Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
With an increasing number of endovascular interventions replacing open vascular reconstruction, the management of open surgical complications may become less familiar to surgeons currently finishing training. Complications after aortoiliac and peripheral arterial reconstruction often develop and progress rapidly to produce disastrous consequences, including major organ failure or loss of limb or life.
These complications may be the result of technical errors, the extent of the pathologic process, or one or more frequently associated diseases. A timeworn surgical principle applies, especially to vascular surgery: “A complication not anticipated is sure to be experienced.” There are currently fewer and more complex open operative procedures being performed by surgeons who lack the suitable training to undertake these operations. Since the introduction of endovascular aneurysm repair (EVAR), the number of patients undergoing open aneurysm repair (OAR) requiring suprarenal clamp placement and adjunctive renal and/or visceral grafts has increased from 6% to 44% and 12% to 44% respectively. Patients undergoing OAR also have a higher incidence of renal vein division (4% to 11% vs. 18%), iliac artery aneurysms (25% vs. 42%), and occlusive disease (12% vs. 20%). Adjunctive renal and/or visceral grafts were required in only 1.9% of patients with infrarenal clamp placement. Although OAR with suprarenal clamp placement can be performed with relatively low mortality rates in major academic centers (0.8% to 6.1%), early recognition and treatment of complications is essential if mortality rates are to be maintained in this range in the future. Additionally, with EVAR becoming the standard of care for aortic aneurysm management, vascular surgeons must become familiar with open conversion in the setting of endograft explant and possible suprarenal clamping.
The primary problems reviewed in this chapter are perioperative bleeding, thrombosis, acute renal injury and failure, spinal cord ischemia, operative embolization, iatrogenic ureteric injury and obstruction, bowel obstruction and intraabdominal compartment syndrome (ICS), chylous ascites, graft deterioration, anastomotic false aneurysms, incisional hernias, and postoperative lower extremity lymphoceles and edema.
Complications of aortoiliac arterial reconstruction are similar regardless of whether the procedure is for abdominal aortic aneurysmal or occlusive disease. A recent comparison of aortofemoral bypass to alternative inflow procedures in the veteran population demonstrated a trend toward higher mortality at 30 days postoperatively (2.7% vs. 0.53%. P = 0.6). Aortofemoral bypass also had higher rates of pneumonia, deep vein thrombosis, pulmonary embolus, postoperative transfusion, and urinary tract infection.
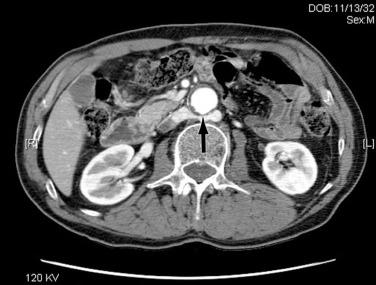
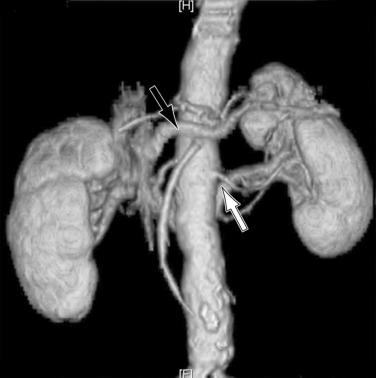
Operative bleeding may occur during dissection of the major vessels from failure to secure hemostasis or from difficulties encountered while sewing in the graft. Venous tears are the most common cause of intraoperative bleeding and usually result from injury to unrecognized venous anomalies and during mobilization of the aorta and iliac arteries from the inferior vena cava (IVC) and iliac veins. The inferior mesenteric, left renal, adrenal, gonadal, and lumbar veins are all at risk of injury during dissection or retractor placement to expose the infrarenal aorta when undertaking aortic bypass procedures for occlusive or aneurysmal disease ( Fig. 60.1 ). A thorough understanding of the anatomy, and familiarity with the characteristics of major venous anomalies and careful operative dissection, are essential to avoid this complication. Venous anomalies can involve the infrarenal, renal, or suprarenal segments of the IVC. Infrarenal segment anomalies include agenesis of the IVC, a left-sided IVC (0.2% to 0.5%), and duplicate IVCs (1% to 3%). Either of these anomalies may be associated with a right or, rarely, left retrocaval ureter. When present, the proximal ureter is dorsal to the IVC and, inferiorly, runs between the vena cava and the aorta. In rare instances, a double right vena cava has been observed. Anomalies of the renal segment of the IVC include a retroaortic renal vein (1.7% to 3.4%; Fig. 60.2 ) and circumaortic venous rings ( Fig. 60.3 ), in which the retroaortic segment is located in a more caudal position relative to the preaortic segment. Anomalies of the suprarenal segment of the IVC are extremely rare and associated with either azygos or hemiazygos continuation and absence of the hepatic venous segment.

The distal IVC and iliac veins are most vulnerable at the level of the posterolateral aspect of the aortic bifurcation in the area of tight adherence of the aorta and common iliac artery to the adjacent wall of the vena cava and right iliac vein. Complete separation of these structures by circumferential dissection is generally unnecessary, because temporary occlusion can usually be achieved by clamp control more distally or with intraluminal balloon occlusion catheters. If the IVC or one of the iliac veins is inadvertently lacerated, bleeding should be controlled by gentle finger pressure or sponge stick tamponade, and the venous laceration closed with interrupted pledgeted Prolene sutures. Application of clamps is hazardous and may enlarge the rent in the vein.
The left renal vein should be routinely identified early during dissection of the aorta above an aneurysm or proximal to the area of major aortic occlusive disease. The caudal border of the left renal vein should be clearly defined so that this structure can be easily retracted cephalad. Division of its adrenal, gonadal, or lumbar branches enhances its mobility and improves exposure. Careful consideration should be given to ligation of these branches if division of the renal vein is contemplated to improve exposure in patients with large or inflammatory aneurysms. Failure to find the left renal vein in its usual position suggests its aberrant location behind the aorta (see Figs. 60.2 and 60.3 ), where it may be readily injured during circumferential dissection of the infrarenal aorta preparatory to application of an occluding clamp.
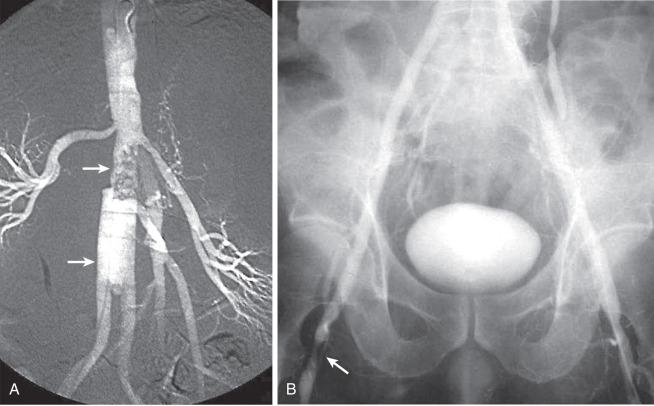
An arteriovenous fistula (AVF) involving the aorta or iliac arteries is an uncommon complication of spontaneous aneurysm rupture into an adjacent vein (about 80% of cases) or of penetrating injury to these major vessels (20%). Aortocaval fistula (ACF) has also been reported after EVAR. The incidence of this complication is quite low; it occurs in less than 1% of all aneurysms and in 3% to 4% of ruptured aneurysms. The presence of an ACF should be suspected when there is a continuous bruit over the aneurysm associated with the sudden onset of abdominal pain, lower extremity venous hypertension, oliguria, hematuria, and congestive heart failure. If suspected, the diagnosis of an ACF or iliac arteriovenous (AV) fistula can be confirmed by color Doppler imaging, computed tomography (CT) scanning, magnetic resonance imaging (MRI), or digital subtraction angiography (DSA). Before the routine use of preoperative imaging studies to evaluate patients with abdominal aortic aneurysms (AAA), the presence of an ACF or ilioiliac AV fistula remained undetected in 25% to 44% of patients undergoing open AAA repair. The patient with an ACF should be rapidly medically optimized before being taken to the operating room. Cardiac catheterization is rarely necessary except in cases of diagnostic uncertainty. The initial medical management should include admission to the intensive care unit (ICU), placement of hemodynamic monitoring lines and catheters, and the use of angiotensin-converting enzyme (ACE) inhibitors and diuretics. Sufficient blood and blood products should be available because intraoperative bleeding may be profuse. The fistula may sometimes be unsuspected intraoperatively because of its small size or its being obscured by the laminated thrombus within the aneurysm, with it becoming apparent only when sudden massive venous hemorrhage occurs within the lumen of the aorta during evacuation of the laminated thrombus from the aneurysmal sac. In all patients undergoing AAA repair without preoperative imaging studies, it is wise to palpate the IVC for the presence of a thrill, indicating an ACF, before opening the aneurysm sac to evacuate the laminated thrombus. If a fistula is suspected, the inferior vena cava should be occluded with a sponge stick or a clamp adjacent to the neck of the aneurysm before the aneurysm is occluded and opened, to prevent embolism of clot or air to the lungs. No attempt should be made to separate the aneurysm wall from the IVC at the fistula site.
