Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The tarsal tunnel is a fibroosseous tunnel within the posteromedial ankle and hindfoot in which the tibial nerve, posterior tibial artery, accompanying veins, posterior tibial tendon, flexor digitorum longus, and flexor hallucis longus tendons pass into the foot. The flexor retinaculum acts as the roof of this tunnel and extends from the medial malleolus to the medial side of the calcaneal tuberosity. The medial distal tibia, talus, and calcaneus make up the tunnel’s floor. Septa, which separate the posterior tibial, flexor digitorum longus, and flexor hallucis longus tendons, project from the fibrous roof to the calcaneus. Between the flexor digitorum longus and flexor hallucis longus tendons, the tibial nerve, posterior tibial artery, and accompanying veins pass to enter the foot.
Before reaching the foot, the tibial nerve divides into three terminal branches: the medial calcaneal nerve (MCN), lateral plantar nerve (LPN), and medial plantar nerve (MPN). Tibial nerve branch morphology varies, but typically the tibial nerve branches within the tunnel just proximal and deep to the upper edge of the abductor hallucis muscle. The MCN branches first, traveling posteriorly to the subcutaneous tissue. Coming off posteriorly, the first branch of the LPN passes under the abductor, over the medial fascia of the quadrates plantae, deep to the plantar fascia, and under the heel to the flexor digitorum brevis, where it sends a sensory branch to the central heel skin, and terminates in the abductor digiti quinti. The first branch of the LPN may branch from the main tibial nerve but still travels under the abductor with the LPN. Anterior to its first branch, the LPN passes deep to the abductor fascia and plantar fascia and over the quadrates plantae. Then it continues distally under the flexor digitorum brevis, terminating in the fourth web space and supplying a branch to the third web space. The LPN also supplies motor branches to the intrinsic muscles. Finally, the MPN innervates the abductor and continues under the abductor and the plantar fascia to form the common digital nerves, which terminate to the first, second, and third web spaces and motor branches to the interossei and lumbricals.
Historically, the term tarsal tunnel syndrome referred to tibial nerve entrapment beneath the flexor retinaculum. In 1987 Heimkes and colleagues first described distal tarsal tunnel syndrome, in which the distal tibial nerve branches are entrapped as they enter the foot. Together, proximal and distal tarsal tunnel syndromes encompass a spectrum of tibial nerve entrapment within the tarsal canal.
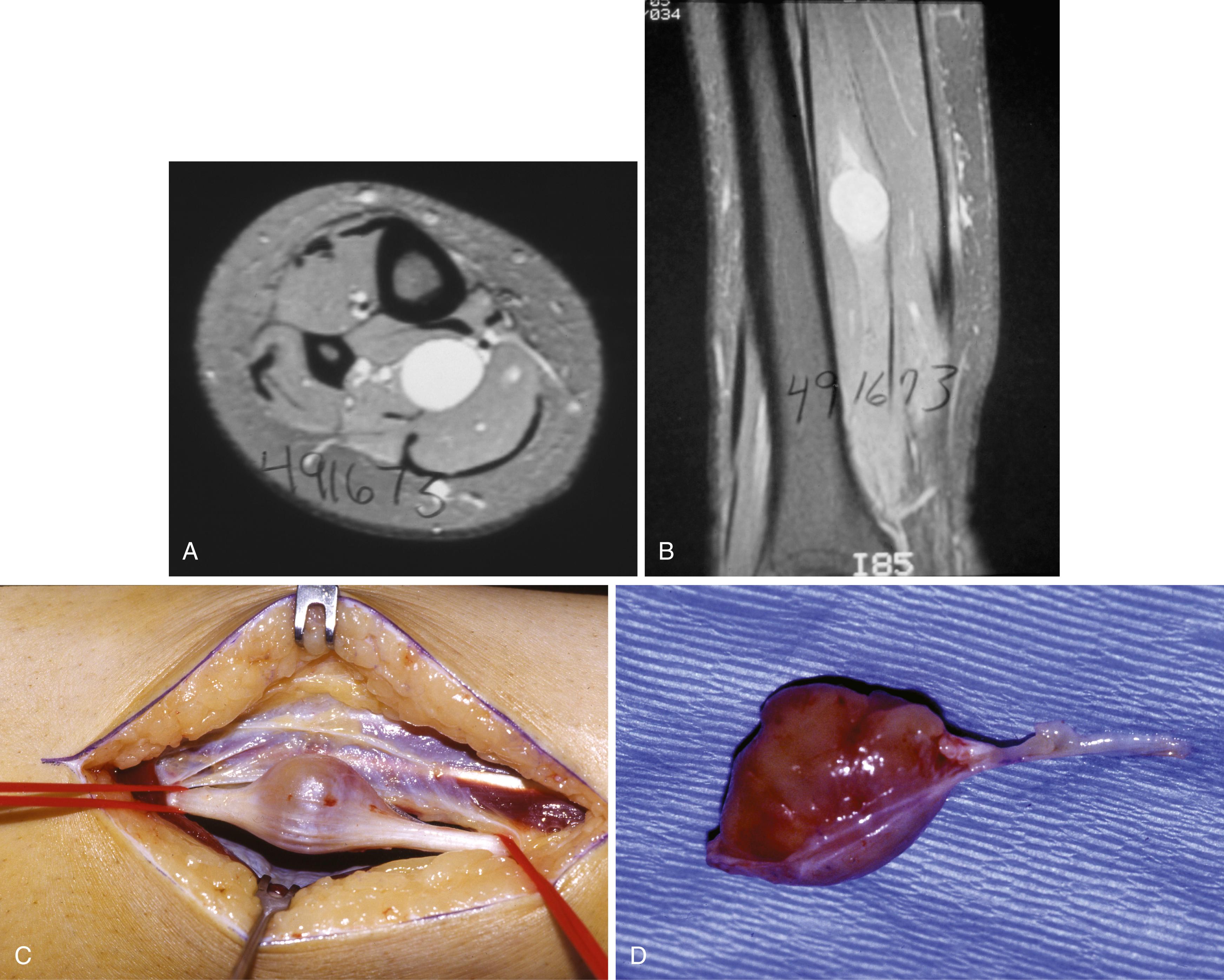
Sources of constriction beneath and adjacent to the tarsal tunnel include bone fragments, tenosynovitis, ganglia, soft-tissue encroachment in inflammatory arthritis, varicosities, neural tumors (neurilemmoma; Fig. 87.1 ), perineural fibrosis, tarsal coalition, and calcaneal osteotomies. Furthermore, a fixed valgus hindfoot can predispose to chronic traction neuropathy of the posterior tibial nerve or one of its branches.
Clinical symptoms of tarsal tunnel syndrome vary, and this disorder should be kept in mind whenever unexplained dysesthesias are present in the plantar aspect of the foot, in the toes, or over the medial distal calf (Valleix phenomenon). Symptoms may be similar to those of plantar fasciitis, but unlike plantar fasciitis, nerve entrapment symptoms do not resolve quickly. Making the diagnosis difficult, typical neurogenic symptoms are not always present and symptoms may be present at night, during exercise, or at rest. Symptoms can be confined to the lateral plantar nerve, medial plantar nerve, or medial calcaneal nerve.
