Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Preterm and term infants are at risk of acquiring brain injury with lasting neurodevelopmental sequelae.
Mechanisms of brain injury in the developing brain are related to unique vulnerabilities due to the maturational stage of the various types of cells in the brain.
The pathogenesis of brain injury in both preterm and term infants provides multiple opportunities for therapeutic intervention, such as addressing excitotoxicity, inflammation, oxidative stress, cytokines, and mechanisms of repair and regeneration.
In the future, it is plausible that a cocktail of medications may be prescribed to address the mechanisms of brain injury at different time points during the injury and repair process.
Antenatal steroids and magnesium sulfate should be administered to women at risk of delivering a premature infant, as they have been proven to reduce the risk of developing intraventricular hemorrhage.
Therapeutic hypothermia is the standard of care for term and near-term infants with moderate to severe hypoxic–ischemic encephalopathy, but its use in infants with mild HIE or in infants at 33 to 35 weeks’ gestation requires further study. Additional drugs such as erythropoiesis-stimulating agents, xenon, melatonin, and allopurinol are being studied as single-agent therapies or as adjunctive treatments to further reduce the risk of death or disability in these infants.
Sick infants are at significant risk for the acquisition of perinatal brain injury. Concerns about long-term neurologic sequelae have increased along with improved survival of extremely premature infants, movement of the limits of viability toward earlier gestational ages, and increased survival among infants with complex medical and surgical conditions. According to the Centers for Disease Control and Prevention, one in 10 infants born in the United States in 2020 was premature. Mortality is highest among the smallest and youngest premature infants, and 40% of survivors have cognitive or physical disabilities. The lifetime cost associated with cognitive and physical disabilities is >$1,000,000 per family, without accounting for parental loss of work to care for a disabled child or family emotional burdens.
Major advances in respiratory and cardiovascular care have not been matched by advances in prevention or treatment of brain injury in premature infants. The best protective strategy remains prevention of preterm birth. Neuroprotective strategies for premature infants focus on antenatal management and postnatal strategies such as providing thermoregulation and maintenance of hemodynamic and respiratory stability, particularly in the first 3 to 7 days after birth. Current regimens thought to further improve neurologic outcomes in preterm infants include antenatal administration of betamethasone or magnesium sulfate, delivery in an appropriate center with a well-trained and experienced resuscitation team, use of delayed cord clamping at birth, postnatal treatment with indomethacin or caffeine, and use of neuroprotective bundles aimed at reducing intraventricular hemorrhage (IVH).
For term infants, there has been significant progress over the last 15 years for those with neonatal encephalopathy due to presumed perinatal asphyxia, also known as hypoxic–ischemic encephalopathy (HIE). Eleven randomized controlled trials (RCTs) that enrolled >1500 infants demonstrated the efficacy of therapeutic hypothermia in reducing the risks of death and neurodevelopmental impairment in infants with HIE. Therapeutic hypothermia is the only clinically available treatment for moderate to severe HIE and is considered the standard of care for this patient population. Several medications, including erythropoietin (EPO), darbepoetin, xenon, topiramate, melatonin, magnesium sulfate, and stem cells are under evaluation to determine if they provide neuroprotection in addition to that of hypothermia. Other agents have been evaluated in animals but are not yet ready for clinical trials. In this chapter, we review neuroprotective strategies and therapies currently in use or being evaluated for use in preterm or term infants.
