Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The two functions of the lower urinary tract are the storage of urine within the bladder and the timely expulsion of urine from the urethra. The precise neurologic pathways and neurophysiologic mechanisms that control these functions of storage and micturition are complex and not completely understood, with our understanding of many of these pathways adapted from animal models. Understanding the interaction of the autonomic nervous system, the peripheral nervous system, and the central nervous system (CNS) in lower urinary tract function is critical to patient care, as modulating those interactions is key to treatment of many lower urinary tract disorders. This chapter reviews the normal function and neurologic control of, and introduces clinical pharmacology concepts regarding, the lower urinary tract in women.
Neurons within the nervous system propagate signals via action potentials, an electrical event. This electrical event results in a chemical event at junctions with other nerves or end organs, relying on chemicals known as neurotransmitters. These neurotransmitters are selectively released from a nerve terminal by an action potential and interact with a specific receptor on an adjacent structure to elicit a specific physiologic response. These chemical events at the synapses can be excitatory or inhibitory.
The nervous system is arranged into the central and the peripheral systems. The CNS includes the brain and spinal cord. Within the brain and spinal cord, nerve cell bodies are arranged in groups of various sizes and shapes called nuclei. Fibers with a common origin and destination are called a tract. Synaptic relationships in the CNS are very complex, with contacts occurring between axons and cell bodies, axons and dendrites, cell body and cell body, or dendrite and dendrite.
Twelve pairs of cranial and 31 pairs of spinal nerves with their ganglia compose the peripheral nervous system. Synaptic relationships in the peripheral nervous system involve only neuron–neuron or neuron–effector interactions. The somatic component of the peripheral system innervates skeletal muscle and receives somatic sensory input.
The autonomic division innervates cardiac muscle, smooth muscle, and glands. The autonomic nervous system consists, in part, of general visceral efferent fibers, supplying the smooth muscle of viscera. Afferent fibers are closely associated with the autonomic efferents, and both the motor and sensory visceral neural activities ordinarily function at a subconscious level. Unlike the somatic motor system, the peripheral efferent autonomic fibers reach the effector organ by at least a two-neuron chain, constituting a preganglionic and a postganglionic neuron. Fibers arising from neurons of the intermediolateral cell column of the 12 thoracic and first two lumbar segments of the spinal cord constitute the sympathetic division (thoracolumbar) of the autonomic nervous system. The parasympathetic division (craniosacral) consists of fibers arising from the neurons of the second through the fourth intermediolateral cell column sacral segments and from cranial outflows.
Sympathetic nerves to the pelvic cavity originate in cord levels T10 to L2 ( Fig. 3.1 ). Generally, their two-neuron chain consists of short preganglionic fibers and long postganglionic fibers. Some preganglionic axons pass via white rami communicantes to the paravertebral sympathetic chain, synapse, and pass by gray rami communicantes to the skeletal nerves. These constitute the paravertebral sympathetics, and they generally follow the segmental nerves to somatic structures. The other preganglionic sympathetic axons pass directly through the paravertebral ganglia to the prevertebral ganglia located at roots of arteries, for which they are named (e.g., lumbar splanchnic nerves terminate in inferior mesenteric and hypogastric ganglia). After synapsing in these ganglia, the postganglionic nerves travel through the right or left hypogastric nerve to join the pelvic plexus and follow the visceral arteries to the organs of the lower abdomen and pelvis. These constitute the prevertebral sympathetics. In addition to these two pathways, preganglionic sympathetic fibers may also ascend or descend within the paravertebral ganglia chain before synapsing or passing through to the prevertebral ganglia.
