Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The prevalence of gallbladder disease makes the goal of understanding gallbladder function a clinically significant one. Gallstone disease in the United States afflicts some 6.3 million men and 14.2 million women aged 20–74 years. In other words, ~ 10%–15% of the adult US population has gallstones or have had a cholecystectomy. The direct costs associated with biliary disease (~$5.5–6.5 billion/year in the United States) are the second highest for any gastrointestinal ailment, with gastroesophageal reflux disease being the costliest, and peptic ulcer disease and colorectal cancer ranking behind gallbladder disease at 3rd and 4th. The costs of gallstone disease exceed the combined totals for chronic liver disease and cirrhosis, chronic hepatitis C, and pancreatic disease. Its frequency and costs make gallbladder disease a public health issue.
It is taken for granted that gallbladder disease is curable by cholecystectomy. Now that laparoscopic surgery is commonly available, and the recovery period is more tolerable as compared to the open laparotomy approach, an increase in inappropriate surgical interventions, including overuse of surgery occurs, and less effort is being expended toward understanding and preventing gallbladder disease. Very few laboratories in the United States, and for that matter, throughout the world, are now focusing on gallbladder motility. However, a sheer number of gallbladder surgeries that are performed (> 700,000/year in the United States alone), the risks and costs associated with these surgeries, and the side effects of gallbladder removal make the goals of developing nonsurgical approaches to prevent gallstone disease and/or restore gallbladder function very important.
A hallmark feature of gallbladder disease is decreased contractility of gallbladder smooth muscle (GBSM). Therefore, understanding GBSM physiology and the regulation of gallbladder contractility in normal and pathological conditions are very worthwhile. This chapter provides an overview of neuromuscular function in the gallbladder, along with a summary of neuromuscular control in the sphincter of Oddi, which works in concert with the gallbladder in the processes of interprandial bile storage and postprandial bile flow.
The gallbladder wall consists of three layers: mucosa, muscularis, and serosa. A ganglionated plexus that lies at the interface between the muscularis and serosal layers provides the local innervation of the muscular and epithelial tissues of the organ, and in some species, ganglia are also sparsely distributed in the lamina propria of the mucosal layer ( Fig. 20.1 ). The main neuronal plexus is comprised of an array of small, irregularly shaped ganglia that are arranged in no discernible pattern. Extrinsic nerves, which include sensory fibers, vagal preganglionic fibers, and sympathetic postganglionic fibers, enter the gallbladder by following blood vessels in peri- and paravascular nerve bundles that interconnect with the ganglionated plexus. The muscularis and mucosal layers also contain nerve fibers that represent a mixture of projections from intrinsic neurons, sympathetic postganglionic nerves, and sensory fibers.
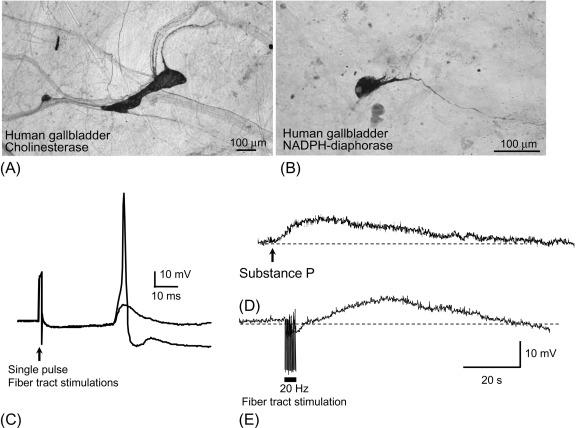
The neurophysiological properties of gallbladder neurons have been determined by intracellular recording techniques in whole mount preparations of guinea pig, opossum, and human gallbladders. Gallbladder neurons rarely exhibit spontaneous activity and they are rapidly accommodating, regardless of the amplitude or duration of the current pulse. These properties suggest that extrinsic nerves, hormones, or inflammatory mediators drive gallbladder neurons. Therefore, gallbladder neurons only release their neurotransmitters onto target tissues in response to incoming commands.
Gallbladder neurons receive fast and slow excitatory synaptic inputs. The fast excitatory postsynaptic potential (EPSP) is mediated by the release of acetylcholine from vagal preganglionic nerve endings, and often causes action potential generation. The slow EPSP is a prolonged depolarization that is not associated with neuronal firing. Therefore, the vagal preganglionic axons represent the principal driving force for generating neuronal output from gallbladder ganglia to the smooth muscle. As described below, the efficacy of ganglionic transmission can be modulated by physiological signals that act presynaptically on vagal terminals or postsynaptically on gallbladder neurons.
