Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pain, defined as the conscious subjective experience associated with real or potential tissue damage, is a product of coordinated activity across large portions of the brain. In contrast, the concept of nociception refers to the objective neural processes triggered by nociceptive stimuli and does not imply consciousness. Therefore one important goal of neuroimaging is to understand how nociception leads to pain and explain what happens when pain appears to be dissociated from nociception, as observed in many chronic pain syndromes. This chapter discusses the non-invasive imaging modalities that can depict the structural and functional central nervous system (CNS) features associated with experimentally induced and chronic pain. The main objective of this chapter is to survey the brain imaging literature on these highly prevalent functional chronic pain syndromes, for which it is difficult to identify a clearly defined pathology. A more in-depth treatment of radiologic imaging techniques routinely used to identify CNS damage can be found in Chapter 20.
An overview of the most commonly used neuroimaging modalities can also be found in Box 10.1 , where we briefly survey the techniques routinely used to identify damage to the CNS, as well as functional techniques that can assess CNS activity as a function of experimentally induced or chronic pain, which will be the focus of this chapter. We will begin with a brief historical overview of functional magnetic resonance imaging (fMRI) studies of acute experimentally induced pain before examining how chronic pain differs from pain evoked by nociceptive stimuli. We will then turn to positron emission tomography (PET) studies of endogenous opioidergic and dopaminergic systems in chronic pain syndromes. Finally, we will review recent electroencephalographic (EEG) studies of acute and chronic pain. Overall, this chapter should provide the reader with a good overview of the cerebral underpinnings of pain, as well as how this knowledge may have contributed to change our conception of pain and how it could be harnessed to guide certain clinical decisions.
Neuroimaging techniques are defined by the obligatory processing of the data after acquisition to be interpreted visually as an image by a human clinician. The amount of processing required for the following imaging modalities is listed in approximately ascending order, starting with plain radiographs, which is the only modality that does not absolutely require processing.
Radiographs (XR): Penetrating ionizing radiation is used to distinguish radiopaque tissues from those that are radiolucent in a two dimensional view. Although simple radiographs of the cerebrum and spinal column can be used clinically to evaluate structural abnormalities in the osseous structures that house neural pathways, they do not reveal disruption of the neural soft tissues. These are most often used clinically to evaluate injury following trauma or to assess prior surgical interventions for hardware integrity and osseous fusion.
Computed Tomography (CT): Tomography is defined as an image produced by a penetrating wave, and in medicine, CT scans are a fast and relatively inexpensive method of imaging the brain and spinal cord. Although CT can visualize solid or aqueous structures with much higher resolution than a plain radiograph, it is still difficult to discern the characteristics of the soft tissues, and the amount of post-processing that can be conducted is limited. Resolution can be improved by the addition of intravenous or intrathecal contrast. The use of CT with intrathecal contrast is most commonly used when patients have spinal hardware in situ , which obscures the relevant anatomy under MRI.
Positron electron tomography (PET): This 3D imaging modality uses intravenous radionuclide tracing to assess tracer uptake in different organs or estimate neurotransmitter activity or receptor availability for ligands (e.g. opioids). Once taken up by tissues, the tracer decays into positrons that react with electrons in the body and emit photons. This is detected by sensors, and the processed images are often coupled with CT to form clinically relevant images and have recently been paired with magnetic resonance for improved resolution.
Magnetic resonance imaging (MRI): Structural MRI evaluates the white matter, gray matter, and cerebrospinal fluid of the cerebrum and spinal cord. In the presence of the magnet, the random motion of atoms within the body is brought into alignment. The radiofrequency coils of the MR machine produce a pulse of energy that slightly tilts the atoms away from their original alignment with the magnetic field. As they recess back to their original orientation, they emit a pulse of energy, which is captured by sensors and processed into an image. MRI can be used to form images with or without the use of paramagnetic contrast dye, and indications for contrast include suspicion for metastasis or hardware in situ that will cause non-contrast MRI to obscure relevant anatomy. Most MR machines in clinical use are those with a 1.5 Tesla or 3 Tesla magnetic field, with a higher Tesla strength correlating to higher image resolution.