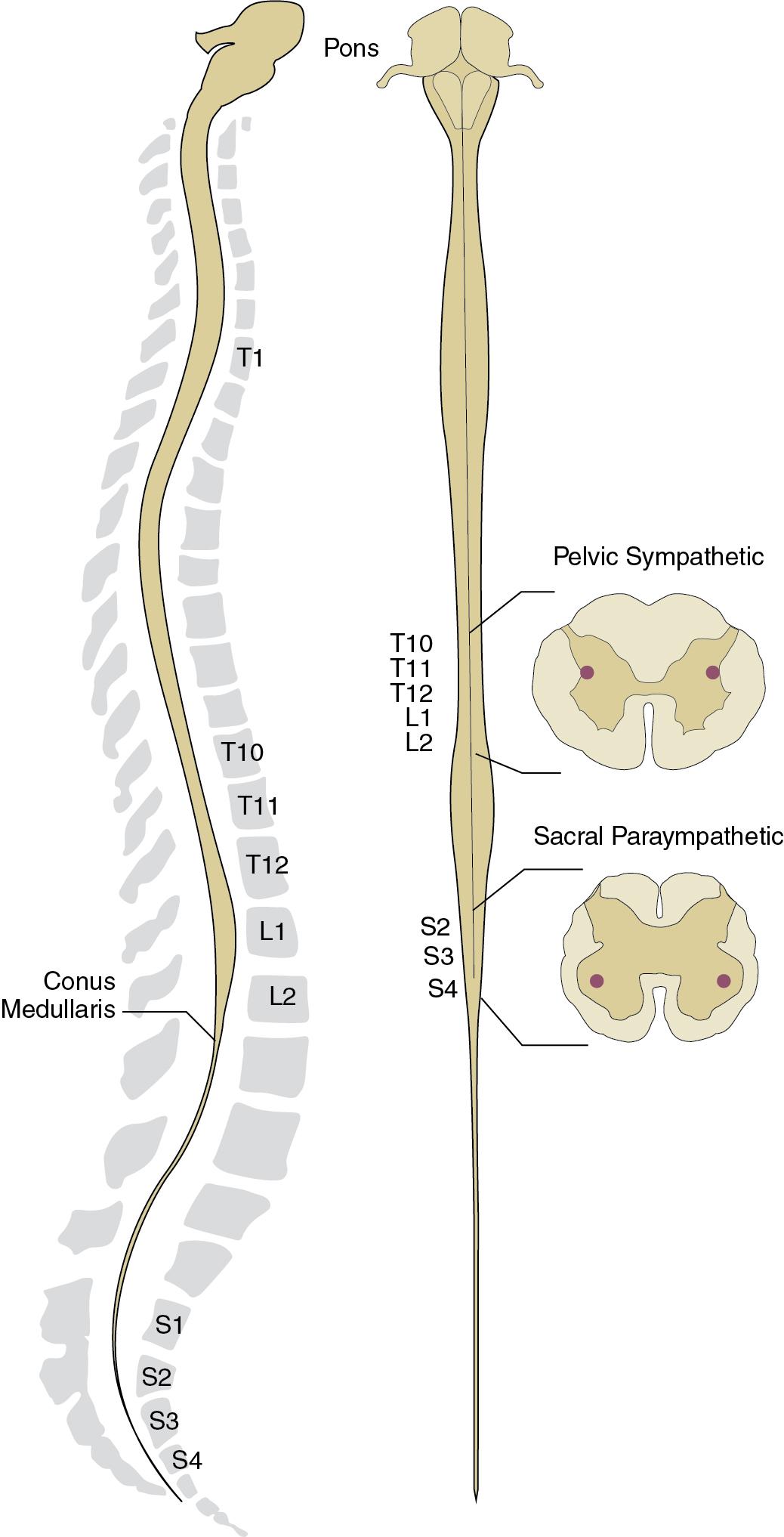
Pelvic parasympathetic system preganglionic fibers originate in spinal segments S2 through S4. The long preganglionic fibers travel through the pelvic nerve to join the hypogastric nerve in forming the pelvic plexus. These fibers continue to ganglia located within or very near the organs that they supply, thus having very short postganglionic fibers and much longer preganglionic fibers.
