Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Nodal marginal zone lymphoma (NMZL) is a primary nodal B-cell neoplasm derived from post–germinal center B cells. This lymphoma shares morphologic and immunophenotypic similarities with other marginal zone lymphomas, particularly extranodal marginal zone lymphoma (EMZL) of mucosa-associated lymphoid tissue (MALT) type and splenic marginal zone lymphoma (SMZL). Thus, secondary lymph node involvement by EMZL and SMZL should be excluded to establish the diagnosis with certainty. NMZL may show some evidence of plasmacytic differentiation but generally less than that seen in lymphoplasmacytic lymphoma (LPL), which is often included in the differential diagnosis.
NMZL is a relatively uncommon lymphoma, accounting for only 1.5% to 1.8% of all lymphoid neoplasms. It is primarily a lymphoma of adults, but a pediatric variant of the disease exists, with some distinguishing morphologic and clinical features. The median age of patients is between 50 and 60 years, with a female predominance in some but not all series. An association with hepatitis C infection has been suggested in some studies but is lacking in others. These discrepant results may relate to overlap among a variety of B-cell neoplasms and different diagnostic criteria. The distinction among NMZL, EMZL, and SMZL can be difficult, and hepatitis C has been linked to EMZL and SMZL as well. Alternatively, these differences could relate to different risk factors in different populations of patients or geographic regions.
Underlying autoimmune disease has been implicated in a variety of EMZLs, including Sjögren's syndrome and Hashimoto's thyroiditis. However, a history of autoimmune disease is lacking in most patients with NMZL. Nodal involvement may be prominent in many patients with salivary gland MALT lymphoma, with a predominance of monocytoid B cells. Therefore, a careful clinical history is important in the evaluation of these cases. Both autoimmune hemolytic anemia and cryoglobulinemia have been reported in a subset of patients with NMZL. However, the incidence is much less than that seen with LPL. Recent molecular studies have identified a high incidence of mutations in MYD88 at L265P in Waldenström's macroglobulinemia and LPL but only infrequently in NMZL, facilitating this distinction.
The majority of patients have generalized peripheral lymphadenopathy, although two series found a higher proportion of localized stage I or stage II disease. B symptoms are present in a minority. Clinical evaluation should be undertaken to rule out secondary nodal involvement by EMZL or SMZL, given the significant overlap in the morphologic features in lymph nodes. Most investigators require the absence of extranodal sites of disease (other than bone marrow, liver, or spleen) in making the diagnosis. Similarly, patients presenting with marked splenomegaly and bone marrow involvement with only minimal lymphadenopathy most likely fall into the category of SMZL. These imprecise diagnostic criteria make it difficult to compare clinical features and outcome.
Bone marrow involvement is relatively uncommon in most series, occurring in less than half of the patients. Peripheral blood involvement is generally rare, and the presence of circulating cells should at least raise the suspicion of other forms of B-cell neoplasia, including SMZL, splenic lymphoma with villous lymphocytes, and atypical chronic lymphocytic leukemia (CLL). According to the International Prognostic Index (IPI) or the modified Follicular Lymphoma IPI (FLIPI), most patients are in the low or low-intermediate risk groups. Elevations in lactate dehydrogenase are common but are usually modest.
Although plasmacytoid differentiation can be observed histologically, it is uncommon to find monoclonal gammopathy in the serum. One exception was the French series reported by Traverse-Glehen and colleagues, in which 33% of patients had an M component or monoclonal spike. These distinctions may relate to differences in diagnostic criteria and the overlap between NMZL and LPL in tissue biopsies.
