Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This study was supported in part by USPHS NIH Grants RO1 DK68055 (AEB) from the National Institutes of Health.
The human colon absorbs water and electrolytes and stores contents until elimination is socially convenient. It also scavenges nutrients produced by bacterial metabolism of undigested carbohydrates. The colon works slowly, taking an average of 65 h to transfer contents from the cecum to the rectum. However, transit can be much slower, without causing any symptoms. Disturbances of colonic motility and sensation, which primarily manifest as constipation, diarrhea, and abdominal discomfort in patients with disorders of function [e.g., irritable bowel syndrome (IBS)], are major reasons for physician visits. These common disorders can be debilitating and last for years. In addition, colonic sensorimotor dysfunctions also occur in organic disorders such as inflammatory bowel disease.
This medical need has stimulated considerable research into normal and disordered colonic functions. Despite this, our understanding of many conditions remains rudimentary. Treatment is often suboptimal because of the complexities. Colonic motility is characterized by patterned contractions of longitudinal and circular muscle layers at each independent point along the organ. Moreover, contractions can influence pressure and flow at some distance. The machinery that drives motility is distributed along the length of the tubular organ. In addition to multiple enteric cell types [e.g., neurons, glia, interstitial cells of Cajal (ICC), smooth muscle cells, enteroendocrine cells, resident white blood cells, and fibroblast-like cells], the extrinsic nervous system significantly affects colonic motility. Muscle contractions act on a non-Newtonian material that changes its fluid content and viscosity as it is propelled along the bowel. Motility is also closely integrated with colonic secretion and absorption. Motility also varies considerably between different parts of the large bowel and there are significant interspecies differences. Finally, the techniques used to measure colonic motility can affect motility themselves and only detect some of the components of colonic motility. These factors confer formidable mechanical complexity to colonic activity; the cardiovascular system, with its four-chambered remote pump and modestly contractile conduits seems relatively simpler by comparison.
Over recent decades, excellent techniques have been developed to analyze receptor function, ion channels, and second messenger pathways in enteric cells. However, it has proved difficult to understand how these components are integrated to generate motor patterns. While we understand many mechanisms of smooth muscle contraction, their interactions with mechanically activated enteric neuronal circuits are still largely a mystery. Smooth muscle action potentials are readily recorded, but their relationship to the mechanics of mixing and propulsion are still obscure. Relating patterns of contraction to their fluid-mechanical consequences is often a matter of speculation or guesswork.
Here, we review our current understanding of colonic motility and, where possible, relate the gross anatomy, coordinated motor patterns, and propulsion of material to their underlying cellular and molecular mechanisms. We will speculate on how gaps between different approaches may be bridged. Because the colon varies so much in its structure between different species and because there are species differences in the effects of mediators, this chapter will focus, where possible, on colonic function in humans. For example, 5-HT 3 receptor antagonists modulate peristaltic reflex activity in in response to mucosal stroking in guinea-pig intestinal preparations, but not in the human gut. Enteric neuronal recordings reveal distinctive features of human enteric neurons, such as the potent postsynaptic excitatory action of histamine through H 3 receptors or the prominent role of protease-activated receptor 1. However, studies in dogs and other large animals have provided valuable data that have not yet been replicated in humans. Furthermore, studies on small laboratory animals, particularly at the cellular and molecular level, have characterized fundamental mechanisms that may also be very germane to human motility.
Patterns of smooth muscle contraction and relaxation facilitate the storage, mixing, and propulsion of content. The muscle cells are the final common effectors in this system. Motility can be monitored either by direct measurement of muscle activity or by indirect measurements (e.g., intraluminal pressure or propulsion). Direct measurements include electrical recordings of membrane potential (e.g., intracellular or whole-cell clamp recording) or extracellular recordings of membrane-associated currents (e.g., action potentials). Intracellular calcium or membrane potential can be measured in vitro using calcium- or voltage-sensitive dyes. In addition, force can be measured with isometric transducers and shortening can be recorded with isotonic transducers or with computerized spatiotemporal mapping. These measures may be supplemented by recordings of intraluminal impedance, which reflect the distribution of content. Finally, a major limitation to understanding visceral afferent processing was overcome by studies demonstrating direct electrophysiological recordings from human visceral afferents ex vivo.
In humans, intraluminal pressure changes can be recorded at multiple sites using perfused tube manometry, solid-state pressure transducers or, more recently, with fiber-Bragg grating based, high-resolution fiber optic catheters. A barostat can measure tone, mechanical properties (i.e., pressure/volume relationships) at a single point and correlate these with perceptions (e.g., of distention). Propulsion or transit of intraluminal content can be measured either ex vivo or in vivo (i.e., with dye-marked content, radioopaque beads, or scintigraphy).