There are varying sizes and types of nerves. They are classified generally by size and myelination ( Table 3.1 ) The largest, myelinated, fast-conducting Aα sensory nerves convey touch, or proprioception, within skeletal muscles. The smallest, nonmyelinated C nerve fibers are slow-conducting nerves that convey pain and temperature on the sensory side or act as postganglionic autonomic nerves on the motor side. The bladder afferent nerves are largely C fibers at birth, until maturation changes the afferents to Aδ (lightly myelinated, small) fibers. It is now appreciated that bladder insults, such as obstruction, bladder inflammation, or spinal cord disease, affecting pathways involved in lower urinary tract function can lead to neuroplastic changes in which some afferents again become C fibers as the bladder’s response to the insults. Understanding the anatomy and physiology of the basic reflex pathways and central voluntary control involved in lower urinary tract function is necessary but appreciating the dynamic ability of the neurons to modify these pathways will provide important insights into evaluation of novel therapies.
| Fiber Type | Location | Function | Role in Normal Bladder Function |
|---|---|---|---|
| Aα (myelinated) | Skeletal muscle | Proprioception | None |
| Aβ (myelinated) | Cutaneous | Mechanoreceptor | None |
| Aδ (finely myelinated axons) | Smooth muscle (detrusor) | Touch/pressure | Bladder fullness and wall tension |
| C (unmyelinated axons) | Mucosa (urothelium) | Nociception | Nociception |
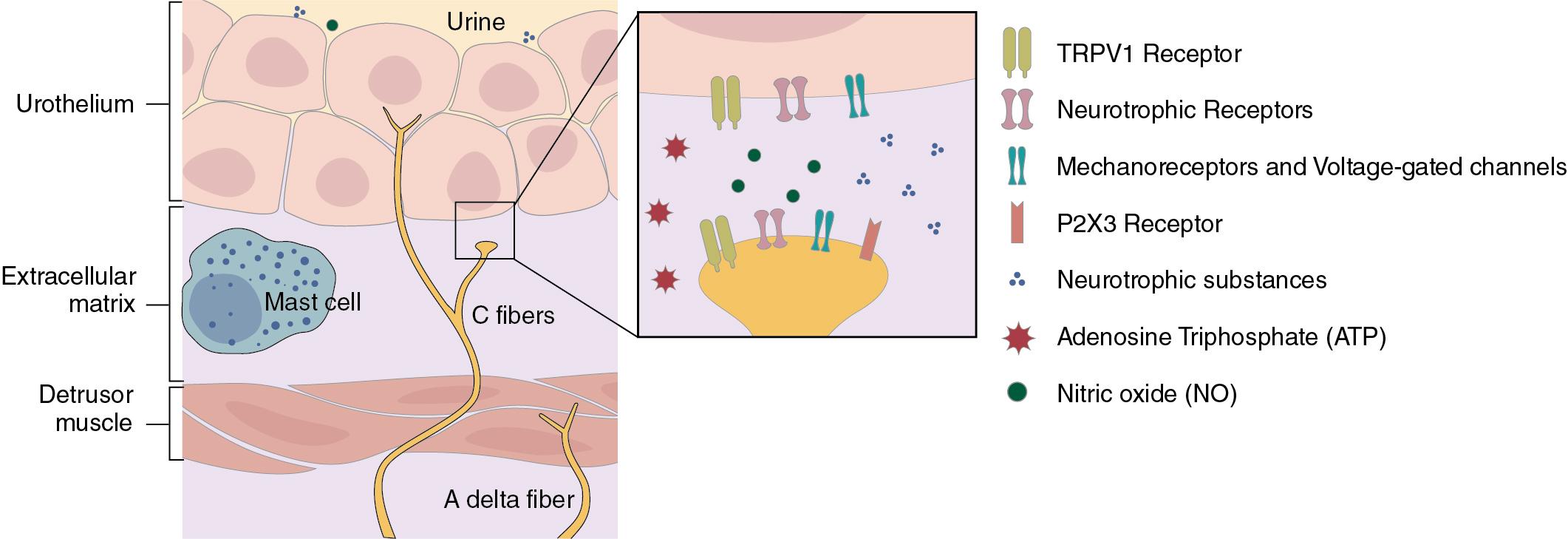
The sensory innervation of the lower urinary tract is intricate and complex. A urothelial and suburothelial network has been described. Interactions involve cell-to-cell communication as well as neural pathways. The sensory nerves are denser in the urethra and trigone and sparser in the dome of the bladder. The afferent axons join their respective efferent nerves in the paths for the autonomic and somatic neural pathways. Controversy exists concerning where the major afferent supply travels. Some claim that the majority of afferent nerves travel with the hypogastric nerve to the thoracolumbar region of the spinal cord, while others state that they travel through the pelvic nerve and enter at the sacral levels. A specific nucleus, Gert’s nucleus, is located ventrolateral to the dorsal horn cells of S1 to S2 and receives Aδ input from the bladder. Ascending projections from here reach to the periaqueductal gray (PAG) area in the mesencephalon. Both Aδ and unmyelinated C fibers provide the majority of this sensory innervation. Detrusor proprioceptive endings (Aδ fibers) exist as nerve endings in collagen bundles. They are stimulated by stretch or contraction and are responsible for the feeling of bladder fullness. Pain and temperature nerve endings (C fibers) are free in bladder mucosa and submucosa.
