Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the early days of vascular surgery, patient assessment was based on a careful history and physical examination. Although a few clinicians used the Collins oscillometer to estimate the pulse pressure in an extremity, there was little help available in terms of quantitative assessment of arterial or venous disease. Angiography provided the only objective determination of pathologic changes. Early experience with arteriography and venography highlighted some of the limitations of these techniques, especially the problem of underestimating the severity of stenotic lesions on single-plane studies. In addition, the cost, patient discomfort, and risk of complications associated with contrast studies precluded their routine use for screening evaluations and follow-up.
The growing interest in more accurate differential diagnosis, localization of disease, determination of its severity, and documentation of progression stimulated the development of objective measurement techniques. In the 1960s, investigators started working with different plethysmographic techniques to quantify arterial occlusive disease in the leg. Modification of ultrasound equipment to measure blood flow by the Doppler shift principle represented an important step forward in instrumentation and led to the rapid development of noninvasive studies. Additional techniques were designed to evaluate carotid artery disease, as well as deep venous occlusion and insufficiency. This chapter describes the main diagnostic techniques used in the noninvasive laboratory and discusses their clinical application for patients with vascular disease. With an understanding of the merits and limitations of each method, clinicians can make the best use of these tests.
High-frequency sound waves (1 to 12 MHz) penetrate soft tissues and are reflected by the different interfaces encountered. Reflection from a moving interface results in the reflected frequency being increased if the motion is toward the point of observation and decreased if the motion is away from it. The magnitude of the shift is determined by the following Doppler equation:
where f s is the frequency shift, V the velocity, f 0 is the transmitted frequency, φ is the angle between the ultrasound beam and the velocity vector, and C is the speed of sound in tissue (1540 m/s). For a given velocity, a greater frequency shift is obtained with a higher transmitting frequency. In contrast, tissue penetration varies inversely with probe frequency, so the selection of a frequency for a given application is a balance between depth and velocity requirements.
In some applications, it is more useful to have a measure of velocity rather than the raw frequency data. If the probe angle relative to the direction of flow can be measured, the velocity is estimated using the Doppler equation. The accuracy of the estimate depends greatly on the accuracy of the angle measurement. Errors are greatest when the probe is at a right angle to flow and least when it is at a low angle. Whenever possible, velocities should be measured with an angle equal to or less than 60 degrees.
Continuous-wave detectors are the simplest systems. The probe has two separate crystals—one transmitting and one receiving continuously. This system detects all velocities within the intersecting paths of the sound beams. If this zone includes more than one vessel (e.g., an artery and a vein), the resulting signal represents a combination of both velocities. The commonly used bedside Doppler pencil is an example of a continuous wave device. Pulsed wave Doppler systems use a single crystal that repeatedly transmits a short burst of sound followed by a waiting period, during which the crystal functions in a receiving mode. By selecting the time and duration of the listening phase, one can define a sample volume, or the portion of the vessel from which velocity is to be measured. Modern duplex scanners use complex scan probes made up of many elements in an array, but the principle of focal sampling is the same.
The shifted frequency obtained from a vessel is within the audible range, so the data can be presented to the examiner as an audio signal. Although qualitative interpretation is helpful in some patient examinations, quantitative measurements provide objective testing. Spectral analyzers are used to determine the main frequency components obtained from a given vessel. This information is usually displayed on a sonogram, which shows the frequency content in time ( Fig. 14.1 ).
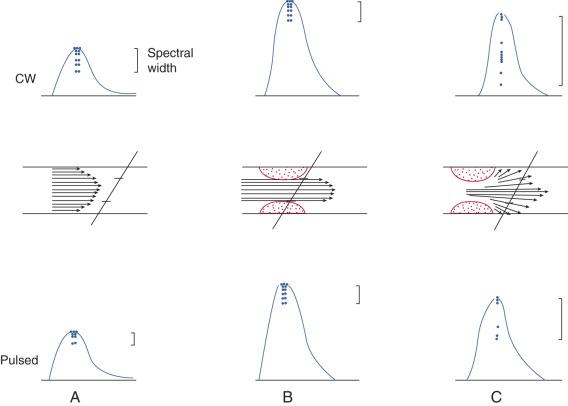
The computational algorithm used to perform the conversion of the amplitude of received frequencies to a frequency over time display is called a fast Fourier transform. This process involves the grouping of frequencies based on quantity for each time unit. This is then converted to a frequency (or proportional velocity) versus time plot where the intensity of the curve (Doppler waveform) corresponds to the amplitude or amount of any given frequency. This has implications in the interpretation of spectral broadening, which implies more turbulent flow with a variety of different frequencies/velocities compared with a more discreet waveform that represents laminar flow with a uniform frequency/velocity for the sampled area.