T1 sequence : The spin-echo (T1) sequence is clinically preferred for MR studies with contrast in evaluation of perfusion of spinal tissues, most commonly to assess primary tumors for vascularization, indicative of high tumor grade, or to evaluate metastases for vascularity that would be amenable to endovascular ablation. It is also used for cerebral imaging in the evaluation of neurologic disorders and supratentorial lesions.
T2 Sequence : Fast spin-echo (T2) is the most common MRI sequence examined by pain physicians evaluating spinal anatomy. In this sequence, the tissues with high water content (e.g. the cerebrospinal fluid) are white/bright on the image, making areas of spinal stenosis or disc herniation highly visible.
STIR : Short tau inversion recovery has higher sensitivity but lower specificity than other conventional sequences in assessing spinal abnormalities because of infectious or neoplastic etiology in the spinal column. Clinically, this is most useful in the evaluation of the spinal cord for multiple sclerosis lesions, and in the vertebral bodies for compression fractures.
FLAIR sequence : The fluid attenuated inversion recovery (FLAIR) sequence is a further modification of the traditional spin-echo (T2) technique that suppresses the CSF signal and minimizes the contrast between gray and white matter. This improves the detectability and visibility of pathologic lesions adjacent to the CSF. This sequence is considered more sensitive in the evaluation of ischemia, neoplastic disease, and multiple sclerosis in the neuraxis.
Angiography : Both CT and MR angiography can be used to assess spinal blood supply for surgical planning or in the diagnosis of vascular pathologies, such as spinal dural arteriovenous fistulas.
Functional MRI (fMRI) and diffusion MRI (dMRI) have been increasingly used in research studies targeting acute and chronic pain populations. These methods represent the only objective method of measuring the subjective experience of pain, as it changes the mentation of the individual. Although these techniques show great promise in future clinical applications, at the time, they are purely used in investigational pursuits.
fMRI : fMRI involves the acquisition of T2*-weighted images of the brain that are sensitive to the proportion of oxygenated and deoxygenated hemoglobin (also called BOLD signal), which is related to brain activity. In task-based fMRI , experimental pain stimuli (thermal, mechanical, electrical, or chemical) can be repeatedly administered to participants for several tens of minutes. The general linear model is then used to estimate the amplitude of the hemodynamic response function, which is the influx of oxygen that follows neuronal activity in small parcels of the brain called voxels. Each voxel had a volume of approximately 8-64 mm 3 . A typical 3 × 3 × 3 mm voxel (27 mm 3 ) contains about 5.5 million neurons. In resting state fMRI (rs-fMRI), participants are scanned while still lying in the scanner for periods of 5–30 min or more. Then, correlations in the time series of functional images can be computed for one or several pairs of brain regions to assess their functional connectivity. Finally, in ASL , brain perfusion is measured by “tagging” water molecules with a radiofrequency pulse in certain slices of the brain and then tracking them as they release their energy in other regions of the brain. Therefore ASL can track metabolic activity related to more diffuse states such as chronic pain, but one current problem is its relatively low signal-to-noise ratio.
Diffusion-weighted imaging (DWI) : DWI measures the diffusion of water molecules in the brain. Diffusion tensor imaging (DTI), a special type of DWI, has been used extensively to map white matter tracts in the brain. Metrics derived from DTI include mean diffusivity (MD), which is the degree to which water diffuses at each location, and fractional anisotropy (FA), which is the coherence of this diffusion in a particular direction.