Afferent nerves are influenced by transient receptor potentials (TRPs), transmembrane cation channels that influence cytosolic ion concentration, mainly calcium and magnesium, as well as affecting other intracellular pathways. These channels have been divided into six subfamilies, with each subfamily having several unique channels. These channels are affected by many different stimuli (both chemical and physical) and play a major role in afferent activity, including detection and integration of noxious stimuli. They achieve this by a dual mechanism whereby their intracellular actions (cation concentrations) are combined with a second mechanism of influencing neurotransmitter release. Activation of C fibers plays a major role in bladder inflammation and overactivity and in conditions where C fiber neuroplastic changes have taken place. Purinergic receptor stimulation (chemical, C fiber) in an animal model enhances the spinal neuronal activity already seen with intravesical filling (Aδ physical stretch), and intravesical adenosine triphosphate (ATP) has been shown to stimulate detrusor overactivity. Purinergic signaling is also involved in the voiding reflex, as studies have shown that altered purinergic receptor expression is found in patients with detrusor overactivity.
Other neurotransmitters involved with bladder sensation include ATP, adenosine, nitric oxide (NO), vasoactive intestinal polypeptide, substance P, and pituitary adenylate cyclase–activating peptide. Some of these act simply as neurotransmitters, but others like NO are also released from the urothelium and act as modulators and messengers. The complex interactions of these neurotransmitters are still being investigated, but their role in conditions involving bladder pain may help influence future therapies ( Fig. 3.2 ).
CNS neurons affecting bladder function may be spinal or supraspinal, with extensive dendritic communications. The chief excitatory neurotransmitter in the CNS is glutamate, frequently acting on n -methyl-d-aspartate receptors. The chief inhibitory CNS neurotransmitters are γ-aminobutyric acid (GABA) and glycine.
The detrusor and the periurethral striated muscle mechanisms have separate cortical and other higher-center regulation pathways. The higher centers chiefly affect the brainstem for the detrusor, whereas they act on the sacral cord for the periurethral mechanisms. The brain pathways known to be associated with bladder and pelvic floor activity include cortical pathways originating in the precentral gyrus, lateral prefrontal cortex, and anterior cingulate gyrus (ACG). Subcortical pathways originate in basal ganglia, brainstem raphe nuclei, locus ceruleus, hypothalamus, and the midbrain PAG, and affect the brainstem, specifically the pontine micturition center (PMC), and lateral pons. Extensive communication takes place with all these structures to control storage and voiding.