These techniques all have their strengths and limitations. Some must be used in isolated preparations while others are usable both in vitro and in vivo. Spatial and temporal resolution are variable. Some are invasive and modify normal physiology. The great challenge for students of colonic function is to integrate observations from these different techniques into a single conceptual whole. This attempt toward that ambitious goal starts with a brief summary of the anatomy of the colon and then describes the effector cells (i.e., smooth muscle), and intrinsic and extrinsic neural circuitry. Thereafter, we characterize major motor patterns, concentrating on human colonic motor patterns, adding material from studies in animals where necessary. Finally, the endogenous and exogenous factors that modulate motor patterns in normal physiology and major colonic sensorimotor disorders will be outlined.
In adult cadavers, the colon is approximately 1.5 m long. Anatomically, different regions can be distinguished; the cecum; ascending, transverse, descending, and sigmoid colons; and the rectum, which lies between the rectosigmoid junction and the anal canal. There are interregional differences between the right and left colon, which are derived from the embryological mid- and hindguts, respectively. Their junction is between the right two-thirds and left third of the colon. The right colon is supplied by the vagus nerve, superior mesenteric vessels, and superior mesenteric ganglion. In contrast, the left colon is largely supplied by pelvic nerves from sacral S 2-4 segments, inferior mesenteric vessels, and from the inferior mesenteric ganglion (IMG). The ascending and transverse colons are relatively compliant and well suited to act as a reservoir. In contrast, the descending and sigmoid colons are less compliant and largely function as a conduit, although sometime material may remain in these distal regions for extended periods.
The external smooth muscle of the colon consists of an outer longitudinal and inner circular layer. The circular layer comprises thick bundles of cells, separated by connective tissue septa. In most mammals, the longitudinal muscle layer forms a thin uniform layer. In humans, other primates, horses, guinea pigs, and rabbits the longitudinal muscle in some colonic regions is gathered into thicker taeniae, separated by thinner intertaenial muscle. In the human descending colon, the taeniae thicken and in the sigmoid colon, the longitudinal fibers become more scattered. At the rectum, bundles of longitudinal muscle spread out to encircle the gut, but with thicker anterior and posterior bands. In the anal canal, the longitudinal muscle layer merges with the striated muscle of the external anal sphincter while the circular muscle layer extends into the smooth muscle of the internal anal sphincter.
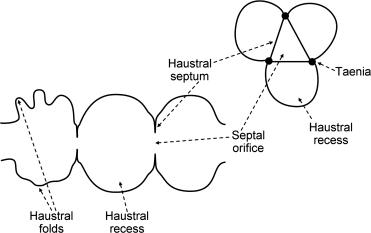
In much of the human colon, the circular smooth musculature forms arches spanning the taeniae, rather than circular rings. Thus, the colon has a triangular cross section with taeniae at the corners ( Fig. 23.1 ). The taenia coli can be considered to function as cables upon, which arcs of circular muscle are suspended, facilitating efficient contraction of the circular muscle. Thus, a 17% contraction of circular muscle reduces luminal diameter of the colon by 59%. If the longitudinal muscles were of uniform thickness, a 17% contraction of circular muscle would only reduce luminal diameter by 31%. Because the longitudinal smooth muscle is much thinner between taeniae, the intertaenial colonic wall is puckered and thrown into sacculations called haustrations. The haustral septae are not fixed structures; they are sustained contractions of circular muscle, which move slowly. Myogenic activity is not sufficient to explain haustra; neural input contributes too. Haustra move, disappear, and reform during mass propulsion of colonic contents. It has been suggested that haustral out-pouchings may have slower flow than the central lumen and may facilitate colonic fermentation and absorption of water and electrolytes. Changes in the septal orifices may regulate flow between segments. Relaxation of longitudinal muscle and circular muscle layers allows the colon to accommodate content, facilitating storage. It has been suggested that the longitudinal and circular muscle layers are reciprocally innervated, so that contraction of the circular muscle layer is always associated with inhibition of the longitudinal muscle layer. However, such associations may be better explained by mechanical interactions between the two layers of muscle. When the circular muscle shortens, elongation of the segment of intestine must occur to preserve the volume of the tissue making up the colonic wall. It has also been shown that longitudinal and circular muscles can contract synchronously during peristaltic activity; however, the extent to which they contract synchronously or out of phase during other motor patterns is less certain.