Immunocytochemical and histochemical studies have demonstrated that a variety of neuroactive compounds are expressed by gallbladder neurons. In every species studied to date, including human, dog, guinea pig, opossum, and Australian possum, all gallbladder neurons are immunoreactive for choline acetyltransferase (ChAT), the biosynthetic enzyme for acetylcholine, indicating that all gallbladder neurons are cholinergic. Other additional neuroactive compounds and associated synthetic enzymes have been detected in these neurons, including tachykinins, neuropeptide Y (NPY), nitric oxide synthase (NOS), vasoactive intestinal peptide (VIP), pituitary adenylate cyclase activating peptide (PACAP), orphanin FQ (OFQ), cocaine and amphetamine regulated transcript peptide (CART), galanin (GAL), and somatostatin (SOM). The coexpression patterns of these compounds among gallbladder neuronal subpopulations are species specific. For a detailed overview, and appropriate references, the reader is referred to Balemba et al.
Although the gallbladder is a tonic organ, individual GBSM cells have phasic electrical and contractile properties ( Fig. 20.2 ). Gallbladder tone appears to result from the collective action of independently paced contractions of individual bundles of GBSM cells. Within the muscularis layer, smooth muscle cells are not arranged in sheets; rather, the muscularis consists of smooth muscle bundles that are interposed and oriented in various directions like a ball of string. The smooth muscle cells within a given bundle are arranged in parallel, and they appear to be electrically coupled since they have a very low-input resistance when evaluated with intracellular electrodes in ex vivo preparations, and they exhibit rapid Ca 2 + transients, called flashes, that occur simultaneously in all of the smooth muscle cells of a given bundle.
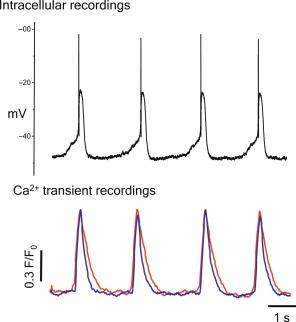
The electrical and ionic properties of GBSM cells have been elucidated by intracellular microelectrode recording studies of intact preparations and by patch clamp recording studies involving isolated myocytes ( Fig. 20.3 ). Rhythmic spontaneous action potentials are a feature of GBSM cells, and these events consist of a rapid spike followed by a plateau phase. GBSM action potentials are briefer and occur at a higher frequency than the slow wave potentials of gastrointestinal smooth muscle, with a plateau duration of 300–500 ms and a frequency of about 0.4 Hz. A number of ionic currents have been detected in GBSM, but two currents that provide the major influence on the contour of the action potential are the large conductance, dihydropyridine-sensitive (L-type) Ca 2 + channel, and the delayed rectifier K + (K V ) channel. While dihydropyridine-sensitive Ca 2 + channels and K V channels are clearly involved in the action potential of GBSM, the generation and pacing of the action potentials involve specialized pacemaker cells [see subsection below on interstitial cells of Cajal (ICC)].
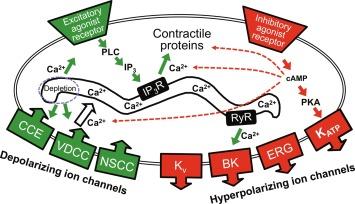
Inhibition of ether-a-go-go-related gene (ERG) K + or nonselective cation channels also affects GBSM activity. Transcript and immunoreactivity for ERG channels are detected in GBSM, and in the presence of ERG channel blockers, the membrane potential is depolarized, the plateau phase of the action potential is prolonged, and multiple spikes are generated.
Action potential generation in GBSM is critically dependent on the resting membrane potential (about − 50 mV), which is ~ 35 mV more positive than the K + equilibrium potential, and a steady state, nonselective cation current contributes to the maintenances of the membrane potential of these cells. With L-type Ca 2 + and K V channels inhibited, we identified a novel spontaneously active cation conductance in GBSM that is mediated primarily by Na + influx. GBSM cells are hyperpolarized, and action potentials are eliminated when this current is inhibited by Na 2 + substitution, indicating that this nonselective cation conductance contributes to the regulation of GBSM excitability and contractility.