During the 1960s, B-mode ultrasound imaging (B = bright mode, a static image of the tissue) was used for visualization of soft tissue structures. Although early devices had only crude resolution, equipment has improved to the point that clear, detailed images of vessels can be produced in real time ( Fig. 14.2 ). In general, experience shows that when high-quality imaging is obtained, the diagnostic accuracy is very high; however, in patients with advanced atherosclerosis, it is difficult to obtain optimal studies, and diagnostic accuracy is lower. A common problem is incomplete imaging of the vessel wall as a result of calcification, which is present in varying degrees in up to half of patients studied. The extent of interference may be limited, but in some vessels there is no visualization of substantial portions of the artery. Although calcified plaques stand out sharply in the ultrasound image, some atheromas are visualized poorly or not at all. A major source of error is that recent thrombus may have the same echo density as flowing blood, so that an occluded vessel may look normal on the ultrasound image.
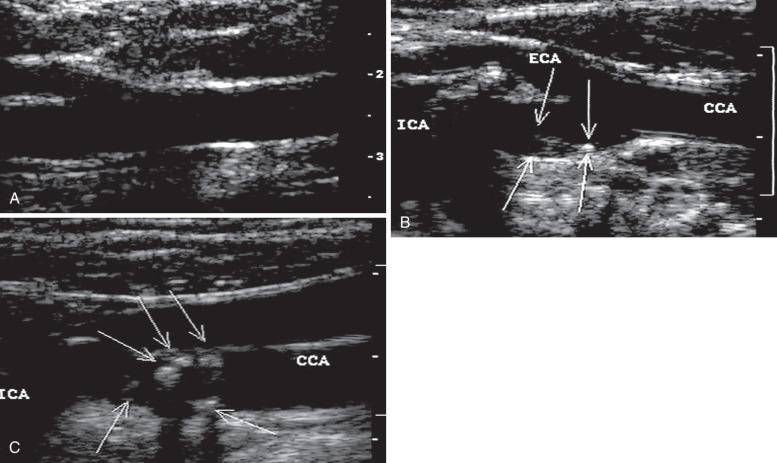
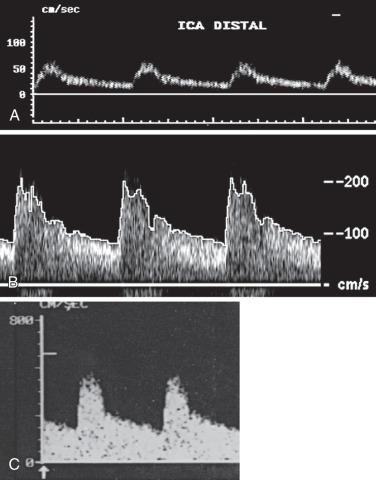
To overcome the limitations of ultrasound imaging, the research team at the University of Washington developed the duplex scanner (also called duplex ultrasound), combining a real-time B-mode ultrasound image system with a pulsed wave Doppler detector. The ultrasound image shows not only the vessel under study but also the location of the sample volume of the Doppler beam, so that the examiner can position it to study velocity patterns at specific locations in the vessel. The device can study calcified vessels by analyzing the Doppler velocity signal distal to the areas of calcification. The evaluation of the Doppler signal from the common carotid artery (CCA) and its branches is performed using spectral analysis ( Fig. 14.3 ). Based on the peak systolic velocity (PSV), end-diastolic velocity, velocity ratios, and degree of spectral broadening, a category of stenosis is assigned to the vessel segment.