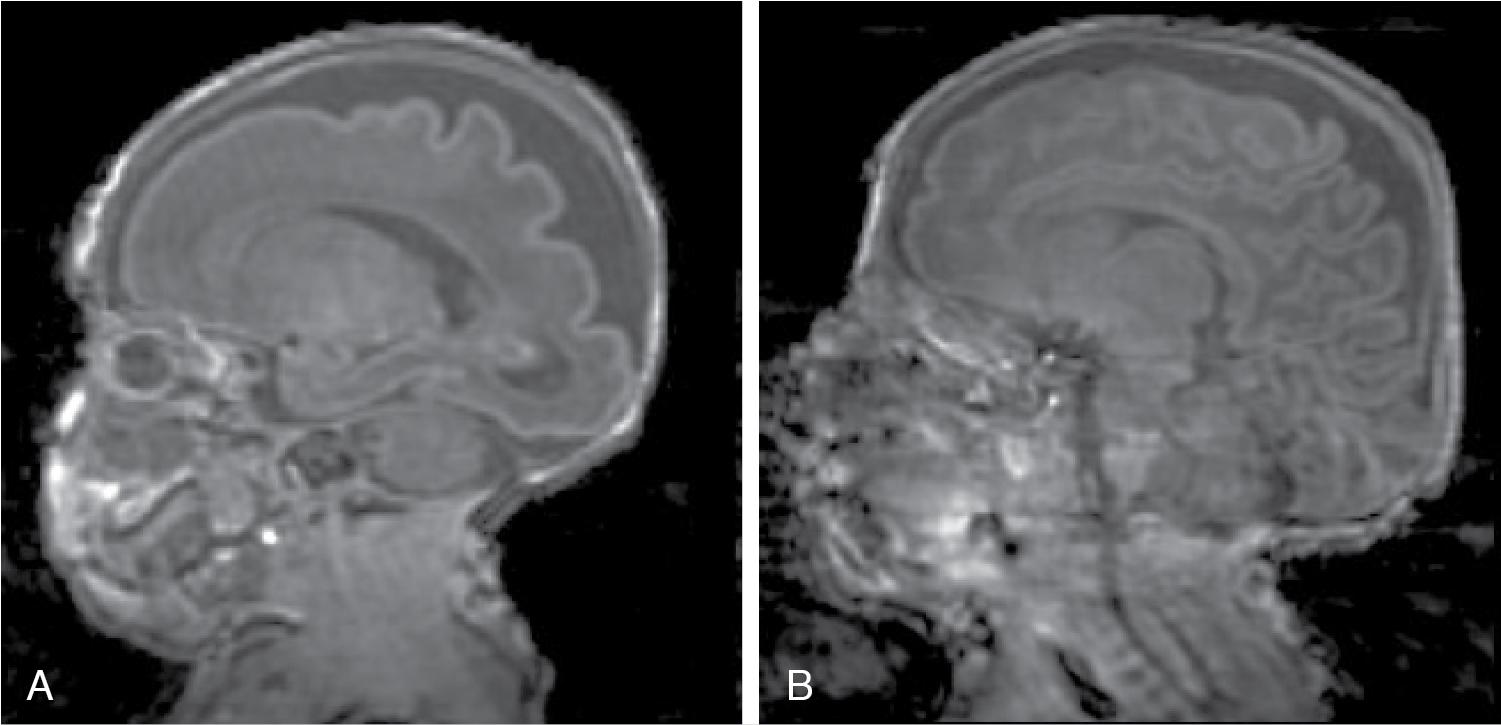
The pathophysiology of brain injury in premature infants is complex, reflecting developmental susceptibility of the immature and rapidly changing preterm brain, , fragility of the vascular germinal matrix (where IVHs originate), and the impacts of various measures needed to sustain life outside of the womb. , The chronic inflammatory state often associated with life-sustaining intensive care is postulated to interfere with normal brain development and may account for the focal injuries to and abnormal maturation of white matter detected using advanced magnetic resonance and diffusion tensor imaging techniques. Chronic mechanical ventilation, oxygen exposure, sepsis, necrotizing enterocolitis, surgery (e.g., ligation of a patent ductus arteriosus), and suboptimal nutrition all likely contribute to the brain pathology observed in this population. At 24 weeks’ gestation, the white matter is populated predominantly by immature oligodendrocytes and lacks myelination, and the cortex is undergoing development and reorganization via neuronal migration and synaptogenesis. Over the next 12 to 16 weeks, while the extremely premature infant is cared for in the neonatal intensive care unit (NICU), the brain undergoes tremendous growth and development ( Figure 14.1 ). Abnormal development of the brain may be caused by direct damage to the brain tissue (as occurs in periventricular hemorrhagic infarction, or PVHI, previously known as grade 4 IVH) or by interruption of normal development without tissue disruption. Between 24 and 32 weeks’ postmenstrual age, preoligodendrocytes and the subplate neurons are highly vulnerable to oxidative injury, hypoxia, and excitotoxicity. Impaired maturation of preoligodendrocytes leads to abnormalities of the white matter on ultrasound and magnetic resonance imaging (MRI), and later poor head growth, motor abnormalities such as cerebral palsy, and other neurodevelopmental disabilities.