A thorough, detailed examination, aided with a good patient history, improves diagnostic accuracy. Careful examination for subtle sensory abnormalities or differences in temperature, sweating pattern, and skin abnormalities may lead to the correct diagnosis. Although a common complaint, sensory abnormalities often are difficult to detect. Dryness and scaliness of the skin may be present over only the lateral or medial plantar nerve distribution. Atrophy of the abductor hallucis, abductor digiti minimi, or both, often a difficult finding to detect, may be obvious compared with the asymptomatic foot. Point tenderness of the medial heel in the soft spot at the lower edge of the abductor hallucis may indicate distal entrapment of the tibial branches, and a Tinel sign over the flexor retinaculum may indicate proximal entrapment.
Abouelela and Zohiery described a “triple compression test” for diagnosing tarsal tunnel syndrome: the ankle is plantarflexed and the foot is inverted (increasing the tarsal tunnel compartment pressures), then digital compression is applied over the tibial nerve. Fifty patients with symptoms suggestive of tarsal tunnel syndrome and 40 asymptomatic patients were tested, and the tarsal tunnel diagnosis was confirmed with electrophysiologic testing. Test sensitivity was 86% and specificity was 100%.
Kinoshita et al. described another provocative maneuver to elicit symptoms suggesting tarsal tunnel syndrome. The dorsiflexion-eversion test is done by maximally everting and dorsiflexing the ankle passively, while all the metatarsophalangeal joints also are maximally dorsiflexed. This position is held for 5 to 10 seconds. In a comparison of 100 feet in asymptomatic volunteers with 44 feet in symptomatic patients, the authors found that the patients’ symptoms were exacerbated in 36 of the 44 feet. None of the feet in the control group exhibited symptoms with this maneuver. After release of the tarsal tunnel, the dorsiflexion-eversion test elicited tarsal tunnel symptoms in only three feet, all of which had calcaneal fractures.
Imaging modalities can assist the evaluation of tarsal tunnel syndrome. Plain radiographs demonstrate osseous abnormalities (e.g., fractures or talocalcaneal coalitions) that may contribute to tarsal tunnel symptoms. The use of ultrasound in the evaluation of tarsal tunnel has been reported; however, we prefer MRI because of the improved detail of the tarsal contents. MRI has been shown to identify the cause of the tarsal tunnel syndrome in up to 88% of patients. More important, MRI aids with surgical planning by providing detailed characteristics and location of space-occupying lesions.
Electrodiagnostic testing is indicated for any patient suspected of having compression of the tibial nerve beneath the flexor retinaculum. In an evidence-based review of the usefulness of electrodiagnostic testing in suspected tarsal tunnel syndrome, Patel et al. recommended the use of nerve conduction studies but found insufficient data to recommend the use of electromyography. Electrical studies also are useful in identifying an unsuspected peripheral neuropathy suggestive of a systemic, rather than localized, nerve injury. Despite evidence demonstrating their usefulness, electrodiagnostic studies can be normal in patients with tarsal tunnel syndrome, and negative electrodiagnostic testing does not provide a contraindication for surgery.
Initially, 6 to 12 weeks of ankle immobilization in a night splint, antiinflammatory agents, and a wide, cushioned, comfortable shoe are recommended. If distal tarsal tunnel syndrome is suspected, an orthosis with a relief channel within the medial arch may be effective; a standard orthosis with a longitudinal arch may worsen symptoms. Caution is recommended in advising surgical treatment of tarsal tunnel syndrome in patients who are older (60 to 80 years old), have posttraumatic scarring within the tarsal canal, have no objective cause for symptoms (idiopathic), and those with protracted psychiatric illness. Symptoms caused by space-occupying lesions should be treated surgically. When conservative treatment fails, surgical treatment is indicated.
If surgical treatment is indicated, the tibial nerve and its branches must be meticulously exposed and unroofed. The release must include incision of 1 to 2 cm of the deep fascia above the proximal edge of the flexor retinaculum and following the medial and lateral plantar nerves beneath the abductor hallucis because one or both of these branches may pass through fascial slings as they enter the plantar surface of the foot. The dissection is refined by the use of magnification, a tourniquet, small dissecting scissors, and nontoothed forceps. Space-occupying lesions should be excised and alignment disorders corrected.
Patients with definite lesions generally respond better to surgical decompression than those with idiopathic or traumatic etiologies. With no identifiable cause, however, the relief of symptoms is less predictable, with approximately 25% of patients achieving little or no relief. Rarely, symptoms may even worsen after surgical release. Symptom duration of less than 1 year also has been cited as a predictor of better outcomes. There are few outcome studies of tarsal tunnel release, and the evidence generally is level IV or V.
Even closer scrutiny of the clinical factors that can influence a surgical decision is required for revision tarsal tunnel release because diagnosing an “inadequate release” often is difficult, even with knowledge of the original procedure. Failures can occur from incorrect diagnosis, inadequate release, poor technique, or a combination of any of these. To minimize the probability of inadequate release, a complete release of the tibial nerve and its branches is recommended, except in the case of a space-occupying lesion, for which a smaller, lesion-specific release is acceptable. Nerve scarring is likely in patients who had relief after their initial surgery but then experienced a slow return of symptoms. Neurolysis with saphenous vein or collagen wrapping to prevent nerve adherence has been recommended in this situation. Worsening symptoms immediately after surgery may indicate an iatrogenic nerve injury, from which a painful neuroma may develop. Despite careful attention to patient history and physical examination findings, combined with meticulous surgical technique, the outcomes of revision tarsal tunnel release are unpredictable, and appropriate counseling and caution are recommended before treatment.
Extend the incision from 1 cm plantar to the navicular tuberosity in a proximal direction, bisecting the area between the medial malleolus and the medial aspect of the tuberosity of the calcaneus, and ending 1 cm anterior to the Achilles tendon. With the foot in the gravity equinus position, this is almost a straight line ( Fig. 87.2A ). Do not undermine the incision.