In the past 20 years, there has been extensive improvement in duplex scanners in terms of both image resolution and Doppler signal processing. The early devices were limited to the study of superficial vessels; however, the availability of low-frequency probes (1 to 5 MHz) permits the evaluation of abdominal vessels, including the aorta, vena cava, and main visceral branches. Study of intracranial artery branches is also possible.
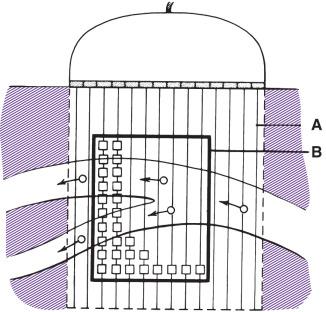
An important later development was the color-coded Doppler system. A linear array transducer composed of many separate elements is used to produce a grid of sample volumes encompassing the area covered by the B-mode image ( Fig. 14.4 ). A portion of the grid is selected for color-coding of velocity information. Each of the sample volumes within the area is examined. If the returning ultrasound signal has no change in phase or frequency, the amplitude information is used to create the gray-scale image at that point in the matrix. If there is a change in phase or frequency, the information is analyzed in terms of velocity. A color is assigned to represent an approximate mean velocity occurring at that point in the field. Red and blue represent flow toward and away from the transducer. The magnitude of the velocity is represented by the hue of the color: a dark shade indicates slow flow, and a lighter shade or white indicates high flow. The aggregate of the color representation from the sample volumes detecting motion produces a real-time representation of the flow patterns within the vessels superimposed on the grayscale image of the stationary tissue. Fig. 14.5 illustrates examples of the advantages of color duplex scans. An additional tool is color-coding of the Doppler power (as opposed to velocity) detected. Power is proportional to the square of the velocity; therefore, this measurement provides more sensitive detection of very slow flow or flow in small vessels. A good example of the benefit of power imaging is the detection of an internal carotid string sign. Squaring the velocity eliminates the positive or negative value, so that power values have no directional representation. Power is represented in a single color, usually orange.
The internal carotid artery (ICA) poses a unique challenge to physical examination, because it is impossible to palpate a distal pulse. It is not uncommon to find a patient whose carotid pulse in the neck is normal to palpation but who has occlusion of the ICA. This limitation stimulated the development of physiologic tests to assess the status of the ICA. Most of the early tests provided indirect measurement by detecting distal changes in blood flow characteristics produced by advanced stenosis. Common features of the indirect methods are that they detect only lesions that are sufficiently advanced to reduce mean blood flow, and they cannot separate a tight stenosis from an occlusion because the physiologic changes in the distal bed may be indistinguishable. These methods achieved a variable degree of clinical use in the 1970s and 1980s, but were ultimately replaced by duplex scanning.
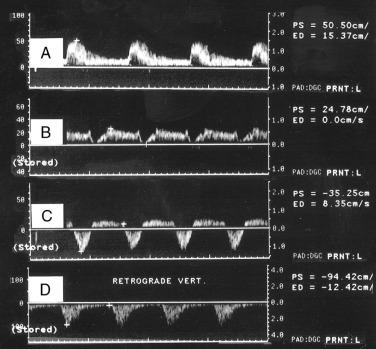
The routine examination covers as much of the CCA and its branches as can be visualized with the configuration of the transducer used. In some patients, the origins of the CCAs can be visualized. Figs. 14.2A and 14.5A show normal carotid bifurcations. The color image demonstrates the reverse velocity detected in the carotid bulb, as a result of the pattern of flow separation at the bifurcation. Many older patients have tortuosity that precludes the CCA, the bulb, and the branches from being visualized in a single plane; in such cases, careful scanning is required to obtain satisfactory imaging. Fig. 14.5B shows an example of tortuosity in an elderly patient. Although such arteries can be studied with a conventional scanner, the application of color flow and power Doppler simplifies the examination. The scan usually identifies the pathologic regions, but with advanced atherosclerosis, it is often difficult to get an adequate image to accurately estimate the degree of stenosis. Much of the classification of stenosis is based on interpretation of the Doppler signal. The two branches are distinguished by the image and the Doppler waveforms. The ICA has a low peripheral resistance at all times, resulting in forward flow throughout diastole, whereas the high resistance in the external carotid artery results in a diastolic flow of zero. Stenoses produce an increased velocity at the site of the lesion and turbulence beyond it (see Fig. 14.1 ). The turbulence is identified as spectral broadening on the sonogram (see Fig. 14.3 ). Mild stenoses may not produce a significant increase in PSV but are identified by a moderate degree of spectral broadening.