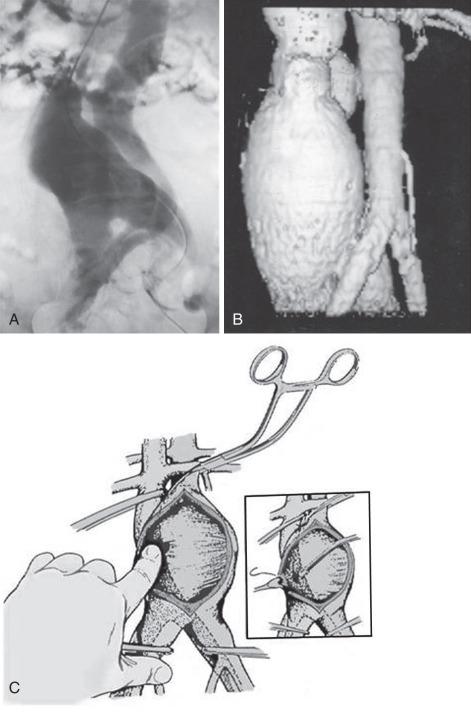
Occlusion of the fistulous defect by direct finger pressure over the fistula, followed by proximal and distal caval compression with sponge sticks or insertion of balloon occlusion catheters, are among the maneuvers used to control the venous bleeding so that the defect can be visualized and closed with Prolene pledget sutures (92%) or patch angioplasty (8%) from within the aortic sac ( Fig. 60.4 ). Intraoperative bleeding from iliac AVFs is often more difficult to control because of their location deep within the pelvis, the size of the defect, and the intimate relationship between the vein and artery. Elective balloon catheter occlusion or placement of a covered stent to occlude the defect in the femoral vein before entering the aneurysm may control venous bleeding and permit closure of the defect without massive blood loss. Autotransfusion is a useful adjunct in the management of ACF and iliac fistulas. Surgical treatment of ACF includes intraaneurysmal closure of the defect and reconstruction of the aorta with a tube or a bifurcated graft. Although endovascular repair with an aortic stent-graft may be the optimal method of treating ACF and iliac AVFs with suitable anatomy, rupture of an enlarged aneurysm sac into the IVC after EVAR is a recognized cause of ACF. Open repair, relining the endoprosthesis, and percutaneous closure of the defect with an Amplatzar ventricular septal defect (VSD) muscle plug are among the therapeutic options available for treating patients with ACF after EVAR. Prophylactic placement of an IVC filter is rarely necessary. Complications of surgical repair, including excessive intraoperative blood loss, myocardial infarction, respiratory failure, stroke, colonic ischemia, paraparesis, and renal failure, occur in 39% of patients. Mortality rates, ranging from an average of 34% (6% to 71%) have declined to 12% in the most recent report by Davidovic and coworkers. Myocardial infarction and multiple-system organ failure remain the major cause of mortality. Long-term survival after repair is greater than 90%.
Arterial bleeding usually arises from the lumbar, anomalous renal, inferior mesenteric arteries, or posterior aortic wall during circumferential dissection or after the aneurysmal sac has been opened. Lumbar vessel injury can be avoided in aneurysm surgery by limiting the dissection to the anterior surface of the aorta and by suture ligation of the lumbar orifices from within the aneurysmal sac after the aneurysm has been opened and the thrombus evacuated. Precaval location of the right renal artery (5%) and multiple renal arteries (30% to 40%) should be identified. The inferior mesenteric artery should also be suture-ligated from the interior or close to the wall of the aneurysm (if there is pulsatile backflow) to avoid injury to mesenteric collaterals. The posterior aortic wall is often heavily calcified or attenuated in patients with AAA. Calcified plaque at the site of the proposed anastomosis may require an endarterectomy, which further attenuates the aortic wall. The preemptive use of a Teflon buttress will reduce the risk of bleeding from tears at the anastomotic suture line from the placement of sutures in a fragile aortic wall. When anastomotic bleeding is encountered, the aorta should be clamped briefly while additional sutures, frequently with PTFE pledgets, are placed to control the bleeding and avoid further tears in the aortic wall. In patients with unfavorable aortic tissues, the use of biological tissue glues may sometimes be helpful. These agents are not a substitute for careful hemostasis, however, and their use is associated with more frequent reoperation for bleeding and the potential for infection. Reinforcement of the aortic anastomosis with a cuff of graft material is another option.
Continued intraoperative bleeding may result from failure to reverse or discontinue anticoagulants or platelet inhibitors preoperatively or the administration of too large a dose of heparin. The massive blood transfusion requirements during repair of thoracoabdominal or ruptured abdominal aneurysms result in the dilution of coagulation factors and platelets. In patients undergoing repair of pararenal aneurysms or the need for supraceliac clamping to facilitate repair, unrecognized fibrinolysis may be the cause of continued bleeding. Only rarely is the bleeding caused by an unrecognized congenital coagulation factor deficiency. The cause of the coagulopathy is often difficult to determine intraoperatively. Because of the higher mortality rates associated with increased intraoperative blood loss and the delay in obtaining the results of laboratory studies, it is recommended that blood, fresh frozen plasma (FFP), and platelets be administered concomitantly to improve the survival rate of these patients. If supraceliac clamping is anticipated or repair of a thoracoabdominal aneurysm is being undertaken, the transfusion of FFP should be started before clamp placement. Antifibrinolytic agents such as aminocaproic acid (Amicar) or tranexamic acid should be considered if activation of the fibrinolytic pathway is suspected. It should be noted, however, that there are no prospective data available for the clinical use of these agents in vascular surgery patients.
Bleeding in the immediate postoperative period usually comes from suture lines, inadequately ligated lumbar vessels, or the inferior mesenteric vein. This is manifested by a continuing need for blood replacement and the development of a retroperitoneal hematoma. This can be identified by palpating the flank—usually the left—which loses its normal soft concavity and becomes distended and tense. When aortic or iliac suture line bleeding is rapid, shock is more obvious, and the patient complains of severe backache similar to the pain of a ruptured aortic aneurysm. Postoperative hemorrhage is treated by immediate return to the operating room for identification and control of the bleeding site under fully monitored general anesthesia. The surgeon should search for the potential bleeding site when blood volume and pressure are at the patient's normal level before closing the retroperitoneum over the aortoiliac reconstruction.
Prevention of this complication requires thorough inspection of the intraabdominal anastomoses and the periaortic area, with special attention given to the orifices of the lumbar or anomalous renal vessels, the inferior mesenteric artery, and the ligated or oversewn stumps of the aorta or iliac vessels.
Hypothermia and acidosis are correctable causes of persistent coagulopathy and postoperative bleeding. Prevention of hypothermia by using heating blankets, increasing room temperature, and infusing adequate volumes of warm blood and fluids to optimize cardiac output are essential. A recent study by Samoila and colleagues reviewed the effects of hypothermia on outcomes following both open and endovascular aneurysm repair. They reviewed eight studies involving 765 patients. Normothermic patients had a shorter length of hospital stay in the intensive care unit. Hypothermia was associated with higher rates of organ dysfunction, higher in-hospital mortality, and prolonged hospital stay.