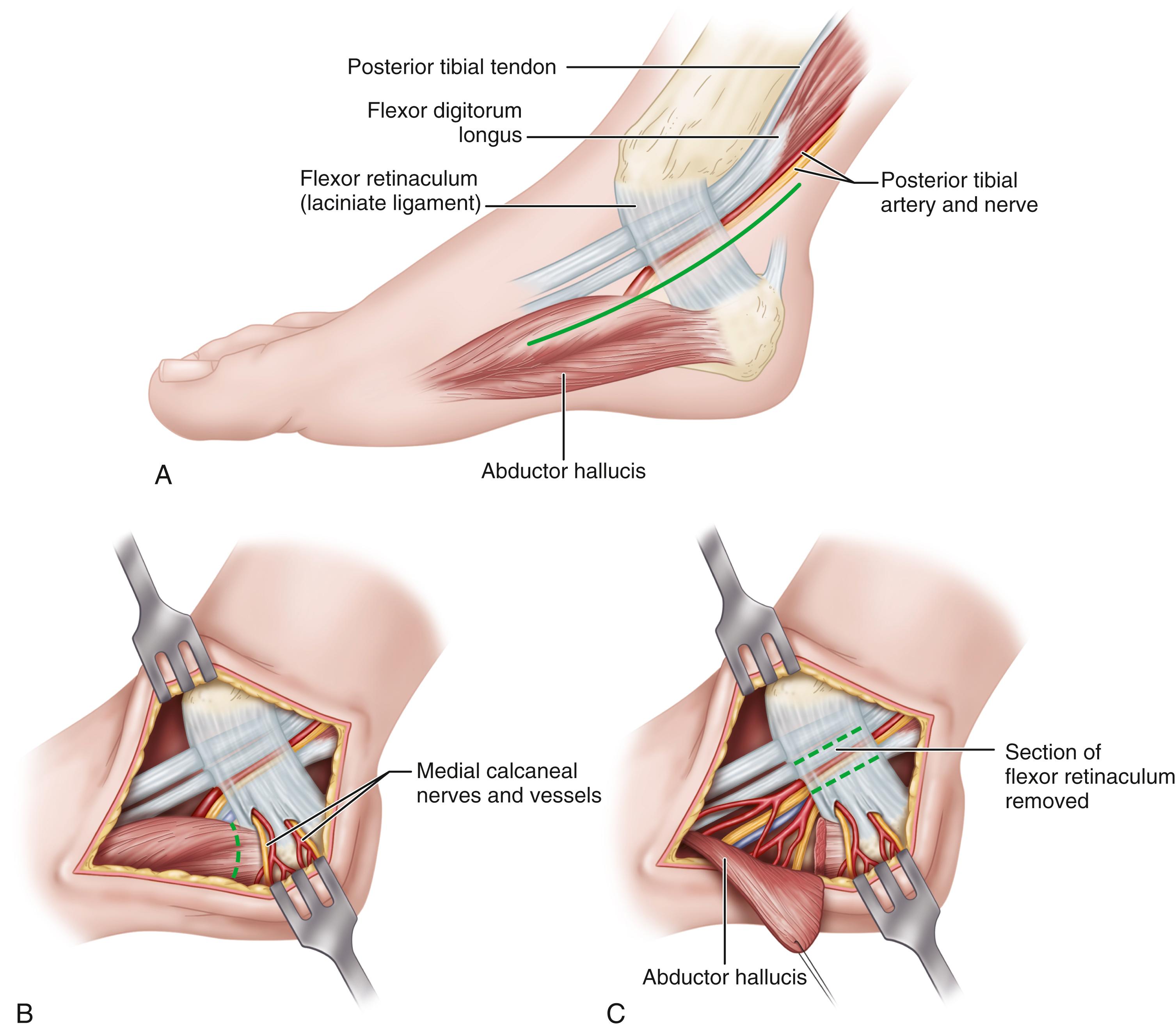
Coagulate or tie the superficial veins connecting the plantar and saphenous systems and deepen the incision through the investing fascia of the calf proximally and the medial side of the foot distally. This allows identification of the proximal and distal (posterior and anterior) borders of the flexor retinaculum and the neurovascular bundle before the bundle disappears beneath the retinaculum.
Occasionally, the nerve is enlarged at the upper border of the retinaculum. Release the retinaculum from a proximal to a distal direction until the muscle fibers of the abductor hallucis are reached.
Sometimes a medial calcaneal branch penetrates the retinaculum, and care must be taken to avoid severing one or more branches of this nerve (medial calcaneal) to prevent a painful neuroma ( Fig. 87.2B ).
The tibial nerve divides beneath the flexor retinaculum into the medial and lateral plantar branches. The medial calcaneal branch may arise from the main tibial nerve or its lateral plantar branch ( Fig. 87.3A and B ). The tibial nerve bifurcates into its medial and lateral components beneath the laciniate ligament in most individuals. When the medial and lateral plantar nerves reach the medial border of the abductor hallucis, they turn plantarward and lateral deep to this muscle.
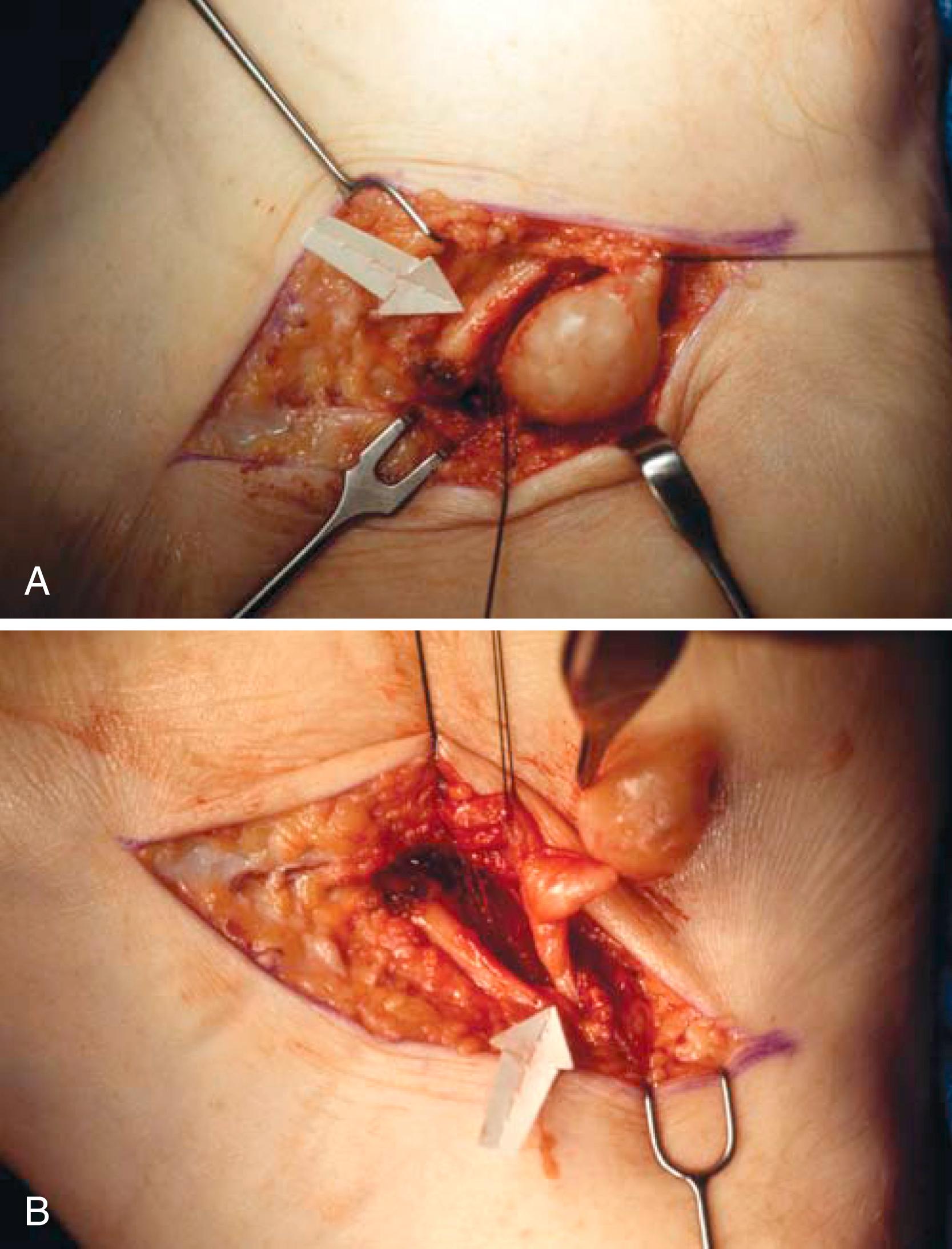
Trace each nerve well distal to the inferior edge of the flexor retinaculum until it is certain that no tethering by the fascial origin of the abductor hallucis exists. This is made easier by releasing part of the origin of the abductor hallucis.
