branchial arch syndrome characterized by ear tags and preauricular pits and fissures, epidermoids and lipodermoids, and vertebral column abnormalities.
Of the approximately 2000 to 3000 profoundly deaf infants born yearly in the United States, 35% to 50% have a defined genetic origin for their hearing impairment.
In approximately one third the hearing loss is syndromal or associated with other anomalies.
In adults, approximately 20% of sensorineural hearing loss has a genetic cause.
Etiologic factors vary with age.
More than 50% of early-onset hearing loss is due to genetic factors, of which:
60% to 70% are autosomal recessive (both parents carry the affected gene, involvement of the offspring is 25%)
20% to 30% autosomal dominant (one parent carries the affected gene and the incidence of involvement of the offspring is approximately 50%)
2% are X-linked
In patients with late-onset hearing loss:
Approximately 20% are due to infections
About 7% due to trauma
35% due to old age, heredity, and noise trauma
Approximately 35% of unknown cause
Hereditary sensorineural hearing loss includes the following categories:
Autosomal-recessive hereditary sensorineural hearing loss:
Hereditary recessive sensorineural hearing loss without associated defects (about 50% of recessive hereditary deafness), including recessive congenital severe deafness, high-frequency deafness, mid-frequency deafness)
Inborn errors of metabolism and deafness:
Albinism and deafness
Hurler syndrome (abnormality of mucopolysaccharide metabolism)
Tay-Sachs disease (ganglioside lipidoses and deafness)
Wilson disease (hepatolenticular degeneration and deafness)
Degenerative diseases of the nervous system and deafness:
Friedrich ataxia (spinocerebellar ataxia, deafness)
Congenital heart disease and deafness:
Jervell and Lange-Nielsen syndrome (cardiac conduction anomaly, deafness)
Endocrine system disorders and deafness:
Pendred syndrome (nonendemic goiter and deafness)
Ocular diseases and deafness
Usher syndrome (retinitis pigmentosa, deafness)
Cockayne syndrome (retinitis pigmentosa, mental retardation, dwarfism, retinal atrophy, deafness)
Alström syndrome (retinitis pigmentosa, obesity, diabetes mellitus, deafness)
Autosomal-dominant hereditary sensorineural hearing loss:
Inborn errors of metabolism and deafness:
Tietze syndrome (albinism, deafness, abnormality of tyrosine metabolism)
Waardenburg syndrome (partial albinism, deafness, abnormality of tyrosine metabolism)
Schäfer syndrome (hereditary mental retardation, deafness, abnormality of tyrosine metabolism)
Hereditary mental retardation, homocystinemia, deafness, abnormality of methionine metabolism
Nephropathies and deafness:
Alport syndrome (hereditary nephritis, deafness)
Muckle-Wells syndrome (hereditary nephritis, urticaria, amyloidosis, deafness)
Herrmann syndrome (hereditary nephritis, mental retardation, epilepsy, diabetes, and dominant nerve deafness)
Ectodermal defects and deafness:
von Recklinghausen disease (vestibular schwannomas [unilateral, bilateral] and deafness)
Dysplasia of the inner ear may be inherited, sporadic, or the result of chromosomal aberrations:
Typically, both ears are involved but perhaps not to a similar extent.
The classification of dysplasias of the osseous and membranous cochlea and vestibular labyrinth are divided into eight types:
Type I: isolated aplasia or dysplasia of the lateral semicircular canal
Type II: type I plus cochlear dysplasia
Type III: type I plus aplasia or rare club-shaped distension of the vestibule with rudimentary superior or posterior semicircular canals; a normal cochlea is present
Type IV: aplasia or dysplasia of all three semicircular canals plus severe cochlea dysplasia
Type V: aplasia or dysplasia of all three semicircular canals plus normal cochlea
Type VI: aplasia of all three semicircular canals plus cochlea aplasia
Type VII: normally configures vestibular labyrinth with aplasia of the cochlea
Type VIII: aplasia of the vestibular labyrinth and cochlea
Above subclassifications of dysplasias of the osseous and membranous cochlea and vestibular labyrinth are based on earlier reports detailing the morphologic changes involving the cochlear modiolus, osseous spiral lamina, and contents of the vestibule and endolymphatic sac and duct. The original designations for the dysplasias of the cochlea and vestibular labyrinth in patients with profound congenital hearing loss include Michel type, Mondini type or Mondini-Alexander type, Scheibe type, and Siebenmann-Bing type:
Michel type includes bilateral aplasia of the cochlear and vestibular capsule with bilateral aplasia of the eighth cranial nerve.
