Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Malignant hyperthermia (MH) is a pharmacogenetic disorder inherited primarily in an autosomal dominant pattern.
MH susceptibility is linked to 230 mutations in the skeletal muscle ryanodine receptor (RyR1) and four mutations in the calcium voltage-gated channel subunit alpha1 S (CACNA1S) genes that encode two Ca 2+ channels necessary for skeletal muscle excitation-contraction coupling.
Physical interactions between L-type Ca 2+ channel (Ca v 1.1) and RyR1 tightly regulate initiation and termination of skeletal muscle excitation-contraction coupling.
Skeletal muscle accounts for approximately 40% of body weight and inherent changes in its metabolism have profound impacts on whole-body metabolism and physiology.
Carriers of MH mutations can exhibit mild to moderate muscle impairments in the absence of triggering agents but are rarely diagnosed.
Carriers of MH mutations are susceptible to anesthetic-triggered runaway skeletal muscle metabolism, which if not promptly treated is lethal.
S igns of MH, including increased end-tidal CO 2 , increased core temperature, muscle rigidity, tachycardia, and more , are consequences of the fulminant hypermetabolic crisis.
Exposure to triggering agents or heat stress leads to acute loss of RyR1/Ca v 1.1 channel regulation, rapid accumulation of Ca 2+ within the sarcoplasm, and a hypermetabolic crisis that stimulates adenosine triphosphate (ATP) utilization by pumps attempting to restore resting Ca 2+ balance among sarcoplasmic reticulum, mitochondrial, and extracellular compartments.
Dantrolene markedly attenuates myoplasmic calcium (Ca 2+ ) concentrations and thereby restores resting Ca 2+ balance and metabolism, with reversal of clinical signs.
Evaluation of persons susceptible to MH includes an in vitro contracture test (IVCT) and caffeine/halothane contracture test (CHCT), and evaluation of DNA to identify mutations.
Currently DNA testing alone can be used to evaluate 42 human mutations and all swine, equine, and canine MH.
Future MH goals include advancement of genetic evaluations in North American and European medical programs and stronger finances to support genetic studies, the identification of the mode of action of dantrolene, a determination of the immediate cause of triggering MH, and the development of effective, noninvasive tests for MH susceptibility.
The absence of mutations in dystrophin, along with dystrophin-associated glycoproteins, is involved in sarcolemmal stability. Its defects are responsible for Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD).
Whereas the risk for an MH mutation in DMD and BMD patients is similar to that in the general population, the incidence of MH-like anesthetic events has been reported to be 0.002 with DMD and 0.00036 with BMD.
Succinylcholine is contraindicated in DMD and BMD patients because of the risk of rhabdomyolysis and hyperkalemia as a result of their unstable sarcolemmal membrane.
Reversal of neuromuscular blockade with sugammadex is a practical alternative to the management of many of these disorders, if rocuronium or vecuronium is used. The combination of rocuronium and sugammadex has improved the anesthetic management for some of these challenging disorders.
This chapter is a consolidation of two chapters in the 8th edition, Chapter 42 , Neuromuscular Disorders and other Genetic Disorders and Chapter 43 , Malignant Hyperthermia and Muscle-Related Disorders. The editors, publisher, and the returning authors, would like to thank the following authors: Aranya Bagchi, Richa Saxena, and Diptiman Bose for their contributions to the prior edition of this work. It has served as the foundation for the current.
Malignant hyperthermia (MH) is one of the most devastating anesthesia-related complications. The fulminant MH syndrome is elicited by the administration of triggering anesthetic agents, such as volatile halogenated anesthetics or depolarizing neuromuscular blocking agents (NMBAs). MH has been and continues to be a life-threatening complication of anesthesia if the diagnosis is not made promptly and treatment is not begun in a timely fashion. Unlike other disorders described in this chapter, MH has virtually no characteristic phenotype before exposure to the triggering agent and is truly an example of the interaction of genes and the environment. Also covered in this chapter are some of the neuromuscular disorders, although rarely encountered in a routine anesthetic practice. This group of disorders challenges both perioperative management and intensive care. They affect the normal function of peripheral nerves, the neuromuscular junction, and/or muscles. Although such diseases are thought to be rare, the number of patients that a clinician may encounter is increasing because of better medical care, increasing longevity, and other possible unidentified factors. Neuromuscular disorders have significant potential to interact with an improper anesthetic plan, and all affected patients require special perioperative attention for anesthetic management. In this area, the armamentarium of invasive and noninvasive diagnostic tools is being developed, especially in genetics.
MH is a pharmacogenetic clinical syndrome that, in its classic form, occurs during anesthesia with volatile halogenated alkanes such as halothane, isoflurane/sevoflurane, /desflurane, and/or administration of the depolarizing muscle relaxant succinylcholine. The fulminant MH episode observed clinically produces muscle hypermetabolism with rapidly increasing body temperature, by as much as 1°C in 5 minutes, and extreme acidosis as a result of acute loss of control of intracellular ionized calcium (Ca 2+ ). It is the sustained high levels of sarcoplasmic Ca 2+ that rapidly drives skeletal muscle into a hypermetabolic state that may proceed to severe rhabdomyolysis. Although MH was initially associated with a mortality rate of 60%, earlier diagnosis and the use of dantrolene have reduced the mortality to less than 1.4%. Current cases of MH are restricted in severity because of diagnostic awareness, early detection through end-expired carbon dioxide (CO 2 ), the use of less potent anesthetic triggers, and prior administration of drugs that attenuate the progression of the fulminant episode. Estimates of the incidence of fulminant MH vary widely from one case per 10,000 to 1:250,000 anesthetics administered. The prevalence of MH events in Japan was calculated to be between 1:60,000 and 1:73,000. However, the prevalence of MH mutations within kindred known to transmit MH-susceptibility (MHS) mutations may be as high as 1:2000. Males appear to be more susceptible to developing a clinical MH episode than females. A gender difference in MHS has also been demonstrated in knock-in mice expressing human MH mutation RyR1-T4825I. The pediatric population accounts for 52.1% of all MH reactions.
Between 50% and 80% of genotyped patients who have had a clinical MH syndrome and a positive muscle biopsy have had their disease linked to one of more than 230 mutations in the type 1 ryanodine receptor (RyR1; sarcoplasmic reticulum [SR] Ca 2+ release channel) gene and four mutations in L-type Ca 2+ channel (Ca V 1.1), the pore subunit of the slowly inactivating L-type Ca 2+ channel encoded by Calcium Voltage-Gated Channel Subunit Alpha1 S (CACNA1S) (also referred to as the dihydropyridine receptor [DHPR]). The genetics of MHS and the related abnormal function of RyR1, the DHPR, and associated proteins are being investigated at the molecular biologic level, with a porcine model and several new mouse models providing intricate details about the etiology of the disorder. Parallel studies in humans are limited by scarce material for scientific study and are complicated by the fact that phenotypes within a single genotype vary as a result of sex, age, genetic, epigenetic, and environmental modifiers.