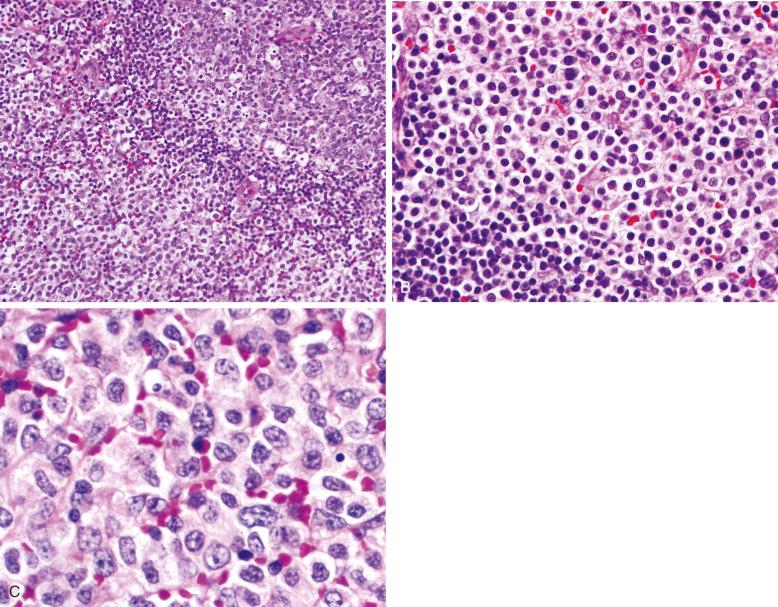
The neoplastic cells in NMZL are heterogeneous in appearance and have been described as monocytoid, centrocyte-like, and plasmacytoid. These smaller cells are usually admixed with a variable number of transformed cells or blasts. Monocytoid cells have round to irregular nuclei with condensed nuclear chromatin, inconspicuous nucleoli, and abundant pale cytoplasm with distinct cytoplasmic membranes. This cell type is commonly seen in MALT lymphomas associated with Sjögren's syndrome, and a dominant population of monocytoid cells should prompt a clinical evaluation for EMZL either concurrently or sometime in the distant. Late relapses of EMZL have been described, sometimes many years after the primary diagnosis. Centrocyte-like cells resemble their counterparts in EMZL. These small to medium-sized cells have coarsely clumped chromatin, irregular nuclei, and sparse cytoplasm. Plasmacytoid cells exhibit varying degrees of plasmacytoid differentiation. They are often slightly larger than the other cell types present, with an ample rim of basophilic cytoplasm. The nuclear chromatin is generally more dispersed than that of mature plasma cells, and small nucleoli may be present. Dutcher bodies may be seen but are generally less common than in LPL. Larger lymphoid cells or blasts, reminiscent of centroblasts, are present in variable proportions but should not constitute the majority of cells present. The overall impression is that of a heterogeneous population of cells that are medium in size, generally round but irregular, and lacking the monotony seen in some other B-cell lymphomas such as CLL or mantle cell lymphoma.
Other inflammatory cells, particularly epithelioid histiocytes, may be present. However, well-formed granulomas are usually absent. Eosinophils may be noted, particularly in cases with plasmacytoid differentiation.
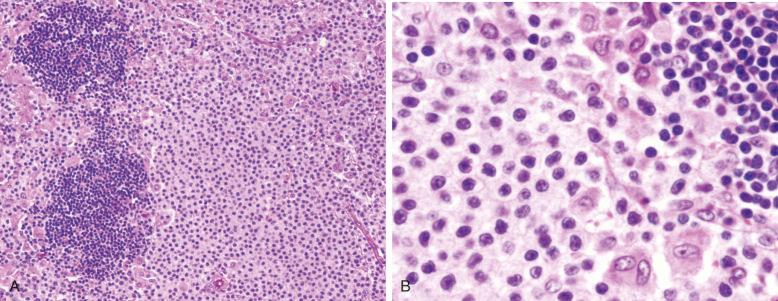
Varied architectural patterns are seen in NMZL. In many instances, the infiltrate is diffuse. However, often there is some semblance of residual follicles that can be expanded, regressed, or, in some instances, colonized by neoplastic cells. These different patterns may correlate to some extent with the nature of the neoplastic cells in different variants. Campo and associates described a “MALT type” of NMZL in which the follicles are generally well preserved, with reactive germinal centers and intact lymphoid cuffs ( Fig. 21-1 ). In this histologic variant, monocytoid B cells are generally abundant. In almost 50% of these cases, further investigation discloses evidence of EMZL at some point in the patient's history ( Fig. 21-2 ).
In the “splenic type” of NMZL described by Campo and colleagues, residual lymphoid follicles are typically regressed, lacking well-formed germinal centers. The mantle cuff might be present but is usually attenuated ( Fig. 21-3 ). The cellular infiltrate is heterogeneous, comprising all the cell types described earlier. The term splenic type was applied to this subset because the tumor cells are typically weakly immunoglobulin (Ig) D positive, resembling the phenotype of SMZL. In addition, the lymph nodes show some resemblance to cases of SMZL with regional lymph node involvement. However, in most of these cases, no connection to SMZL is shown.