An activated clotting time (ACT) is done and specimens sent for a platelet count, fibrinogen, prothrombin time (PT), and partial thromboplastin time (PTT); these tests may not be helpful in the acute setting. Fibrinogen is the first clotting factor to fall in this situation and should be replaced. A platelet count of 100,000 or greater should not negate the need for platelet infusion because the platelets may not be functional. Congenital coagulation factor deficiency is rare and can usually be ascertained by careful preoperative history and evaluation. Preliminary screening studies do not reliably identify the need to search for precise factor deficiencies and direct their replacement before and during surgery. Intraoperative monitoring of the ACT before and after heparin administration is the most effective means of identifying variations in the individual response to intraoperatively administered unfractionated heparin and to determine the adequacy of its reversal before closing the abdomen. Proper management of bleeding caused by congenital or acquired deficiencies requires repeated monitoring of pertinent coagulation parameters during and immediately after the operative procedure.
The rising number of cardiac and peripheral percutaneous and endovascular interventions being performed has resulted in antiplatelet agents being administered to increasing numbers of patients. It is not uncommon for patients undergoing coronary or peripheral vessel angioplasty or stenting to receive a combination of drugs, including aspirin; clopidogrel; heparin; glycoprotein (GP) IIb/IIIa inhibitors such as abciximab, eptifibatide, and tirofiban; and direct thrombin inhibitors such as bivalirudin. Emergency surgical procedures to control bleeding from retroperitoneal hemorrhage or expanding groin hematomas from cannulation sites can be associated with significant perioperative blood loss into the thigh or retroperitoneum.
Although thromboelastography may be a useful adjuvant to therapy, it is not universally available. Discontinuation of the antiplatelet agent or anticoagulant therapy and delay of surgery when feasible is sometimes the preferred option. However, should emergency intervention be required, red blood cells, FFP, fibrinogen concentrate, and platelets should be infused. Cryoprecipitate and specific factor replacement should be reserved for patients with specific coagulation factor defects.
Initially approved for treating patients with hemophilia who had antibody inactivating factors V111 and 1X, recombinant activated factor V11 (rFV11a) is used extensively in patients with massive bleeding from surgery or trauma and may be beneficial in patients undergoing ruptured AAA (rAAA) repair. Recombinant activated factor V11a binds locally to the tissue factor at the site of vessel injury, generating small amounts of thrombin sufficient to activate platelets. The activated platelet surface membranes form a template on which rFV11a directly or indirectly mediates further activation of the coagulation pathway, ultimately generating more thrombin and the conversion of fibrinogen to fibrin. Recombinant factor V11a also stabilizes clot by activation of a thrombin-mediated fibrinolysis inhibitor. Clinical studies in patients undergoing prostatectomy, liver transplantation, noncoronary cardiac surgery, and trauma have shown that rFV11a minimizes operative blood loss and reduces transfusion requirements. A major concern with the use of this agent is the risk of thrombosis. The incidence is believed to be low because of the dilution of coagulation factors in patients from massive blood loss. One recent study analyzing outcomes following AAA repair and the use of rFVII demonstrated a lower mortality in patients who responded to treatment, however the rate of thromboembolic adverse events was 14%.
It is estimated that there are approximately 2.5 million patients on long-term vitamin K therapy and 40% of the US population over 40 years of age is on antiplatelet therapy. The need for temporary interruption of these therapies for surgical and other invasive procedures is therefore not infrequent. In a recent study, approximately 15% of patients on long-term vitamin K therapy required a major surgical procedure within a 4-year follow-up period. Recommendations for the discontinuation of vitamin K and antiplatelet agents preoperatively or preprocedurally must be balanced with the risk of thrombotic occlusion of recently placed stents or stent-grafts, the type of surgery including the risk of hemorrhagic complications, and the pharmacodynamic/kinetic profile of the therapy used to reverse the vitamin K therapy.
Neilipovitz and colleagues, using decision analysis, showed that the continued use of aspirin in vascular surgery patients reduces perioperative mortality, despite a 2.5% increase in hemorrhagic complications. A more recent study by Khashram and colleagues demonstrated that the perioperative use of aspirin was a significant predictor of improved survival following aortic aneurysm repair. The antiplatelet effects of clopidogrel persist for 7 to 10 days, so this drug should be discontinued 1 week before surgery. These recommendations are based on observations from case studies of patients undergoing coronary artery bypass grafting who received aspirin and clopidogrel or aspirin and placebo. Patients receiving both drugs had a higher rate of major postoperative bleeding (9.6% vs. 6.3%), reoperation (9.8% vs. 1.6%), and blood transfusion (3.0 units vs. 1.6 units) compared with patients receiving aspirin and placebo. The effects of GP IIb/IIIa inhibitors on platelets are quite variable. The antiplatelet effects of eptifibatide are usually abated within 8 hours of cessation of therapy in patients with normal renal function. Abciximab has a biological half-life of 8 hours, but its effects on the surface of circulating platelets can be detected for up to 2 weeks after discontinuing the drug.
The risk of hemorrhage and thromboembolism varies with the international normalized ratio (INR) in patients on vitamin K therapy. With INR ratios between 2 and 3, the relative risk of hemorrhagic events is 2.7 compared with 21.8 at INR ratios between 3 and 5. The risk of thromboembolism increases significantly with low INR; compared with INR ratios between 2 and 3, the relative risk of thromboembolism associated with INR below 2 was 3.5; within the range of 2 to 3, there is still a higher risk of thromboembolic than hemorrhagic events (2.6%/ year vs. 1.4%/year). Patients with mechanical heart valves or chronic atrial fibrillation and those at high risk of recurrent deep vein thrombosis who are on long-term vitamin K therapy should have the vitamin K agonist reversed and receive bridging low-molecular-weight heparin (LMWH). Therapeutic options for acute reversal of vitamin K agonists include vitamin K, FFP, prothrombin complex concentrate (PCC), and, possibly, rFVIIa. PCC is obtained from plasma; 60 mL of PCC is equivalent to 1500 mL of FFP, reducing volume requirements.
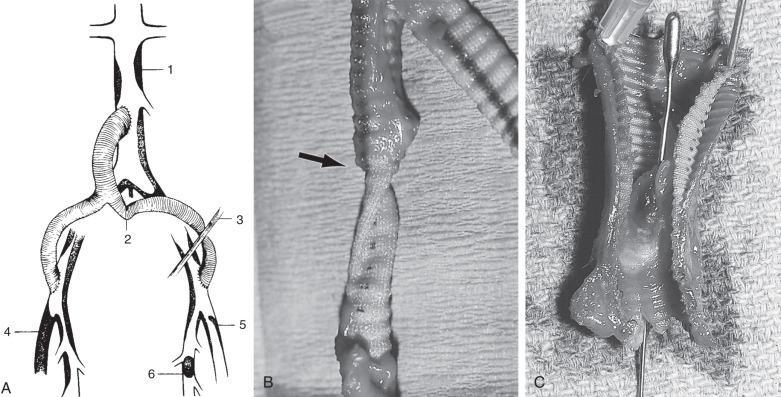
Graft thrombosis in the early postoperative period is almost invariably caused by technical problems ( Fig. 60.5 ) that usually occur at the distal anastomoses. These include an elevated intimal flap, narrowing of the artery at the anastomotic suture line, failure to remove a clot adherent to the inner wall of the graft before completion of the anastomosis, twisting or kinking in the retroperitoneal tunnel, compression of the femoral limb of the graft by the inguinal ligament, unrecognized inflow disease, or inadequate runoff secondary to unappreciated iliac, deep femoral, superficial femoral, or infrapopliteal disease. Rarely, thrombosis after aortofemoral bypass or aneurysm replacement is caused by hypercoagulability from inadequate doses or resistance to heparin, antithrombin III deficiency, protein C or S deficiency, a mutation in factor V Leiden or prothrombin genes, homocysteinemia, anticardiolipin antibodies, heparin-induced thrombocytopenia (HIT) and thrombosis, or stasis caused by reduced cardiac output.