Mondini type includes anomalies of the cochlea; this may include the presence of a single coil and/or flattening and underdevelopment
Scheibe type, referred to as cochleosaccular dysplasia, includes morphologic changes limited to the membranous cochlea and saccule. The utricle and semicircular canals are not involved.
Siebenmann-Bing type includes dysplasia of the membranous cochlea and vestibular labyrinth with a well-formed bony labyrinth (cochlear capsule).
Definition: Heterotopias include the presence of normal-appearing tissue(s) in an anatomic location in which they normally are not found.
Synonyms: Choristomas; ectopias
Heterotopias that occur in the middle ear include salivary gland tissue and neuroglial tissue:
Presence of glial tissue in the middle ear may represent acquired encephalocele rather than heteropia; see next section.
May present with unilateral conductive hearing loss, tend to occur more often in women and occurs over a wide age range
Often arise in conjunction with facial nerve and ossicular chain anomalies:
The combination of facial nerve and ossicular chain anomalies may be explained on the basis of a second branchial arch developmental abnormality.
Salivary choristomas of the middle ear may be associated with branchial arch abnormalities, most commonly the second arch, as well as abnormalities of the facial nerve, including:
Bilateral preauricular pits, conchal bands, an ipsilateral facial palsy, and bilateral Mondini-type deformities
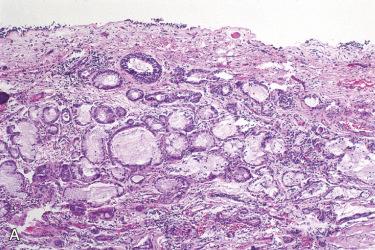
Choristomas appear as a lobulated, nonpulsatile soft tissue mass lying in the middle ear space with an intact tympanic membrane.
Salivary gland tissues include an admixture of seromucous glands and adipose tissue.
Conservative surgical removal is the preferred treatment.
Choristomas often adhere to dehiscent facial nerve; if complete surgical resection will compromise the integrity of the facial nerve, then incomplete resection is justified.
Biopsy for diagnostic purposes followed by observation is an alternative to surgical excision.
Rarely, salivary gland ectopia may produce a salivary gland neoplastic proliferation (e.g., pleomorphic adenoma).
True neuronal heterotopias in which isolated neuroglial tissue is located in the middle ear and temporal bone without continuity with the central nervous system is rare.
More common occurrence in which glial-type tissue is present within the middle ear/temporal bone is seen in association with an acquired encephalocele.
Represent herniation of the brain into the middle ear and mastoid via compromise of the tegmen, a thin bony shell that separates the middle ear and mastoid cavity from the temporal lobe:
Tegmen may be compromised or destroyed secondary to trauma or prior surgery, by a complication of otitis media, or due to a congenital defect.
Fracture of the temporal bone may also result in herniation of the brain into the middle ear and mastoid.
Some cases appear to have occurred spontaneously without known association with an underlying cause and/or without a history of trauma, tumor, cholesteatoma, or surgery of the mastoid or cranium.
No gender predilection; may occur over a wide age range but tends to occur in older individuals
Often represent an incidental finding in patients requiring surgery for chronic otitis media
Associated symptoms may include unilateral conductive hearing loss, persistent otorrhea, leakage of CNS tissue, and/or recurrent episodes of meningitis.