If the epineurium appears unequally thickened, it should be incised.
Remove a section of the flexor retinaculum over the neurovascular bundle ( Fig. 87.2C ).
Remove the tourniquet and secure hemostasis before closing the wound (skin and subcutaneous tissue only). Apply a sterile compression dressing.
Apply a short leg posterior splint to “rest” the wound while the incision is in the initial stages of healing (10 to 14 days).
A bulky compression dressing and a short leg plaster splint are applied with the foot in mild equinovarus for 10 to 14 days. After sutures are removed, a walking boot for 2 to 4 weeks allows the patient to bear weight to tolerance. Ankle edema persisting for many weeks is common if the dissection has been extensive, and complete recovery may require 6 to 12 months.
The anterior tarsal tunnel syndrome, denoting entrapment of the deep peroneal nerve beneath the inferior extensor retinaculum, also has been described ( Fig. 87.4 ). The symptoms include pain and dysesthesias over the dorsum of the foot and first web space. Examination demonstrates decreased touch and pinprick in the first web space, a possible Tinel sign over the deep peroneal nerve beneath the inferior extensor retinaculum, and rarely, atrophy of the extensor digitorum brevis muscle if the motor branch of the deep peroneal nerve arises more distally than normal (i.e., distal and deep to the inferior extensor retinaculum). Proximally radiating dysesthesias into the anterior compartment of the leg also may occur. The examination should include the assessment of extensor digitorum brevis and extensor hallucis brevis muscles, and sensation should be compared with that of the opposite, asymptomatic foot. If bilateral anterior tarsal tunnel syndrome is present, a diffuse peripheral neuropathy must be suspected.
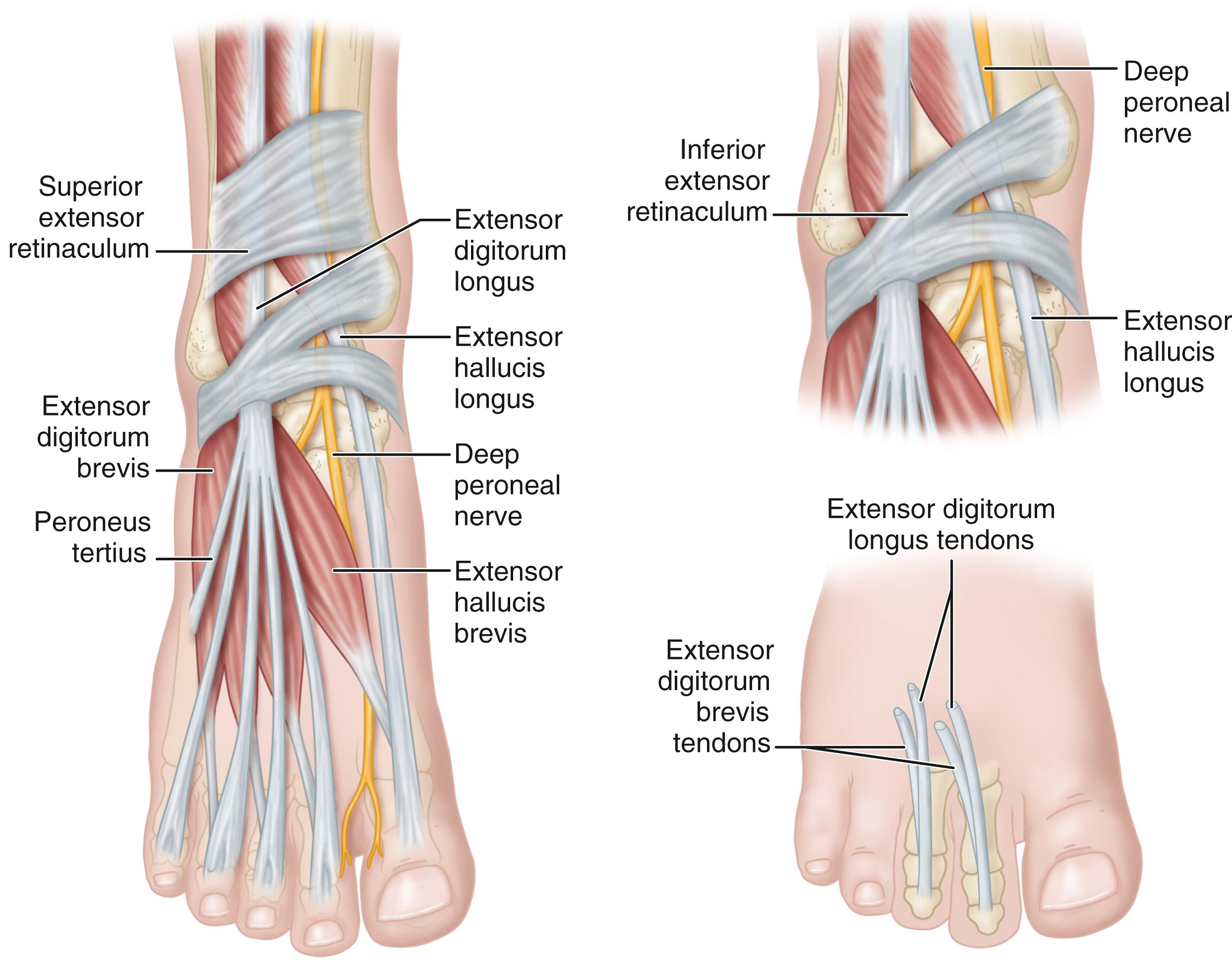
Mann and Baxter stated that this syndrome most commonly occurs in runners or in patients with dorsal osteophytes at the ankle, midtarsal, or metatarsocuneiform articulation. The most common areas of nerve entrapment are shown in Fig. 87.5 . Extensor hallucis brevis hypertrophy in ballet dancers, tight-fitting shoes, hooking the feet under a bar when doing sit-ups, and ganglion cysts, with or without the presence of traumatic osteophytes, also have been reported as causes.