The adequacy of pulsatile blood flow through the graft or endarterectomy should be evaluated in the operating room before the incisions are closed, by palpation of the graft and the arteries immediately distal to the anastomoses and by direct inspection of the pedal circulation beneath the drapes and palpation of distal pulses. If necessary, noninvasive measurements such as Doppler flow or pulse-volume recording tracings can be obtained intraoperatively. Intraoperative completion angiograms or color-flow Doppler imaging should be obtained in all patients who have had extensive reconstructive procedures of the common femoral, superficial femoral, or deep femoral arteries to ensure the adequacy of the repair. Noninvasive studies should be performed routinely in the recovery room when pulses cannot be felt distal to the repair or when the anticipated improvement in circulation has not occurred. Objective information obtained from these easily performed studies is particularly valuable in the immediate postoperative period, when patients are frequently hypothermic and peripherally vasoconstricted. Detection of unsatisfactory graft function mandates immediate direct evaluation of the involved anastomoses before wound closure or prompt return to the operating room if graft flow subsequently deteriorates.
An ACT should be obtained and additional systemic heparin given as necessary. This is especially important if protamine has been given at the initial operation. Treatment of immediate postreconstructive thrombosis consists of thorough inspection of the intraluminal aspect of the involved anastomosis. This is best accomplished through an incision in the distal end of the graft or by takedown of the anastomosis to directly view the intima and the runoff vessels adjacent to the arteriotomy. Effective revision may require stabilization of an elevated plaque, extension of an iliac limb to the common femoral artery, or patch angioplasty of a deep or proximal superficial femoral stenosis. Complementary bypass from the femoral to the popliteal or infrapopliteal vessels is only infrequently required when runoff through the deep femoral artery is inadequate. The lie of the graft should always be inspected throughout its length to ensure that there is no kinking, twisting, or external compression within the retroperitoneal tunnel.
Prevention of early graft thrombosis depends on an accurate evaluation of the distal runoff bed by preoperative noninvasive hemodynamic testing and imaging; computed tomography angiography (CTA), magnetic resonance angiography (MRA), or DSA. The iliac artery, rather than the femoral, should be palpated throughout its length before the selection of this vessel as the site for distal anastomosis. The orifices of the runoff vessels should be inspected and calibrated with dilators. Special attention should be given to the deep femoral orifice, which often requires endarterectomy or extension of the graft over its orifice when the distal anastomosis is performed at the common femoral level. Tacking sutures may be required to prevent distal intimal dissection. Finally, technical perfection in the performance of anastomoses is mandatory to avoid narrowing of the runoff vessels.
The widespread use of unfractionated heparin and LMWH has resulted in an increased frequency of HIT, reported to occur in 5% of patients receiving unfractionated heparin and in 0.5% receiving LMWH. The heparin/platelet factor 4 (PF4) complex generates predominantly IgG and to a lesser extent IgM antibodies, which activate prothrombotic platelet microparticles, resulting in platelet consumption, increased thrombin generation, and the risk of venous and arterial thrombosis. A milder form of HIT that is neither immune-mediated nor prothrombotic occurs more often in patients receiving heparin. Heparin-induced thrombocytopenia with thrombosis (HITT) should be suspected when the platelet count decreases by more than 50% from baseline or drops to 150,000 or less in patients receiving heparin therapy. Thromboembolic complications usually become evident between 5 and 14 days after heparin therapy and can involve the deep veins of the lower extremity, the pulmonary arteries, and major cerebral veins. Arterial thrombotic complications may involve the lower extremities and the coronary, mesenteric, renal, or cerebral arteries. When HITT is encountered intraoperatively, the surgeon has to act without confirmatory tests. If HITT is suspected because of the presence of “white clots” or unexplained intraoperative thrombosis, blood should be drawn immediately for diagnostic confirmation and a specimen of the thrombus sent to the laboratory for a touch prep to confirm the predominance of platelets in the clot. The administration of all unfractionated heparin should be discontinued and the ACT normalized with protamine if heparin has been administered recently.
Laboratory confirmation of HITT using the PF4/polyanion immunoassay is specific if strongly positive but has low specificity (as a result of IgM, IgG, and nonspecific antibodies) if the enzyme-linked immunosorbent assay is weakly positive. The platelet serotonin release assay is more specific if there is greater than 80% serotonin release. The principles of treatment include discontinuation of all heparin, and administration of direct thrombin inhibitors such as argatroban, lepirudin, or bivalirudin to maintain the PTT between 1.5 times and 3.0 times baseline. Prophylactic platelet transfusions should be avoided. Because warfarin may predispose to microvascular thrombosis in patients with acute HITT receiving direct thrombin inhibitors, it should be reversed with vitamin K if a dose has already been given and should be reintroduced only when the platelet count is greater than 150 × 10 4 /L.
Thrombosis caused by the progression of downstream atherosclerosis or anastomotic intimal hyperplasia (AIH) is the most frequent late complication of aortoiliac and aortofemoral procedures. The impaired outflow through the external iliac artery or the branches of the common femoral usually manifests as unilateral limb ischemia ( Fig. 60.6 ). Anastomotic intimal hyperplasia causes stenosis, usually at the graft-artery interface of the distal anastomosis: occlusion occurs when flow diminishes sufficiently to result in stasis thrombosis. The majority of patients initially treated with aortofemoral bypass have stenosis/occlusion of the superficial femoral artery at the time of the primary procedure. Therefore, an adequate lumen at the origin of the deep femoral artery is the most significant factor in ensuring long-term patency of these grafts. Underestimating the severity of outflow disease at the time of primary reconstruction is an important cofactor in progressive atherosclerosis that increases susceptibility to late graft limb occlusion. Despite an adequate primary procedure, progression of femoral or infrapopliteal atherosclerotic disease is more likely to occur in patients with continued exposure to atherogenic risk factors, particularly patients who continue to smoke. Impaired inflow is the second most common cause of late postrevascularization thrombosis. Although it is four to nine times less frequent than impaired outflow, it is the most common cause of simultaneous bilateral postreconstructive lower limb ischemia after aortoiliac or femoral surgery. The most common mechanism is obstruction from progressive infrarenal aortic atherosclerosis proximal to the site of previous repair (see Fig. 60.5A ). This is usually the consequence of placing the proximal anastomosis too low on the aorta (i.e., at or below the inferior mesenteric artery; see Fig. 60.6A ). The area between this site and the renal arteries is an active site of progressive atherosclerosis. Likewise, after aortoiliac endarterectomy, late occlusion is more likely if the proximal infrarenal aorta is not included in the endarterectomy. The use of an end-to-end rather than an end-to-side aortic anastomosis may be associated with fewer thrombotic failures, although this has not been clearly defined. Superior hemodynamic flow characteristics, the absence of competitive flow, less chance of embolization from the host aorta, and less angulation of the limbs as they arise from the body graft have been cited as the advantages of the end-to-end aortic anastomosis.
Angulation of the graft limb at the bifurcation may produce kinking because of failure to pull the graft limb out to full length before the distal anastomoses are performed or excessive length of the graft body, resulting in too wide a bifurcation angle (see Fig. 60.5B ). Inadequate retroperitoneal tunneling of the graft limbs may promote thrombosis as a consequence of extrinsic compression from the mesentery of the sigmoid colon or the recurrent portion of the inguinal ligament (see Fig. 60.5C ).
Less frequent causes of late thrombosis of aortoiliac and femoral reconstructions include accumulation of mural thrombus and false aneurysms. Mural thrombus develops when the graft diameter is significantly larger than the outflow artery. The flow pattern of the larger graft adjusts itself to the smaller outflow artery, leaving a peripheral layer of slowly moving blood that clots to form the mural thrombus. The normal, smooth, firmly adherent fibrous neointima becomes lined with a thick, gelatinous, loosely adherent mural thrombus that reduces the functioning lumen to the diameter of the outflow vessel. Fragmentation with distal embolization or progressive narrowing of the graft lumen with secondary acute thrombotic occlusion may then occur. Anastomotic false aneurysms, although relatively rare causes of late limb ischemia, may also produce peripheral embolization or thrombosis of the aneurysm and the adjacent vessel lumen. Finally, aortoiliac and femoral reconstructions can progress suddenly to thrombosis from percutaneous catheter interventions, cardiac embolization, or decreased cardiac output secondary to myocardial infarction or congestive heart failure. Rarely, no apparent cause for late thrombosis can be identified, implicating thrombogenicity of the graft surface or degeneration and disruption of the neointima.