Public education and communication in the United States are provided by Malignant Hyperthermia Association of the United States (MHAUS, 11 E. State Street, P.O. Box 1069, Sherburne, NY 13460, U.S.A.; telephone: (+1) 607-674-7901; fax: (+1) 607-674-7910; e-mail: info@mhaus.org ; website: http://www.mhaus.org ), and by emergency consultation with the MH Hotline (1-800-MHHYPER, or 1-800-644-9737). The North American Malignant Hyperthermia Registry (NAMHR), a professional subsidiary of MHAUS, collates findings from muscle biopsy centers in Canada and the United States (NAMHR, 1345 SW Center Drive, P.O. Box 100254, Gainesville, FL 32610, U.S.A.); telephone: (+1) 888-274-7899; fax: (+1) 352-392-7029.; website http://anest.ufl.edu/namhr/ ).
Between 1915 and 1925, one family experienced three anesthetic-induced MH deaths with rigidity and hyperthermia and was puzzled for decades regarding the cause of these deaths. MHS was eventually confirmed in three descendants by in vitro muscle biopsy tests. In 1929, Ombrédanne described anesthesia-induced postoperative hyperthermia and pallor in children accompanied by significant mortality but did not detect any familial relationships. Critical worldwide attention to MH began in 1960 when Denborough and associates reported a 21-year-old Australian with an open leg fracture who was more anxious about anesthesia than about surgery because 10 of his relatives died during or after anesthesia. Denborough and colleagues initially anesthetized him with the then-new agent halothane, halted it when signs of MH appeared, successfully treated the symptoms, aborted the syndrome, and subsequently used spinal anesthesia. Further evaluations by George Locher in Wausau, Wisconsin, and Beverly Britt in Toronto, Canada, led to the discovery that MH risk was indeed familial. It was also found that the cause of the syndrome was the result of skeletal muscle involvement rather than central loss of temperature control by the recognition of increased muscle metabolism or muscle rigidity early in the syndrome, low-threshold contracture responses, and elevated creatine kinase (CK) values.
Interestingly, a similar syndrome was discovered in swine inbred with breeding patterns designed to produce a rapid growth rate and superior muscle development (e.g. , Landrace, Piétrain, Duroc, and Poland China). Porcine stress syndrome , which is associated with increased metabolism, acidosis, rigidity, fever, and death from rapid deterioration of muscle and results in pale, soft, exudative pork, can be triggered by any stress, such as separation, shipping conditions, weaning, fighting, coitus, or preparation for slaughter, and had become a significant problem for meat production. In 1966, Hall and coworkers reported that a syndrome that appeared to be identical to MH could be induced in stress-susceptible swine by the administration of halothane and succinylcholine. The cause of this syndrome in pigs was discovered to be a single missense mutation in RyR1 , and all susceptible swine have the same Arg615Cys mutation in the SR calcium release channel RyR1.
In 1975, Harrison described the efficacy of dantrolene in preventing and treating porcine MH, which was rapidly confirmed in humans by a multihospital evaluation of dantrolene used to treat anesthetic-induced MH episodes. Today, dantrolene still remains the primary pharmacologic approach for successful MH therapy.
MH is a syndrome caused by dysregulation of excitation-contraction (EC) coupling in skeletal muscle. Normal muscle contraction is initiated by nerve impulses arriving at the neuromuscular junction (i.e. , the motor end plate) that trigger the release of acetylcholine from the nerve terminal. Acetylcholine activates nicotinic cholinergic receptors (nAChR), nonselective cation channels located at the postsynaptic neuromuscular junction, that are essential for local depolarization of the surface muscle membrane (sarcolemma) and initiating action potentials that propagate rapidly along the sarcolemma of muscle cells. Invaginations of the sarcolemma (termed transverse or T tubules) act as conduits to rapidly and uniformly direct-action potentials deep within the myofibrils where they transduce a conformational change in the “voltage sensor” protein Ca V 1.1. A central T-tubule is flanked on both sides by a terminal cisternae element from the SR that contains the Ca 2+ release channels (RyR1). Conformational changes in Ca V 1.1 residing within the T-tubule are mechanically transmitted to RyR1 residing in the junctional face of the SR. More specifically, physical coupling of four Ca V 1.1 (dihydropyridine receptor) units to every second RyR1 channel form linear arrays at specialized junctions ( triadic junctions ) that are essential for linking electrical signals at the T tubules with the release of Ca 2+ stored within the SR. Release of SR Ca 2+ causes the free, cytoplasmic (sarcoplasmic) Ca 2+ concentration to increase from 10 –7 M to about 10 –5 M. This released Ca 2+ binds to contractile proteins (troponin C and tropomyosin) in the thin filament to expose myosin’s actin binding sites which allow shortening and force development by the muscle fibers (i.e. , muscle contraction). The entire process is termed excitation-contraction coupling (EC coupling) Intracellular Ca 2+ pumps (i.e. , sarcoplasmic/endoplasmic reticulum Ca 2+ -adenosine triphosphatase [ATPase], or SERCA) rapidly sequester Ca 2+ back into the SR lumen, and muscle relaxation begins when the Ca 2+ concentration falls below 10 –6 M and ends when the resting sarcoplasmic Ca 2+ concentration is restored to 10 –7 M. Because both contraction and relaxation are energy-related processes that consume adenosine triphosphate (ATP), knowing the molecular events contributing to EC coupling and the subsequent relaxation phase is essential to understanding the cause of MH ( Fig. 35.1 ). Clinical and laboratory data from humans, swine, and mice with knock-in mutations indicate that the fulminant MH syndrome is associated with a persistent increase in the concentration of sarcoplasmic Ca 2+ . The increased activity of pumps and exchangers trying to correct the increase in sarcoplasmic Ca 2+ associated with triggered MH increases the need for ATP, which in turn produces heat. Thus the common etiological feature of the disorder is hyperthermia. The rigidity that is frequently seen during a fulminant MH episode is the result of the inability of the Ca 2+ pumps and transporters to reduce the unbound sarcoplasmic Ca 2+ below the contractile threshold (10 –6 M). Dantrolene is an effective therapeutic for treatment of fulminant MH because it reduces the concentration of sarcoplasmic Ca 2+ to below contractile threshold. However, the pathway by which dantrolene lowers sarcoplasmic Ca 2+ is complex and still not fully understood. Dantrolene’s ability to suppress Ca 2+ release from SR appears to depend on elevated sarcoplasmic Mg 2+ concentration ; however the drug also attenuates depolarized-triggered Ca 2+ entry mediated by Ca V 1.1, which is exacerbated in MHS muscle cells and MH normal muscle cells exposed to ryanodine. Thus whether dantrolene directly inhibits RyR1 or requires additional intermediates within the triad junctions remains to be clarified.
