Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
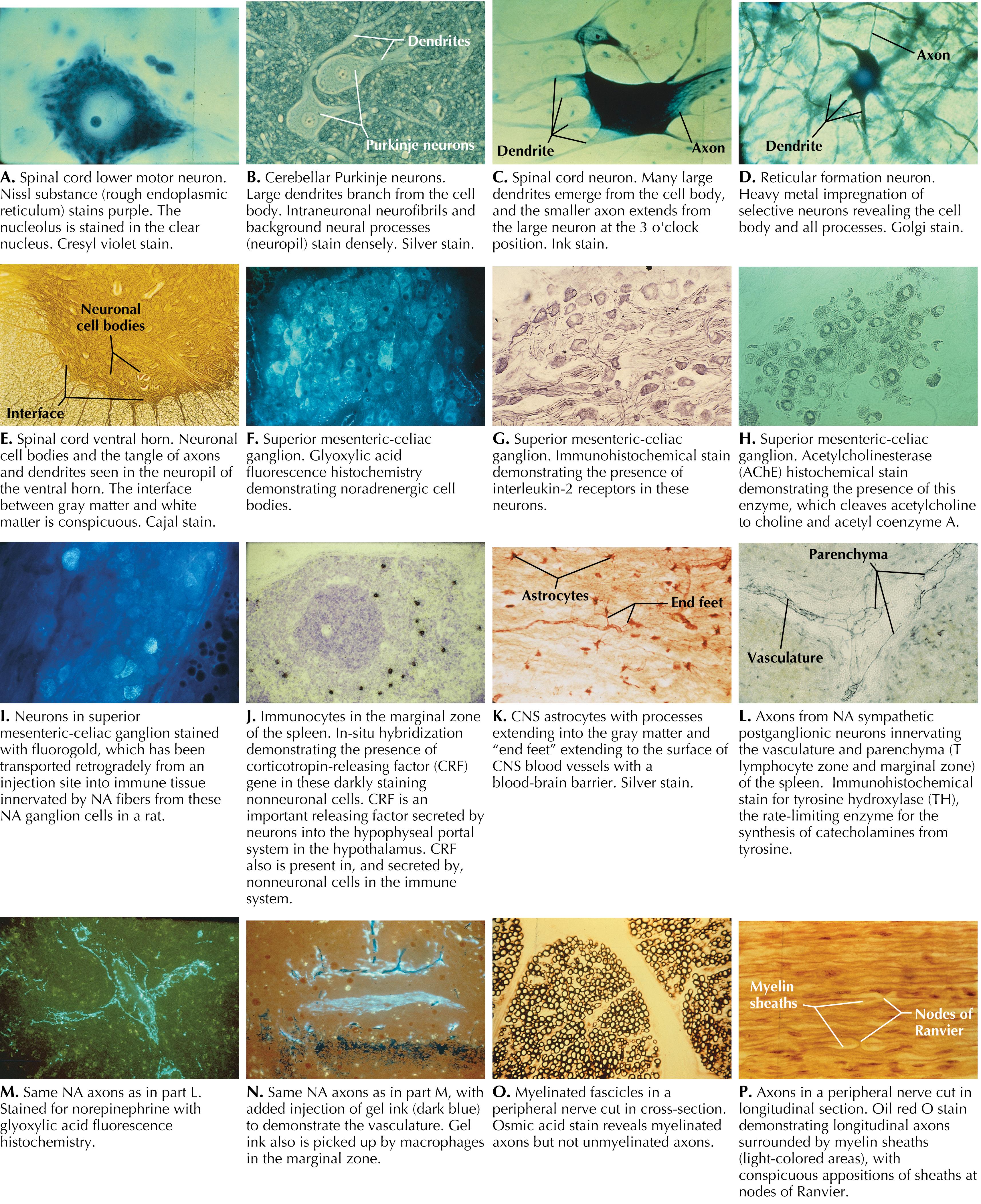
Neuronal structure reflects the functional characteristics of the individual neuron. Incoming information is projected to a neuron mainly through axonal terminations on the cell body and dendrites. These synapses are isolated and are protected by astrocytic processes. The dendrites usually make up the greatest surface area of the neuron. Some protrusions from dendritic branches (dendritic spines) are sites of specific axodendritic synapses. Each specific neuronal type has a characteristic dendritic branching pattern called the dendritic tree, or dendritic arborizations. The neuronal cell body varies from a few micrometers (μm) in diameter to more than 100 μm. The neuronal cytoplasm contains extensive rough endoplasmic reticulum (rough ER), reflecting the massive amount of protein synthesis necessary to maintain the neuron and its processes. The Golgi apparatus is involved in packaging potential signal molecules for transport and release. Large numbers of mitochondria are necessary to meet the huge energy demands of neurons, particularly those related to the maintenance of ion pumps and membrane potentials. Each neuron has a single (or occasionally no) axon, usually emerging from the cell body or occasionally from a dendrite (e.g., some hippocampal CA neurons). The cell body tapers to the axon at the axon hillock, followed by the initial segment of the axon, which contains the Na + channels, the first site where action potentials are initiated. The axon extends for a variable distance from the cell body (up to 1 m or more). An axon larger than 1 to 2 μm in diameter is insulated by a sheath of myelin provided by oligodendroglia in the central nervous system (CNS) or Schwann cells in the peripheral nervous system (PNS). An axon may branch into more than 500,000 axon terminals and may terminate in a highly localized and circumscribed zone (e.g., primary somatosensory axon projections used for fine discriminative touch) or may branch to many disparate regions of the brain (e.g., noradrenergic axonal projections of the locus coeruleus). A neuron whose axon terminates at a distance from its cell body and dendritic tree is called a macroneuron or a Golgi type I neuron; a neuron whose axon terminates locally, close to its cell body and dendritic tree, is called a microneuron, a Golgi type II neuron, a local circuit neuron, or an interneuron. There is no typical neuron because each type of neuron has its own specialization. However, pyramidal cells and lower motor neurons are commonly used to portray a so-called typical neuron.
Neurons require extraordinary metabolic resources to sustain their functional integrity, particularly that related to the maintenance of membrane potentials for the initiation and propagation of action potentials. Neurons require aerobic metabolism for the generation of adenosine triphosphate (ATP) and have virtually no ATP reserve, so they require continuous delivery of glucose and oxygen, generally in the range of 15% to 20% of the body’s resources, which is a disproportionate consumption of resources. During starvation, when glucose availability is limited, the brain can shift gradually to using beta-hydroxybutyrate and acetoacetate as energy sources for neuronal metabolism; however, this is not an instant process and is not available to buffer acute hypoglycemic episodes. An ischemic episode of even 5 minutes, resulting from a heart attack or an ischemic stroke, can lead to permanent damage in some neuronal populations such as pyramidal cells in the CA1 region of the hippocampus. In cases of longer ischemia, widespread neuronal death can occur. Because neurons are postmitotic cells, except for a small subset of interneurons, dead neurons are not replaced. One additional consequence of the postmitotic state of most neurons is that they are not sources of tumor formation. Brain tumors derive mainly from glial cells, ependymal cells, and meningeal cells.