Based on the PSV and the degree of spectral broadening, the ICA is placed into one of six diagnostic categories. There are two sets of criteria that have been used for many years, and although some laboratories have made modifications or adjustments, the basic principles continue to be applied. The criteria developed at the University of Washington use primarily ICA velocity parameters ( Table 14.1 ). Further improvements in accuracy can be obtained using ratios of ICA velocities to CCA velocities in normal portions of the artery ( Table 14.2 ). The diagnosis of ICA occlusion must be based on image and Doppler information, because the low flow found with some “string signs” is below the velocity detection threshold of many scanners. Contemporary scanners have improved our ability to find small residual flow channels, especially using power Doppler imaging. Both the stippled appearance of chronic thrombus and a small diameter of the ICA indicate occlusion. Overall, low-grade plaques are best assessed with the image, whereas advanced lesions are best evaluated with the Doppler information.
| ICA Stenosis | ICA Velocity | Spectrum |
|---|---|---|
| Normal vessel | Peak systolic velocity <125 cm/s | No broadening |
| 1%–15% | Peak systolic velocity <125 cm/s | Limited broadening in late systole |
| 16%–49% | Peak systolic velocity <125 cm/s | Broadening throughout systole |
| 50%–79% | Peak systolic velocity >140 cm/s; end-diastolic velocity <140 cm/s | Broadening throughout systole |
| 80%–99% | End-diastolic velocity >140 cm/s (severe stenosis may have very low velocity) | Broadening throughout systole |
| Occlusion | No ICA Doppler signal; flow to zero in common carotid artery |
| ICA Stenosis | Peak Systolic Velocity (cm/s) | Diastolic Velocity (cm/s) | ICA/CCA Velocity |
|---|---|---|---|
| Normal vessel | <110 | <40 | <1.8 |
| 1%–39% | <115 | <40 | <1.8 |
| 40%–59% | <130 | >40 | <1.8 |
| 60%–79% | >130 | >40 | >1.8 |
| 80%–99% | >250 (severe stenosis may have very low velocity) | >100 | >3.7 |
| Occlusion | No ICA Doppler signal | No ICA Doppler signal | No ICA Doppler signal |
Duplex scanning has been adopted as the standard for carotid disease diagnosis and screening. Several studies have demonstrated that the technique can be highly accurate. These studies have shown rates of 92% to 96% accuracy in the identification of severe stenosis. When these studies are analyzed in terms of correct category of stenosis, exact agreement is found in 77% to 87%, with poor agreement in only 1% to 2%. Of particular importance is the fact that experienced laboratories make few errors in separating severe stenosis from occlusion. Mansour and coauthors reported a 98% positive predictive value and 99% negative predictive value in the correct determination of ICA occlusion.