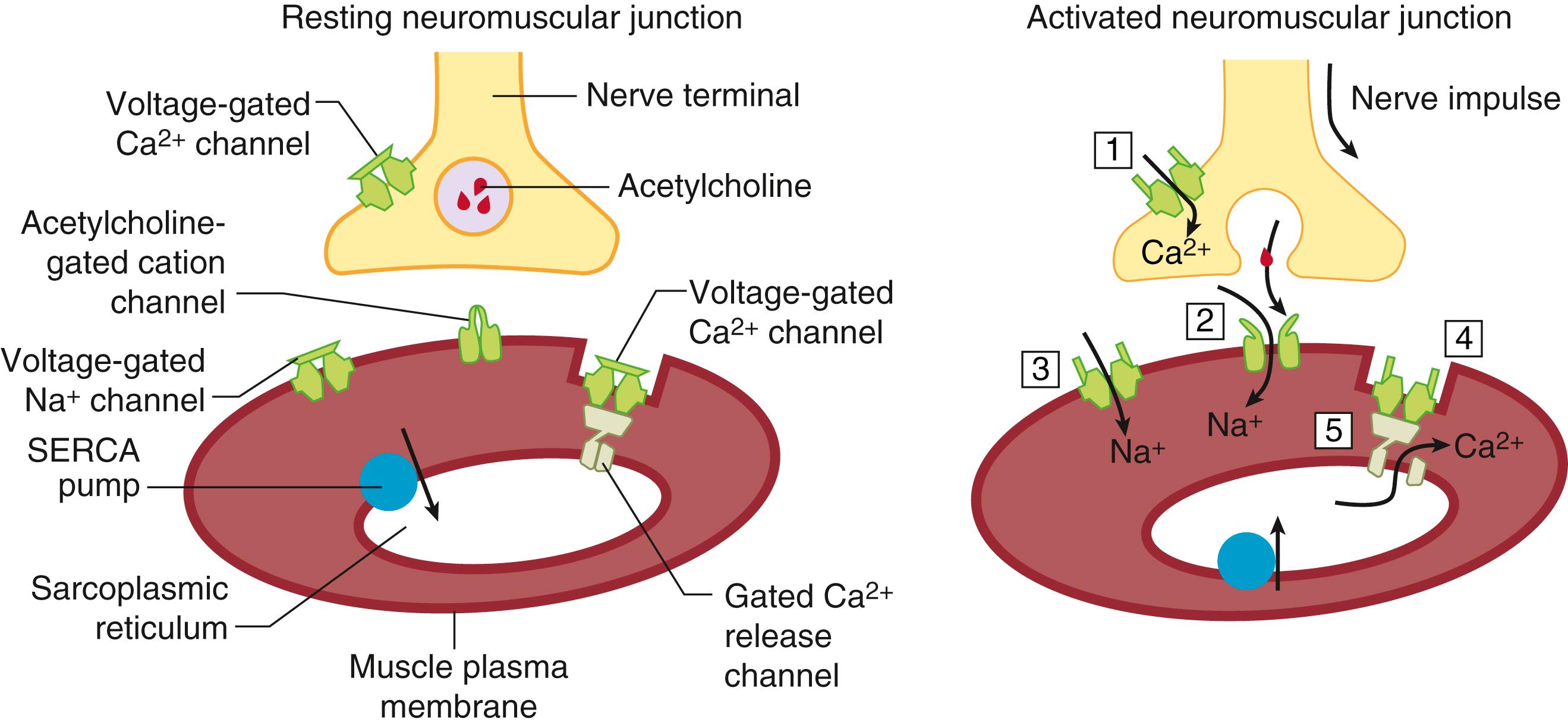
Ryanodine receptors (RyRs) within the muscle are synonymous with the junctional foot protein/SR calcium release channel, and are so named because they specifically bind the toxic plant alkaloid ryanodine, which can activate or inhibit the channel depending on its concentration. In all mammals there are three RyR isoforms. In humans, they are encoded by three genes located on chromosomes 19q13.1, 1q42.1-q43, and 15q14-q15, for the “skeletal” (RyR1), “cardiac” (RyR2), and “brain” (RyR3) isoforms, respectively. Each functional RyR is a homotetramer consisting of four identical subunits (∼5000 amino acids each), and an accessory protein, calstabin 1 (FK506 12-kd binding protein [FKBP12]). . The total mass of the tetramer exceeds 2 mega-Daltons. Thus it is one of the largest known proteins and the largest known channel in mammalian species. Evidence of direct coupling of Ca V 1.1 and RyR1 has been demonstrated both by expressing chimeric Ca V 1.1/Ca V 1.2 cDNA in dysgenic myotubes that lack constitutive expression of Ca V 1.1 and chimeric RyR1/RyR2/3 cDNA in dyspedic myotubes that lack constitutive expression of RyR1, 2, and 3. Such studies have provided compelling evidence that the cytoplasmic region between repeats II and III (i.e. , cytosolic II-III loop) of Ca V 1.1 contains a stretch of 46 amino acids (L720 to Q765) and multiple regions of RyR1 that are essential for engaging bidirectional signaling between Ca V 1.1 and RyR1.
In the last two decades, our understanding of EC coupling has increased significantly by identifying protein-protein interactions that regulate both the release and sequestration of Ca 2+ within skeletal muscle. The elemental unit of function has been named the Ca 2+ release unit (CRU), and it is localized within junctional regions of T-tubule and SR membranes. The CRU is a macromolecular assembly of interacting proteins that participate in regulating EC coupling. RyR1 is a high-conductance channel that regulates release of SR Ca 2+ and is the central component of the CRU. The functional RyR1 tetramer anchored within the SR membrane physically spans the junctional space to interact with tetrads composed of four voltage-activated Ca V 1.1 subunits within the T-tubule membrane. This physical interaction engages a form of bidirectional signaling that tightly regulates the function of both proteins. Moreover, interaction of Ca V 1.1 and RyR1 does not occur in isolation, but are further subject to regulation by a number of proteins localized within the triad junction, including Homer 1, which physically binds and functionally couples target proteins, calstabin 1, triadin, junctin, Mg29, junctophilin 1 and 2, calsequestrin, calmodulin, STAC 3, the catalytic and regulatory subunits of protein kinase A, and protein phosphatase 1. . It is likely that this list is not complete and that there are other critical components which make up this tightly regulated macromolecular complex. More importantly, there is increasing experimental evidence that mutations found in RyR1 ( MH RyR) or Ca V 1.1 ( MH Ca V 1.1) can alter protein-protein interactions in the CRU, as well as alter the functional fidelity of bidirectional signals.