The diagnosis of late thrombosis is suggested by the sudden or progressive recurrence of symptoms, a decrease or loss of previously present distal pulses, and a concomitant reduction in ankle pressure indices, Doppler flow, or pulse-volume recording waveforms. The degree of ischemia after thrombosis of a reconstruction is usually more severe than before the primary revascularization procedure. The frequency of late thrombosis increases from 5% to 10% in the first 5 years to 20% to 30% at 10 years. Therefore, routine and long-term follow-up of these patients at regular intervals is required to monitor the adequacy of graft function. If significant stenosis can be demonstrated before complete thrombosis, surgical correction is simplified. When either abrupt or gradual change is apparent, prompt imaging studies should be performed to determine the status of the graft, the anastomoses, the inflow, and the runoff bed.
The severity of recurrent ischemia may range from minimal to severe claudication to rest pain to gangrene, depending on the extent of compensating collaterals and the vigor of the patient's normal activity. Imaging studies are required to determine whether further surgery is feasible and to guide the surgeon in the selection of the most appropriate reoperative or interventional procedure, considering the patient's age, health status, and level of activity.
Correction of late thrombosis requires preoperative delineation of the underlying anatomical problem, followed by appropriate corrective maneuvers. The occlusion of one limb of an aortoiliac bifurcation graft is usually caused by overlooked or progressing disease in the external iliac and femoral arteries. Thrombolytic therapy may be useful in delineating the artery involved by the progression of atherosclerosis. Balloon catheter angioplasty with stenting is not usually indicated because of the extent of the disease or location beneath the inguinal ligament. One reliable solution consists of retroperitoneal exposure of the occluded limb, balloon catheter thrombectomy, and graft extension to the femoral level. Femorofemoral bypass is an alternative if the donor iliofemoral inflow is satisfactory, especially in a high-risk patient. Axillofemoral bypass may be required if neither of the preceding methods is feasible.
The most commonly encountered situation is a thrombosed aortofemoral graft limb with impaired outflow. Inflow can usually be restored by the use of catheter-based thrombolysis, or surgical or percutaneous graft thrombectomy using a balloon thromboembolectomy catheter or mechanical thrombectomy device with or without regional lytic agents ( Fig. 60.7 ). A thromboendarterectomy stripper or adherent clot catheter is often required to complete the extraction of adherent clots and old pseudointima. The Fogarty occlusion catheter is passed through the ring of the stripper into the patent aortic portion of the graft, and its balloon is fully inflated and pulled down to occlude the proximal end of the limb to control bleeding and prevent crossover embolization. The stripper is passed back and forth and rotated around the catheter within the occluded graft limb up to the distended balloon to scrape thrombus from the graft wall. Thereafter, the balloon is deflated just enough to permit its tight withdrawal through the graft limb along with the stripper and detached thrombus (see Fig. 60.7 ). Use of the Fogarty adherent clot catheter obviates the need for the thromboendarterectomy stripper. The patient is systemically heparinized (100 to 125 units/kg) during all these maneuvers.
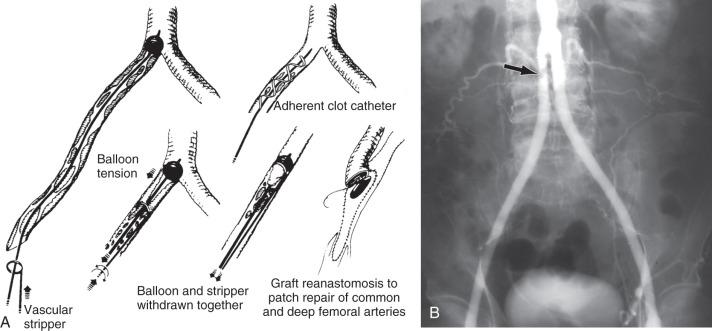
Thrombectomy is usually combined with a profundoplasty of varying extent to provide outflow. However, femoropopliteal or femorodistal bypass may be required, depending on the extent and location of outflow disease ( Fig. 60.8 ). If an occluded graft limb cannot be reopened by thrombectomy, a femorofemoral graft can be inserted. Replacement of the graft limb is another alternative but is technically more difficult. Endovascular options include thrombus removal by catheter aspiration, thrombus dissolution with hydrodynamic catheters such as the Cordis Hydrolyser, the BSIC Oasis, and Angiojet systems. These mechanical therapeutic modalities are often combined with the use of thrombolytic agents. Percutaneous thrombectomy is more frequently used to treat lower extremity graft or native arterial occlusions. The use of these mechanical and aspiration thrombectomy catheters to treat aortofemoral limb occlusion is limited to small series and isolated case reports.
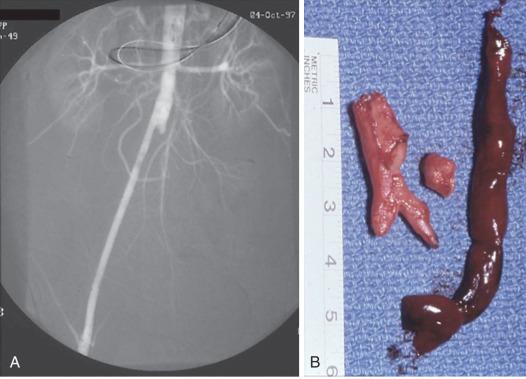
If an entire bifurcated graft is thrombosed, a problem at the proximal anastomosis such as low placement of the graft with progression or unrecognized proximal disease, kinking, or anastomotic aneurysm, or cardiac embolization is a likely cause. Imaging with CTA, MRA, or DSA is required to identify proximal progression of disease. If no proximal problem can be demonstrated, thrombectomy with a balloon or an adherent clot catheter can be attempted but is usually not successful. The alternatives are to replace the original prosthesis or insert an axillobifemoral bypass. The latter procedure is less technically demanding and less hazardous and is the reoperation of choice in a physiologically compromised patient. When groin scarring is especially intense, bypass to the mid-deep femoral artery simplifies the outflow repair of the reoperative procedure by avoiding a tedious and hazardous dissection in the area of a previous femoral anastomosis.
Endovascular treatment of this complication may be feasible in selected patients by establishing inflow with covered stents or stent grafts after dissolution or removal of the thrombus. An aggressive attitude toward reoperation after thrombotic failure of aortoiliac reconstruction is warranted, especially if the patient will derive sustained benefit from long-term patency and improved limb function. Operative morbidity and mortality rates are low. Reoperative mortality rates of 3% and cumulative 3-year patency rates of 68% to 75% have been reported. Judicious use of extraabdominal approaches has contributed significantly to reduced reoperative morbidity and mortality.
Although thrombectomy has been the treatment of choice in the management of occluded aortofemoral and femoropopliteal bypass grafts, incomplete removal of thrombotic material and the difficulties associated with reoperation have led to the evaluation of direct intraarterial infusion of thrombolytic agents, either preoperatively or intraoperatively, for the management of this problem. The potential benefits of lytic therapy include delineation of the cause of the graft thrombosis (most commonly distal occlusion caused by intimal hyperplasia or progression of disease) and, as a consequence, shorter operation time, reduced blood loss, ease of extracting any residual thrombus, and reduced wound complication rate. Potential disadvantages and complications of thrombolytic therapy include the need for monitoring in an intensive care unit, delay in surgical intervention, and risk of bleeding or renal impairment from the contrast load required for frequent angiographic evaluation. Further, mechanical thrombectomy for aortofemoral graft limb occlusion is at least as effective as clot lysis and adds little to the operative procedure required to restore outflow.