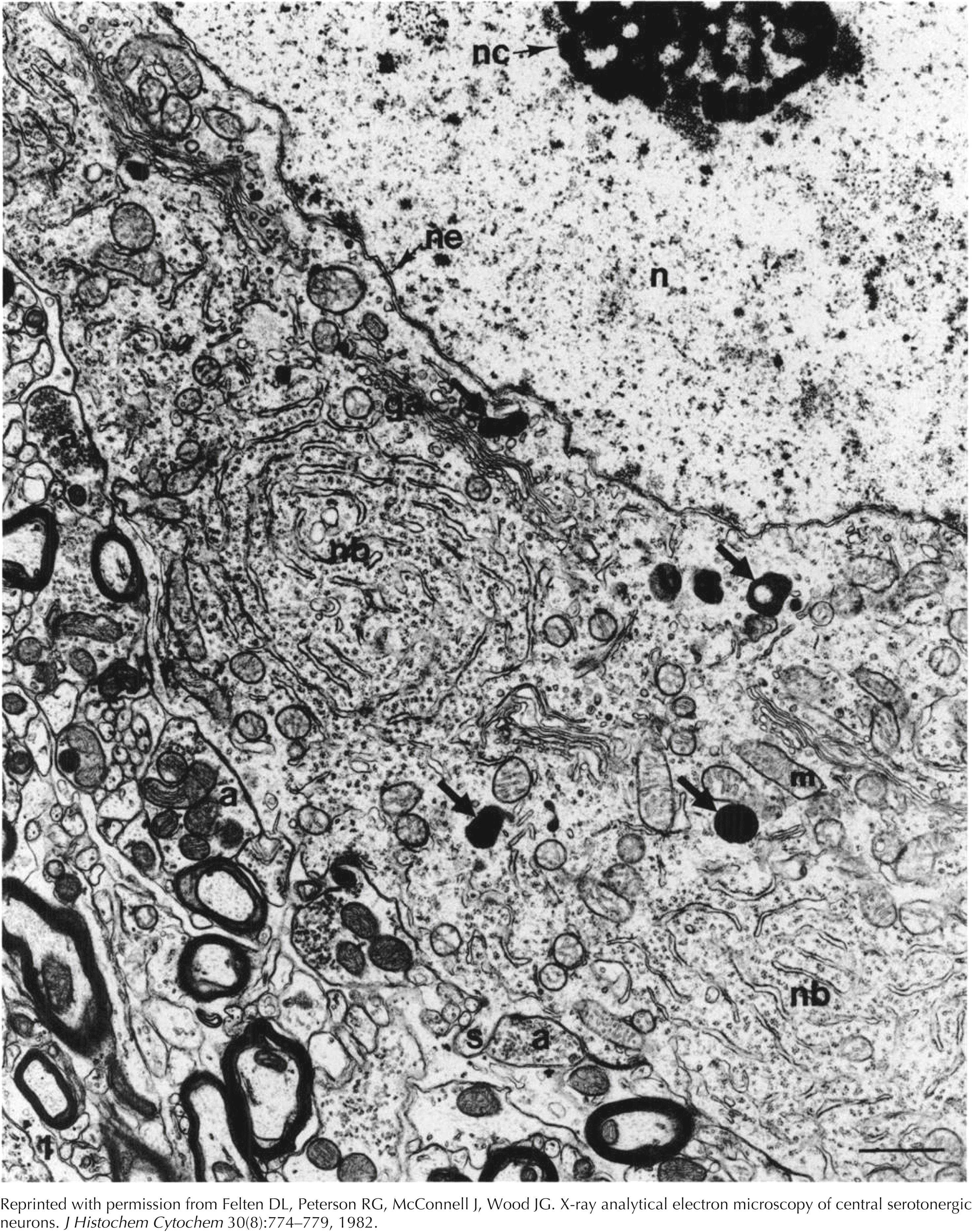
Neuron of nucleus raphe obscurus, standard transmission electron microscopy. The nucleolus (nc) is prominent and the chromatin is dispersed throughout the nucleus (n). The bounding nuclear envelope (ne) follows an uneven contour. Within the cytoplasm of the cell body are Nissl bodies (nb) with their characteristic lamellar organization of rough endoplasmic reticulum, scattered ribosomes, arrays of Golgi apparatus (ga), mitochondria (m), and dense particles (large arrows). These dense particles demonstrate the presence of serotonin when stained with chromium and examined with x-ray analytical electron microscopy. Axon terminals (a) synapse on the neuronal membrane. A small somatic spine (s) is present near one of these synapses. This specimen was fixed in 4% glutaraldehyde, osmicated, and stained with uranyl acetate and lead citrate. Bar = 1 um. Original magnification × 15,000.
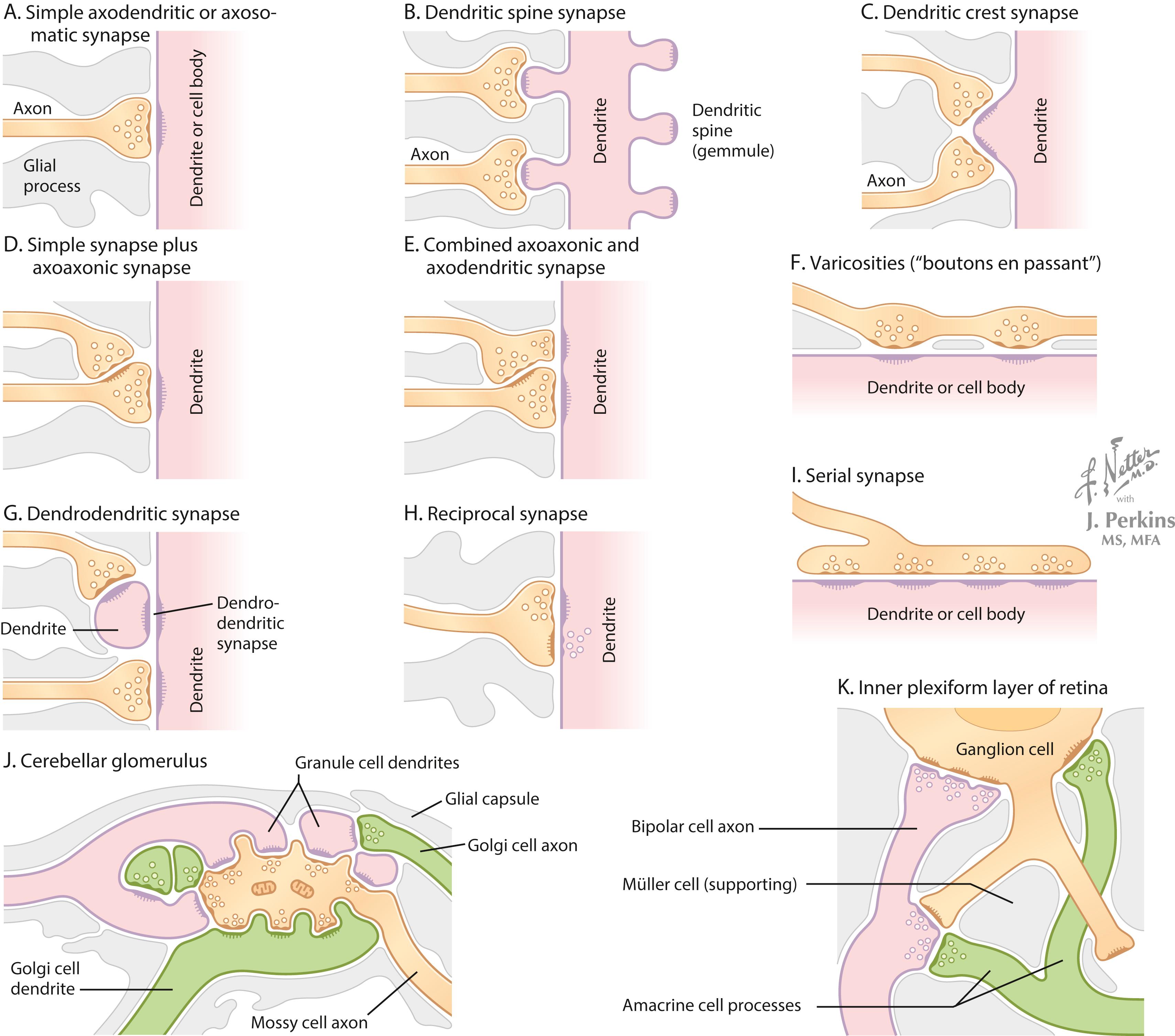
A synapse is a site where an arriving action potential, through excitation-secretion coupling involving Ca 2+ influx, triggers the release of one or more neurotransmitters into the synaptic cleft (typically 20 μm across). The neurotransmitter acts on receptors on the target neuronal membrane, altering the membrane potential from its resting state. These postsynaptic potentials are called graded potentials. Most synapses carrying information toward a target neuron terminate as axodendritic or axosomatic synapses. Specialized synapses, such as reciprocal synapses or complex arrays of synaptic interactions, provide specific regulatory control over the excitability of their target neurons. Dendrodendritic synapses aid in the coordinated firing of groups of related neurons such as the phrenic nucleus neurons that cause contraction of the diaphragm.