In the presence of certain chemical substances, MH mutations in RyR1 or DHPR cause severe dysregulation of RyR1 channel function. This can be seen in vitro as a heightened sensitivity to volatile anesthetics, 4-chloro- m -cresol, caffeine, ryanodine, and potassium depolarization. Chemically induced dysfunction of the RyR1 complex appears to be the principal cause of triggering uncontrolled skeletal muscle metabolic acidosis (aerobic and glycolytic), rigidity, and hyperkalemia, but the mechanisms governing the syndrome are unclear. Also unclear is the relationship among exertional heat illness, exertional rhabdomyolysis, and MHS, an area that requires more investigation and, if possible, controlled clinical studies.
Two essential cations greatly shape the kinetics and magnitude of Ca 2+ release in response to depolarizing triggers: Ca 2+ itself and Mg 2+ . The normal RyR1 complex responds to Ca 2+ in a biphasic manner. First, Ca 2+ activates the channel in a graded manner between 100 nM and 100 μM, whereas higher concentrations inhibit channel activity. This biphasic action is thought to occur via binding of Ca 2+ to two classes of regulatory sites on RyR1, a high-affinity stimulatory site and a low-affinity inhibitory site. Mg 2+ -induced inhibition is the second important physiologic regulator of RyR1 activity in skeletal muscle. Mg 2+ inhibits RyR1 in a cooperative manner (n H ≈ 2; 50% inhibitory concentration [IC 50 ] ≈ 650 μM). It is likely that Mg 2+ acts by competing with Ca 2+ at its activator sites and by binding to yet unidentified low-affinity inhibitory sites. It is possible that MH mutations introduce allosteric instability into the RyR1 complex which leads to a reduction of inhibition rather than directly altering the binding properties of Ca 2+ or Mg 2+ , or both, at the activator or inhibitor sites. Therefore hypersensitivity to pharmacologic agents is likely to be closely tied to altered responses to physiologic ligands. However, whether MHS channels are primarily hyposensitive to inhibition by Mg 2+ or Ca 2+ (or both), are hypersensitive to activation by Ca 2+ , or exhibit altered sensitivities in both directions to both ions seems to be highly dependent on the location of the MH mutation. Studies have also pursued the “leaky channel” hypothesis by examining SR preparations from homozygous R615C MHS pigs and heterozygous R163C and C512S mice. They observed a significantly lower Ca 2+ loading capacity (38%, 23%, and 22% lower than matched wild type mice, respectively) primarily mediated by the presence of leaky channels that remain active even with 100 nM extravesicular Ca 2+ . Recent studies indicated that expression of Ca V 1.1 represses the basal activity of the ryanodine-insensitive RyR1 leaky state. It is important that MHS mutations appear to not only alter bidirectional signaling during EC coupling and inherent regulation of RyR1 channel functions, but also weaken negative regulation conferred by Ca V 1.1 on RyR1 Ca 2+ leak under nontriggering conditions. These findings at the molecular and cellular level using knock-in MHS mice confirm earlier measurements made in porcine and human MHS muscles, myotubes, and myoball preparations and in dyspedic myotubes expressing MH RyR1 cDNAs, all of which have been shown to have chronically elevated resting cytoplasmic [Ca 2+ ] i .
Results from both functional and structural evidence suggests that long-range interdomain interactions between regions of RyR1 are involved in channel regulation by stabilizing protein conformations critical for normal channel transitions. A three-dimensional reconstruction of RyR1 by Samso and coworkers shows that the RyR1 architecture is designed to support long-range allosteric pathways such as coupling with Ca V 1.1 and binding to ligands such as calmodulin and FKBP12. This structural model for gating has been recently confirmed at molecular scale resolution by several laboratories.
Although the majority of mutations that confer MHS reside in the RyR1 gene, three mutations in the CACNA1S gene encoding for the Ca V 1.1 subunit of skeletal muscle have been linked to human MHS. The Arg1086His mutation in the intracellular loop connecting homologous repeats III and IV of Ca V 1.1 represented the first MH-causing mutation so far identified in a protein other than RyR1. Physiological characterization of the R1086H mutation further demonstrated that sensitivity of RyR1 activity was significantly enhanced by membrane depolarization or by pharmacologic activators of RyR1 (e.g. , caffeine). In addition, Pirone and associates have identified an MHS-causing Thr1354Ser mutation in the S5-S6 extracellular pore-loop region of the homologous repeat IV of Ca V 1.1 Expression of the T1354S mutation also accelerated L-type Ca 2+ current kinetics and also contributed to an increase in RyR1-mediated Ca 2+ release. The Arg174Trp Ca V 1.1 MH mutation occurs at the innermost basic residue of the IS4 voltage-sensing helix, a residue conserved among all Ca V channels. Unlike the other Ca V 1.1 MHS mutations, homozygous expression of R174W completely ablates the L-type current, but despite this, has no influence on normal EC coupling. In murine studies, muscle fibers from Het R174W animals verify the increased sensitivity of Ca 2+ release to caffeine and halothane compared with myotubes expressing wild type Ca V 1.1, but whether this mutation is sufficient to confer anesthetic- or heat-triggered fulminant MH remains to be tested.
Other cellular processes can affect MH episodes. It has been demonstrated that concurrent administration of nondepolarizing neuromuscular blocking drugs at the same time as triggering agents can delay or prevent the onset of clinical MH syndrome. Pretreatment of MHS pigs with sufficient nondepolarizing neuromuscular blocking agent, which is used to completely abolish muscle twitch elicited by electrical stimulation of the nerve, prevented halothane from triggering the clinical syndrome for 90 minutes, the longest time point tested. However, in the continued presence of halothane, when function of the neuromuscular junction was restored by administration of the cholinesterase inhibitor neostigmine, clinical MH was triggered immediately. This suggested a close relationship between functional neuromuscular junctions or depolarization of the sarcolemma (or both) and the clinical syndrome.
In myotubes, sarcolemmal excitation-coupled Ca 2+ entry (ECCE) is sensitive to the conformation of the RyR1 and is enhanced by several mutations in RyR1, including MH mutations. ECCE appears to be an inherent property of Ca V 1.1 during long or repetitive depolarization of myotubes, possibly mediated by shifting Ca V 1.1 to the mode 2 gating conformation. Nevertheless, enhanced ECCE in MHS muscle may contribute to an increased sensitivity to depolarization and appears to be one target for dantrolene’s abrogation of responses to both electrical and potassium chloride depolarization. Although CACNA1S expression undergoes developmental switching to a splice variant that downregulates Ca 2+ current density of Ca V 1.1 channels in adult fibers, mutations that maintain Ca 2+ current density more similar to those measured with embryonic myotubes has recently been shown to promote muscle pathology.