In patients suspected of having ectopic neuroglial tissue in this site, radiologic imaging (e.g., CT scans) may prove valuable in identifying a defect and/or connection to the CNS.
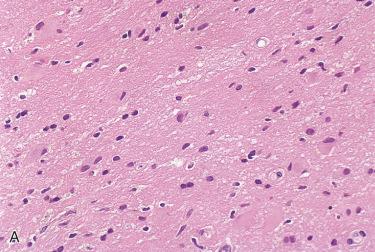
Includes a heterogeneous population of cells, including glial cells, histiocytes, and mature lymphocytes; reactive alterations of the neuroglial tissue (gliosis) may be present
In addition, granulation tissue and keratinizing squamous epithelium (cholesteatoma) may be present
Immunohistochemistry:
Confirmation of neuroglial tissues includes reactivity with glial fibrillary acidic protein (GFAP).
Chronic otitis media:
Fibrillary appearing stroma that are often present may simulate the appearance of neurofibrillary matrix
GFAP will assist in confirming or excluding the presence of neuroglial tissues.
Glial neoplasms
Management for acquired encephalocele is surgical:
For defects smaller than 1 cm in diameter, the transmastoid approach can be used.
For defects larger than 1 cm, the combined transmastoid-minicraniotomy approach provides good access.
Potential complications include brain abscess.
Definition: Nonneoplastic dermal fibroproliferative process representing exaggerated tissue response to trauma representing one extreme of the spectrum of reparative reactions of the skin.
“Keloid” derived from the Greek word chele, meaning “claw-like,” describing the tendency for these lesions to extend beyond the site of injury
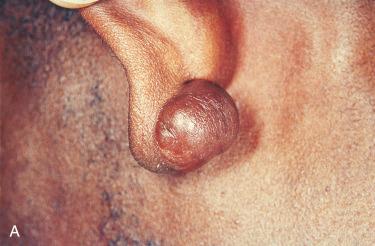
Equal gender predilection; can occur at any age but is most common in young adults under 30 years of age
Common in dark-skinned people, especially in young black women who have had their ears pierced
Most common sites include presternum, limbs, neck, and face; the latter include the earlobes and pinna.
Most often are asymptomatic but may be associated with pruritus, paresthesia, and pain
Often associated with a variety of cutaneous injuries, including surgery, ear piercing, BCG vaccinations, injections, burns, lacerations, insect bites; the development following injury may occur anywhere from weeks up to a year.
Often polypoid in appearance covered by thin, glistening, hairless skin; size is variable, usually measuring less than 2 cm; however, they may attain a diameter of several centimeters.
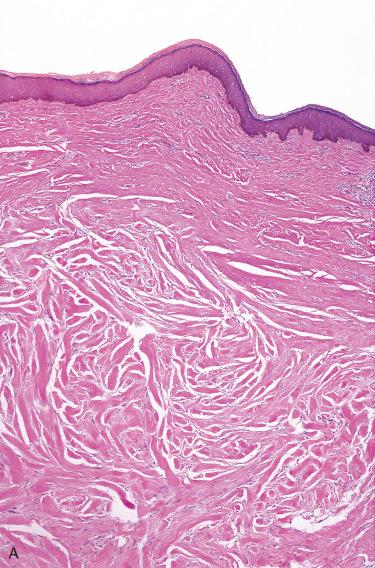
Haphazardly arranged fascicles of hyalinized collagenous fibers with scattered fibroblasts and myofibroblasts
Proliferation is not encapsulated but blends subtly with the surrounding dermal fibrous tissue:
Collagen bundles are often separated by dermal mucosubstance, creating an “edematous” appearance.
Poorly vascularized with widely scattered dilated blood vessels
Overlying epidermis is thin and atrophic, without dermal adnexal structures.
Foreign body giant cell reaction is uncommon, except in patients treated with corticosteroid injection.