Kuhn and colleagues, in a series of 129 patients with acute and subacute occlusions of native arteries (77) and bypass grafts (55), reported recanalization with r-tPA thrombolysis in 73.6% of cases. There was no statistically significant difference in primary therapeutic success rates between native arteries and bypass grafts. The morbidity rate was 31% (minor complications, 20.2%; major complications, 10.9%), and the mortality rate was 2.3%. Twenty-seven patients required radiologic and surgical interventions within 12 months, with a limb salvage rate after primary successful recanalization of 89.5%. Successful lysis, which does not appear to be affected by the duration and cause of the graft thrombosis, can be achieved in 50% to 90% of occluded prosthetic graft limbs, 50% to 77% of saphenous veins, and 38% to 71% of prosthetic grafts. Despite successful clot lysis, however, a recent study by Schrijver and colleagues suggested that long-term amputation-free survival at 1 and 5 years is still lower than expected (76% and 65% for native arteries and 78% and 51% for bypass grafts).
The thrombolytic agents used clinically are recombinant thrombolytic peptides represented by recombinant tissue plasminogen activator (r-tPA), which enzymatically breaks down cross-linked fibrin strands within the thrombus by converting plasminogen to plasmin. The three recombinant thrombolytic peptides that are clinically relevant are alteplase (r-tPA), reteplase (r-PA), and tenecteplase (TNK). r-PA and TNK have been structurally modified from native r-tPA to increase their half-life and fibrin specificity. TNK possesses a longer half-life, improved fibrin specificity, and increased resistance to plasminogen activator inhibitor and has been shown to have a better safety profile compared with t-PA in patients with acute myocardial infarction (MI). A metaanalysis of studies comparing thrombolysis with surgical intervention concluded that catheter-directed peripheral intraarterial thrombolysis (PIAT) resulted in higher limb salvage rates and lower mortality rates than surgical intervention. Limb salvage rates at 30 days in patients receiving PIAT were 93% compared with 85.5% with surgery. These higher limb salvage rates were maintained at 6- to 12-month follow-up (89% vs. 73%). Mortality at 30 days was 4% for PIAT compared with 15% for surgical patients. This trend in mortality rates was maintained at 6- to 12-month follow-up (8% vs. 29%), respectively.
Infusion of the thrombolytic agents is usually accomplished by a multi–side-hole catheter or an infusion wire placed so that it covers the extent of the thrombus. There are multiple dosage regimens advocated for the use of these agents. Usually a loading dose followed by a continuous infusion is given: t-PA at 0.25 to 10 mg/hour, r-PA at 0.25 to 1 units/hour, and TNK at 0.25 to 0.5 mg/hour. Heparin in a dose of 300 to 500 units/hour is usually administered through the access sheath to prevent pericatheter thrombosis.
Patients are monitored carefully in the ICU for evidence of bleeding. Blood is drawn for hemoglobin, hematocrit, platelet count, fibrinogen, PT, and activated PTT (aPTT) every 6 hours. After successful lysis, the underlying lesion is treated with catheter-based techniques or surgery. Long-term anticoagulation with warfarin is usually indicated.
Bleeding, the major complication of lytic therapy, occurs after 7% to 48% of infusions. The most common sources of bleeding are angiography or venous puncture sites, the interstices of prosthetic grafts, and systemic bleeding at remote sites. Central nervous system bleeding is the most lethal complication. Bleeding from a groin arterial puncture site may also result in femoral pseudoaneurysm or retroperitoneal hematoma, which may compress the femoral nerve within the iliac fascia or in the thigh. The resulting femoral neuralgia, reported to occur in up to 30% of patients, may persist for as long as 1 year. Rarely, a femoral nerve palsy develops in patients; this may be extremely debilitating, especially in patients with claudication or amputation of the contralateral limb. There is some evidence that the risk of bleeding may be higher with t-PA than with r-PA or TNK.
The most important determinant of the long-term success of lytic therapy is the presence of a lesion correctable by surgical revision or balloon catheter dilatation. Such lesions responsible for the occlusion can be identified in approximately 21% to 30% of patients with 86% to 89% patency at 1 year compared with 37% of a similar number of grafts without correctable lesions.
The results of three prospective studies comparing the efficacy of intraarterial thrombolysis and surgery in patients with lower limb ischemia reported successful catheter placement in 72%, clot dissolution in greater than 70%, and limb salvage at 1 year in greater than 80%. In a study of 114 patients with acute lower limb ischemia of less than 7 days' duration who were randomized to thrombolytic therapy or surgery, Ouriel and colleagues achieved clot dissolution in 70% and observed equivalent limb salvage rates (82%) and improved patient survival (82% vs. 58%) in the patients receiving thrombolytic therapy at 1 year. They attributed the improved survival in the thrombolytic group to the more frequent occurrence of cardiopulmonary complications in the patients undergoing surgery. In a subsequent study, Ouriel and associates randomized 213 patients with acute limb ischemia of less than 14 days' duration to recombinant urokinase or surgery. Clot lysis was achieved in a similar number of patients (71%) but there was no difference in mortality rate (14% vs. 16%) or amputation-free survival rate (75% vs. 65%) between the two groups at 1 year. In the Surgery versus Thrombolysis for Ischemia of the Lower Extremity (STILE) trial, the efficacy of rt-PA and urokinase was compared with that of surgery in 393 patients with limb ischemia of less than 6 months' duration. Failure of catheter placement occurred in 28% of patients. Patients with ischemia of less than 14 days' duration receiving lytic therapy had lower amputation rates than did surgical patients, whereas patients with ischemic symptoms of more than 14 days' duration fared better with surgery. The results of these studies suggest that in selected patients, thrombolytic therapy may be a useful adjunct or alternative to surgical therapy. However, long-term patency rates of 28% to 37% of thrombolysed grafts are clearly inferior to those obtained with surgery. Thrombolytic therapy may be extremely valuable in patients with limb-threatening ischemia secondary to thrombosed popliteal aneurysm. Thrombolysis may improve the chances of achieving long-term patency and limb salvage.
Reteplase differs from alteplase both in its structure and biochemical composition and in its lower affinity for thrombin. McNamara reported a 34% incidence of bleeding in a series of 40 patients treated with t-PA compared with 3% among those receiving r-PA at doses of 2 to 8 mg/hour. Even reducing the dosage of t-PA to 0.5 to 1 mg/hour was still associated with a 25% incidence of bleeding requiring transfusion.
In all cases, the risks and benefits of the use of lytic therapy in the treatment of patients with graft limb occlusions must be evaluated carefully.
The relatively low incidence of complications, improved technique of administration, and efficacy of thrombolytic agents have reduced the need for urgent surgical thrombectomy in patients with noncritical limb ischemia. Successful lytic therapy readily identifies the cause of the graft limb occlusion and may allow a less extensive repair. In addition, lytic therapy may reduce the risk of wound and graft complications and reduce the incidence of reperfusion edema and compartment syndrome associated with extensive redo procedures.
Mechanical thrombectomy devices that theoretically permit rapid revascularization of an ischemic extremity using minimally invasive techniques are gaining in popularity. The use of mechanical energy to cause fragmentation, dissolution, and aspiration of thrombus is appealing. These devices can be classified broadly into (1) aspiration thrombectomy catheters that remove the thrombus by steady manual suction through a large-lumen aspiration catheter; (2) pull-back thrombectomy catheters that withdraw the thrombus with a balloon catheter or basket into a trapping device, allowing the clot to be removed; (3) recirculation thrombectomy devices that ablate the thrombus by hydrodynamic vortices, which pulverize the thrombus into microscopic fragments; (4) nonrecirculation thrombectomy devices, which macerate the thrombus mechanically into fragments that are larger than those produced by recirculation catheters; and (5) energy-assisted devices that use ultrasound, laser, or radiofrequency to lyse the thrombus or enhance the effects of pharmacologic agents. Several of these devices are currently being evaluated clinically, with complete angiographic success reported in approximately 50% of patients and partial success in an additional 27%. Concomitant lytic therapy or balloon angioplasty is often a necessary adjunct. The most extensively studied device is the AngioJet rheolytic thrombectomy system, which is approved for peripheral arterial and coronary applications. Chief limitations of the current devices include catheter size and working length, the possibility of fluid overload, hemolysis, and cost effectiveness. The major treatment limitation of these devices is their lack of efficacy against organized thrombotic or embolic material.