In addition to ECCE, classic store-operated capacitive Ca 2+ entry pathways similar to the store-operated Ca 2+ entry (SOCE) seen in nonexcitable cells have been shown to be present in skeletal muscle and appear to be more active in MHS muscles both at rest as a response to chronic store depletion and during an MH crisis. These SOCE channels have also been suggested to be a target for dantrolene, but this has not been validated by other studies. Together, these data suggest that MH RyRs or MH Ca V 1.1 assume a conformation that enhances Ca 2+ entry via ECCE or SOCE (or both). This enhanced entry, when combined with decreased sensitivity of MH RyRs to Ca 2+ and Mg 2+ inhibition, could provide cellular conditions that heighten sensitivity to triggering agents and perpetuate the fulminant clinical MH syndrome.
Dantrolene is the only medication that has been shown to be effective in reversing the symptoms of MH. Preadministration of dantrolene will also prevent the development of fulminant MH in homozygous pigs or MH mice when exposed to a triggering stimulus. Dantrolene sodium is a hydantoin derivative (1-[5-(4-nitrophenyl)-2-furanyl]methylene]imino]-2,4-imidazolidinedione) that does not block neuromuscular transmission, but causes muscle weakness by direct muscular action. The properties of dantrolene have been closely correlated with its ability to reduce efflux of Ca 2+ from the SR in vitro. Dantrolene (20 μM) counteracts the effect of reduced Mg 2+ inhibition in MH-affected muscle. Dantrolene (20 μM) can inhibit the enhanced sensitivity to caffeine seen in MH muscles, and both dantrolene and its more water-soluble analog azumolene (150 μM) have been shown to reduce depolarization-induced release of Ca 2+ , both in muscle and in triadic vesicles. The idea that dantrolene suppresses SR Ca 2+ release as a result of direct interactions with RyR1 is somewhat controversial. Paul-Pletzer and associates demonstrated that [ 3 H]azidodantrolene specifically labels the amino terminus of RyR1 defined by the 1400-amino acid residue N-terminal calpain digestion fragment of RyR1. More detailed analysis further localized the [ 3 H]azidodantrolene binding site to a single domain containing the core sequence corresponding to amino acid residues 590 through 609 of RyR1. However, to date, we lack evidence of a direct action of dantrolene on single RyR1 channels studied in lipid bilayers, even though they are reconstituted with calstabin 1, ATP, and activating concentrations of Ca 2+ , which suggests that dantrolene’s main action is to alter key protein-protein interactions. The recent discovery that inhibition of SR Ca 2+ release by dantrolene requires Mg 2+ may help resolve the controversy of the conflicting observations on dantrolene inhibition of RyR1 channel activity in Mg 2+ -free bilayer experiments.
RyR1 mutations have been found in 50% to 80% of patients and relatives who are labeled MHS by positive contracture tests and in almost all families with central core disease (CCD) and King-Denborough syndrome (KDS). More than 210 missense mutations and 8 deletions associated with MH have thus far been detected. Another 29 missense mutations are associated with CCD and multiminicore disease ( MmD ) in patients with unknown MH testing status. Interestingly, 40% of missense RyR1 mutations occur at CpG dinucleotide sequences. Five other loci (17q21-24, 1q32, 3q13, 7q21-24, and 5p) have been linked to families with both positive contracture tests and an unusual response to anesthesia, and have been designated MHS loci 2 through 6, respectively. However, of these five, the only gene that has been shown to be associated with MH is CACNA1S , which codes for Ca V 1.1 (the α 1S -subunit of DHPR) in the MHS3 locus. Two causative mutations in this gene are linked to less than 1% of MHS families worldwide. In some of the other loci, all genes within the locus have been ruled out as causing susceptibility to MH. Hence for practical reasons, the RyR1 gene remains the primary target for current clinical genetic analysis.
The missense mutations associated with MHS, CCD, or, in some cases, both, are dispersed throughout the coding region of the RyR1 gene, and all allow transcription of a protein that is putatively functional. Until recently, it was thought that most RyR1 mutations were clustered in three “hot spots:” between amino acid residues 35 and 614 (MH/CCD region 1), between amino acid residues 2163 and 2458 (MH/CCD region 2) in the sarcoplasmic foot region of the protein, and between amino acids 4643 and 4898 in the carboxyl-terminal transmembrane loop or pore region (MHS/CCD region 3) ( Fig. 35.2 ). It appears that the supposition that there were “hot spots” was simply due to bias in sample analysis inasmuch as the missense mutations associated with MH or CCD (or both) are scattered over 54 of the 107 exons of RyR1 . Approximately 41% of reported MH mutations are found in multiple families. CCD mutations are predominantly found in the C-terminal region of the gene (exons 85-103), and only 10 mutations (17%) have been described in more than one family: R4861H ( n = 14), V4849I ( n = 9), I4898T ( n = 7), L4824P ( n = 4), A4940T ( n = 4), G4638D ( n = 3), R4893W ( n = 3), R4861C ( n = 2), R4893Q ( n = 2), and G4899E ( n = 2).
![Fig. 35.2, Schematic representation of the triad junction of skeletal muscle shows the junctional foot protein (ryanodine [RyR1] receptor) and its associated proteins. In skeletal muscle, the α 1S -subunit of the dihydropyridine receptor (DHPR) participates in excitation-contraction coupling. These physical links transmit essential signals across the narrow gap of the triadic junction that activate the RyR1 and release Ca 2+ from the sarcoplasmic reticulum. Fig. 35.2, Schematic representation of the triad junction of skeletal muscle shows the junctional foot protein (ryanodine [RyR1] receptor) and its associated proteins. In skeletal muscle, the α 1S -subunit of the dihydropyridine receptor (DHPR) participates in excitation-contraction coupling. These physical links transmit essential signals across the narrow gap of the triadic junction that activate the RyR1 and release Ca 2+ from the sarcoplasmic reticulum.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/NeuromuscularDisordersIncludingMalignantHyperthermiaandOtherGeneticDisorders/1_3s20B9780323596046000353.jpg)
The true ethnic distribution of MH and CCD is difficult to ascertain. MH and CCD have been reported in Western populations predominantly, but this is likely the result of the manner and frequency in which cases are reported. It does appear that some mutations are clustered in a given region of the world, but the distribution and frequency appear to be somewhat population specific. In the United Kingdom, 69 RyR1 mutations have been discovered, 25 of which are found only in a single family. G2434R is found in approximately 40% of the 434 mutation-positive MH families investigated in the United Kingdom, with the next most common mutations being T2206M (10%) and G341R (8%). In Switzerland, V2168M and I2336H are the predominant mutations, and in Germany, R163C (MH and CCD), R614C (MH), T2206M (MH), G2434R (MH), and R2454H (MH) have each been detected in five or more independent cases. G341R and R614C are common in France, and R614C has also been found in several MH families from Italy and Canada. G341R has been found frequently in Belgium. The mutation common to Europe and North America is G2434R, which occurs in 4% to 7% of European and 5.5% of North American families. Single-family mutations are the most common mutations reported in Japanese, Chinese, Taiwanese, Australian, and New Zealand MH families with the exception of the R163C mutation reported in a large population in rural New South Wales in Australia and the T4826I mutation which is found in numerous families in the Maori population of New Zealand. However, despite these two exceptions, it is likely that the reason for unique family mutations in Asia and Australasia may reflect the small number of cases investigated there. Because genetic screening in European and North American studies has predominantly targeted only regions 1 and 2 of the original hot spots in the gene, the absence of RyR1 mutations in some of the screened population could be explained by RyR1 mutations located outside these two regions or by involvement of other genes.