Pools of amorphous mucin-like material may also be seen following steroid injection.
Hypertrophic scar:
Lack the dense hyalinized collagenous fibers, have more delicate fibrillar collagen, and have a more orderly arrangement of the collagen and fibroblastic cells often with a parallel orientation to the skin surface
Mature hypertrophic scars generally do not have an abundance of mucosubstances and therefore have a more compact microscopic appearance.
Hypertrophic scars usually do not recur following excision.
Dermatofibroma (DF) and dermatofibrosarcoma protuberans (DFSP):
Extremely low cellularity of keloids distinguishes it from DF and DFSP.
In contrast to keloids there is hyperplasia of the overlying epidermis in DF and DFSP.
Unusual variant of dermatofibroma characterized by keloidal-type changes and termed keloidal dermatofibroma has been described:
Clinically appear similar to usual DF
Characterized by keloidal-like collagen admixed with elements typically present in DF
DFSP shows immunoreactivity for CD34.
Surgical excision is the preferred treatment.
Recurrence rates of 40% following simple surgical resection have been reported.
Intralesional steroid (triamcinolone) injections alone provide response rates of 50% to 100% with recurrence rates of 5% to 50% at 5 years:
Intralesional triamcinolone injections considered gold standard in nonsurgical management
Intralesional verapamil, independent of or in conjunction with triamcinolone, has shown efficacy in treatment of keloids (and hypertrophic scars) with flattening of the raised scars and regaining normal pigmentation.
When surgery is followed by steroid injection or radiation therapy (10 Gy), recurrence rates are consistently below 50%.
Intralesional injection of interferon or bleomycin has shown 50% reduction in size, and response appears to be limited to the area treated.
Intralesional cryosurgery has emerged as a safe and effective new treatment by destroying the hypertrophic scar tissue with minimal damage to the skin surface.
TGF-β (transforming growth factor beta) and PDGF (platelet-derived growth factor) play an integral role in the formation of keloids, and future development of selective inhibitors of TGF-β might produce new therapeutic tools with enhanced efficacy and specificity for treatment of keloids.
Definition: Benign cystic degeneration of the auricular cartilage of unknown cause.
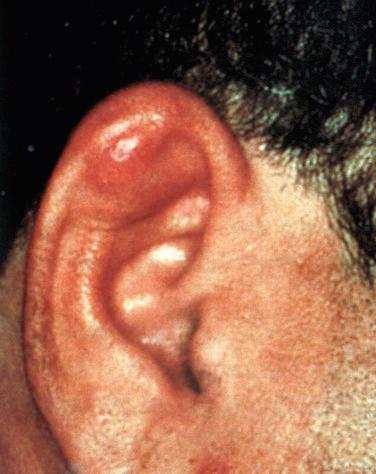
Synonyms: Pseudocyst of the auricle; auricular or endochondral pseudocyst
Typically affects men and rarely seen in women; generally affects young and middle-aged adults
Can occur along any portion of the auricle with the most common site adjacent to the helix
Symptoms include unilateral, painless swelling of the auricular cartilage without overlying ulceration or erythema; symptoms develop over a period of weeks to years; may occur anywhere on the auricle but the scaphoid fossa (80%) is the most common site; occasionally lesions may be bilateral.
Markedly elevated lactate dehydrogenase (LDH) levels may be found in aspirated fluid.
Although trauma has been implicated in causing these lesions, there is no definitive connection to a prior traumatic event and the cause(s) for this condition remains unknown:
Belief that repeated trauma stimulated by such phenomena as sleeping on hard pillows, the wearing of motorcycle helmets, stereo headphones, or the Italian birthday custom of having one's auricle pulled
Ischemic necrosis of the cartilage or the abnormal release of lysosomal enzymes by chondrocytes may also be cofactors.
May arise within a potential plane left during embryonic fusion of the auricular hillocks
Fluid-filled distended mass
Excised tissue may include only a fragment of the cyst wall or, less often, a full-thickness excision of the ear.