Neurotransmission from active enteric excitatory and inhibitory motor neurons sums with myogenic mechanisms to regulate the excitability and contractility of smooth muscle cells, as in most regions of gut. Resting membrane potential which averages approximately − 50 mV in the colon is not uniform across the thickness of the circular muscle layer, but rather is characterized by a gradient, being up to 36 mV more hyperpolarized in the inner circular smooth muscle region than at the myenteric border. This gradient, which is generated by carbon monoxide, causes a graded contractile response to excitatory inputs, so that weak excitation recruits only the more depolarized smooth muscle and stronger input recruits more hyperpolarized smooth muscle. Studies in heme oxygenase-2 knockout (HO-2-KO) mice, which lack the constitutive form of heme oxygenase, suggest that in the colon the gradient is maintained by carbon monoxide derived from submucosal neurons.
Resting membrane potential is determined by multiple types of K + channels, nonselective cation conductances, possibly mediated via TRP channels, an electrogenic sodium pump, and sodium channels, which all shift resting membrane potential away from the equilibrium potential of K + ions. When muscle is hyperpolarized by neurotransmitters, slow waves reach lower levels of depolarization, evoke fewer Ca 2 + action potentials, less Ca 2 + entry and less forceful contractions. In contrast, excitatory transmitters activate inward currents, depolarize membrane potential, and increase peak depolarization of slow waves. At very depolarized potentials, continuous, low probability opening of Ca 2 + channels (window currents) can occur, producing continuous modest influx of Ca 2 + . Calcium influx via voltage-dependent calcium channels contributes to excitation-contraction coupling and possibly other Ca 2 + -sensitive cell signaling pathways. Both L- and T-type calcium channels are involved. [While CaV1.2 channels are important in excitation–contraction coupling, gastrointestinal (GI) smooth muscle cells express additional types of voltage-dependent calcium channels, as detailed elsewhere in this volume.]
Three major types of myogenic smooth muscle electrical activity primarily contribute to the patterning of motility in the human colon: (i) slow-wave activity with a mean frequency of 2–4 contractions/min, but with intermittent longer duration myogenic potentials; (ii) prepotential oscillations similar to membrane potential oscillations (MPO) in canine colonic muscles; and (iii) action potentials superimposed upon slow waves.
In the human colon, electrical slow waves, which average 12 mV in amplitude, cause Ca 2 + entry by activating voltage-dependent dihydropyridine-sensitive (L-type) Ca 2 + channels; Ca 2 + entry induces electromechanical coupling and segmental contractions. When action potentials are superimposed upon slow waves, Ca 2 + entry is greatly augmented. Between slow waves, the open probability for Ca 2 + channels is low, so action potentials and powerful muscle contractions do not occur. In older studies, it was suggested that contractions were never evoked by slow waves alone; action potentials were always required. However, it is now clear that slow waves in the colon may trigger sufficient Ca 2 + influx to activate the contractile apparatus causing small contractile events that may not have been detected by the strain gauge transducers used in older studies.
Calcium entering smooth muscle cells via voltage-dependent calcium channels leads to phosphorylation of the myosin light chains in the contractile apparatus, which triggers cross bridge cycling and contraction. Phosphorylation is activated when intracellular [Ca 2 + ] rises, primarily via calcium/calmodulin activation of myosin light chain kinase and/or inhibition of myosin light chain phosphatase. However, other kinases, including Rho kinase, can also phosphorylate myosin light chains in a calcium-independent manner and may regulate the calcium sensitivity of the contractile apparatus. Intracellular calcium levels are primarily determined by opening of voltage-dependent calcium channels, especially CaV1.2, other channels may also contribute, including stretch-sensitive nonselective cation channels and channels coupled to G-protein coupled receptors. Influx of Ca 2 + from extracellular fluid may be supplemented by the release of calcium from inositol trisphosphate receptor-coupled intracellular stores, via G q/11 . This pathways is activated via excitatory neurotansmitters from enteric motor neurons, including acetylcholine acting via muscarinic receptors and tachykinins acting on neurokinin receptors.
In 1911, Ramon y Cajal originally described the cells that now bear his name and speculated that they might modify smooth muscle contraction. However, the contribution of ICC to normal GI motility was uncovered much later by two seminal findings. First, neonatal BALB/c mice injected peritoneally with a monoclonal antibody ACK2 lost ICC and developed lethal intestinal dilatation and hypomotility. ACK2 is a neutralizing antibody to Kit, a type 3 receptor tyrosine kinase which is expressed on ICC. Second, mice with spontaneous mutations in the Kit gene resulting in the loss of function in one allele and decreased function in the other (W/W v mice) lacked intestinal electrical slow waves and myenteric small intestinal ICC, supporting the concept that ICCs were responsible for generating slow waves.