Atherothrombotic debris is present to varying degrees in most atherosclerotic arteries but especially in the distal aorta. Protruding atheromas of the aortic arch and descending aorta have assumed increasing importance as potential sites for embolization during catheter manipulation in the aorta for cardiac catheterization, carotid stenting placement of thoracic endografts, or bypass surgery. Embolization may also occur spontaneously or with the use of anticoagulants and thrombolytic therapy. Readily detectable by transesophageal echocardiography, lesions greater than 0.5 cm are most likely to be associated with embolic events. Evidence of spontaneous embolization has also been demonstrated at autopsy studies, but the incidence appears to be low (0.3% to 0.7%). The effects of spontaneous embolization may be exacerbated by dislodgement of atheromatous debris during intraoperative manipulations.
Variable amounts of atherothrombotic material may be dislodged and carried to a downstream territory as a consequence of manipulation during arterial dissection and clamp placement. After cardiac operative procedures, 66% of emboli lodge in the gastrointestinal (GI) tract and 48% lodge in the kidneys. Visceral embolization occurs in 40.9% of patients undergoing aortic reconstruction with the “shaggy” aorta syndrome. Embolization may also occur on reestablishment of circulation as a result of the accumulation of fresh thrombus in the temporarily static column of blood above or below the clamps if it is not carefully evacuated before circulation is restored. In the immediate postoperative period, atheroembolism should be suspected if the blotchy areas of discoloration of the skin of the abdominal wall and extremities and toes and impaired renal function do not improve with warming and optimization of cardiac output. A nonspecific systemic response characterized by fever, eosinophilia, elevated erythrocyte sedimentation rate (ESR), and highly specific C-reactive protein (hsCRP) may accompany an embolic episode.
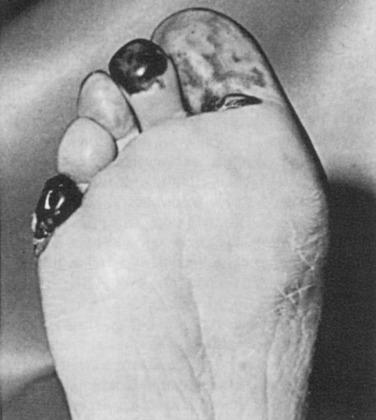
Larger emboli lodging in major vessels can usually be retrieved with a balloon thrombectomy catheter. Smaller embolic particles that cannot be retrieved will be flushed into end arteries of the feet or toes, leading to the “trash foot” syndrome. The end result is the appearance of patchy areas of painful skin gangrene at these sites ( Fig. 60.9 ). This may be a minor and self-limited problem, or it may produce extensive gangrene of all the digits and the forefoot, leg, buttocks, and rarely the abdominal wall.
Prevention is key because it is frequently difficult or impossible to treat this complication. Careful review of preoperative imaging studies will determine the site of clamp placement. A variety of technical maneuvers has been used to prevent or minimize operative embolization. Unnecessary and overly vigorous handling of vessels before the application of clamps should be avoided. Effective preclamping heparinization, monitored by intraoperative measurement of the ACT, reduces stasis thrombus formation above the proximal clamp and in the sluggish circulation distally. In patients with suspected or demonstrable atheromatous debris within the aorta on CT scans, the distal clamps should be applied to the common femoral or iliac arteries before proximal occlusion to avoid downstream displacement of debris when the aortic clamp is placed. The proximal clamp may need to be placed at the level of the diaphragm if the pararenal segment of the aorta appears to be involved. The lumen of the aortic prosthesis should be thoroughly aspirated to remove blood and debris after testing the proximal suture line, and efforts should be made to prevent the accumulation of blood within the prosthesis while distal iliac or femoral anastomoses are being performed. Vigorous antegrade flushing of the proximal vessel and retrograde flushing from the distal arteries as the last few stitches are being placed in an anastomosis, before the restoration of circulation, is the most reliable maneuver to ensure that retained debris and clot are effectively removed.
Treatment depends on the severity of embolization. Minor patchy areas of cyanosis or necrosis can be observed, with spontaneous recovery anticipated. More extensive involvement with threatened viability of the distal foot requires attempted removal of embolic material with small Fogarty balloon catheters passed into the distal vessels through the patent popliteal artery. Distal intraarterial infusion of r-tPA may be applicable in selected patients. Occasionally, when there is severe ischemia of a single or multiple digits, lumbar sympathectomy or amputation may be necessary. Low-dose corticosteroids may offer short-term benefit in patients with acute or subacute renal failure with systemic symptoms but have no favorable effect on long-term renal outcome. Statins, which stabilize atherosclerotic plaques and potentially improve outcomes, should be offered to all patients. Whether intensive lipid-lowering statin therapy for 3 to 6 months before surgery in patients at known risk of embolization will prove beneficial is presently unknown.
A sudden decrease in blood pressure should be anticipated after removal of the aortic clamp to restore flow to one or both extremities after aortoiliac reconstruction. The cause is hypovolemia resulting from incompletely replaced blood loss and fluid sequestration during surgery, compounded by a variable degree of preoperative dehydration that is usually present. Contributing factors are peripheral vasodilatation secondary to limb ischemia during the period of aortic occlusion and a decrease in cardiac output caused by a sudden return of acidic blood and other vasoactive metabolites to the central circulation on restoration of limb perfusion. The major consequences are significant reduction in coronary perfusion, which may promote myocardial injury, especially in patients with significant coronary artery disease, and temporary renal ischemia, which may contribute to renal failure. Prevention is preferable to treatment after a hypotensive insult has already occurred and depends on adequate hydration and effective restoration of intravascular volume during the procedure, and especially before clamp release. Effective volume replacement requires careful monitoring of blood loss and accurate estimation of the extracellular fluid shifts caused by sequestration and loss from evaporation. The extent of intravascular depletion is directly related to the duration of intraperitoneal and retroperitoneal exposure during surgery.
The most reliable guide to ensuring adequate volume replacement without circulatory overload is the use of a Swan-Ganz catheter to monitor left-heart filling pressures and myocardial performance. Cooperation between the surgeon and the anesthesiologist is essential during the critical moments before clamp release. Left atrial filling pressures should be optimized before release of the clamps. The arterial pressure must be continuously observed while blood flow is slowly restored to the extremities by gradual release of the clamps until full flow can be tolerated without hypotension. Finally, when a bifurcated graft is inserted, it is best to complete the anastomosis to one limb and restore its circulation immediately so that lower body perfusion can be resumed with the least amount of delay; this avoids washout acidosis and reduces declamping hypotension.
Renal impairment in patients undergoing abdominal aortic surgery is an important cause of morbidity and mortality and can range in severity from acute renal injury (ARI) to acute renal failure (ARF). Acute renal injury, manifested by the occurrence of albuminuria (93%) and proteinuria (22%) in patients undergoing aortic surgery, is the mildest form of impairment. Albuminuria usually occurs before cross-clamping and peaks between 0 and 6 hours after declamping. Acute renal dysfunction according to the RIFLE criteria (risk, injury, failure, loss of renal function, and end-stage renal disease), defined as an increase in serum creatinine (sCR) of 50% from baseline or oliguria of less than 0.5 mL/kg per hour for 6 hours, may progress to acute renal failure, which manifests as a 200% increase in sCR and oliguria of less than 0.5 mL/kg per hour for 24 hours or anuria.