The configurations of the synapses of key neuronal populations in particular regions of the brain and of target cells in the periphery determine the relative influence of that input. At the neuromuscular junction, a sufficient amount of acetylcholine is usually released by an action potential in the motor axon to guarantee that the muscle end plate potential reaches threshold and initiates an action potential. In contrast, the neuronal inputs into reticular formation neurons and many other types of neurons require either temporal or spatial summation to allow the target neuron to reach threshold; this orchestration involves coordinated multisynaptic regulation. In some key neurons such as lower motor neurons (LMNs), input from brainstem upper motor neurons (UMNs) is directed mainly through spinal cord interneurons and requires extensive summation to activate the LMNs; in contrast, direct monosynaptic corticospinal UMNs input onto some LMNs, such as those regulating fine finger movements, terminate close to the axon hillock/initial segment; and can directly initiate an action potential in the LMNs. Some complex arrays of synapses among several neuronal elements, such as those seen in the cerebellum and retina, permit modulation of key neurons by both serial and parallel arrays of connections, providing lateral modulation of neighboring neuronal excitability.
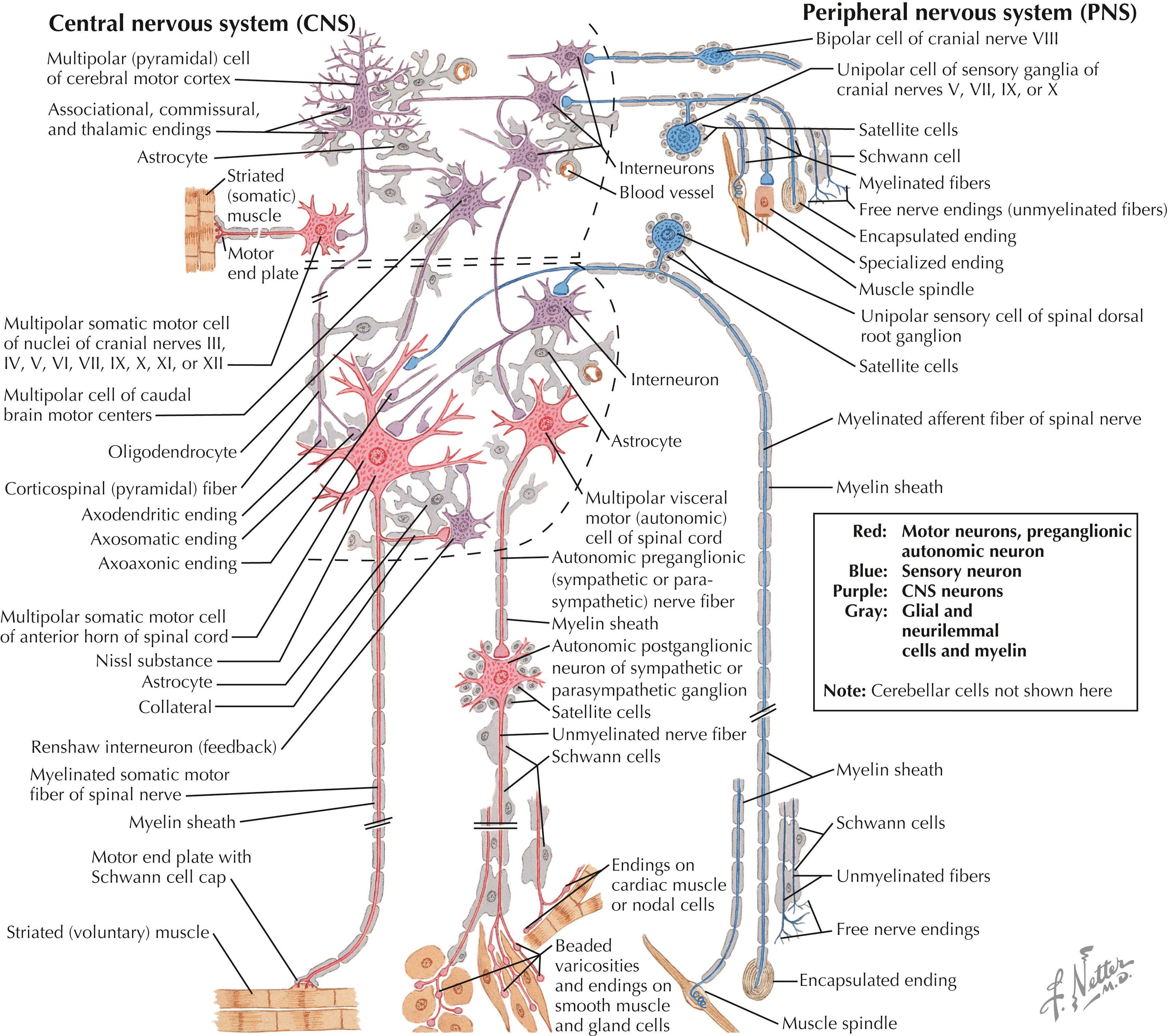
Local interneurons and projection neurons demonstrate characteristic size, dendritic arborizations, and axonal projections. In the CNS (denoted by dashed lines), glial cells (astrocytes, microglia, oligodendroglia) provide support, protection, and maintenance of neurons. Schwann cells and satellite cells provide these functions in the PNS. The primary sensory neurons (blue) provide sensory transduction of incoming energy or stimuli into electrical signals that are carried into the CNS. The neuronal outflow from the CNS is motor (red) to skeletal muscle fibers via neuromuscular junctions, or is autonomic preganglionic (red) to autonomic ganglia, whose neurons innervate cardiac muscle, smooth muscle, secretory glands, metabolic cells, or cells of the immune system. Neurons other than primary sensory neurons, LMNs, and preganglionic autonomic neurons are located in the CNS in the brain (enclosed by upper dashed lines) or spinal cord (enclosed by lower dashed lines). Neurons and glia are not drawn to scale.
Neuronal form and configuration provide evidence of the role of that particular type of neuron. Dorsal root ganglion cells have virtually no synapses on the cell body; the sensory receptor is contiguous with the initial segment of the axon to permit direct activation of the initial segment upon reaching a threshold stimulus. This arrangement provides virtually no opportunity for centrifugal control of the initial sensory input; rather, control and analysis of the sensory input occurs in the CNS. Purkinje neurons in the cerebellum have huge planar dendritic trees, with activation occurring via hundreds of parallel fibers and the background excitability influenced by climbing fiber control. This type of array allows network modulation of Purkinje cell output, via neurons of the deep cerebellar nuclei, to UMNs, a control mechanism that permits fine-grained, ongoing adjustments to smooth and coordinated motor activities. Small interneurons in many regions have local and specialized functions that have local circuit connections, whereas large isodendritic neurons of the reticular formation receive widespread, polymodal, nonlocal input, which is important for general arousal of the cerebral cortex and consciousness. Damage to these key neurons may result in coma. LMNs and preganglionic autonomic neurons receive tremendous convergence upon their dendrites and cell bodies to orchestrate the final pattern of activation of these final common pathway neurons through which the peripheral effector tissues are signaled and through which all behavior is achieved.