By contrast to smooth muscle, ICC have multiple processes and typically few thick filaments. They regulate gut motility via several mechanisms. They generate electrical slow waves that then propagate through smooth muscle cells via gap junctions (reviewed in Ref. ); they influence the smooth muscle membrane potential and membrane potential gradient ; and they mediate some of the mechanosensitivity of smooth muscle. It has also been suggested that they play an important role in mediating neurotransmission from axons of enteric motor neurons to the smooth muscle although this has recently been questioned.
In addition to ICC, other classes of interstitial cells include ICC-like (ICC-LI) cells, fibroblast-like platelet-derived growth factor receptor-α (PDGFRα +) cells, fibroblast-like cells, and teleocytes. In several species, including primates, ICC, and PDGFRα + form gap junctions with smooth muscle cells and function as a multicellular syncytium referred to as the SIP syncytium. Indeed, nerve varicosities form more junctional connections with ICC than with smooth muscle. Electrical coupling ICC and PDGFRα + also express a variety of receptors for neurotransmitters, hormones, paracrine substances, and inflammatory mediators.
In large animals, several types of ICC in the colon are located between the layers of longitudinal and circular muscle layers, in the plane of the myenteric plexus (MP) (ICC MY , also referred to as ICC AP ) and in the submucosa adjacent to the circular muscle (submuscular ICC, ICC SM , which may also be referred to as submucosal ICC). ICC are also found within the muscle layers (ICC IM ) and a few can be found along the serosa (serosal ICC). In large animals, such as human and dog, a subclass of ICC IM is also found in the septa (septal ICC) that separate bundles of circular muscle cells. ICC MY and ICC SM form extensive networks along the colon and are electrically coupled to one another and to the smooth muscle cells by gap junctions. They also make close appositions and synapse-like contacts with varicose axons of enteric motor neurons ( Fig. 23.2 ). A recent study has suggested the existence of another class of ICC distinguished by close associations with microvessels in several layers of the human colon.
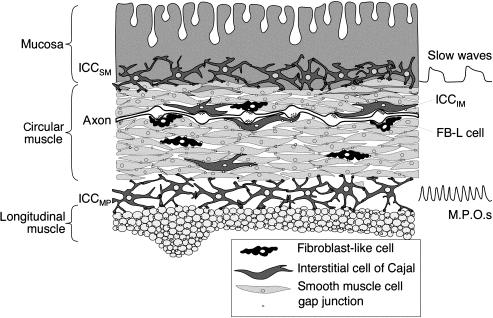
Enteric motor neurons innervate ICC, can influence slow-wave frequency, and influence ICC excitability. In the canine and feline colon, the major slow-wave activity and predominant contractile rhythm (2–4/min) recorded in vitro are associated with the intact submucosal border and hence ICC SM . Similarly, slow-wave pacemaker activity in human colon originates at the submucosal border. At the myenteric border, associated with ICC MY are the pacemakers for the small, rapid (12–20/min) oscillations in membrane potential (MPOs) of longitudinal and circular smooth muscle layers in dog and human. Slow waves and MPOs summate in the central region of circular muscle producing a complex pattern of activity that regulates contractile amplitude and frequency. These cyclic depolarizations then produce action potentials in smooth muscle cells especially when excitatory motor neurons are active ( Fig. 23.3 ).
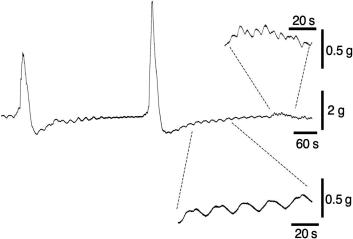
The rat colon also has several pacemakers albeit organized differently compared to associated humans and larger mammals, such as dogs. The submucosal border is associated with slow-wave activity occurring at around 14/min. The myenteric border is associated with very slow complexes, occurring at a mean frequency below 1/min. These slow depolarizations evoke bursts of smooth muscle action potentials at high frequency and corresponding, large-amplitude prolonged contractions. They are not blocked by tetrodotoxin (which blocks neuronal activity) but are blocked by nifedipine, consistent with their action potentials playing a key role in excitation-contraction coupling. These slow complexes may be related to colonic migrating motor complexes (CMMCs) of rodent colon, although the latter require intact neuronal activity. Alternatively, they may be the rodent equivalent of the long-duration myogenic contractions reported in the canine colon.