Intact cyst usually contains fluid described as “olive-oil-like.”
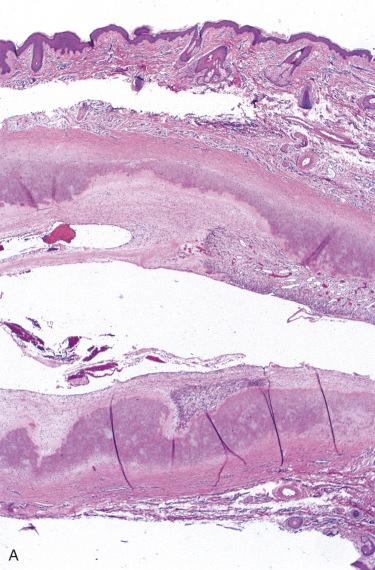
Cyst wall consists of a 1- to 2-mm rim of cartilage.
Cyst lining may be a smooth and glistening cartilaginous surface or may include roughened rust-colored patches.
Cyst is usually an elongated cleft, but multifocal cystic degeneration may be seen.
Changes are restricted to the cartilage within which irregular-shaped cystic areas are seen; cyst is the result of loss of cartilage.
Cysts lack an epithelial cell lining (pseudocyst) and generally are devoid of content.
Cystic cleft is often centrally placed in the cartilaginous plate.
Rim of fibrous tissue may be seen along the inner portion of the cyst or a granulation tissue reaction composed of fibrovascular tissue, and scattered chronic inflammatory cells can be seen in association with the cysts.
In long-standing cases fibrous tissue may essentially obliterate the cystic space.
Some examples are characterized by a distinctly proliferative cartilaginous response, developing a thickened cartilaginous wall.
Surrounding cartilage is unremarkable.
Relapsing polychondritis
Subperichondrial hematoma
Chondrodermatitis nodularis chronicus helices
Complete surgical excision without distortion of the underlying cartilaginous plate is the preferred treatment and is curative.
Due to the potential for surgical-related deformity, full-thickness resection is not advocated.
In addition to the obvious cosmetic concerns, long-standing lesions may result in deformity of the ear.
Steroid injection alone has been unsuccessful and may result in cartilage deformity.
Incision and drainage or curettage have shown variable success; needle aspiration alone results in rapid reaccumulation of fluid but, when combined with bolster suture compression, long-term follow-up has shown an absence of recurrences.
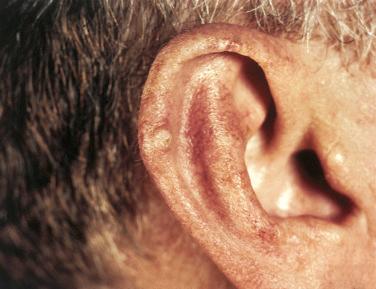
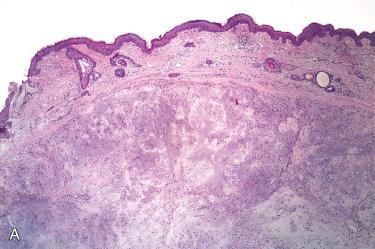
Definition: Idiopathic nonneoplastic ulcerative lesion of the auricle.
Synonyms: Winkler disease or nodule
More common in men in late middle age and older age groups; considered uncommon in women
Most frequently occurs along the superior portion of the helix; lateral helical, antihelical, and antitragal involvement also seen
Spontaneously occurring unilateral painful nodule is the most common clinical presentation; manipulation of the lesion results in intense pain; pain is thought to result from the perichondrial involvement.
Clinically, the lesions appear as round, reddish areas usually measuring less than 1 cm in diameter; many cases are clinically considered to be carcinomas.