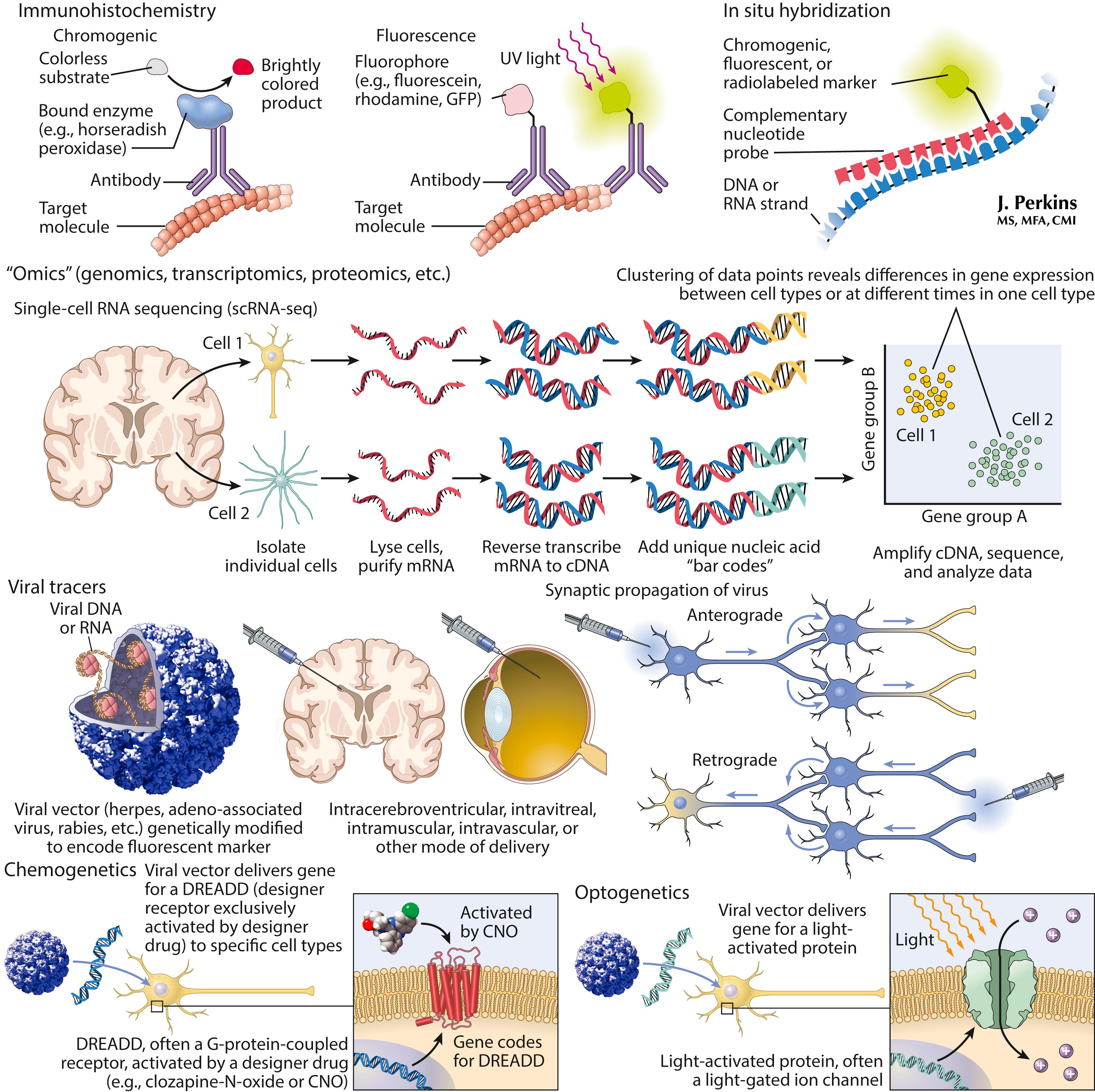
Multiple molecular approaches are used to investigate neurons and their complex interactions. Traditional methods localize specific proteins or mRNA species using immunohistochemistry and in situ hybridization, respectively. New technologies (OMICS) can tag RNA from single cells to investigate patterns of gene expression and their changes in disease states. Some viruses can transduce specific cell types, particularly neurons, and provide precise and efficient means to express molecules in the nervous system. This technology is used to (1) label neuronal pathways, including transsynaptic connections, and (2) deliver engineered G-protein-coupled receptors or light-sensitive ion channels to modulate neural function directly with exogenous stimuli. Viral vectors are being developed for gene replacement or gene targeting in human disease; some of these therapies are already approved, such as a treatment for spinal muscular atrophy .
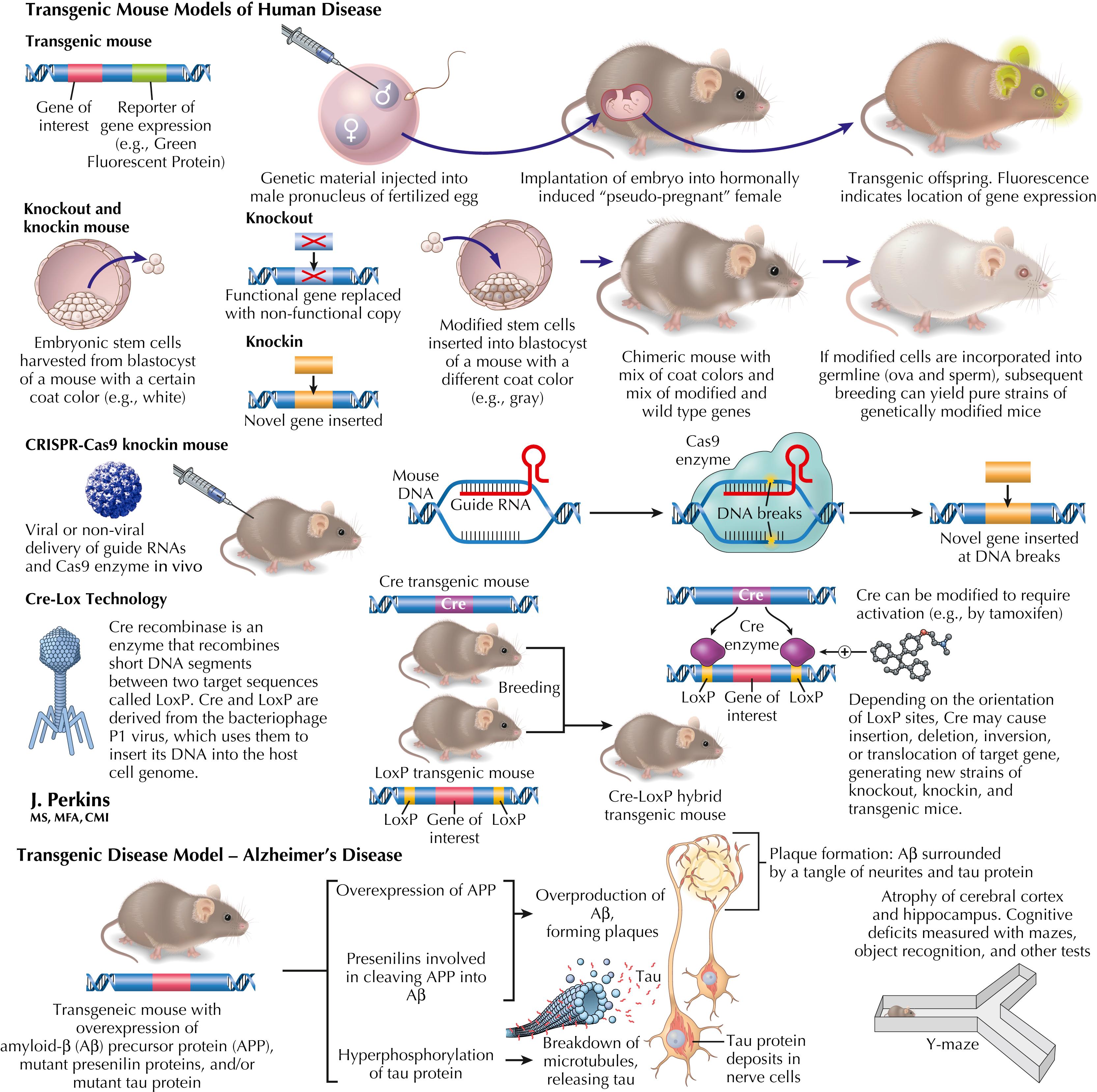
Genetic manipulation of mammalian DNA, initially in mice, is a powerful experimental tool to explore the effect of the introduction or deletion of specific genes. Transgenic mice, in which a new gene or set of genes is introduced, are widely used in models of human disease. Gene function or disease phenotypes can also be investigated using knockout or knockin technologies, which have been made increasingly efficient by the discovery of systems such as CRISPR-Cas9. This system provides direct, targeted gene insertion or deletion. Other genetic technologies such as Cre-Lox, adapted from bacteriophages, provide methods for cell-specific expression or deletion of genes, depending on which cells express Cre, as well as temporal control of expression or deletion with the use of modified Cre, which can be activated by a drug or hormone. The latter approach is especially useful for avoiding the impact of genetic manipulation during development.