ICC-generated slow waves are triggered by pacemaker currents, which evolve into a rapid upstroke when they exceed a depolarization threshold. This rapid upstroke is insensitive to dihydropyridines, which block L-type calcium channels, thereby distinguishing them from smooth muscle action potentials, which are predominantly mediated by L-type calcium channels. The upstroke potential is followed by a plateau potential that is also generated by ICC. While smooth muscle cells can modify the shape of slow waves propagated from ICCs, they do not generate de novo slow waves because they do not have a pacemaker channel. Slow waves from ICCs spread into smooth muscle via gap junctions and activate a variety of smooth muscle ion channels, including L-type calcium channels, contributing powerfully to the contractile response. A Ca 2 + -activated chloride conductance, attributable to the ANO1 channel, plays a key role as a pacemaker current in ICCs. Genetic deactivation of ANO1 abolished slow-wave activity.
Structural and functional evidence suggests that cholinergic and nitrergic neurotransmission involves ICC IM in the stomach and ICC DMP in the small intestine. In the colon, close appositions between motor neuron axons and interstitial cells have been reported, suggesting a similar functional arrangement. However, recent analysis has raised doubts about the essential role of ICCs in neurotransmission ; this matter is yet to be settled. It appears that some of the tonic effects of nitric oxide may be mediated via pacemaker ICCs or intramuscular ICCs, although nitric oxide acutely released by enteric motor neuron terminals may not be so dependent on ICCs.
Distension of the colon activates inhibitory enteric reflexes, which cause accommodation of the colonic wall. With further distension, excitatory enteric reflexes are activated, leading to peristalsis. However, when all neural responses are blocked, gut smooth muscle still responds to stretch with depolarization and action potentials. Three mechanisms have been proposed that involve ICC. One is the physical transfer of mechanical information at specialized junctions known as “peg and socket junctions” in which the peg forms a narrow projection of part of the cytoplasm of ICC into smooth muscle cells (the socket). The second is the regulation of the slow wave by mechanical force through the production of prostaglandins, and the third is mechanosensitivity through shear-sensitive ion channels. Sodium channels, which have an established role in cardiac pacemaking, have also been discovered in ICC. A sodium current is expressed in human and dog intestinal circular smooth muscle and ICC but not in smaller animals. This current is mechanosensitive and is activated by membrane shear stress. It is carried by the channel Nav1.5, encoded by SCN5A. The window current of Nav1.5 overlaps with ICC membrane potentials observed during slow waves, suggesting a baseline flow of sodium through Nav1.5. Pharmacologic blockade of the sodium current decreases slow-wave frequency and hyperpolarizes smooth muscle. Stretch of intestinal muscle strips in neuronal blockers and nifedipine (to block L-type Ca 2 + channels) increases the slow-wave frequency. Mutations in SCN5A are associated with GI symptoms in patients with IBS.
In health, ICC in the human colon and other regions of the GI tract undergo complete turnover every few months. Hence, functional ICC networks are maintained by tightly regulated loss and replacement of ICC. Apoptosis and trans/dedifferentiation, contribute to ICC loss while repair from injury, and differentiation from ICC stem cell precursors replenish ICC. Adult ICC also divide. Factors that affect ICC survival in vitro and in vivo in other tissues include Kit-ligand (stem cell factor or steel factor), nitric oxide (mouse stomach), serotonin via the 5-hydroxytryptamine (5-HT)2B receptor (mouse jejunum), interleukin-9 (cultures), insulin and insulin-like growth factor (IGF)-1 signaling through stem cell factor (mouse stomach), and oxidative stress (mouse stomach).
ICC are reduced in several motility disorders, including slow transit constipation and chronic megacolon. However, the contribution of ICC loss to colonic sensorimotor dysfunctions is unclear since enteric neurons are also depleted in these conditions. It is not known how much damage ICC networks can withstand before functional abnormalities develop. The published literature is conflicting, perhaps because ICC cannot be reliably visualized in formalin-fixed, paraffin-embedded tissue. Human gut tissue requires careful handling because ICC are particularly susceptible to ischemia. In addition, there are limited quantitative data on ICC populations in normal human colon. Antibodies to Ano-1 may be particularly useful in future studies to identify ICC abnormalities since unlike Kit, Ano-1 is not expressed on mast cells.