The study of the cerebral correlates of pain began with the investigation of patients with brain lesions causing abnormal pain perception. , The main conclusion from these studies was that pain was principally perceived in the thalamus, with a limited role in the cortex. However, as neurophysiologists began to follow the projections of nociceptive pathways up to the brain, the role of cortical structures in pain perception has gradually become more important. With the advent of functional neuroimaging techniques in the late 1980s—first PET and then fMRI—researchers became endowed with a means to non-invasively probe the human brain in search for areas responding to noxious stimuli. Using these techniques, several studies found that high-intensity noxious stimuli (thermal, electrical, mechanical, or chemical) systematically produced more activity than low-intensity innocuous stimuli in a set of regions comprising the thalamus, anterior cingulate cortex (ACC), insula (Ins), primary and secondary somatosensory cortices (S1 and S2), and prefrontal cortex (PFC) ( Fig. 10.1 ). ,
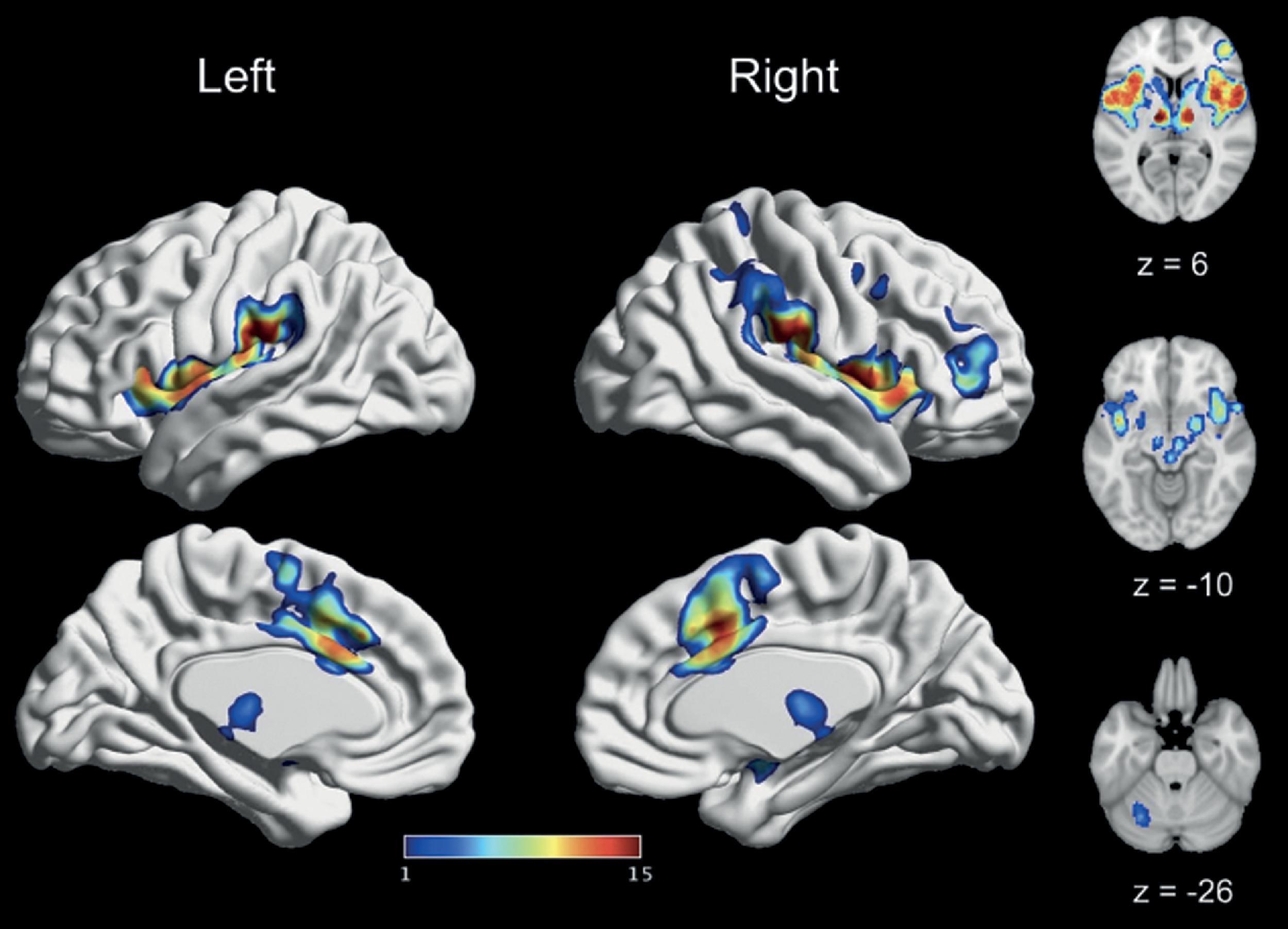
Inspired by Ronald Melzack’s terminology, researchers soon started to refer to this collection of brain regions as the “pain matrix.” , Melzack’s original “Neuromatrix” theory of pain proposed that the conscious experience of pain was generated by the flow of activity within a widespread network of convergent/divergent loops between the thalamus, cortex, and limbic system. He thought that the general function of this network, which he called the “body-self neuromatrix,” was to produce a unified representation of the body and self. Moreover, he suggested that the various bodily states generated by the neuromatrix each had a particular “neurosignature” (i.e. a characteristic pattern of neuronal activity stemming from the interaction between environmental inputs and the pre-existing state of the neuromatrix). Therefore according to Melzack, there is no “pain center” in the brain, but rather a general-purpose “body-self neuromatrix” capable of representing a wide array of bodily states, of which pain is just one particular example. Eventually, Melzack’s idea of a “body-self neuromatrix” was simplified, and the term “pain matrix” began to be used to refer to the brain regions generally activated by pain.