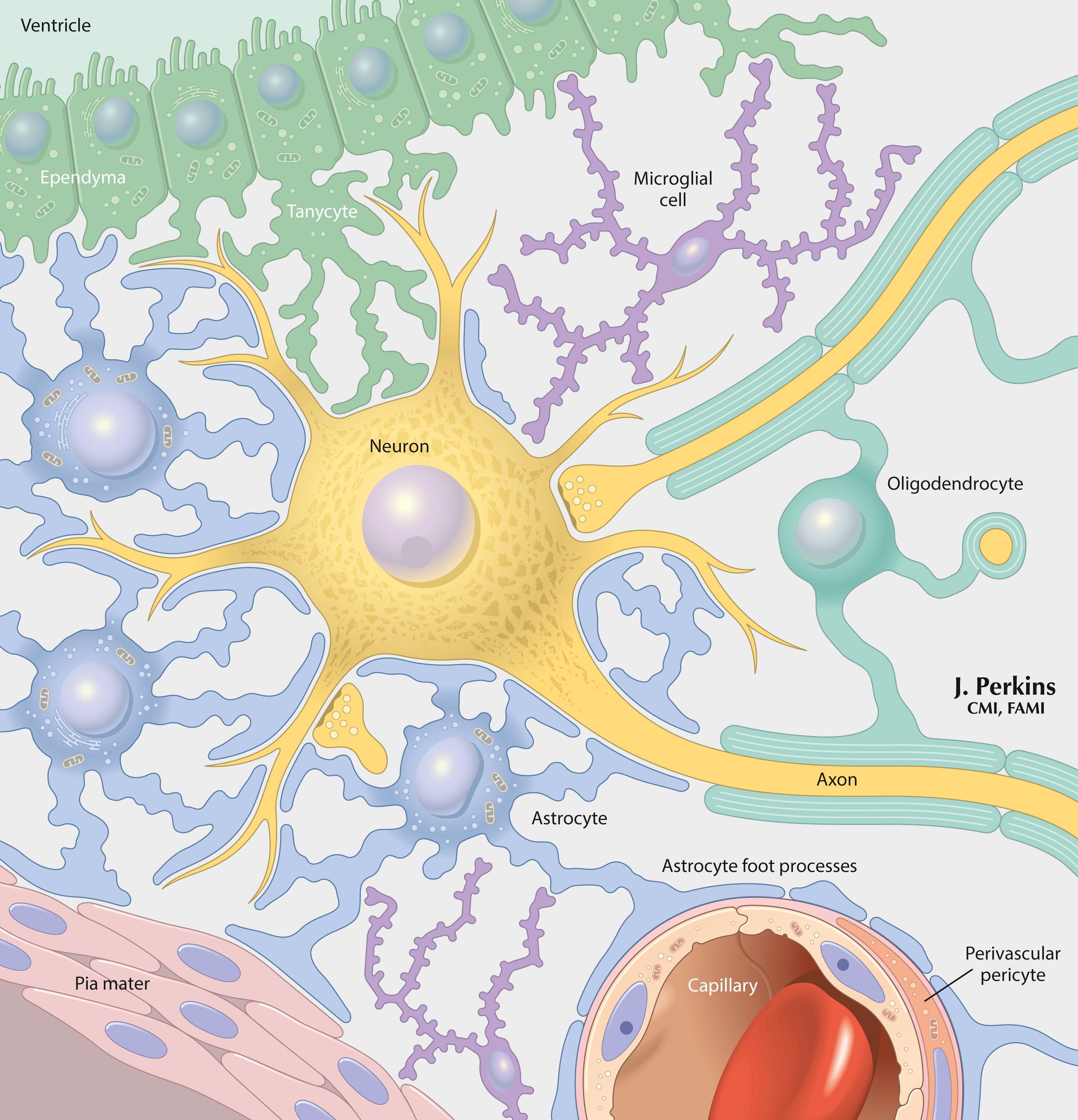
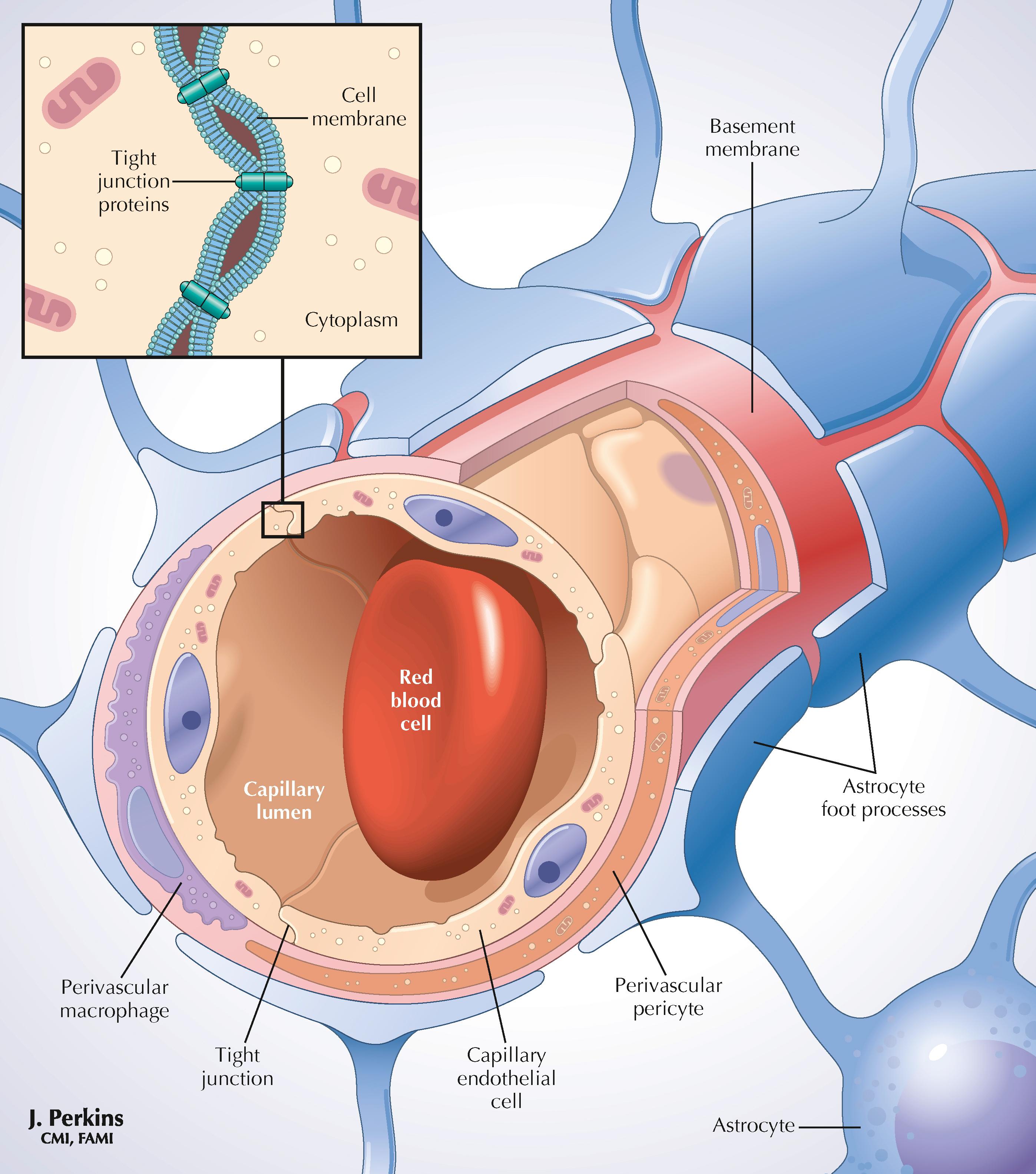
Astrocytes provide structural isolation of neurons and their synapses and provide ionic (K + ) sequestration, trophic support, and support for growth and signaling functions to neurons. Oligodendroglia (oligodendrocytes) provide myelination of axons in the CNS. Microglia are scavenger cells that participate in phagocytosis, inflammatory responses, cytokine and growth factor secretion, and some immune reactivity in the CNS. Perivascular cells participate in similar activities at sites near the blood vessels. Schwann cells provide myelination, ensheathment, trophic support, and actions that contribute to the growth and repair of peripheral neurons. Activated T lymphocytes normally can enter and traverse the CNS for immune surveillance for a period of approximately 24 hours.