Fibroblast-like cells were originally distinguished by features shared with true fibroblasts (e.g., abundant rough endoplasmic reticulum). Now, they can be precisely defined by specific labeling with antibodies for PDGFRα. These cells are intertwined with ICC and axons of excitatory and inhibitory neurons. These cells appear to mediate electrical components of purinergic inhibitory neurotransmission to GI smooth muscle from enteric inhibitory motor neurons. Activation of purinergic P2Y1 receptors on PDGFRα cells leads to opening of small conductance Ca 2 + -activated K + (SK3) channels, also expressed on the same cells. Electrical coupling via gap junctions then causes hyperpolarization and inhibition of smooth muscle cells. The role of PDGFRα cells in colonic motility is currently unknown. Due to the close coupling of smooth muscle cells, interstitial cells, and PDGFRα cells function together as the “SIP” syncytium (after the initial letter of the three cell types ). Recent data suggest interactions between these cell types since nitric oxide causes prejunctional inhibition of purinergic neurotransmitter release in the mouse and human colon
The enteric nervous system (ENS) contains sensory neurons, interneurons, and the motor neurons that provide input into the smooth muscle apparatus of the colon. This motor input interacts with myogenic mechanisms to create regional patterns of contraction and relaxation which mix and propel content. Sympathetic and parasympathetic neural pathways, which are modulated by inputs from the central nervous system, modulate colonic motility according to the broader needs of the organism. Extrinsic sensory pathways provide a continuous stream of information to the central nervous system about the mechanical and chemical state of the gut wall and luminal contents. 1
The ENS is the largest single division of the autonomic nervous system. The cranial and sacral parasympathetic innervation of human gut is mediated by a few tens of thousands of neurons; the sympathetic innervation of the gut amounts to tens of thousands to one hundred thousand neurons. It has been estimated that there may be between 200 and 600 million enteric neurons in the whole GI tract. The ENS directly controls important gut functions by innervating effector tissues, including the smooth muscle layers, epithelial cells and, to a lesser extent, intramural blood vessels, enteroendocrine cells, and lymphoid tissue. Sympathetic and parasympathetic neurons modulate gut functions largely via their inputs onto enteric neurons; though in some cases, they directly innervate effector tissues (see below).
The cell bodies of most enteric neurons are localized in two extensive networks: the MP (sandwiched between the circular and longitudinal muscle layers of the muscularis externa) and the submucous plexus which lies within the submucosal connective tissue. In the human colon, the MP consists of irregularly spaced stellate ganglia joined by thick interganglionic connectives. Each ganglion contains, on average, 70–80 nerve cell bodies and glial cells. In both myenteric and submucous plexuses, human ganglia contained more glia per neuron than in the intensively studied laboratory animal, the guinea pig. Together, the myenteric ganglia and connectives comprise the primary plexus ( Fig. 23.4 ). A secondary plexus (nonganglionated) consists of nerve trunks aligned with circular muscle bundles that innervate the muscle layer and penetrate through it, en route to the submucosa. A tertiary plexus is associated with the longitudinal muscle layer and contains axons of motor neurons that innervate it.
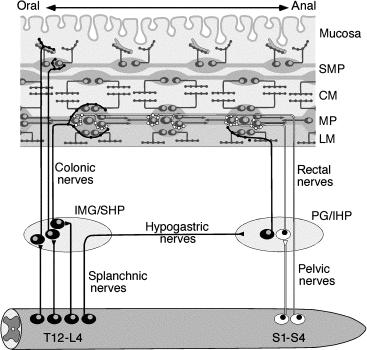
The submucous plexus in human colon actually consists of several distinct, though interconnected layers. These are referred to as Meissner’s plexus (referring to the innermost layer, closest to the mucosa) and Henle’s plexus or Schabadasch’s plexus (referring to the outermost layer nearest the circular muscle). Some authors have distinguished an intermediate plexus. These plexuses contain different populations of nerve cells that can be distinguished on the basis of their size, immunohistochemical coding, and projections. In humans and larger animals, the outer submucous plexus contains motor neurons that project to the external muscle layers and presumably control motility. In contrast, the inner submucosal plexus contains more neurons that project to the mucosa and likely regulates secretomotor and vasomotor activity. In most individuals, there are also a small population of “ectopic” nerve cell bodies in the mucosal plexus that lie between the muscularis mucosa and the glandular tissue; these have coding similar to neurons in the inner submucosal plexus.
Ideally, to understand how the human ENS controls colonic motility, we would have a comprehensive account of all classes of enteric neurons, their properties, connections and their activity during different behaviors. No such account currently exists. For the rather patchy and inconsistent data that are available, the reader is referred to extensive reviews and the present volume. Much of our current understanding of the innervation of the gut comes from the upper gut of laboratory animals; our understanding of colonic innervation in humans is limited. In addition to species differences, techniques vary between laboratories. Our ability to relate events at the cellular level to the real-time behavior of the whole organ is in its infancy although computer modeling can be valuable.