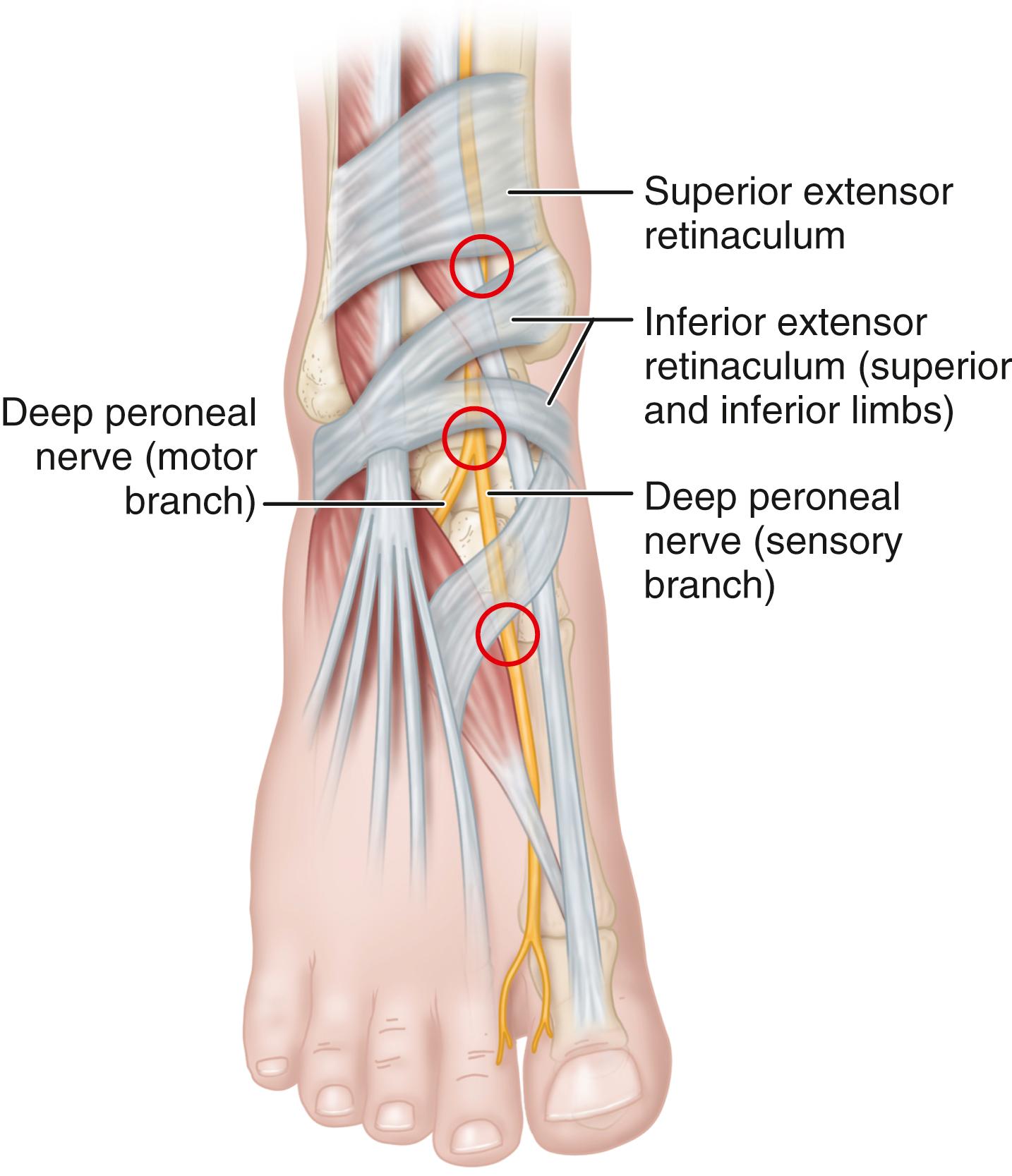
Electrical studies may confirm the diagnosis with fibrillations, positive sharp waves, and reduced motor action potentials in the extensor digitorum brevis and increased distal motor and sensory latencies in the presence of a normal nerve conduction velocity in the deep peroneal nerve from the fibular neck to the ankle. The entrapment often occurs distal to the motor branch to the extensor digitorum brevis.
Successful outcomes have been reported with both operative and nonoperative treatments. Similar to tarsal tunnel syndrome, nonoperative management is the first line of therapy, with the exception that space-occupying lesions can be removed before an extensive course of nonoperative management. Conservative treatment consists of immobilization, identification and removal of external pressure etiologies (e.g., overly tightened shoelaces), neurologics (e.g., gabapentin), and occasional corticosteroid injections. If conservative treatment fails, surgical management is indicated; several small studies have reported good results in 80% to 100% of patients after surgical decompression for anterior tarsal tunnel syndrome. Surgical management includes removing the offending structures, such as resecting impinging osteophytes, excising ganglion cysts, and partial release of the extensor retinaculum or extensor hallucis brevis tendon as needed for decompression. Surgical release most commonly includes an open approach, but authors have described success with endoscopic techniques as well.
(Mann)
Before surgery, locate the area of compression at the anterior ankle joint or the dorsal talonavicular joint.
Make a longitudinal incision 5 to 7 cm long over the dorsum of the foot from the talonavicular joint to the first intermetatarsal space.
Identify the deep peroneal nerve and dorsalis pedis artery. Identify the deep peroneal nerve as it courses beneath the extensor hallucis brevis and release the constricting portion of the inferior extensor retinaculum.
Mann and Baxter recommend releasing only the portion of the retinaculum that seems to be constricting the nerve.
Remove any underlying lesion, such as a ganglion cyst or osteophyte.
The patient is placed in a cast or removable walking boot and begins weight bearing to tolerance. Once the incision has healed and sutures are removed after 2 weeks, then the patient can resume non-compressive footwear and physical activity as pain and swelling allow.
Arising posteriorly off the LPN, the first branch of the LPN passes deep to the abductor hallucis, over the medial fascia of the quadratus plantae, deep to the plantar fascia, and under the calcaneus to the flexor digitorum brevis, terminating in the abductor digiti quinti (ADQ). Entrapment occurs between the lateral border of the abductor hallucis and the medial border of the quadratus plantae, producing chronic heel pain symptoms. Distinguishing this pathologic process from plantar fasciitis can be difficult, and the two entities may coexist. Electrodiagnostic studies can help with the diagnosis. Advanced imaging may show atrophy of the ADQ, but this atrophy commonly occurs in asymptomatic patients, rendering this finding nonspecific. Patients will have tenderness over the first branch of LPN deep to the abductor hallucis, which is in close proximity to plantar fascia origin. Unlike proximal tarsal tunnel symptoms, patients do not typically complain of numbness. Most patients improve with nonoperative treatment, consisting of heel cups, nonsteroidal antiinflammatory drugs, rest, ice, physical therapy, or steroid injections. However, for those with persistent symptoms, an open or endoscopic release is described, with 83% to 88% good to excellent results.