The reported incidence of ARF after elective aortic aneurysmectomy is 1% to 8%, with a mortality rate of 40%. However, if the aneurysmectomy is emergent, the reported incidence of ARF is 8% to 46%, with a mortality of 57% to 95%. A more recent study by Siracuse and colleagues reported the incidence of acute renal failure following open repair of ruptured AAA to be 17.3%. The major cause of ARI is reduced renal perfusion resulting from decreased cardiac output, decreased blood volume, and dehydration. A contributing factor is renal cortical vasospasm produced by infrarenal application of the aortic clamp, which stimulates the renin-angiotensin mechanism. Other promoting factors include: underlying renal artery stenosis; suprarenal aortic cross-clamping (which totally eliminates renal perfusion); ischemia reperfusion injury; ligation of the left renal vein; intraoperative embolization; older age; hypertension; and drugs such as diuretics, β-blockers, calcium antagonists, nephrotoxic antibiotics, nonsteroidal antiinflammatory drugs, cyclooxygenase 2, and angiotensin-converting enzyme inhibitors. Cholesterol embolization to the kidneys may originate from debris and clot accumulating proximal to the aortic clamp or from manipulation of the juxtarenal aorta. Preoperative contrast (CTA, DSA) and endovascular catheter manipulations may produce a mild to moderate degree of renal dysfunction, which can be compounded by blood loss, hypotension, and dehydration during the operative procedure. Renal artery obstruction may be produced by displacement of large atherosclerotic plaques, thrombus, or dissection at the orifices of the renal arteries when an aortic clamp is applied. Myoglobinemia can occur after restoration of circulation to limbs that have been severely ischemic for an extended period. Finally intraabdominal hypertension resulting in ICS, especially in patients with ruptured aneurysms, must be considered.
Although the consequences of ischemic injury to the kidney are complex, injury to the tubules, especially the S3 segment of the proximal tubule and the thick ascending limb of the loop of Henle, is central to the development of oliguria. Obstruction of the tubular lumen by cellular debris and casts results in a reduction of the ultrafiltration pressure and sequestration of tubular fluid within obstructed tubules, in addition to back-leakage of fluid into the interstitium.
The critical issue in ARF is prevention, which is related primarily to the maintenance of an effective circulating blood volume and adequate hydration in the immediate perioperative period. It is essential that the patient be well hydrated and have a good urinary output at the commencement of surgery. Any significant extracellular fluid volume deficits should be restored the evening before surgery, especially if CTA, DSA, or mechanical bowel preparation has recently been performed. The sCR level should be measured after procedures requiring contrast administration, and if a decrease in renal function is identified, surgery should be delayed, if possible. Central filling pressures should be monitored perioperatively to ensure that volume replacement is optimal in relation to cardiac output and myocardial performance. It is appropriate to give mannitol and commence an infusion of renal-dose dopamine (2 to 3 µg/kg) just before cross-clamping the aorta to promote an osmotic diuresis and reduce the effects of renal cortical vasospasm. Bicarbonate is given to alkalinize the urine if there is any question of significant contrast-related nephrotoxicity or myoglobin washout from renewed perfusion of limbs that have undergone prolonged ischemia. Renal insufficiency is a significant complication of surgical procedures requiring cross-clamping of the thoracic or suprarenal aorta. Currently, a number of therapeutic maneuvers including preoperative statins, N -acetylcysteine, heme-oxygenase, ischemic preconditioning, fluid loading, renal cooling, calcium-channel blockers, atrial natriuretic peptide, endothelin antagonist, and fenoldopam are being evaluated in cardiac patients to determine their efficacy in reducing the incidence of ARF. The data regarding renal protection in vascular surgery patients are conflicting and have been extrapolated primarily from patients undergoing cardiac surgery procedures.
During dissection required to gain proximal control of large infrarenal or juxtarenal aneurysms, the left renal vein and its branches are vulnerable to injury. Access to this portion of the aorta is facilitated by division of the left renal vein close to its origin from the IVC, thus preserving the tributaries and reducing renal venous congestion if the vein cannot be re-approximated at the completion of the aortic anastomosis. In the past, this maneuver was viewed as one of little long-term consequence. However, Huber and coworkers and Abu Rahma and associates demonstrated increased sCR concentrations in patients who had renal vein ligation. Whether the renal dysfunction after renal vein ligation is solely a consequence of the resultant increased venous pressure or develops from a combination of venous hypertension and transient ischemia from intraoperative suprarenal clamp placement, which is required more frequently than with infrarenal clamp placement (11% vs. 18%), is not yet clear. Nonetheless, it is prudent to repair the renal vein if there is congestion of the left kidney and reanastomosis can be accomplished without undue tension or prolongation of the procedure.
Renal arteries should be dissected free and temporarily clamped in patients with aortic occlusion and thrombus that extends up to the renal orifices. The quality of the renal pulses must be evaluated and the blood flow assessed by Doppler color-flow imaging after restoration of circulation through the aorta in any patient who has had significant juxtarenal aortic manipulation.
Postoperatively, the continued retroperitoneal and intraperitoneal sequestration of extracellular fluid requires replacement with lactated Ringer solution, within the limits imposed by left-heart filling pressures, to ensure adequate renal output. Volume replacement should be reduced after the second postoperative day to prevent fluid overload from the mobilization of large volumes of sequestered extravascular fluid. The urinary output is monitored continuously and should be maintained at or above 0.5 mL/kg per hour. The specific gravity is determined frequently, and the blood urea nitrogen and sCR are measured daily for 2 or 3 days to determine the quality of renal function. Diuretics should not be given until intravascular volume has been fully restored. A rise in sCR, the standard marker used to detect ARF, does not allow for the early diagnosis of ARI because of a lack of specificity for ARI; poor correlation with glomerular filtration rate; and the influence of age, sex, body mass index, hydration, and nutrition. Several biomarkers are currently being investigated in patients undergoing cardiac surgery to improve the detection of early renal dysfunction. Cystatin C, kidney injury molecule-1 (KIM-1), N -acetyl β- d -glucosaminidase (NAG), and neutrophil gelatinase–associated lipocalin (NGAL) are among the early detectable biomarkers in the serum and urine of patients with ARI. However, correlation of individual markers with glomerular filtration rate is variable, and a combination of markers may be required to improve the sensitivity of these molecules in the early diagnosis of ARI. At the onset of ARF, urinary specific gravity increases (>1.015) and sodium and urea decrease. Once tubular necrosis occurs, the urine becomes isosthenuric; the urinary sodium, fractional excretion of sodium, and urea increase to greater than 20 mml/L, greater than 1%, and greater than 35%, respectively; and the ratio of blood urea nitrogen (BUN)-to-creatinine ratio falls from 20 : 1 to 10 : 1. If ARF is diagnosed, fluid replacement should be restricted to maintain central filling pressure in the normal range. Dialysis is used aggressively to control excess volume and relieve azotemia and hyperkalemia. Intravenous hyperalimentation or parenteral nutrition should be instituted early in the clinical course of patients with ARF to minimize protein catabolism.
Renal artery stenosis or impaired renal function is frequently present in patients undergoing aortic reconstruction for occlusive and aneurysmal disease. The liberal use of CTA, MRA, and DSA before surgery is recommended to identify renal anomalies, renal artery stenoses, or suprarenal extension of an aneurysm so that appropriate alterations in operative management can be planned. Often, renal revascularization can be accomplished by balloon angioplasty and stenting, along with operative or endovascular correction of the aortic disease. In this setting, patients often have impaired renal function, and the large volume of contrast agents used for imaging studies or intervention may result in additional nephrotoxicity. The preoperative administration of N -acetylcysteine has been shown to reduce contrast-related ARF from 12% to 2% to 4%. A cautionary note regarding the use of N -acetylcysteine in the immediate perioperative period: Wijeysundera and coworkers have shown that its use is associated with greater transfusion requirements in patients undergoing cardiac surgery procedures. Additional maneuvers to reduce the risk of renal ischemia include the use of temporary renal perfusion with cold lactated Ringer solution containing heparin, mannitol, and methylprednisolone. Postoperatively, a renal perfusion scan or aortography should be performed immediately if total renal shutdown occurs because this suggests a renal artery occlusion. This requires immediate reoperation to restore kidney circulation. The use of statins in the early postoperative period has been shown to lower the incidence of AKI in cardiac surgery patients. Welten and associates, in a study of 2170 patients undergoing lower extremity bypass or abdominal aortic surgery, demonstrated that early statin use increased the odds of recovery if renal function deteriorated postoperatively. In addition, statin use was also associated with improved long-term survival irrespective of changes in renal function.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here