Care practices during the antenatal and perinatal period may affect the pathogenesis of brain injury. Due to the complex pathophysiology that leads to the development of IVH, care bundles based on avoidance of abnormal physiologic or coagulopathic states have been used in quality improvement studies to attempt to reduce IVH and ultimately improve neurodevelopmental outcome. Antenatal steroids and magnesium sulfate are both associated with lower rates of IVH. Management at the time of delivery, including delayed cord clamping, resuscitation by an experienced team, and practices such as early continuous positive airway pressure, slow rates of volume administration of infusion, and gentle or noninvasive ventilation strategies may also reduce brain injury. Midline head positioning, minimal handling, reduction of stress and painful procedures, and addressing nutritional deficiencies have been identified as potentially helpful. , Given the premature infant’s limited ability to autoregulate cerebral blood flow, measures should be taken to avoid hypotension, hypertension, and hypocarbia or hypercarbia. Hyperoxia is also toxic to the developing brain by promoting production of oxygen free radicals. Targeted oxygen saturation goals may help prevent exposure to high oxygen levels. Early introduction of breast milk (mother’s own or donor milk) may improve immune status and early gut function and thus reduce the risk of developing necrotizing enterocolitis. Developmental care practices such as containment devices, protection from noise and light, and kangaroo or skin-to-skin care are also frequently included in care bundles. However, evidence that demonstrates the efficacy of these methods to prevent high-grade IVH, PVHI, periventricular leukomalacia, or long-term motor or cognitive impairment remains limited.
Among very-low-birth-weight infants (<1500 g), the incidence of IVH is about 20%. In extremely low-birth-weight infants (<1000 g), the incidence of IVH is 45%. Increasing use of antenatal betamethasone since the 1980s has been associated with a commensurate reduction in the rate of IVH. The initial studies of antenatal steroids in the 1970s and 1980s, including the landmark study of Liggins and Howie in 1972, had the primary goal of improving respiratory outcomes. The most recent Cochrane Review , which includes data on >8000 infants, shows that antenatal steroid treatment is also associated with decreased risk of perinatal death (relative risk [RR] = 0.72; 95% confidence interval [CI], 0.58–0.89), neonatal death (RR = 0.69; 95% CI, 0.59–0.81), any grade of IVH (RR = 0.55; 95% CI, 0.40–0.76), and severe (grade 3 or 4) IVH (RR = 0.26; 95% CI, 0.11–0.60). Despite these and other benefits, meta-analyses have not shown an improvement in long-term developmental outcomes.
The mechanisms by which antenatal steroid treatment reduces IVH are thought to be similar to those of indomethacin, including structural stabilization and reduced permeability of the basement membrane of the germinal matrix vasculature. In theory, these changes should make the fragile germinal matrix more tolerant to changes in cerebral blood flow caused by episodes of hypoxia, hypercarbia, or hypocarbia or unstable blood pressure.
Recent work has focused on the relationships between the risk of IVH and the proximity of antenatal betamethasone exposure to delivery or repeated courses of antenatal steroids. A recent study of 429 infants <28 weeks at birth evaluated the impact of proximity of steroid exposure and severe IVH (grade 3 or 4). In premature infants born ≥10 days after a course of maternal betamethasone, the rate of severe IVH was 17% compared with 7% for those born <10 days after steroid exposure (adjusted odds ratio [aOR] = 4.16; 95% CI, 1.59–10.87; P = 0.004). This higher risk of IVH potentially may be reversed by a repeat course of steroids, because infants who received a second course had a rate of IVH of 8%, similar to that for infants born <10 days after the first course. Investigators examined the dose-dependent effect of antenatal corticosteroids (ANS) on neonatal mortality, morbidities, and neurodevelopmental outcome by comparing cohorts 401 to 1000 g or 22 to 27 weeks’ gestation with no ANS, partial ANS, and complete ANS. This observational study found significant differences in death (43%, 29.6%, and 25.2%, respectively), severe IVH (23.3%, 19.1, and 11.7%, respectively), death or necrotizing enterocolitis (48.1%, 37.1%, and 32.5%, respectively), death or bronchopulmonary dysplasia (74.9%, 68.9%, and 65.5%, respectively), and the primary outcome of death or neurodevelopmental impairment (68.1%, 54.4%, and 48.1%, respectively). These results emphasize the importance of prompt administration of ANS to women with threatened preterm delivery with a goal of providing a complete course prior to delivery. A recent RCT performed by the National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network in women at 34 to 36 weeks 5 days of gestation at high risk for delivery found a significantly reduced risk of neonatal respiratory complications in the betamethasone-treated group but no difference in IVH. Given the beneficial impact of antenatal steroids on both respiratory and neurologic outcomes, it should be the goal to administer betamethasone to all women at risk of delivery at <37 weeks’ gestation.