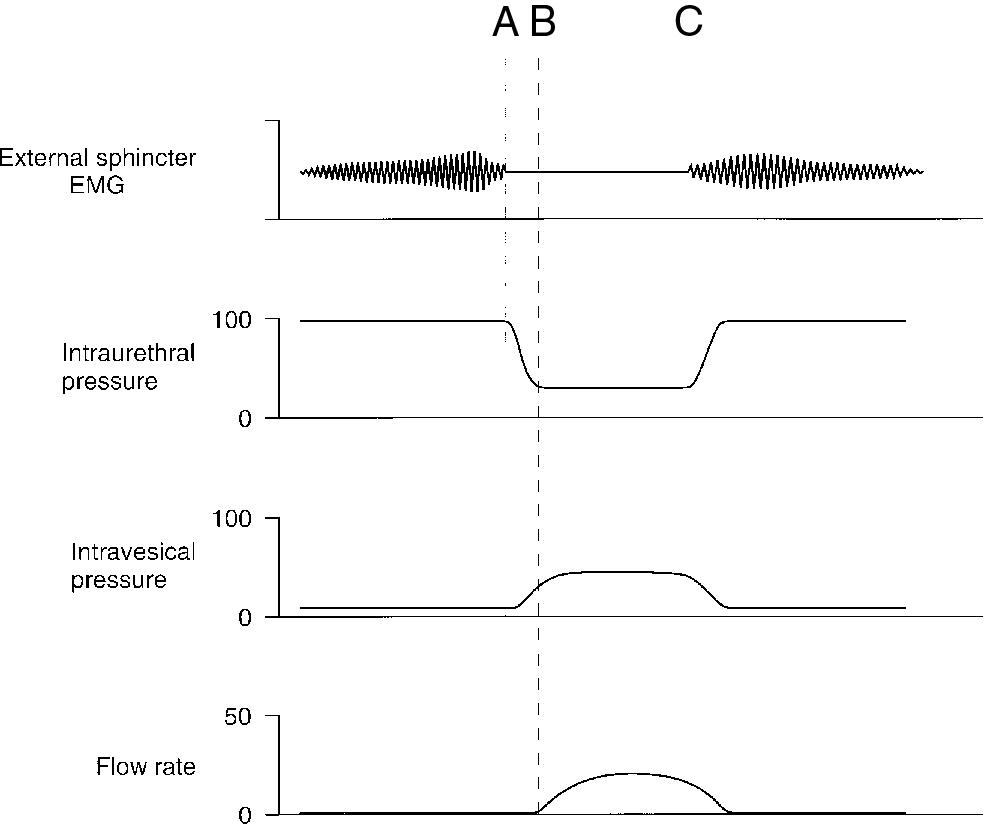
The brainstem’s importance in lower urinary tract function has been known since 1921, when Barrington ablated this area in cats and produced permanent urinary retention. He demonstrated that the middle pons was the brain level from which bladder motor tone arises. This region in the pons has been called the pontine micturition center or the M region. Stimulation results in a decrease in urethral pressure and silence of pelvic floor electromyographic signal, followed by a rise in detrusor pressure ( Fig. 3.3 ). Tracing studies reveal direct projections from the M region to the intermediolateral cell column of the sacral cord and the parasympathetic preganglionic bladder motor neurons. Other projections are to sacral cord interneurons that activate GABA inhibition of Onuf’s nucleus neurons, resulting in relaxation of urethral skeletal muscle. The detrusor motor nuclei in the pons receive input from basal ganglia and coordinating afferents from the cerebellum and the PAG. The PAG then communicates with the M region to stimulate micturition. In addition to these important supraspinal tracts, there is evidence that spinal reflexes may also facilitate voiding. Electrical stimulation of urethral afferents can stimulate detrusor activity in spinal cord patients. This reflex has been postulated to augment bladder emptying by being activated when urine enters the urethra. Table 3.2 summarizes several important regions in the CNS involved in urine storage and voiding.
| Structure | Location/Function |
|---|---|
| PAG |
|
|
|
|
|
| PMC |
|
|
|
|
|
| Onuf’s nucleus |
|
|
|
|
|
| Gert’s nucleus |
|
|
|
|
|
| Anterior cingulate gyrus/cortex |
|
|
Stimulation of the same level of the pons at a more lateral position, the L region, results in contraction of the urethral sphincter by fibers originating from Onuf’s nucleus (neuronal cell bodies for the urethral sphincter and other pelvic floor muscles located in the lateral aspect of the anterior horn of the gray matter of the sacral spinal cord from S2 to S4). Direct cortical relay to the L region gives voluntary micturition control. These brainstem activities, which are important for continence (L region) and micturition (M region) in adults, are replaced by reemerging pathologic primitive reflexes in disease states. Most important in this respect is the C fiber–mediated reflex that emerges following disconnection from pontine regulatory influences as a consequence of spinal cord disease. These effects are mediated by sensory neurotransmitters that are prompted to appear by nerve growth factors. Similar development of C fiber–afferentation is seen with bladder outlet obstruction and with bladder inflammatory states. In addition, with spinal cord injury it appears that the C fibers become sensitized to mechanical distension, a role reserved for Aδ fibers in the normal bladder. This leads to further activation of primitive, ineffective voiding reflexes, which are also modulated by TRP channels acting on the sensory side.