Discussions in the field revolved around how different regions of the “pain matrix” contributed to the unified pain experience. An important distinction was made between the sensory dimension of pain, reflecting the location, intensity, and sensory quality of the experience, and the affective dimension reflecting the intrinsic unpleasantness of pain. The sensory dimension of pain is thought to result from activity in the cerebral targets of the lateral spinothalamic system: ventral posterior (VPI) and ventral posterior lateral thalamic nuclei, S1, S2, and posterior insula (pINS). Studies of patients with brain lesions confirmed that damage to S1/S2 produces impairments in perceiving the intensity and location of pain. Moreover, the parieto-opercular region, which encompasses S2 and pINS, seems to be particularly important for pain. Not only is activity in that region relatively specific to pain, but it is also the only region of the brain where brain stimulation can occasionally provoke painful sensations. However, without further activation of other brain regions associated with affective feelings, activity in these sensory regions does not appear sufficient to produce a full-blown pain experience.
Indeed, nociceptive sensations must also be perceived as unpleasant to really be considered as pain. The affective dimension of pain is thought to depend on activity in the cerebral targets of the medial spinothalamic pathway: the medio-dorsal thalamic nucleus, ACC, and anterior Ins (aINS). Lesions in the ACC, or of the white matter tracts connecting it to the operculo-insular cortex, can produce a condition called pain asymbolia, whereby nociceptive sensations seem to be devoid of negative affect. Finally, activity in the aINS is generally ascribed to interoception, that is, the constant monitoring of the internal state of the body in the service of homeostasis, which includes pain but also other imperative sensations such as hunger or thirst. In addition to activity in core “pain matrix” regions (thalamus, S1, S2, ACC, insula), brain imaging studies of acute experimental pain have often reported activation in several other regions of the brain such as the brainstem (BS), amygdala, hippocampus, and prefrontal cortex (PFC). Activity within these regions tends to be interpreted as either reflecting non-experiential aspects of pain, such as learning or autonomic regulation, or post-experiential processes not specific to pain, such as secondary appraisals and action selection. Therefore an important distinction in the field was between a primary pain effect that would be specific and intrinsic to the pain experience, and a secondary pain effect that would be more optional and the result of secondary appraisals about the significance of pain for oneself.
The concept of the “pain matrix” soon became criticized based on its lack of specificity. Because of its nature, pain results from excessive somatosensory stimulation. Accordingly, brain imaging studies of acute experimental pain have capitalized on the difference between high- and low-intensity stimulation to track regions responding to pain. However, this means that activity in these regions can also be interpreted as reflecting the perceptual salience of high-intensity stimulations, which may or may not be specific to pain. By comparing the patterns of activity elicited by visual, auditory, and tactile non-painful and painful heat stimulations, Mouraux et al. concluded that most regions of the “pain matrix,” such as the Ins and ACC, responded to the salience of external stimulation and were not specific to pain.