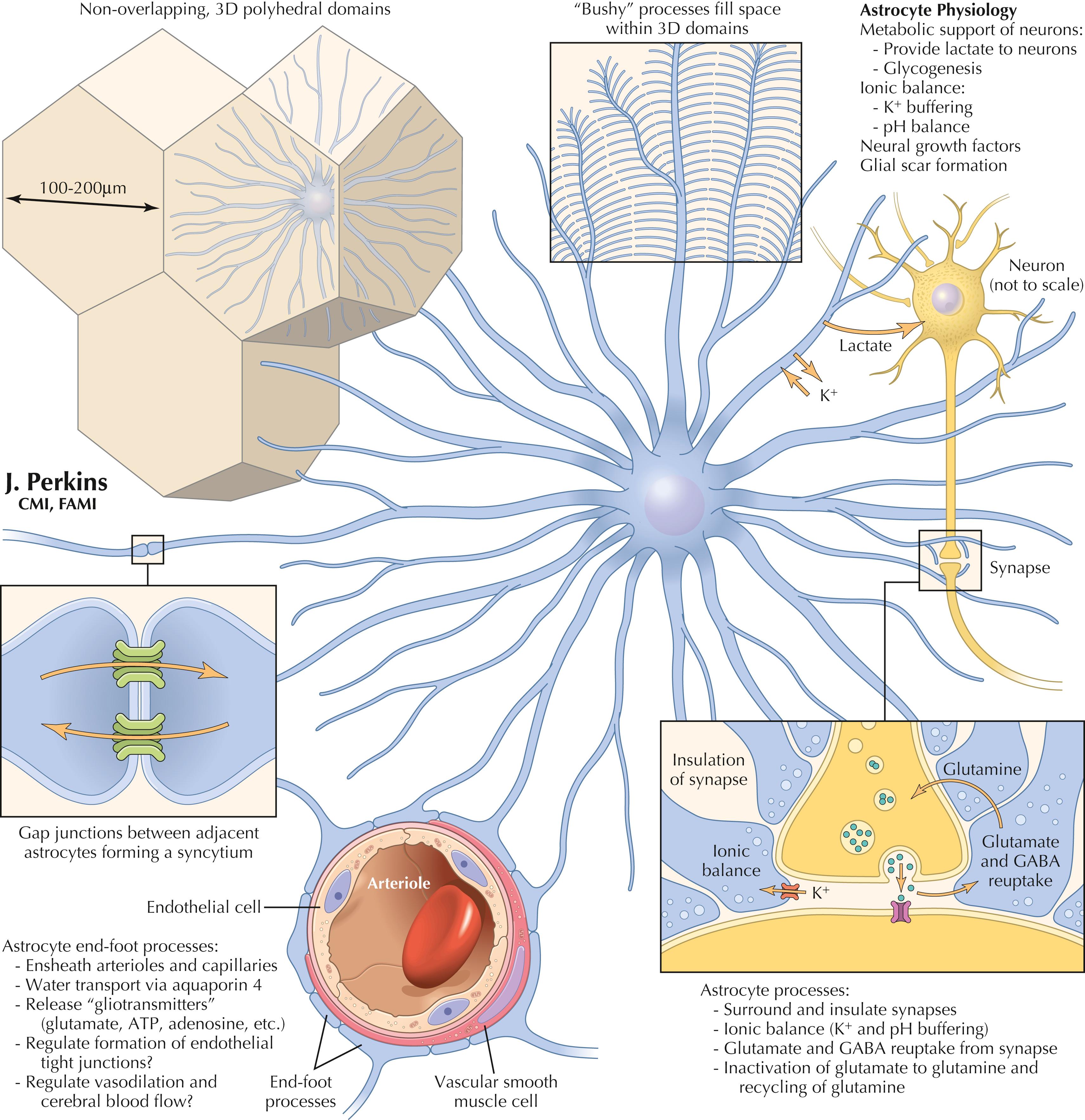
Astrocytes are the most abundant glial cells in the CNS. They arise from neuroectoderm and are intimately associated with neural processes, synapses, vasculature, and the pial-glial membrane investing the CNS. Astrocytes in gray matter are called protoplasmic astrocytes, and in white matter are called fibrous astrocytes. The somas vary in diameter from a few micrometers to 10 or more micrometers. Astrocytes are arrayed in nonoverlapping 3D polyhedral domains of 100–200 μm across (up to 400 μm in hominids). Structurally, astrocytic processes interdigitate, forming a syncytium to protect synapses (as close as 1 μm to these structures). Astrocytic endfeet associate with vascular endothelial cells and associated smooth muscle cells. Astrocytic processes invest the entire pial membrane from the inside.
Physiologically, astrocytic processes affect ion balance (sequester K + ), transport water via aquaporin 4 channels, uptake and recycle glutamate and gamma-aminobutyric acid (GABA), provide metabolic support to neurons, and can become reactive after CNS injury and lay down glial scar tissue. Astrocytes also can release growth factors and bioactive molecules (termed gliotransmitters ) such as glutamate, ATP, and adenosine. In development, specialized astrocytes, called radial glia, provide a scaffold for orderly neural migrations in the CNS. Astrocytes are able to transfer mitochondria to neurons damaged by a stroke.
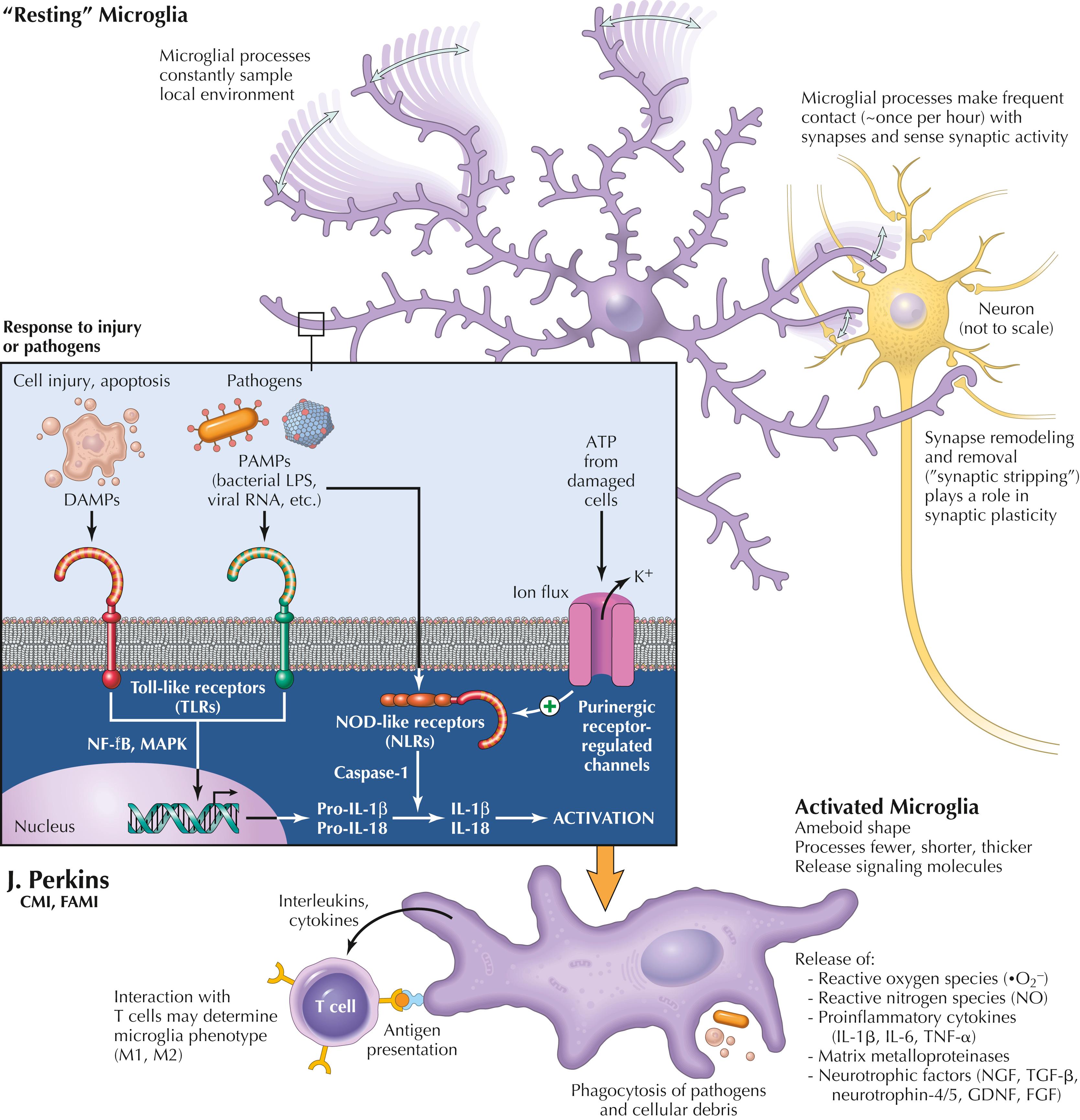
Microglial cells are mesenchymal cells derived from yolk sac that come to reside in the CNS. They are a unique resident population with the capacity for self-renewal. Microglia provide constant surveillance of the local microenvironment, with processes moving back and forth up to 1.5 μm/min. Microglial processes can grow and shrink up to 2–3 μm/min. They have a territory 15–30 μm wide, with little overlap with each other. Resting microglia have soma of 5–6 μm diameter, and activated microglia are ameboid in appearance, with soma of approximately 10 μm diameter.