Inheritance of human MH can no longer be considered to be solely autosomal dominant with variable penetrance because more than one MH-linked mutation has been identified in some probands and families. Six non-consanguineous families harbor at least two RyR1 mutations that have both been linked to MHS, and in two of those families, one is an RyR1 mutation and the second is a Ca V 1.1 mutation. Although MHS homozygotes are common in affected pigs, they are rare in humans and only found in 50% of currently available transgenic mouse populations. The known MHS homozygous humans also appear clinically normal, but exhibit stronger responses to in vitro contracture test (IVCT) and caffeine/halothane contracture tests (CHCTs) than heterozygous individuals do. Homozygosity of two MH mutations in “hot spot” 1 leads to perinatal lethality in mice. Double heterozygous individuals do not appear to show any additive effect of the second mutation on IVCT.
The gold standard for diagnosis of MH is the halothane and caffeine muscle contracture test, also known as the IVCT or the CHCT. There are two protocols developed by the European Malignant Hyperthermia Group (EMHG) and the North American Malignant Hyperthermia Group (NAMHG), respectively. The two protocols are similar, but not identical. For the purpose of differentiation, we designate IVCT to the EMHG protocol and CHCT to the NAMHG protocol.
For IVCT, the muscle biopsy will be performed on the quadriceps (either vastus medialis or vastus lateralis) and consists of three parts: a static caffeine test, a static halothane test, and a dynamic halothane test. For the static caffeine test, a stepwise increased concentration (0.5, 1, 1.5, 2, 3, 4, and 32 mmol/L) is applied. The lowest concentration of caffeine which produces a sustained increase of at least 0.2 g in baseline tension is reported as the caffeine threshold. Then, the halothane threshold is obtained using the same method by exposing the muscle to halothane concentrations of 0.5%, 1%, 2%, and 3% v/v. The dynamic halothane test is performed with the muscle stretched at a constant rate of 4 mm/min to achieve a force of approximately 3 g and held at the new length for 1 min for a 3 min exposure to halothane. For each cycle, halothane concentration will be increased from 0.5%, 1%, 2%, to 3% v/v: volume (solute) per volume (solvent). The concentration of halothane which produces a sustained increase of at least 0.2 g in the muscle tension compared to the pre-halothane control is defined as the dynamic halothane threshold. The IVCT protocol classifies the patients into three groups: MH Susceptible (MHS HC ) group with a caffeine threshold at the caffeine concentration of 2 mmol or less and a halothane threshold of 2% v/v halothane or less; MH Normal (MHN) group with a caffeine threshold at the caffeine concentration of 3 mmol or more without a halothane response at 2% v/v; MHS H and MHS C groups which describe individuals that used to be classified as MHE (equivocal) who are only responsive to either halothane or caffeine alone. The MHE descriptor was dropped because use of the ‘equivocal’ label outside of its laboratory context has the potential to confuse patients and clinicians unfamiliar with its derivation.
For CHCT, a muscle biopsy can be taken from the following sites in the order of preference: (1) the vastus group, (2) the rectus abdominis, and (3) other muscle groups under special circumstances. Required tests include exposure of muscle to 3% v/v halothane alone and to incremental caffeine concentrations (0.5, 1, 2, 4 mmol, and 8.0 mmol if the response at 4 mmol is <1 g, and 32 mmol) alone. Optional tests include exposure of muscle to a combination of both 1% halothane and incremental caffeine concentrations, and to 2% v/v halothane alone. According to CHCT protocol, an individual is MHS when either of the halothane or the caffeine test is positive, and MHN when both tests are negative.
The sensitivity of IVCT was reported to be 99.0% (95% confidence interval [CI] 94.8%-100%) if the MHE group is considered susceptible and the specificity of IVCT was 93.6% (95% CI 89.2%-96.5%), while the sensitivity and specificity of CHCT was 97% (95% CI 84%-100%) and 78% (95% CI 69%-85%), respectively. Recently, fluoroquinolones and statins, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, were found to induce significant contractures in MHS muscle bundles, but not in MHN. Ondansetron and 3,4-methylenedioxymethamphetamine (MDMA) may also dose-dependently induce contracture or increase the sensitivity of the contractile apparatus to calcium in both MHS and MHN fibers. Modifications to the IVCT protocol by adding ryanodine or 4-chloro- m -cresol, a RyR-specific agonist, has been reported, but has not been included in the standard protocol. Furthermore, Metterlein and associates studied the possibility of replacing halothane with newer volatile agents in IVCT. At increasing concentration, except for sevoflurane, all newer volatile agents, including enflurane, isoflurane, and desflurane, induced significantly greater contractures in MHS muscle compared to MHN bundles. However, within the MHS muscle bundles, halothane produced significantly higher contractures, and was considered the strongest discriminator for MH using the IVCT protocol. A direct application of high sevoflurane concentration of 8%, instead of the stepwise application, has been shown to induce significantly stronger contractures in MHS subjects. Nevertheless, from the retrospective analysis of the Japanese MH database, there was no evidence between the severity of MH triggered by sevoflurane and isoflurane, or other agents, suggesting sevoflurane is a weak or weaker MH triggering agent.
Discordance has confounded linkage analysis worldwide. Examples include MHN patients carrying an RyR1 mutation associated with MH and MHS patients who do not carry the familial RyR1 mutation. Several explanations are possible, the most likely being that IVCT/CHCT is not clinically precise and that the thresholds for IVCT or CHCT are inexact. This would lead to errors in determining whether a patient was MHN or MHS. A second possibility is variable penetrance with possible allelic silencing, and a third is that individuals with discordance have mutations in other unknown genes or modifier genes that affect the function of RyR1 and its phenotypic penetrance. The discrepancy between the incidence estimates of MH events and the prevalence estimates also points to possible epigenetic factors at work. Carpenter and associates have suggested that the severity of MHS may be related to the RyR1 variant and mutation found within the highly conserved regions of RyR1 gene. The rarity of large kindreds with MH makes linkage analysis and understanding variability in clinical manifestations difficult. Robinson and associates demonstrated by the transmission disequilibrium test that loci on chromosomes 5 and 7 and, to a lesser extent, loci on chromosomes 1 and 7 influence susceptibility to MH.