Although the majority of attention has been focused on the carotid circulation, laboratories routinely investigate the status of the vertebral arteries as well. The examination seeks two types of problems: stenosis in the vertebral artery itself and the abnormal flow produced by subclavian steal, which has both an occlusion of the proximal subclavian artery and reversal of flow in the vertebral artery. In the majority of cases of significant vertebral stenosis, the lesion is located at the origin of the vessel. In some cases of severe occlusive disease, there is sufficient asymmetry in the waveforms of the two vertebral arteries to point to the problem side. However, a more complete assessment is obtained by examining the origins. Because of its deeper location, the left vertebral artery is more difficult to study than the right. Ackerstaff and associates found that the status of the ostium could be studied satisfactorily in about 80% of patients. When adequate evaluation of the prevertebral portion was possible, a sensitivity of 80% and a specificity of 97% were achieved in the detection of reductions greater than 50% in diameter. Most clinical cases of subclavian steal are demonstrated by a reverse flow in the vertebral artery on the affected side. Von Reutern and Pourcelot demonstrated that in some cases of subclavian stenosis there is distortion of the waveform rather than complete reversal of flow. The abnormal waveforms may have attenuation of the systolic component or an alternating pattern with reverse flow in systole and forward flow in diastole ( Fig. 14.6 ). Such cases can be assessed more fully by recording the Doppler signal after arm exercise or the induction of reactive hyperemia. In the presence of advanced subclavian stenosis, this stress test produces full reversal of flow.
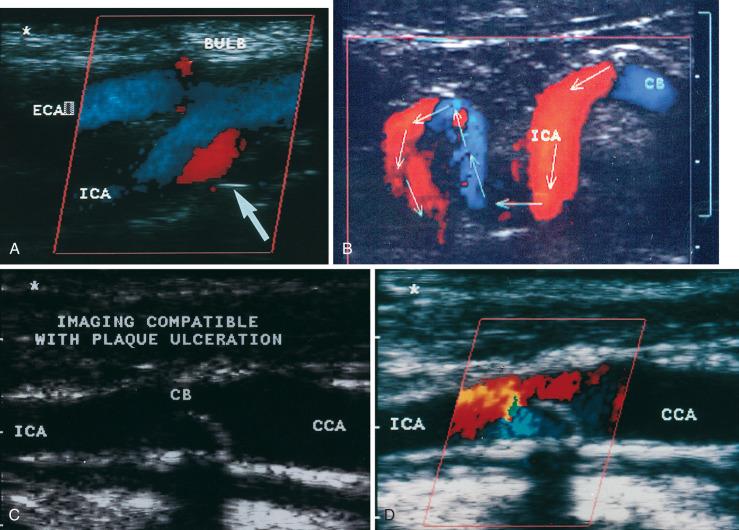
In addition to estimating the severity of a stenosis, ultrasound can be used to study the plaque itself. Most investigators merely distinguish between homogeneous- and heterogeneous-appearing plaques and describe the surface as either smooth or irregular. More elaborate approaches to the description of morphology are being evaluated, but no single approach has been widely adopted.
A large number of transient ischemic attacks and strokes are caused by thromboembolization from plaques in the carotid bifurcation. In most situations, a duplex scan is the initial workup, identifying the location and severity of lesions in the carotid system. Many centers use the ultrasound study as the definitive test on which to base the decision to treat. Having an experienced vascular laboratory with a validated record of high accuracy in carotid scanning is the critical element in using duplex scanning as the definitive test. In other settings, additional confirmation is obtained with magnetic resonance or computed tomography angiography (CTA).
Many asymptomatic patients are being referred to vascular laboratories for the evaluation of cervical bruits. Although some of these patients have bruits radiating from the heart or the great vessels, in a considerable number the sound originates from the carotid bifurcation. Duplex scanning can provide accurate separation according to category of stenosis (see Tables 14.1 and 14.2 ). Patients with severe stenosis are considered at increased risk of stroke and are evaluated for optimal medical and/or surgical treatment for stroke risk reduction. Lesions that fall in the moderate category (>50% stenosis) should have follow-up testing to detect those that progress into the high-risk group. Most people with normal vessels or early disease (<50% stenosis) do not require routine follow-up imaging.
Another indication is the screening of patients with advanced atherosclerotic disease in the coronary or peripheral vessels. Owing to the diffuse nature of atherosclerosis, some of these patients have occult carotid bifurcation lesions, with a resulting increased risk of stroke. Screening is performed most often in patients who are being considered for cardiac or major peripheral arterial operations to detect carotid lesions that may substantially increase the risk of perioperative stroke. Although screening may be appropriate for patients with multiple risk factors or severe occlusive disease in other arteries, routine testing of large populations results in a low yield of stenoses in the 80% to 99% category and is not cost-effective.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here