The basal ganglia are associated with the production of dopamine, one of the catecholamine neurotransmitters that is largely inhibitory to bladder activity. Nearly 75% of patients with decreased dopamine resulting from Parkinson disease have slowness of movement, gait disturbance, and tremor, as well as bladder overactivity. Serotonin (5-HT; from the raphe nuclei) acts to inhibit reflex bladder and pelvic nerve activity by suppressing afferent bladder information. The sympathetic autonomic nuclei and the sphincter motor nuclei also receive a serotonergic input from the raphe nucleus. Fibers from the raphe nuclei of the reticular formation may moderate responsiveness to different phases of the sleep–wake cycle or emotional states.
The locus ceruleus is the brainstem’s CNS container of norepinephrine cell bodies. Norepinephrine acts to tonically facilitate continence-related reflexes.
The hypothalamus and midbrain PAG are activated during micturition. The anterior hypothalamus and PAG project to the pons M region and can induce bladder contraction via pontine parasympathetic pathways, whereas the posterior hypothalamus has sympathetic inhibitory pathways. The hypothalamus function, although poorly delineated, is known to involve β-endorphin neurotransmitters. Interneurons in the lumbosacral cord project strongly to the PAG, which in turn projects to the pons M region, thus making the PAG an important component in bladder reflex activities.
Pudendal cortical pathways affect periurethral striated muscle innervation by direct descending paths. At this level, the pudendal motor nuclei act as described to affect lower urinary tract skeletal muscle activity.
The limbic system in the temporal lobes exerts controls affecting all autonomic functions and is a favored site for epileptiform activity. Enkephalin is a notable neurotransmitter here as well as in the reticular formation. The cerebellum, where GABA is prominent along with standard neurotransmitters, regulates muscle tone and coordinates movement. Disease in this area produces spontaneous, high-amplitude detrusor overactivity.
The ACG and the prefrontal cortex have rich communications with the brainstem areas concerned with lower urinary tract function. Imaging studies show increased activity of the ACG during filling and voiding. It projects heavily to the PAG, which in turn connects to the M region in the pons. Depending on the area affected within the ACG, lesions may lead to bladder overactivity. The prefrontal cortex is thought to play a major role in volitional voiding and deciding the social appropriateness of voiding. Lesions here lead to inappropriate bladder overactivity and voiding.
There has been increased interest in neural circuits, as opposed to isolated centers of cortical brain activity, as they relate to the process of bladder filling, storage, and voiding. This has led to a number of studies intended to better characterize the connectivity to support the integrity of these circuits as they relate to bladder function. Functional MRI studies in continent women demonstrate activation of a large number of brain regions that change significantly between full versus empty bladder in regions including the medial frontal gyrus, posterior cingulate, inferolateral temporal and postcentral gyrus, amygdala, and caudate. In women with incontinence, very few regions show different levels of activation when the bladder is full versus when it is empty. The changes in functional connectivity when the bladder is full suggest that there is a central process responsible for control in the full bladder state, and that this relies on how brain systems are functionally integrated.
By adolescence, disparity in growth of the spinal cord and the vertebral column leads to the cord terminating around the first lumbar vertebra ( Fig. 3.1 ). The adult conus medullaris is quite short and contains the entire S1 to S5 segment. Because the spinal cord terminates well above the respective segmental foramina, the cauda equina describes the terminal ventral and dorsal nerve roots as they travel through the spinal canal to exit. The cauda equina is subject to various spinal pathologies, including lumbar disc disease, trauma, tethered cord, and spinal stenosis. Although the thoracolumbar levels are important in sympathetic autonomic influence of the lower urinary tract, the conus medullaris has greater significance, because autonomic detrusor nuclei and pudendal somatic nuclei are housed in the intermediolateral and ventromedial anterior gray matter, respectively. The conus medullaris also houses neurons involved with defecation and sexual function, with relays for cortical separation of these visceral functions (encephalization) developing after birth.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here