It is only with recent advances in machine learning that more accurate and fine-grained multivariate pain-predictive patterns could be devised. Machine learning departs from traditional statistics by assessing the prediction accuracy on a different sub-partition of the data than that used for developing the predictive model. From this perspective, predictive models can be said to be “trained” to minimize their out-of-sample error, thereby maximizing the prediction accuracy for new unseen data points. This makes machine learning an ideal technique for optimizing the predictions of individual patients. Before being applied to pain, machine learning has already been used in cognitive neuroscience to make different types of predictions based on patterns of brain activity, such as identifying which letters participants are seeing , or the content of their dreams. Using a similar approach, Wager et al. developed an fMRI-based brain signature to predict the pain produced by thermal stimuli of different intensities ( Fig. 10.2 ). This signature pattern, called the neurologic pain signature (NPS), also proved to be highly sensitive to the presence of diverse types of pain in new individuals tested on different scanners. The NPS also distinguished pain from other salient and unpleasant events, such as social rejection, or vicarious pain. Finally, although the NPS was shown to be highly sensitive to pain caused by the intensity of noxious inputs, it appeared to be insensitive to the impact of psychological interventions, such as cognitive self-regulation.
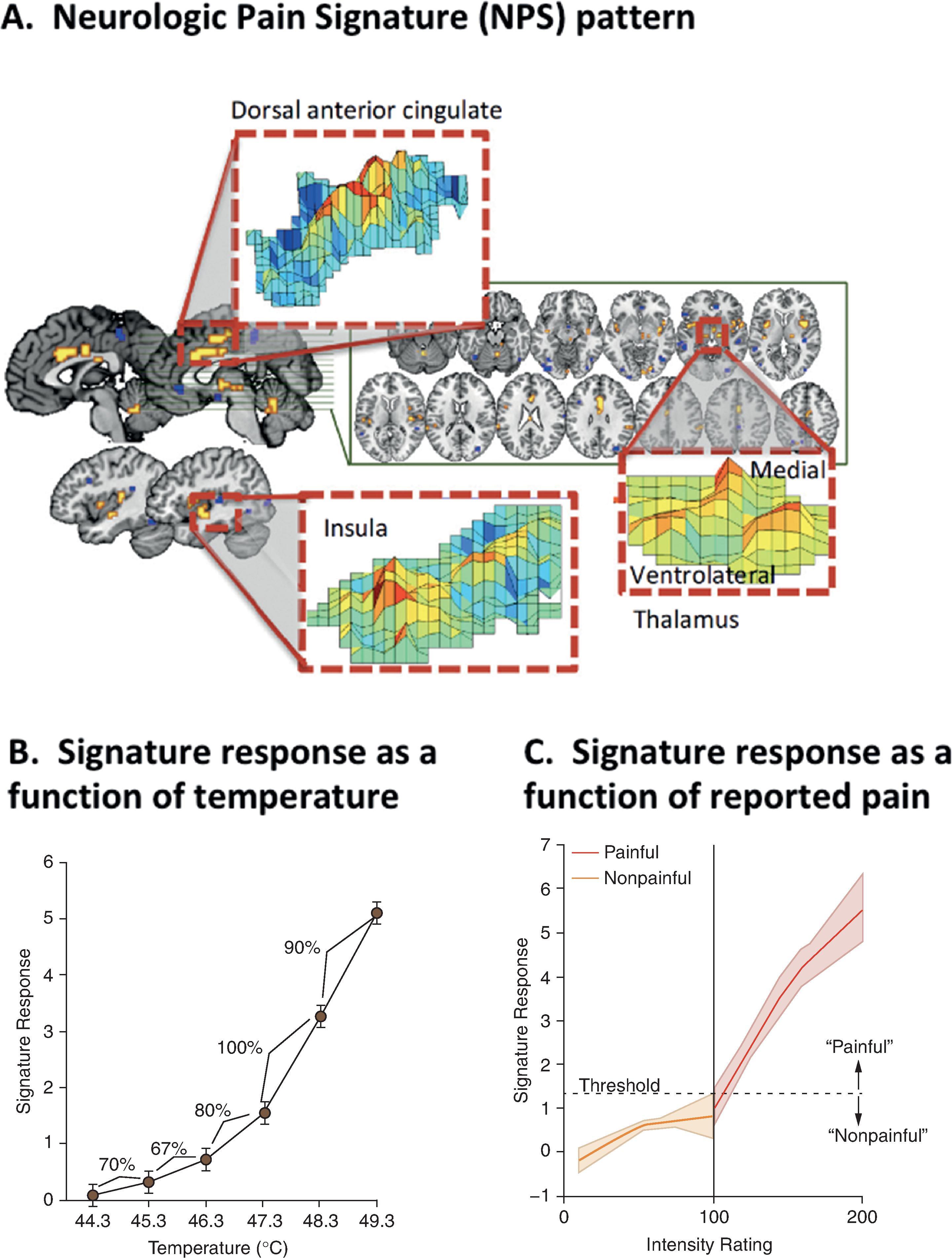
To capture the additional, non-nociceptive source of variation in pain ratings, a second signature was trained to predict pain after controlling for stimulus intensity and NPS pattern expression. The resulting signature, called the stimulus intensity independent pain signature (SIIPS; Fig. 10.3 ), was shown to explain approximately 16% of the variation in pain ratings for single pain trials, which is comparable to the variance explained by the NPS. When combining both signatures, predictions account for 25% of the explained variance for single trials and over 80% of the explained variance when averaging over several trials. Eventually, other signatures were also trained to predict social rejection, vicarious pain, negative affect, and various other subjective experiences related to pain. Therefore it seems possible to use measures of brain activity to predict the presence of pain or other phenomena closely related to pain.