Ca 2 + -activated K + (BK) channels and ATP-sensitive K + (K ATP ) channels are also expressed by GBSM. While BK channel blockers do not appear to affect the membrane potential or action potential contour in GBSM, BK channels have been identified in patch clamp studies of GBSM, and they are responsible for spontaneous transient outward currents that are easily detectable in these cells. BK channel currents are activated by local release of Ca 2 + through ryanodine receptor channels (RyR) of the endoplasmic reticulum. While the functional relevance of these Ca 2 + “sparks” and associated transient outward currents in GBSM are not completely understood, they likely contribute to the regulation of GBSM excitability as they are inhibited by cholecystokinin (CCK).
K ATP channels appear to play a critical role in GBSM hyperpolarization and relaxation, and activation of their current mediates the action of inhibitory inputs to the GBSM. Exposure of intact GBSM to the K ATP channel activators lemakalim or pinacidil leads to a prolonged hyperpolarization that is associated with elimination of spontaneous action potentials, and the K ATP channel blocker, glibenclamide, blocks this effect. In isolated cells, the K ATP channel openers activate a glibenclamide-sensitive current. These channels appear to mediate the actions of inhibitory agonists in gallbladder muscle because the inhibitory effects of calcitonin gene-related peptide (CGRP) and histamine H 2 receptor agonists are blocked by glibenclamide. Activation of the K ATP channel involves the activation of the cyclic AMP-adenylate cyclase-protein kinase A signal transduction cascade, whereas activation of protein kinase C inhibits K ATP channel function.
Influx of Ca 2 + into GBSM, and Ca 2 + release from intracellular stores are critical events for normal GBSM function since gallbladder excitability and contractility depend on [Ca 2 + ] i increases. Two types of rhythmic spontaneous Ca 2 + transients have been identified in Ca 2 + imaging studies of intact whole mount preparations of GBSM cells, Ca 2 + flashes, and Ca 2 + waves. Ca 2 + flashes are rapid Ca 2 + transients that are associated with the Ca 2 + influx that occurs during spontaneous action potentials in GBSM. Flashes occur simultaneously in all of the smooth muscle cells within a given bundle, but flashes in nonintersecting bundles exhibit asynchronous pacing.
Ca 2 + waves are intracellular Ca 2 + transients that propagate within a given smooth muscle cell, and these events appear to correspond to subthreshold depolarizations of GBSM cells. Like the spontaneous action potentials of GBSM, Ca 2 + flashes and waves are sensitive to L-type Ca 2 + channel and inositol triphosphate (IP 3 ) inhibitors. Excitatory agonists enhance Ca 2 + flash and wave activity in a phospholipase C-dependent manner, suggesting that they play a role in spontaneous excitability and pacemaking in GBSM. Interestingly, while Ca 2 + flashes are typically asynchronous among separate muscle bundles, they become synchronized in the presence of excitatory agonists. The spatiotemporal patterns of these events support a model in which asynchronous electrical and contractile activity of GBSM bundles throughout the muscularis layer is responsible for maintenance of net tone in the organ. Furthermore, synchronous global electrical rhythms that likely result from excitatory agonist stimulation may contribute to gallbladder emptying.
There are functional studies indicating that Ca 2 + entry through L-type Ca 2 + channels is required for GBSM to respond to excitatory neuro-hormonal inputs. However, CCK-induced contraction involves Ca 2 + release from intracellular stores, which have both inositol triphosphate (IP 3 ) receptors and RyRs, and are dependent on sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) pumps for refilling and maintenance of intracellular Ca 2 + stores. As described above, Ca 2 + release from intracellular stores can also cause relaxation via release of Ca 2 + through RyR, as sparks, and activation of BK channels, resulting in membrane hyperpolarization and relaxation. These opposing actions of Ca 2 + , which is stored in a single intracellular domain, are likely to involve separate localizations of the IP 3 and RyR Ca 2 + release channels in GBSM cells. This allows for a more versatile and complex role of intracellular Ca 2 + mobilization in this cellular model. L-type Ca 2 + channels appear to be important for the maintenance of intracellular Ca 2 + stores in GBSM since emptying of these stores induces the activation of L-type Ca 2 + , as well as capacitative, channels. This could account for the inhibitory effect of Ca 2 + channel blockers on the actions of excitatory agonists in gallbladder muscle. Alterations in Ca 2 + handling (related to both Ca 2 + influx and release mechanisms) can be responsible for gallbladder stasis in inflammation.