(Baxter, Pfeffer, Watson et al.)
Starting distal to the medial malleolus but in line with its posterior border, extend a 4-cm oblique incision distally over the proximal abductor hallucis muscle.
Carry the dissection down to the superficial fascia overlying the abductor hallucis muscle. Divide this superficial fascia and retract the muscle belly distally to allow exposure of the deep abductor fascia.
Decompressing the nerve, release the deep fascia from proximal to plantar to the level of the plantar fascia.
Identify the plantar fascia and transect the medial half of its width under direct vision.
Close the wound with interrupted nylon sutures and apply a short leg splint.
To minimize incisional complications, patients should remain non–weight bearing for 2 weeks. Once the sutures are removed, patients can weight bear as tolerated with a walking boot for another 4 weeks. After weaning out of the boot, supportive footwear is recommended.
The MPN typically arises within the tarsal tunnel as the anterior division of the tibial nerve. Within the tarsal tunnel, the MPN runs between the tunnels for the flexor hallucis longus laterally and flexor digitorum longus anteromedially. Passing deep to the abductor hallucis, it enters the plantar foot posterior and plantar to the flexor digitorum longus tendon, continuing superficial (plantar) to that tendon but deep to an interfascicular septum, and connecting the deep abductor fascia to the upper border of the flexor accessorious immediately plantar to the flexor hallucis longus tunnel. At the knot of Henry, the nerve passes plantar (superficial) to the flexor digitorum longus and flexor hallucis longus. At the base of the first metatarsal, the nerve divides into its terminal medial and lateral branches. The MPN provides sensation to the medial plantar foot, plantar first through third toes, and plantar medial half of the fourth toe, and provides motor innervation to the abductor hallucis, flexor hallucis brevis, flexor digitorum brevis, and first lumbrical muscles. Limited literature and reports describe injury to the MPN. Historically, entrapment of this nerve was reported in runners, hence the term jogger’s foot . Stenosis typically occurs within the knot of Henry. Symptoms consist of medial arch pain and dysesthesias that radiate distally into the medial three toes. Excessive valgus and firm orthotics that apply pressure into the arch may exacerbate symptoms. Treatment consists of eliminating the offending shoes or orthotics, rest, ice, nonsteroidal anti-inflammatory drugs, and steroid injection. Only after failure of conservative treatment is surgical release indicated.
The sural nerve is most commonly formed by the medial sural nerve only, a branch of the tibial nerve; but many variations frequently occur, as the nerve is composed of the medial sural and an anastomotic branch from the lateral sural or common peroneal nerve. Knowledge of these anatomic variations is important when evaluating and treating sural nerve pathology. The nerve provides sensory input to the posterior lateral leg and hindfoot, with variable extension to the lateral forefoot. Injury can occur from inversion of the ankle, instability of the ankle, fractures, ganglion cysts, and, most commonly, iatrogenic trauma. Treatment includes identifying and removing (if possible) the source of injury. Medications, including nonsteroidal antiinflammatories, steroid dose packs, or antiepileptic medications, such as gabapentin or pregabalin, can improve symptoms. Nerve blocks also may be diagnostic and therapeutic. If symptoms persist despite conservative treatment, then successful treatment with surgical decompression or excision has been described. Recently, Lans et al. retrospectively reviewed 49 patients with surgically excised sural neuromas. Consistent with previous reports, 63% of patients had pain improvement, and an additional 8% had improvement after a second surgery. Hence, surgical treatment is beneficial, but patients must be warned of the 20% to 30% risk for persistent neuropathic pain.
Branching from the common peroneal nerve is the superficial peroneal nerve, which runs within the anterolateral leg compartment, innervating the peroneal longus and peroneal brevis. It exits through deep fascia at the distal third of the leg, where it divides into the medial and intermediate dorsal cutaneous nerve branches, supplying sensation to the dorsal foot. The path of the nerve varies significantly, traveling through the lateral leg compartment only, the anterior leg compartment only, or both compartments. Passing through the fascia creates a site for entrapment. This site of stenosis is further exacerbated by muscle hypertrophy, displaced fractures, direct trauma, mass effect, edema, and inversion injuries. Patients describe vague pain or paresthesia that is worsened by activity over the dorsum of distal third of the leg and foot. At the site of entrapment, examination often reveals a positive Tinel sign associated with a fascial defect. To prevent placing the nerve under tension, conservative treatment involves immobilization and limiting inversion and plantar flexion. Corticosteroid injections can be diagnostic and therapeutic. If conservative treatment fails, then surgical treatment involves release of the stenosing fascia.
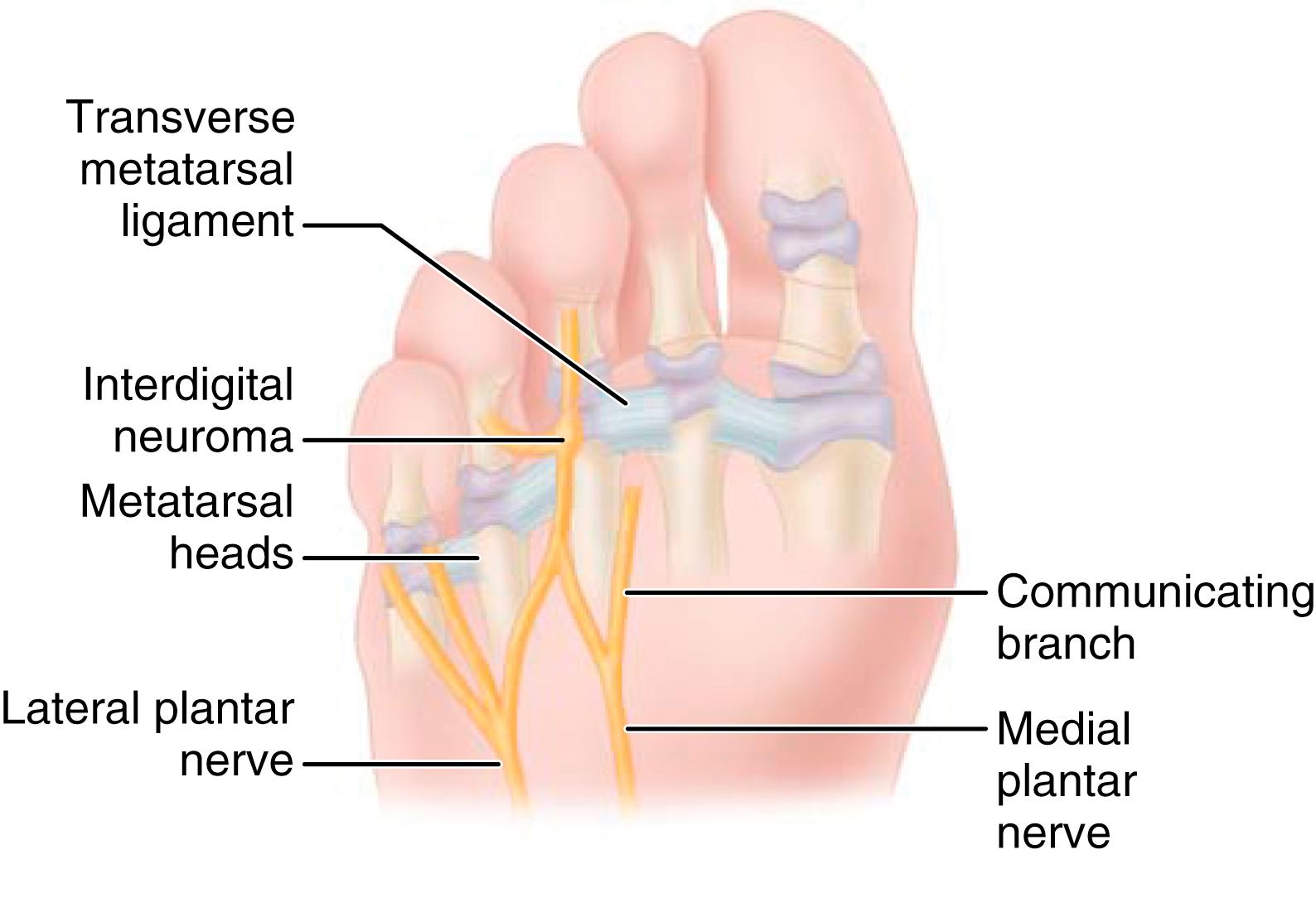
Commonly known by its eponym, Morton’s neuroma, the interdigital neuroma is an entrapment of the interdigital nerve near the distal edge of the transverse metatarsal ligament. It occurs most commonly in the third web space, followed by the second web space. Rarely does it involve other web spaces. Metatarsophalangeal joint instability and metatarsalgia must be carefully considered in the working diagnosis.