In 2000, the European MH group (EMHG) formulated guidelines for RyR1 mutation screening with linkage data to other loci for some MH families, but all MH investigators emphasized the vital role of IVCT in the diagnosis of MH. These guidelines for screening have reduced the number of relatives requiring contracture testing without increasing the risk of misdiagnosis. In 2015, the EMHG published a revision of the guidelines for the investigation of MHS. This updated guideline provided a detailed patient referral criteria and clinical interpretation of IVCT results ( https://www.emhg.org/testing-for-mh-1 ).
Only a small number of MHS families have been investigated extensively in North America by phenotyping, linkage analysis, and screening of specific genes. Collaborative protocols over the past several years between MH biopsy centers and molecular biologists have screened 209 unrelated MHS subjects for mutations in the RyR1 gene (see Distribution of RyR1 Mutations).
Larach and coworkers reported a 34.8% morbidity rate in 181 MH cases reported to the NAMHR between January 1987 and December 2006. They also reported that the occurrence was more frequent in young males (75%) (median age of 22.0) and 75% of these patients had undergone at least one general anesthetic with no observed signs of MHS. This underscores the complication of determining the prevalence of MHS in the absence of an inexpensive MH diagnostic test.
Fulminant MH is rare. Acute episodes of MH depend on four variables: a genetic (perhaps rarely acquired) predisposition, the absence of inhibiting factors, the presence of an anesthetic or nonanesthetic trigger, and the presence of environmental factors that could potentiate the action of one or more of the other three variables.
Anesthetic drugs that trigger MH include ether, halothane, enflurane, isoflurane, desflurane, sevoflurane, and depolarizing muscle relaxants, the only currently used of which is succinylcholine. Desflurane and sevoflurane appear to be less potent triggers than halothane and produce a more gradual onset of MH. The onset may be explosive if succinylcholine is used. MHS swine were traditionally screened by induction with a volatile anesthetic, which led to pronounced hind limb rigidity within 5 minutes, frequently sooner. Prior exercise even an hour before induction of anesthesia increased the severity and hastened the onset of rigidity in swine. Similarly, in the new knock-in mouse models, the onset of limb rigidity after commencing exposure to volatile anesthetics is very rapid. There are also several modifying factors that are more likely to be present in humans than in pigs or mice and can alter (or even prevent) the onset of clinical MH. Mild hypothermia and preadministration of barbiturates, tranquilizers, propofol, or nondepolarizing neuromuscular blockers delay or prevent the onset of MH in MHS humans, thus making them respond less predictably than swine or MH knock-in mice. There have been many instances in which fulminant MH has been reported in patients who have previously tolerated potent triggers without difficulty. The reason behind why this occurs is unknown, but it is likely to be related to prior or concurrent administration of drugs that prevent or delay onset of the syndrome, as described earlier, or unknown environmental influences that help provoke the positive incident. Thus onset of the syndrome in humans is extremely variable both in initial symptoms and in the time of onset of the syndrome. Its onset is so variable that making the diagnosis in the setting of a clinical anesthetic can be quite difficult. Although not perfect, the clinical grading scale developed by Larach and colleagues is a useful way for clinicians to retrospectively determine whether a patient who responded abnormally to anesthesia is in any way likely to actually have had a clinical MH episode. However, MH is most easily diagnosed prospectively by vigilance, recognizing its signs and symptoms, and knowing how to treat the syndrome.
The two classic clinical manifestations of fulminant MH syndrome may start in one of the following two scenarios.
Rigidity after induction with thiopental and succinylcholine, but successful intubation, followed rapidly by the symptoms listed after scenario 2.
Normal response to induction of anesthesia and uneventful anesthetic course until onset of the following symptoms:
Unexplained sinus tachycardia or ventricular arrhythmias, or both
Tachypnea if spontaneous ventilation is present
Unexplained decrease in O 2 saturation (because of a decrease in venous O 2 saturation)
Increased end-tidal PCO 2 with adequate ventilation (and in most cases unchanged ventilation)
Unexpected metabolic and respiratory acidosis
Central venous desaturation
Increase in body temperature above 38.8°C with no obvious cause
The usually muted onset of MH (scenario 2) is in most cases detected quickly by the development of tachycardia, increased levels of expired CO 2 , and muscle rigidity. It can be delayed for several reasons and may not be overt until the patient is in the recovery room. Once initiated, the course of MH can be rapid. When clinical signs such as increased expired CO 2 , muscle rigidity, tachycardia, and fever suggest MH, more than one abnormal sign must be observed before making the diagnosis because according to a metaanalysis of many reported cases, a single adverse sign does not usually indicate MH. The mechanism by which anesthetics and depolarizing muscle relaxants trigger MH is unsolved, but it cannot be ignored that they are etiologic agents and that early diagnosis is critical for successful treatment.
MH can be triggered by stress such as exercise and overheating, known as “awake” MH. Numerous anecdotal reports of MH-like episodes in humans after stressful situations were reported. Measurement of plasma catecholamine levels during exercise showed no differences between MHS and normal individuals. Therefore it is unlikely that these responses were provoked by sympathetic overdrive or catecholamine surge.
Wappler and associates reported RyR1 mutations in three of twelve unrelated patients with exercise-induced rhabdomyolysis (ER); and 10 of those same 12 patients produced abnormal contracture response with IVCT. One had an equivocal response. In susceptible swine, environmental stress such as exercise, heat, anoxia, apprehension, and excitement triggers fulminant MH (see History). These responses are related to muscle movement or to increased temperature. Increased ambient temperature triggers fulminant MH in four strains of heterozygous MH mice and in two homozygous strains. Epidemiologic studies have shown that exercise-induced symptoms, including rhabdomyolysis, may occur more frequently in MHS patients ; and an Arg401Cys RyR1 mutation was present in three cases of exercise-induced rhabdomyolysis. Other reports are largely anecdotal and relate heat stroke, sudden and unexpected death, unusual stress and fatigue, or myalgias to possible “awake” MH episodes. Stresses associated with these episodes include exercise and environmental exposure to volatile nonanesthetic vapors. In the United States, MHAUS provided recommendations of adverse effects of heat and exercise in relation to MHS.