Microglia can carry out phagocytosis of debris and apoptotic cells, remodel and remove synapses in developing and adult CNS, and respond to injury or pathogens. Microglia have receptors for multiple types of stimuli, such as ATP (indicator of local damage), toll-like receptors (TLRs) that respond to molecules released from dying cells (DAMPS: damage-associated molecular patterns) or pathogens (PAMPS: pathogen associated molecular patterns) such as LPS on gram-negative bacteria, or double-stranded RNA in viruses. Reactive microglia produce reactive oxygen species (ROS), reactive nitrogen species (RNS, such as NO), proinflammatory cytokines (IL-1β , IL-6, TNF-α), matrix metalloproteinases (MMPs), and neurotrophic factors (such as NGF, TGF-β, neurotrophin 4/5, GDNF, FGF). Such signal molecules from activated microglia can affect neurons and astrocytes, inducing dysfunction. Recent evidence suggests that peripheral macrophages can transfer mitochondria to assist primary sensory neurons in inflamed tissue.

Oligodendrocytes are neuroectodermally derived glial cells that have the major role of myelinating central axons. The trigger for myelination may include associated axonal size and signal molecules (such as ATP, K + , glutamate, GABA, and some cell adhesion molecules). Each oligodendrocyte can myelinate individual intermodal segments of an average of 30 separate axons (as high as 60 axons); adjacent internodal segments are myelinated by different oligodendrocytes. This pattern of central myelination leaves periodic nodes of Ranvier bare, with sodium channels, at which action potentials (APs) are reinitiated as they travel down the myelinated axon and its branches (called saltatory conduction ). Oligodendrocytes can be attacked by antibodies directed at specific oligodendrocyte proteins in multiple sclerosis, leading to oligodendrocyte death and axonal dysfunction. Oligodendrocyte precursor cells can replicate following such insults and remyelinate the denuded central axon segments. Oligodendrocyte membranes possess monocarboxylate transporter 1 (MCT 1), which can deliver lactate, pyruvate, and ketone bodies to the axon. Oligodendrocyte precursor cells (OPCs) are present in the adult CNS and have NG2 and PDGFα receptors.
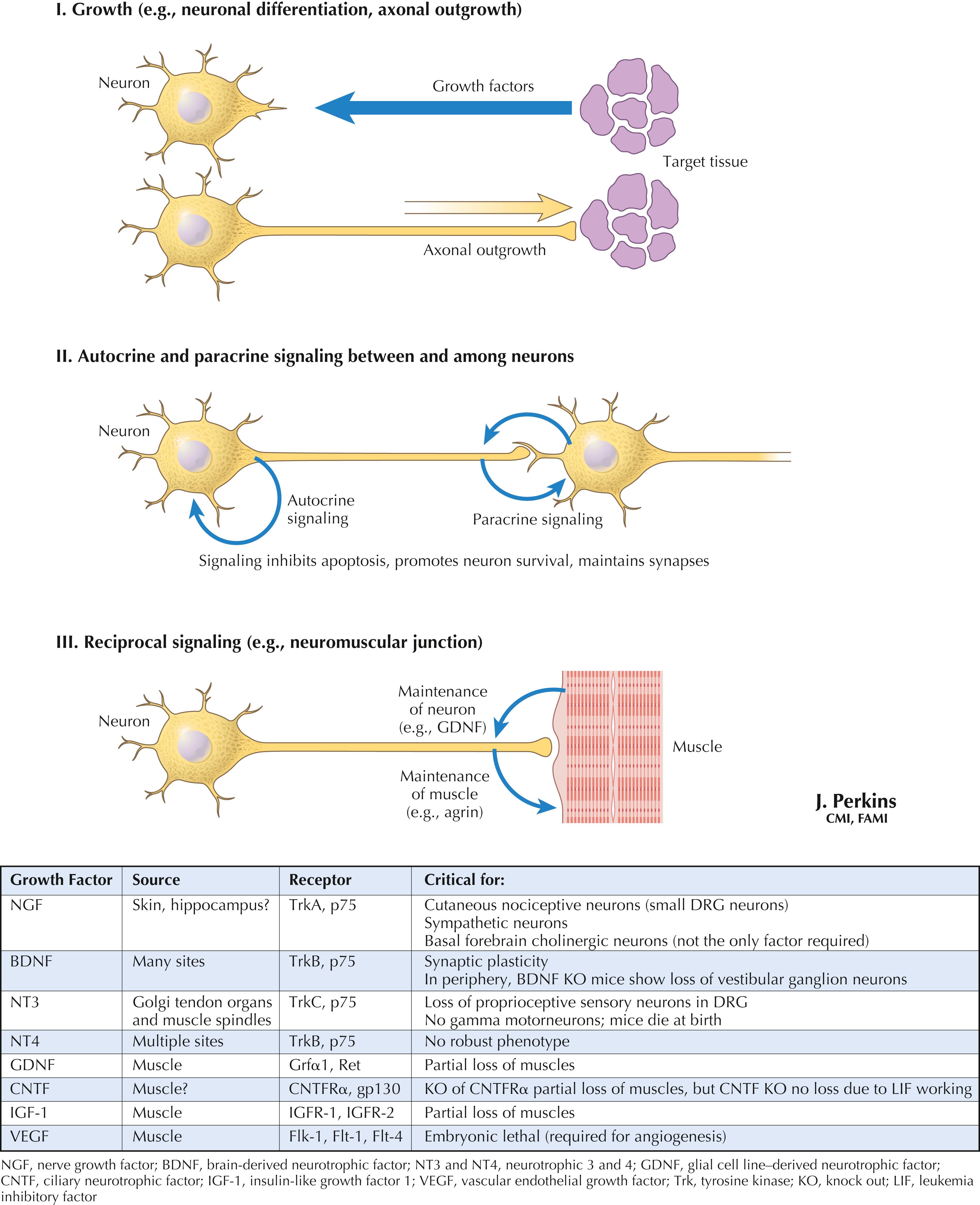
Neuronal growth factors and trophic factors are signal molecules produced by neurons, glia, and target tissues that can influence neuronal differentiation, growth of neurites, establishment of contacts for signaling, maintenance of neural contacts with their central or peripheral targets, and other functions. These factors act through specific receptors and can induce the production of specific molecules, such as agrin for the maintenance of nicotinic cholinergic receptors at the neuromuscular junction. Several identified growth factors, along with their sourced receptors and possible roles, are provided in the table above.