The true etiology of this entrapment remains controversial. The unique anatomy of the third web space has been cited as a factor contributing to the entrapment. Unique to the third web space, a communicating branch from the common digital branch of the LPN joins the common digital branch from the MPN to innervate the third web space ( Fig. 87.6 ). Because of this communicating branch, the common digital nerve to the third web space has been suggested to be thicker and more likely to be compressed against the unyielding transverse intermetatarsal ligament dorsal to it. Levitsky et al., however, found this communicating branch to be absent in 73% of cadaver feet, and neuromas were identified in almost equal distribution between the second and third web. Other anatomic theories include increased mobility of the fourth tarsometatarsal joint, but this mobility would not explain the incidence in the second web space. Thus the etiology remains debatable, and opinions even differ in regard to the cellular pathologic processes in neuroma specimens.
In a strict sense, the term neuroma is incorrect because the proliferation of axons seen in a traumatic neuroma is absent. Instead, the enlargement results from the deposition of hyaline and collagenous material, making this pathologic process most likely degenerative rather than proliferative. The repetitive trauma against the deep transverse intermetatarsal ligament is the most likely source for the degenerative process, but even this is uncertain. Repetitive microtrauma, perineural fibrosis, ischemia from vasa nervorum occlusion, and endoneural edema are likely responsible for the symptoms of interdigital neuroma. Because this is not a proliferative process, the term interdigital neuritis or neuralgia, rather than interdigital neuroma, has been suggested.
The following list summarizes reported pathologic findings:
Perineural fibrosis ( Fig. 87.7A )
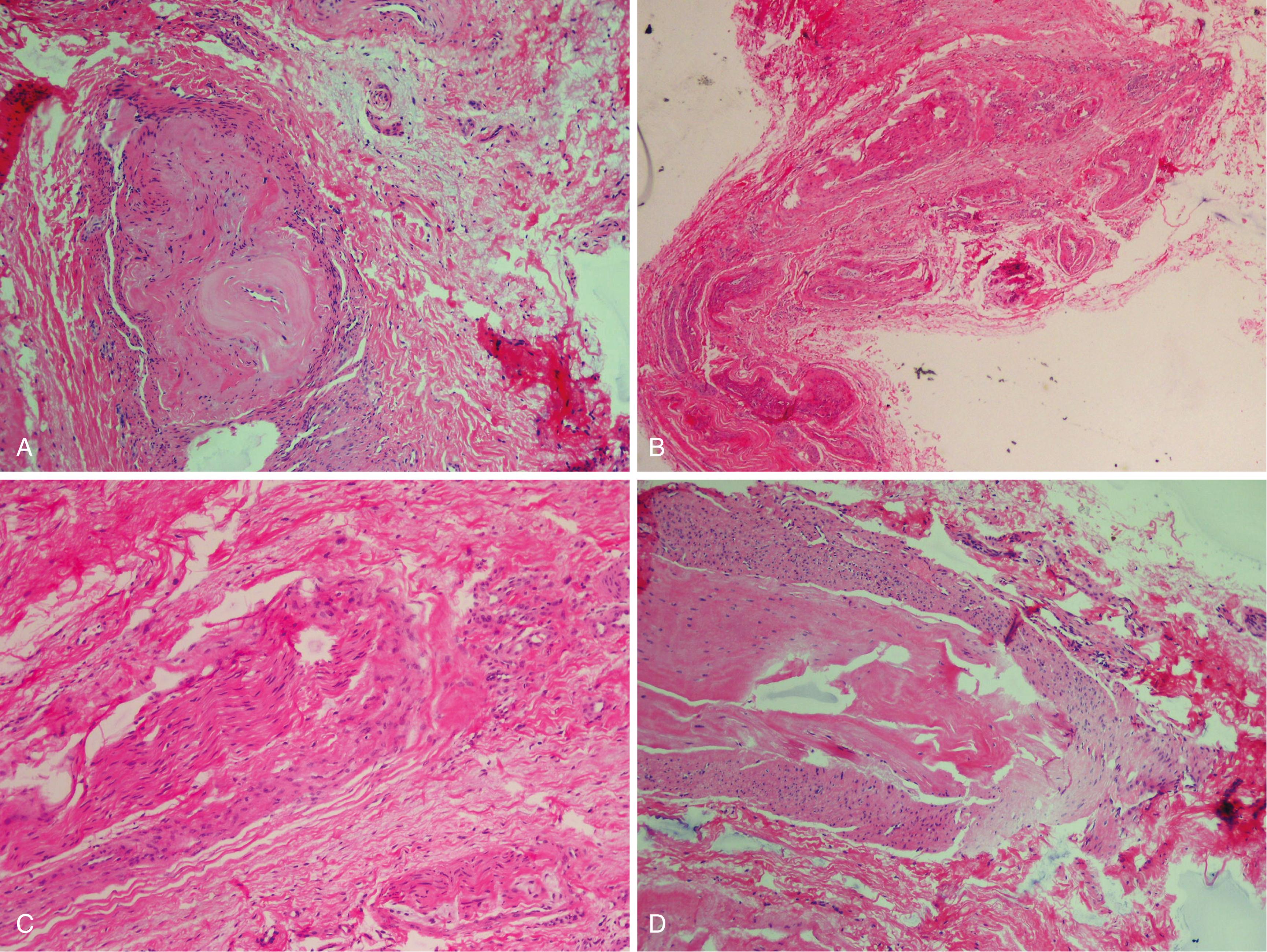
Increased number of intrafascicular arterioles with thickened and hyalinized walls caused by multiple layers of basement membranes ( Fig. 87.7B ). Demyelinization and degeneration of nerve fibers with a decrease in the number of axis cylinders ( Fig. 87.7C )
Endoneural edema
Absence of inflammatory changes
Frequent presence of bursal tissue accompanying the specimen ( Fig. 87.7D )
The primary symptom of interdigital neuroma is pain, which is most often located in the region of the third metatarsal head, and frequently described as burning, aching, or cramping. Trauma may initiate the symptoms, and the duration of pain varies from a few weeks to many years. It is increased with walking and relieved by rest, removing the shoe, or massaging the forefoot. In exploring other possible pain sources, careful examination should include a Lachman test of the metatarsophalangeal joint, dorsal palpation, and palpation under the metatarsal head versus the web space to discern pain etiology. If the tenderness is mostly in the web space and not under the plantar aspect of the proximal phalangeal base or the dorsolateral aspect of the second metatarsophalangeal joint, a neuroma is likely. The palpable and audible click associated with reproducing the patient’s pain is helpful for diagnosis (Mulder’s click). This click frequently is present without pain on the opposite asymptomatic foot. Hence, the click alone is not diagnostic. Simulating weight bearing in narrow shoes can reproduce symptoms. Pressure is placed under the web space with the thumb dorsal followed by gentle medial-to-lateral squeezing of the forefoot by the examiner’s other hand. Repeating this maneuver followed by relaxation will reproduce painful clicking of the neuroma ( Fig. 87.8 ). Subjective numbness of the toes of the involved interspace is common, but objective signs of decreased sensibility are less common. Women are more commonly affected than men (8:1), and the condition usually is unilateral. The use of sensory action potentials to confirm the diagnosis objectively has yielded variable results. Diagnostic injections have been shown to extravasate into the adjacent web space, making them less sensitive for accurate diagnosis. Although MRI and sonography have been reported for diagnosing interdigital neuroma, they have been shown to be of limited value, so the diagnosis of interdigital neuroma is still a clinical one.
Numerous reports have confirmed the efficacy of neuroma excision in the third web space. The results of nonoperative treatment, consisting of metatarsal bars or pads, local injection of a steroid preparation into the affected web space, and wide toe box shoes, have been unpredictable but successful often enough to warrant at least a trial period.