Enteric neurons belong to 15–20 different functional classes. The traditional division of nerve cells as sensory neurons, interneurons, or motor neurons may not be useful for the ENS. For example, neurons characterized by the presence of several long axons arising from the cell body are called the Dogiel-type II neuron. These cells are mechanosensitive and chemosensitive and have therefore been classified as “intrinsic primary afferent neurons” (IPANs). However, these neurons also function as interneurons, since they are potently excited by slow synaptic inputs, which can initiate long trains of action potentials. Moreover, the same cells partly mediate cholinergic secretomotor input to the mucosal epithelium. These cells are sensory, interneurons, and secretomotor neurons. Indeed, many enteric neurons interneurons and motor neurons have direct mechanosensitivity and this may be a widespread characteristic of enteric neurons.
Different classes of enteric neurons can be distinguished by combinations of morphology, histochemical, or immunohistochemical coding and electrophysiological characteristic. The first comprehensive classifications of all functional classes of enteric neurons were in the guinea pig small intestine. This was largely based on combinatorial immunohistochemistry, but has since been supplemented with information from electrophysiological recordings, intracellular dye fills and retrograde tracing studies. The classification scheme has proved very robust and still provides a benchmark for understanding the ENS. Many other studies have compared combinations of markers in enteric neurons without attempting to distinguish all functional classes. This can provide interesting insights. For example, cholinergic and nitrergic neurons account for nearly all myenteric neurons but the proportion of nitrergic neurons (as a percentage of all myenteric neurons is higher in the human colon than the human small intestine. The functional significance of such findings is yet to be established.
Final common effector neurons in the ENS innervate smooth muscle, blood vessels (vasomotor neurons), and epithelia (secretomotor neurons). Most relevant to the control of colonic motility are the excitatory and inhibitory motor neurons to the muscularis externa. The longitudinal and circular muscle layers are known to be innervated by separate populations of motor neurons in lab animals and this is also true of the human colon. Excitatory motor neurons in human colon are cholinergic (i.e., contain choline acetyltransferase immunoreactivity), and usually, but not always, contain tachykinins. The axons of circular muscle excitatory motor neurons typically project into the muscle layers close to their cell bodies or up to 11 mm oral to their cell bodies. Once in the muscle they then branch extensively over many millimeters around the circumference, with numerous varicose swelling that are sites of transmitter release. It appears that the majority of close appositions are with ICCs and PGDFRα cells, rather than with smooth muscle directly, at least in some parts of the GI tract. Many excitatory motor neurons also contain a variety of neuromodulators and transmitters including enkephalins and dynorphins. Inhibitory motor neurons to the circular muscle, on the other hand, generally project aborally, from 1 to 19 mm before their axons enter the muscle layers. They account for just under half the motor neurons. They branch extensively in the circular muscle and release a variety of neurochemicals that inhibit smooth muscle contractility. These include nitric oxide (or a related compound), one or more peptides including vasoactive intestinal polypeptide (VIP), pituitary adenylate-cyclase activating peptide (PACAP) and a purine that may be adenosine triphosphate (ATP) or nicotinamide adenine dinucleotide. Polarized projections of inhibitory and excitatory motor neurons to the circular muscle appear to be a common feature in the innervation of gut of many regions including the human colon.
The organization of longitudinal muscle is slightly different. In the guinea pig small intestine and colon, cholinergic excitatory motor neurons to longitudinal muscle generally have short local projections and do not show a preferential polarity, whereas inhibitory motor neurons to the longitudinal muscle of the colon preferentially project aborally before branching.
The polarized projections of excitatory and inhibitory motor neurons extend only over a distance of a few millimeters to a few centimeters, yet polarized reflex pathways in the intestine extend over much greater distances. This is probably because of the contribution of interneurons in enteric neural circuits. Ascending interneurons and descending interneuronal pathways have been identified, but there do not appear to be specialized circumferentially projecting interneurons. In the guinea pig small intestine, the single class of ascending interneuron has projections up to about 15 mm in length and contain immunohistochemical markers, which suggest that acetylcholine and tachykinins are their major transmitters. They make functional chains running up the gut by synapsing onto other interneurons of the same class. In this way, ascending reflexes activated by local stimuli spread far up the intestine beyond the projections of individual motor neurons or interneurons. In the guinea pig colon, chemical coding studies suggest that there may be several classes of ascending interneuron.
There are several different functional classes of descending interneurons. Descending interneurons often form long functional chains running down the gut, synapsing onto other neurons of the same class but contacting other enteric neurons too. In this way, reflexes may extend up and down the colon for distances greater than the individual neuronal components. To speculate, these interneuronal chains may also contribute to switching between patterns of motility, since activation of a single class of interneurons could then modify the activity of circuits over a considerable length of the bowel. Most, descending interneurons are likely to be cholinergic, but they can be divided into different classes on the basis of cotransmitters and modulators that they contain. In the guinea pig small intestine, at least four classes of descending interneuron have been distinguished by immunoreactivity for 5-hydroxytryptamine, somatostatin, and vasoactive intestinal polypeptide, with or with nitric oxide synthase and choline acetyltransferase. Similarly, in the human colon, descending interneurons are more numerous and have longer projections than ascending interneurons and there are at least three classes of interneurons characterized by choline acetyltransferase, nitric oxide synthase, and 5-HT. Compatible with these principles, a model has been proposed that rhythmic motor patterns in the colon may be substantially coordinated by activity in 5-HT-containing descending interneuronal pathways.