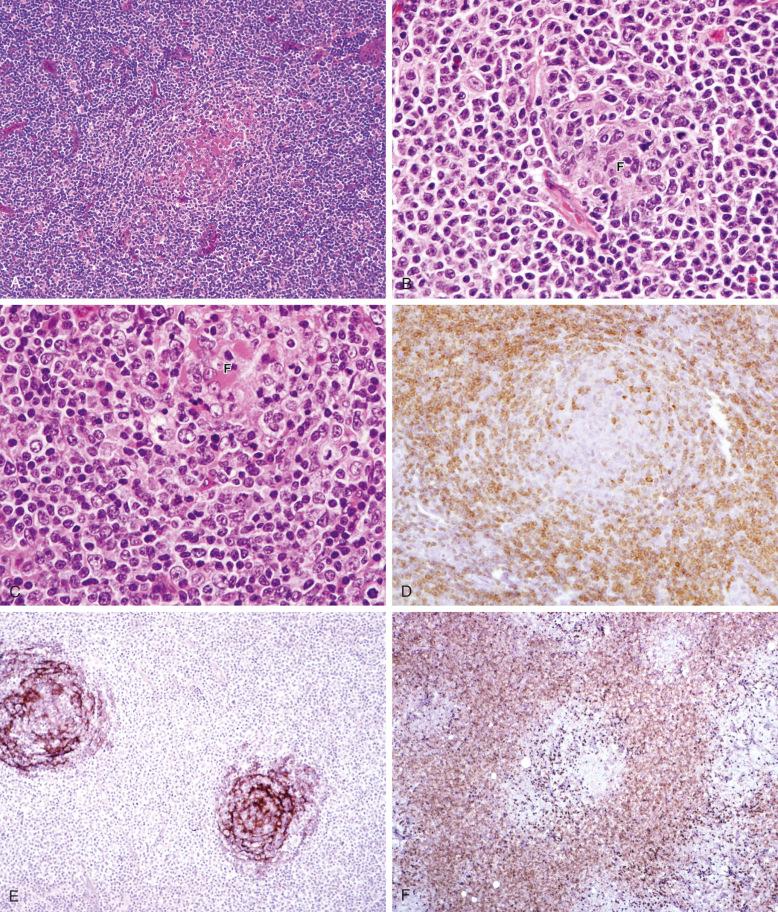
Many cases do not correspond cleanly to either subtype. They have an overall polymorphic cellular composition—the polymorphic subtype of NMZL ( Table 21-1 ). Follicles may be absent or partially preserved and may show prominent regressive features ( Fig. 21-4 ). In these cases, follicular colonization is more common, and plasmacytoid differentiation is often present, either within or outside the follicles. Eosinophils may be abundant and tend to correlate with plasmacytoid differentiation.
| Feature | MALT Type | Splenic Type | Polymorphic Type |
|---|---|---|---|
| Hyperplastic germinal centers | ++ | +/– | + |
| Prominent mantle zones | ++ | – | –/+ |
| Intrafollicular T cells | – | ++ | + |
| IgD expression | – | + | +/– |
| Plasmacytoid differentiation | – | +/– | ++ |
| Risk of extranodal disease | ++ | – | – |
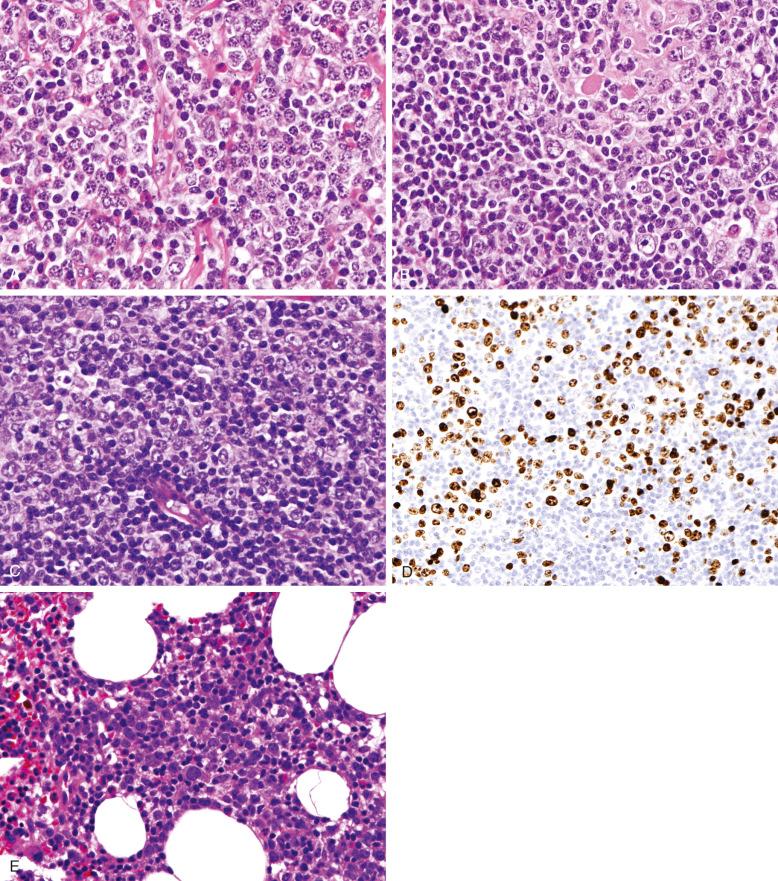
Follicular colonization can be a prominent feature in NMZL, and extensive infiltration of residual follicles may impart a nodular or follicular growth pattern, mimicking follicular lymphoma. Follicular colonization has also been described in EMZL. In some cases, the cytology of the cells within the colonized follicles may be different from that of the cells in the perifollicular zone. No definite pattern emerges, but plasmacytoid differentiation is often prominent in the colonizing cells. In some cases, a higher proportion of blastic cells is noted within the colonized follicles.
In some cases, particularly in the pediatric variant of NMZL, hyperplastic germinal centers with expanded and irregular configurations may be seen. In pediatric cases, these abnormal follicles resemble the disrupted follicles of progressive transformation of germinal centers. Other authors have described these follicles as “floral” in nature, terming these cases the floral variant of NMZL. The follicles, despite their unusual appearance, do not appear to be neoplastic because they regain expression of BCL6 and CD10 and are negative for BCL2 protein. This unusual morphologic variant of NMZL is most common in children and young adults but can also occur in older age groups. Interestingly, most of these cases present with isolated nodal sites of stage I disease and do well with conservative therapy after simple excision of the nodal mass.