Cause remains unknown; however, several theories have been suggested, including:
Cold exposure
Actinic damage
Local trauma
Degenerative change with pressure necrosis
The skin of the auricle is thin with little subcutaneous fat and therefore may be unusually sensitive to injury; further, the vascular supply to the area is somewhat deficient with the avascular cartilage depending on the dermal circulation for its sustenance; these anatomic features may predispose the auricle to the development of CNCH.
Winkler considered the underlying pathologic event to be a cartilaginous-based process; however, the cause appears to be linked to a primary cutaneous alteration because the cutaneous changes are more significant and a more constant feature.
Likely that the development of CNCH is multifactorial and includes actinic damage
Usually appears as a dome-shaped, discrete nodule with a scaly crust covering a central area of ulceration ranging in diameter from 3 to 18 mm, with an average of 7 mm:
Rarely, CNCH may achieve diameters of 2 to 3 cm.
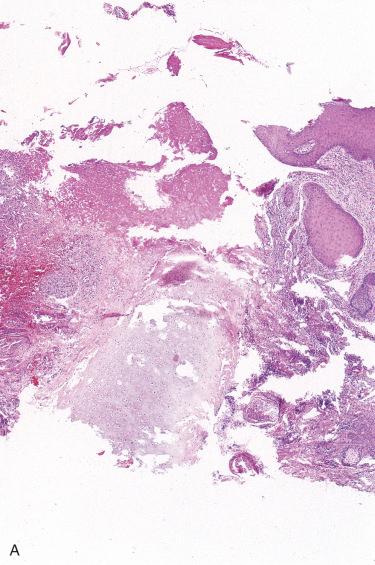
Central portion of the involved epidermis is ulcerated with adjacent epithelium showing acanthosis, hyper- and parakeratosis, and pseudoepitheliomatous hyperplasia.
Base of the ulcer shows granulation tissue, edema, fibrinoid necrosis, and an acute and/or chronic inflammatory cell infiltrate.
Granulation tissue and inflammatory process usually extend to and involve the perichondrium and cartilage.
Basal cell carcinoma
Squamous cell carcinoma
CNCH is frequently misdiagnosed as a cutaneous malignancy, particularly as basal cell carcinoma or squamous cell carcinoma.
Same mistake may be perpetuated by microscopic examination, particularly if the epidermal hyperplastic changes are misinterpreted as representing either squamous cell carcinoma or a hypertrophic actinic keratosis; careful attention to the extensive dermal changes and usually some degree of cartilaginous alterations, along with the well-demarcated nature of the epidermal proliferation and lack of cytologic atypia in the adjacent epidermis, should help in excluding a squamous neoplasm in cases of CNCH.
Complete surgical excision is the preferred treatment and is curative:
Surgery includes wedge excision or cartilage excision alone.
In a minority of patient trials with injection of glucocorticoids directly into the lesion have been shown to be effective in eradicating the lesion.
No malignant potential
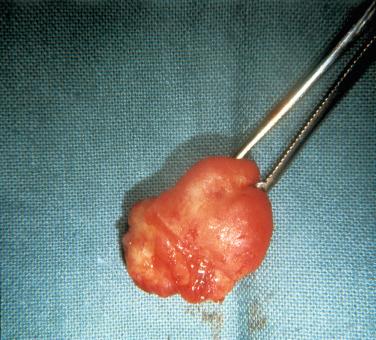
Definition: Localized overgrowth of bone classically described as a reactive lesion consisting of a compact proliferation of layers of bone of varied size and appearance, including nodular, mound-like, pedunculated, or flat protuberances on the surface of a bone.
Synonym: Surfer's ear
NOTE: Broad-based lesions are referred to as exostosis; pedunculated lesions have been termed osteoma.
Broad-based outgrowths of bone arising from the wall of the external auditory canal
Usually are multiple and bilateral
Tend to remain asymptomatic until they reach a size sufficient to interfere with the normal egress of cerumen and exfoliated skin:
Obstruction of the external auditory canal may cause recurrent episodes of external otitis, conductive hearing loss, and tinnitus.