A masseter spasm or trismus is defined as jaw muscle rigidity in association with limb muscle flaccidity after the administration of succinylcholine. The masseter and lateral pterygoid muscles contain slow tonic fibers that can respond to depolarizing neuromuscular blockers with a contracture. This is manifested clinically on exposure to succinylcholine as an increase in jaw muscle tone, and was well defined by van der Spek and associates. There is a spectrum of responses, for example, a tight jaw that becomes a rigid jaw and then a very rigid jaw ( Fig. 35.3 ). This jaw rigidity may occur even after pretreatment with a “defasciculating” dose of a nondepolarizing relaxant. If there is rigidity of other muscles in addition to trismus, the association with MH is absolute; anesthesia should be halted as soon as possible and treatment of MH begun.
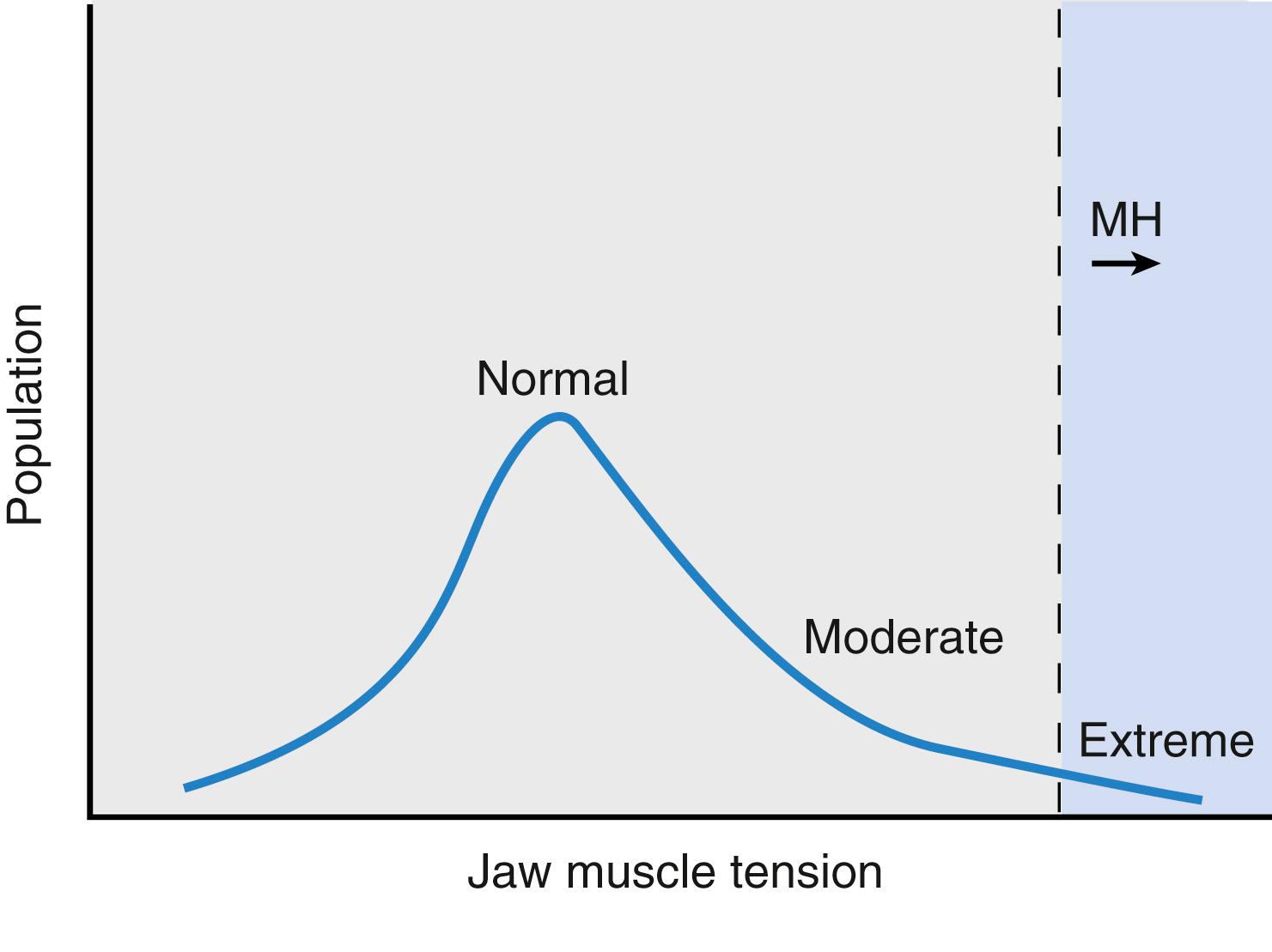
However, in more than 80% of patients with trismus but no rigidity of other muscles, it is a variant found in normal patients. If trismus occurs, proper monitoring should include end-expired CO 2 , examination for pigmenturia, and arterial or venous blood sampling for CK, acid-base status, and electrolyte levels, particularly potassium. Although scientifically unproven, it is thought that the initial tightness of the jaw and its duration may predict the gravity of the response. MHAUS recommends following CK and urine myoglobin for 36 hours with 6-hour intervals. Patients with masseter spasm should be observed closely for at least 12 hours.
CCD is a rare hereditary disease. It was first reported in 1956 by Magee and Shy. A recent population study in northern England revealed a prevalence of 1:250,000. In 1971, Engel and colleagues reported a related congenital myopathy, multicore disease. Subsequently, various designations of the terms were reported for the variations of the disease, including minicore myopathy and multiminicore myopathy. MmD is now the most official term for these variations sponsored by the European Neuromuscular Centre.
As mentioned, most CCD cases are due to dominant missense mutations in the RyR1 gene. Clinically, CCD patients present with muscle weakness of variable degree and histologically with central cores in the skeleton muscle type I fibers. MmD is considered a recessively inherited myopathy with severe axial weakness, while respirator, bulbar, and extraocular muscles are commonly affected. MmD has a heterogenous genetic association with a recessive mutation in the SEPN1 gene on chromosome 1p36 and in the RyR1 gene. Both type 1 and type 2 fibers may be affected.
Serum CK levels in CCD patients are often normal but may be elevated up to 6 to 14 times in rare cases. Muscle ultrasound often demonstrates increased echogenicity in the quadriceps muscle with relative sparing of the rectus muscle. This characteristic pattern of selective involvement can also be seen on the muscle MRI and has been reported in the patients with typical CCD, which seems to be distinctive to conditions linked to RyR1 locus.
The relationship between CCD and MHS is complex. A positive IVCT test has been confirmed in many patients with CCD, whereas MHS has been excluded in some. In consideration of the strong link and potential risk, it is advisable to consider all patients with CCD at risk for MH unless the patient has a negative IVCT. Although MHS has not been reported in SEPN1 -related myopathies, it is prudent to apply a nontriggering approach to MmD patients, given the potential risk in RyR1 -related MmD. Clinical MH reactions have been reported in MmD patients.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here