![Figure 10.3, Stimulus Intensity Independent Pain Signature (SIIPS). The SIIPS was developed to model endogenous cerebral contributions to pain beyond nociception. Some of the cerebral contributions may interact with nociceptive brain systems (red nodes), whereas others contribute to pain independent of nociceptive processing (green nodes). Right , the SIIPS pattern, which is predictive of residual pain ratings after removing the effects of stimulus intensity and NPS response. The map shows thresholded voxel weights (at q<0.05, false discovery rate [FDR]). Some examples of unthresholded patterns are presented in the insets; small squares indicate the individual voxel weights. aINS , Anterior insula; CB , cerebellum; dmPFC , dorsomedial PFC; dpINS , dorsal posterior insula; HC , hippocampus; MCC , mid-cingulate cortex; midINS , middle insula; NAc , nucleus accumbens; SMA , supplementary motor area; TP , temporal pole; vmPFC , ventromedial PFC; vlPFC , ventrolateral PFC. Adapted from Woo et al. 171 Figure 10.3, Stimulus Intensity Independent Pain Signature (SIIPS). The SIIPS was developed to model endogenous cerebral contributions to pain beyond nociception. Some of the cerebral contributions may interact with nociceptive brain systems (red nodes), whereas others contribute to pain independent of nociceptive processing (green nodes). Right , the SIIPS pattern, which is predictive of residual pain ratings after removing the effects of stimulus intensity and NPS response. The map shows thresholded voxel weights (at q<0.05, false discovery rate [FDR]). Some examples of unthresholded patterns are presented in the insets; small squares indicate the individual voxel weights. aINS , Anterior insula; CB , cerebellum; dmPFC , dorsomedial PFC; dpINS , dorsal posterior insula; HC , hippocampus; MCC , mid-cingulate cortex; midINS , middle insula; NAc , nucleus accumbens; SMA , supplementary motor area; TP , temporal pole; vmPFC , ventromedial PFC; vlPFC , ventrolateral PFC. Adapted from Woo et al. 171](https://storage.googleapis.com/dl.dentistrykey.com/clinical/NeuroimagingTechniques/2_3s20B9780323711012000105.jpg)
This new multivariate approach also marks a departure from traditional brain mapping grounded in systems neuroscience. Indeed, instead of tracking pathways that convey nociceptive information from the periphery to the brain, the goal is to identify the patterns of brain activity that may give rise to our subjective experiences of pain. One interesting avenue for understanding mind-brain relationships comes from the field of emotion research, where more recent theories of emotional feelings have stressed the concept of “degeneracy.” Degeneracy refers to the idea that there may be a many-to-one mapping between brain activity and subjective experiences. This suggests that the same subjective experience, such as pain, may be caused by different patterns of brain activity on different occasions. However, this not mean that any pattern of brain activity could produce pain, but rather that there can be substantial variation within a restricted set of possibilities. This is what Melzack had initially proposed, and recent research on pain neurosignatures supports this. We may need to abandon our search for a one-to-one mapping between pain and a specific pattern of brain activity, as this concept is likely fundamentally flawed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here