Since the earliest studies of reflexes in isolated preparations ex vivo, it was deduced there must be sensory nerves that can be activated by distension, stroking, or chemicals stimuli. Studies on isolated preparations (e.g., Refs. ) confirmed the existence of intrinsic sensory neurons. An important insights came with the finding that Dogiel type II neurons in the guinea pig small intestine (neurons distinguished by having multiple long processes arising from their cell bodies) are mechanosensitive and chemosensitive. Since then, the ion channels and conductances that are responsible for the distinctive long-after hyperpolarizations that follow action potentials in these cells have been characterized. Consistent with this, these neurons are also activated by mediators (including 5-hydroxytryptamine and adenosine triphosphate) released by mucosal enteroendocrine or enterochromaffin cells.
Dogiel type II enteric sensory neurons branch extensively in local enteric ganglia and make slow excitatory synaptic contact with most other classes of enteric neurons, including other Dogiel type II neurons and make fast synaptic contacts with other enteric neurons. Neurons with similar morphological and electrophysiological properties are also located in submucosal ganglia, although this population seems preferentially sensitive to mucosal mechanical stimulation. Dogiel type II neurons constitute about 30% of all myenteric neurons in the small intestine and a smaller proportion in the distal colon. The extensive excitatory interconnections between Dogiel type II neurons suggest that populations of these neurons may be activated together and that this could contribute to setting the threshold for activation of specific motor patterns. Direct evidence for such a role for Dogiel type II neurons has been provided by recording calcium transients in myenteric nerve cell bodies in murine colon during colonic migrating myoelectric complexes. In human small intestine, it was estimated that Dogiel type II neurons comprised about 10% of myenteric neurons, but comparable numbers for the human large intestine are not available.
Other classes of enteric neurons also appear to act as primary afferent neurons, capable of transducing mechanical stimuli. The circular and longitudinal muscle layers are both activated via neural pathways when the guinea pig colon is stretched circumferentially. However, during such stimuli Dogiel type II neurons are not significantly excited and therefore cannot provide the synaptic drive for the motor pattern. Instead, mechanosensory activity was detected in one or more classes of interneurons, which drive circuits via fast, rather than slow excitatory synaptic potentials. The possibility that uni-axonal neurons might be directly mechanosensitive has been directly confirmed. Enteric “viscerofugal” neurons have single axons, which innervate sympathetic neurons in prevertebral ganglia. These neurons show mechanosensitivity in the presence of low [Ca 2 + ], which blocks transmitter release, suggesting that they are not activated by other neurons. Direct activation has been demonstrated with application of mechanical stimuli to their cell bodies, which evokes bursts of action potentials. This idea has been extended by studies using potential-sensitive dyes to monitor electrical activity in enteric neurons during mechanical stimulation. Such studies have shown that a significant proportion of uniaxonal myenteric neurons, including probable interneurons and motor neurons, have direct, rapidly adapting responses to mechanical stimuli that persist when synaptic transmission is blocked. These neurons are found in both guinea pig and mouse colon, alongside slowly adapting mechanoreceptors that may correspond to Dogiel type II neurons.
Other classes of enteric neurons include secretomotor neurons and vasomotor (vasodilator) neurons that control epithelial secretion and blood vessel diameter. These types of neurons may supply collaterals that contact other enteric nerve cell bodies and potentially could interact with motility-generating circuits. Another class, the viscerofugal neurons play an important role in coordinating motility reflexes. These neurons project out of the gut and make synaptic contact onto sympathetic neurons in prevertebral ganglia. Here they provide cholinergic synaptic input to postganglionic sympathetic neurons involved in secretomotor and motility pathways, bypassing the central nervous system. These neurons function as interneurons as they receive prominent fast excitatory synaptic inputs and are directly mechanosensitive, primarily to local strain at their cell bodies. These neurons appear to be synaptically activated in parallel with motor circuits as they often fire bursts of action potentials before contractions. Viscerofugal neurons activate extrinsic reflexes that coordinate activity between different regions of gut such as the rectocolonic inhibitory reflex, which utilizes adrenergic sympathetic pathways.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here