As described above, GBSM cells exhibit spontaneous, rhythmic electrical activity and Ca 2 + transients, and these events are associated with contractions of individual muscle bundles. These events are not affected by inhibition of neuronal activity with tetrodotoxin, indicating that they are not neurally mediated.
There are strong morphological and physiological data in support of the concept that specialized cells, comparable to the ICC of the gastrointestinal tract, exist in the gallbladder, and that these cells generate spontaneous rhythmic activity in the gallbladder muscularis. The tyrosine kinase receptor protein, kit, which has been used as a marker of ICC in the gut, is detectable infusiform-shaped cells that are present in GBSM, oriented in parallel to the muscle cells. The gold standard for identification of ICC is their ultrastuctural features, and at the electron microscopic level, cells with features of ICC have been identified. These features include electron dense cytoplasm and an abundance of mitochondria and calveoli, and these cells are observed in direct contact with one another, and with smooth muscle cells.
Physiologically, Ca 2 + imaging studies provide strong evidence for the existence of ICC with a pacemaker function in the gallbladder. Like smooth muscle, these cells exhibit Ca 2 + flashes, but interestingly, the flashes persist in ICC-like cells in the presence of gap junction blockers, whereas they are diminished in smooth muscle cells. This indicates that these cells can generate spontaneous activity, but smooth muscle cells cannot, and it is consistent with our finding that in isolated GBSM cells, we do not detect spontaneous rhythmic activity even when the cells are warmed to 36°C. Collectively, the findings described above indicate that the spontaneous, rhythmic activity that is detected in GBSM, and which corresponds to smooth muscle bundle contractions, is generated by specialized ICC-like cells, and is not an intrinsic property of GBSM. Tan and colleagues have reported a decrease in kit expression in gallbladders from subjects undergoing cholecystectomy for gallstone disease, indicating that a decrease in ICC could contribute to hypomotility in this disorder, as has been reported for slow transit constipation and gastroparesis.
Postprandial gallbladder contraction is triggered by the release of CCK from enteroendocrine cells in the epithelial lining of the duodenum. In 1928, Ivy and Oldberg solidly established the concept that CCK acts as a hormone to cause gallbladder emptying ; however, it is also likely that CCK acts at several sites to promote gallbladder contraction. The most direct means of gallbladder contraction is for hormonal CCK to act on receptors located on GBSM, which expresses the CCK 1 , but not the CCK 2 receptor. It is likely that the low-affinity CCK 1 receptor is present in GBSM, since the EC 50 for the CCK-induced gallbladder contraction is in the 10–50 nM range. It is unclear whether CCK receptors on GBSM are a normal physiological site of action for CCK, because postprandial concentrations of CCK in the serum are in the 10–20 pM range, which is below the threshold for a direct action of CCK on gallbladder muscle strips. Furthermore, meal-induced gallbladder contractions and contractions induced by physiological concentrations of CCK in vivo are significantly attenuated by neural blockade in several species, including human. Consistent with this, hexamethonium, which blocks the vagal preganglionic input to gallbladder neurons, inhibits CCK- and meal-induced gallbladder contractions. Therefore, a neural mechanism is likely involved in the prokinetic effects of postprandial CCK release.
While gallbladder neurons do not respond directly to the peptide, CCK has a potent presynaptic excitatory effect on nerve terminals in gallbladder ganglia. CCK increases acetylcholine release from vagal efferent nerves terminating on gallbladder neurons. The concentration-effect relationship of the presynaptic action of CCK in gallbladder ganglia indicates that CCK can act physiologically at this site.
It is likely that postprandial gallbladder contractions are also promoted by CCK, released from enteroendocrine cells, acting via a paracrine mechanism on vagal afferent nerve fibers in the lamina propria of the duodenum. Subdiaphragmatic vagal afferent fibers are sensitive to CCK, and postprandial physiological responses, such as increased gastric motility and pancreatic secretion, have been attributed to CCK-mediated increases in vagal afferent activity. Following a meal, CCK stimulates vagal afferent nerve fibers, which act in the vagal motor complex to increase the rate of firing of vagal preganglionic neurons.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here