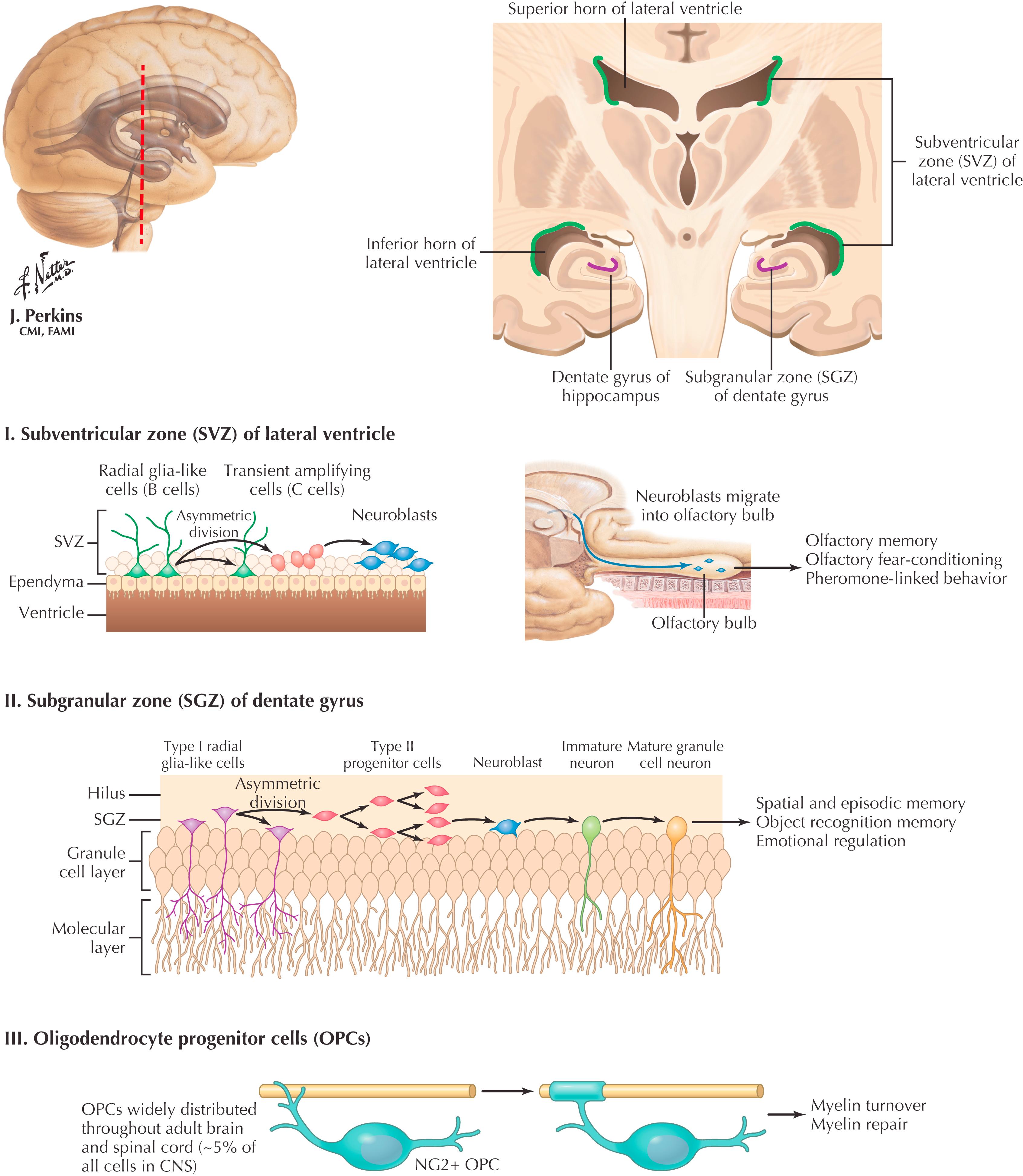
Embryogenesis involves the proliferation of stem cells, followed by differentiation and migration of the resultant cell types. In the CNS, neuronal stem cells, derived from the neural tube, persist in the subventricular (or subependymal) zone of the lateral ventricles (I). Waves of neuronal proliferation, differentiation, and migration occur during prenatal CNS development. After birth, stem cells in the subventricular zone continue to proliferate and produce granule cells (neurons) for many brain regions; this process is driven by postnatal environmental stimuli. Throughout adulthood radial glial-like cells, in the subgranular zone of the dentate gyrus, give rise to neuroblasts that contribute new granule cell neurons (II). In addition, oligodendroglial progenitor cells throughout the CNS can proliferate and then differentiate into mature oligodendrocytes (III). This process can occur after a demyelinating lesion and helps to remyelinate CNS axons (e.g., after a multiple sclerosis lesion).
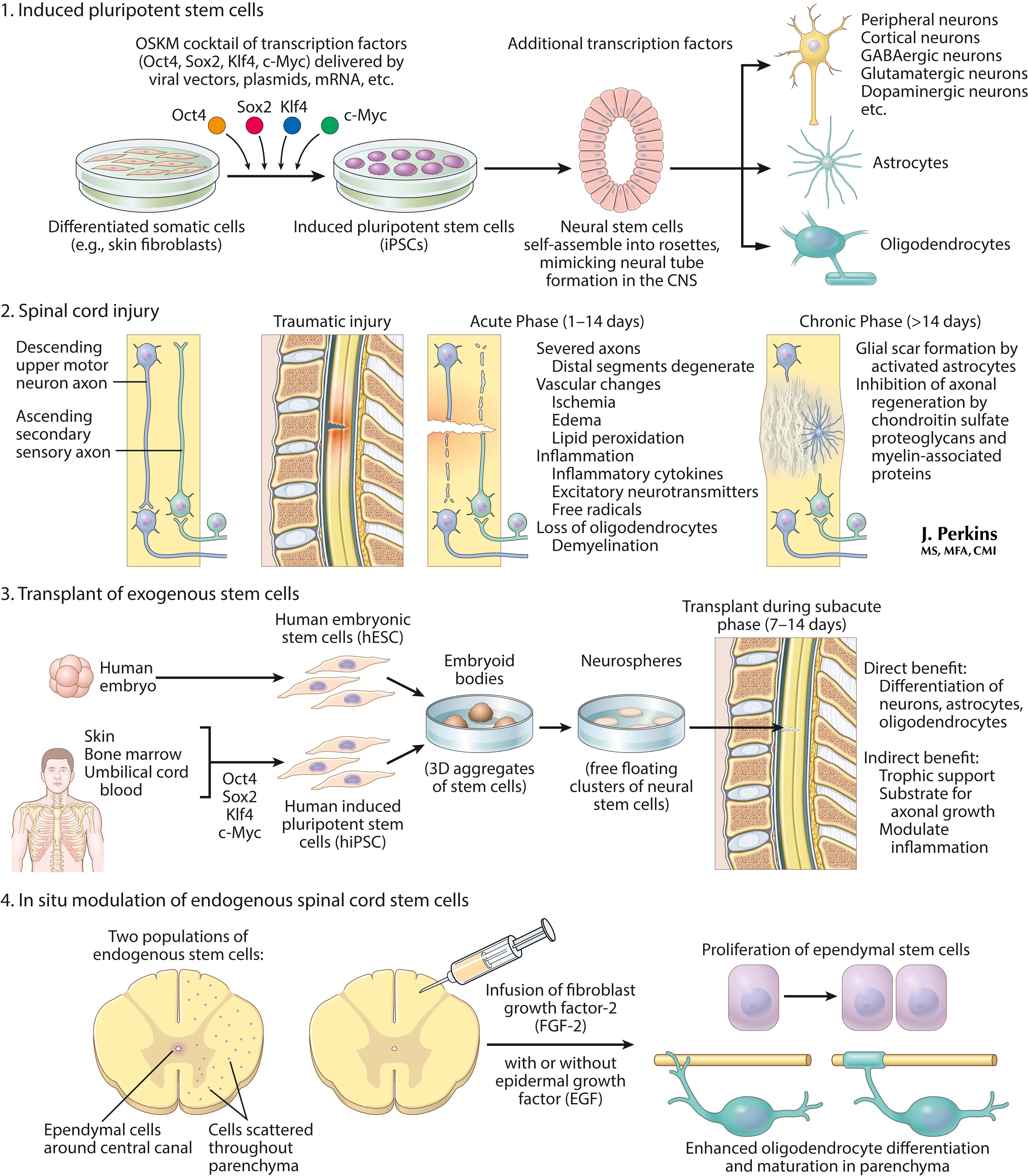
Recent approaches to stem cell therapy after a spinal cord injury are depicted here. 1. Induced pluripotent stem cells can be generated from somatic cells (e.g., skin biopsy) and differentiated into neural lineage cells for therapy. 2. The pathologic process of spinal cord injury shows acute and chronic responses. 3. Use of exogenous stem cells transplanted during the subacute phase leads to differentiation of neurons and glia, and trophic support and modulation of inflammation. 4. In situ modulation of endogenous stem cells uses infusion of growth factors. These approaches remain experimental but offer possible applications of knowledge derived from stem cell biology to treat devastating conditions such as spinal cord injury.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here