Predilect to cold water swimmers and surfers, with the highest incidence being found in Australia and New Zealand:
White water kayakers are the first inland population to experience exostoses at the rates seen in coastal populations (e.g., surfers); earplugs may be protective.
Appearance of exostoses is usually better appreciated by the surgeon, as only fragments are available to the pathologist in most cases.
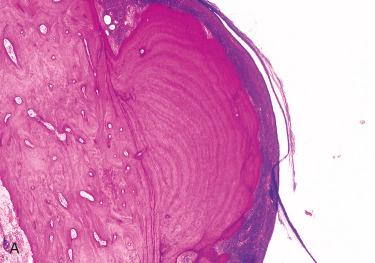
The intact exostosis is a broad-based, mound-like bony proliferation that is similar in color and texture to normal cortical bone.
The bone is covered by a layer of periosteum with overlying thin skin.
The periosteal layers are like the skin of an onion and usually lack trabecular architecture or marrow spaces.
Osteoma:
Much less common lesion in relationship to the external auditory canal
Distinction between exostosis and osteoma is usually readily made on the basis of the clinical presentation; there has been some controversy regarding the ability to distinguish between the two lesions histologically; some authorities consider the lesions histologically different, whereas others do not find the microscopic features sufficiently distinctive to be separated.
Medical treatment resolves the symptomatic external otitis and related hearing loss.
For patients who do not respond to medical treatment, transmeatal surgical excision is the preferred treatment.
Definition: Reactive process of unknown pathogenesis characterized by the formation of multiple cartilaginous nodules in the synovium, with many becoming detached and floating within the joint space.
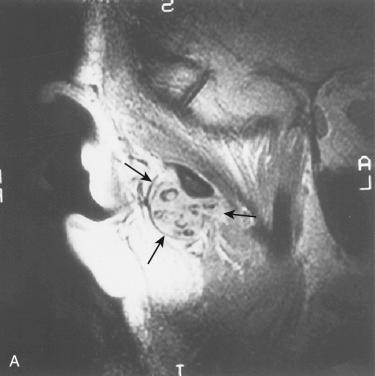
Synonyms: Synovial osteochondromatosis; synovial chondrometaplasia
Predilects to women and generally occurs in adults
May present with preauricular swelling and limited motion of the joint with deviation of the mandible
May involve the external auditory canal, resulting in an asymptomatic mass lesion
Radiographic features include the presence of numerous radiopaque loose bodies within the region of the joint but destruction of bone is absent:
CT scan excellent to define bony surfaces of the articular joints but fails in detection of loose bodies when these are not yet calcified
MRI is gold standard when diagnosis is suspected because it can visualize loose bodies at early stage and also evaluate disk condition and eventual extra-articular tissue involvement
On T2-weighted imaging:
Signs of low signal nodules within amorphous iso-intensity signal tissues; signs of low and intermediate signal nodules within joint fluids used to detect loose cartilaginous nodules
Intraoperatively, the lesion is usually confined to the joint space itself and is easily enucleated; on occasion the lesion may extend beyond the joint capsule into the parotid gland, auditory canal, temporal bone, or cranium.
Rarely occurs in association with pseudogout (calcium pyrophosphate dihydrate deposition disease); see later.
Pathogenesis:
Synovial chondromatosis is a condition in which foci of cartilage develop in the synovial membrane of a joint apparently through metaplasia of the sublining connective tissue of the synovial membrane.
Fibroblast growth factor 2 (FGF-2) found to be expressed in chondrocytes and fibroblast-like cells of loose bodies and believed to be involved in the pathogenesis
Clonal chromosomal alterations (chromosome 6 abnormalities) in synovial chondromatosis suggest this is a neoplastic lesion rather than a metaplastic/reactive process.
Cell proliferation studies have shown intermediate proliferative activity of the cellular composition of synovial chondromatosis between enchondromas and chondrosarcomas.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here