The appearance of NMZL in other anatomic sites is not well described. The incidence of bone marrow involvement has varied in different series, with most centers reporting a range of 20% to 40%. Bone marrow infiltration generally consists of loose non-paratrabecular aggregates or, in some cases, interstitial infiltration. One study also noted paratrabecular aggregates in some cases. As noted earlier, peripheral blood involvement is rare. Involvement of other extranodal sites usually leads to a consideration of the diagnosis of EMZL.
There is no established grading system for NMZL, although considerable variation in the proportion of blastic cells or proliferating cells (as measured by Ki67) may be seen. In general, the proportion of blastic cells is less than 20% of the total cell population. Some authors have reported cases with foci of large cell transformation, but the clinical significance of these foci has not been shown. In a subsequent study, the Lyon group noted that a number of cases contained more than 20% blastic cells and suggested that this feature might account for the more aggressive clinical course in that series. However, the investigators were unable to relate outcome to the proportion of large cells or histologic progression. Specifically, there was no difference in survival between the groups with greater than 20% and less than 20% large cells. Nathwani and coworkers reported a relatively high frequency (20%) of transformation to diffuse large B-cell lymphoma in NMZL, but the criteria for transformation were not delineated. Moreover, no difference in survival for those patients who had “transformed” was shown. One study from Japan found a poorer overall survival for cases of NMZL containing a component of diffuse large B-cell lymphoma, but overall, there was no difference in survival for the four histologic subtypes identified: splenic type, floral type, MALT type, and MALT type plus diffuse large B-cell lymphoma. My practice is to perform Ki67/MIB-1 staining in cases of NMZL and to note in the report if the proliferation rate is especially high (>50% of the nucleated cells), but it is not clear that different therapeutic approaches benefit this subset of cases.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here