Magnesium sulfate was introduced to prevent maternal eclampsia and was then used as a tocolytic agent. It was later recognized in the late 1980s and early 1990s to have neuroprotective effects, with a reduction in the rate of IVH after treatment of mothers with preeclampsia. Concurrently, animal evidence of neuroprotection in age-appropriate models suggested benefit for human fetuses in the range of 26 to 34 weeks’ gestational age. Several subsequent controlled trials, including four in which the primary outcome was neuroprotection of the fetus, have confirmed this effect. Meta-analyses demonstrate a clear reduction in the risk of cerebral palsy at 18 to 24 months of corrected age, but data on whether this benefit is sustained at early childhood are conflicting. Antenatal administration to mothers at risk of preterm delivery reduces the risk of cerebral palsy (RR = 0.68; 95% CI, 0.54–0.97) and the rate of substantial gross motor dysfunction (unable to walk without assistance at age 2 years) (RR = 0.61; 95% CI, 0.44–0.85). Antenatal magnesium sulfate administration is considered standard of care by the American College of Obstetricians and Gynecologists for women presenting with preterm labor at <32 weeks’ gestation and expected to deliver within 7 days. Dosing regimens vary, in the range of a 4- to 6-g loading dose followed by 1 to 2 g/hr by continuous infusion for 12 to 24 hours.
The mechanism of neuroprotective action of magnesium sulfate is not well understood. Magnesium is important for key cellular processes such as glycolysis, oxidative phosphorylation, protein synthesis, DNA and RNA aggregation, and maintenance of cell membrane integrity. Magnesium is also involved in mechanisms of cell death and dysfunction, modulating inflammatory cytokines and free radicals, and preventing excitotoxic calcium injury by reducing calcium entry into cells. Finally, magnesium may have important hemodynamic effects that stabilize cerebral blood flow and thus reduce the risk of IVH.
Apnea, defined as cessation of breathing for more than 15 seconds, occurs in at least 85% of infants born at <34 weeks’ gestation and is frequently accompanied by bradycardia and desaturation. Caffeine, a methylxanthine respiratory stimulant, is one of the most commonly used medications in premature infants. Methylxanthines reduce both apnea and the need for mechanical ventilation. However, methylxanthines inhibit adenosine receptors, and adenosine preserves brain adenosine triphosphate (ATP) levels during experimental hypoxia and ischemia. They also increase oxygen consumption and may diminish growth. Accordingly, there was uncertainty about short- and long-term benefits or risks of methylxanthine treatment.
This uncertainty was addressed by the Caffeine for Apnea of Prematurity (CAP) trial, an RCT in infants with birth weights of 500 to 1250 g ( N = 2006). The primary outcome was a composite outcome of death, cerebral palsy or cognitive delay, deafness, or blindness assessed at 18 to 21 months of age. Caffeine decreased death or survival with neurodevelopmental disability (40.2% vs. 46.2%; aOR = 0.77; 95% CI, 0.64–0.93; P = 0.008). Significant reductions in cerebral palsy and cognitive delay were observed without any difference in the rates of death, deafness, or blindness. The number of infants needed to prevent one adverse outcome was 16 (95% CI, 9–56).
When the CAP trial participants were seen at 5 years of age, caffeine therapy was no longer associated with a significantly lower risk of death or disability (21.1% vs. 24.8%; aOR = 0.82; 95% CI, 0.65–1.03; P = 0.09). Interestingly, the incidence of cognitive impairment at 5 years was similar in the placebo- and caffeine-treated groups and was lower than at 18 months, but gross motor impairment was less severe in the caffeine-treated infants, who had better motor coordination and visual perception. The rate of developmental coordination disorder (a form of motor dysfunction not associated with cerebral palsy or cognitive dysfunction, defined as motor performance less than the fifth percentile on the Movement Assessment Battery for Children) was lower in the caffeine-treated group (11.3% vs. 15.2%; OR = 0.71; 95% CI, 0.52–0.97; P = 0.032). This was felt to be an important finding, because developmental coordination disorder is associated with learning disabilities, poor school performance, behavioral problems, poor social skills, and low self-esteem. The CAP trial has now followed 76% of the original cohort to 11 years of age, when the primary outcome was functional impairment defined as a composite of poor academic performance, motor impairment, and behavior problems. Rates of functional impairment were not significantly different between the groups, but caffeine therapy was associated with a reduced risk of motor impairment (19.7% vs. 27.5%; OR = 0.66; 95% CI, 0.48–0.90; P = 0.009).
Caffeine has been called a silver bullet in neonatology because of its wide therapeutic index, tolerability, and efficacy in reducing bronchopulmonary dysplasia, patent ductus arteriosus, severe retinopathy of prematurity, and neurodevelopmental disability. Few drugs used in the neonatal period have been tested in RCTs with follow-up out to 11 years with continued evidence of benefit. The exact mechanisms responsible for neuroprotection remain incompletely elucidated. Brain microstructural changes consisting of improved myelination have been seen in the caffeine-treated group, and larger MRI studies are underway to better understand this finding.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here