In a level 1 prospective, randomized study, Thomson et al. found that at 3-month follow-up, corticosteroid injections produced significantly improved visual analog scale (VAS) scores compared to the control group. However, Lizano-Díez et al. found no difference between corticosteroid with anesthetic injections and anesthetic injection alone at 3 and 6 months. Roughly half of the patients still requested surgical excision. Furthermore, caution is advised with the repetitive use of steroids, which can cause atrophy of the plantar fat pad or disruption of the adjacent joint capsule with resultant toe deviation.
Through nerve sclerosis, alcohol injections have been reported to improve symptoms in short-term follow-up, but Gurdezi et al. found no improvement at long-term follow-up (average, 61 months) in a study of 45 patients: only 29% reported improvement and 36% proceeded with surgical excision. Furthermore, 12 of the 45 patients had minor complications. Epinosa et al. found similar results: only 22% of their 32 patients reported improvement after alcohol-sclerosing injections. A recent systematic review found insufficient high-quality evidence supporting the use of alcohol injections for interdigital neuromas. Because of these poor or inconclusive results, we do not use sclerosing agents for neuroma treatment.
Other than corticosteroid and alcohol injections, less common nonsurgical treatments also have been described through small series. Reported treatments include extracorporeal shockwave therapy (ECSWT), radiofrequency ablation, cryoneurolysis, hyaluronic acid injections, and capsaicin injections. The sparse literature has not validated the safety and efficacy of these alternative modalities.
Surgical excision remains the most predictable management for successful treatment of an interdigital neuroma; however, an accurate diagnosis and patient counseling are critical to ensure optimal outcomes. In most of the series reported, 80% to 95% of the patients are completely asymptomatic and pleased with the result of surgery. However, Mann and Reynolds reported that 65% of patients still noted some local plantar tenderness after surgery, and 20% subjectively rated their result as less than a 50% improvement in symptoms. Another study reported limitation in footwear in 70% of patients, and Womack et al. found that the long-term outcomes of neuroma excision were not as successful as previously reported. More recently, Kasparek and Schneider reported that 76% of 98 feet had an excellent or good result an average of 15 years after neuroma excision. Detailed preoperative examination and careful patient selection for surgery are recommended. In addition, the patient must be informed before surgery that some symptoms may remain after surgery. The surgeon should look carefully for other causes of metatarsalgia that might coexist in a patient with an interdigital neuroma and should explain that any symptoms other than those caused by the interdigital neuroma would not be improved by its excision.
Opinions vary as to whether excision is the operative treatment of choice for interdigital neuroma. Gauthier performed epineural neurolysis using a longitudinal dorsal approach. The deep transverse intermetatarsal ligament was released to facilitate neurolysis. Overall, 28% of the patients either were unimproved or had enough continued symptoms to warrant further treatment. We have had no experience with this technique. Furthermore, the division of the deep transverse intermetatarsal ligament during this procedure is controversial. Whether its division results in a “dropped” metatarsal or splaying of the forefoot remains uncertain. Through the dorsal approach, the nerve is better exposed in its proximal portion if the ligament is divided. Through the plantar approach, the ligament does not impair exposure of the neuroma in its proximal portion and usually is left intact.
The choice of a dorsal or plantar incision for removing a neuroma probably is determined by the surgeon’s training. Either approach can be satisfactory, but patients with a plantar incision complain of soreness around the wound for many weeks, which gradually resolves. Each approach has advantages and disadvantages. For a recurrent interdigital neuroma, a plantar approach is recommended because the exposure is excellent. With a dorsal approach, identifying the stump of the neuroma amid the scar tissue can be difficult. The plantar approach allows the surgeon to identify the nerve in normal tissue and dissect it out to the involved tissue. Dorsal and plantar longitudinal approaches were compared in one study that assessed sensory loss, number of sick leave weeks, and further treatments. The overall patient satisfaction was not significantly different between the two approaches. Infection, missed nerves, and nerve stump neuroma occurred in the dorsal group, and three minor (2 × 3 mm) intraincisional keratoses occurred in the plantar group. More recently, Nery et al. reported 89% good results, 7% fair results, and 4% poor results at 7-year follow-up of 168 patients who had neuroma excision through a plantar incision.
Regardless of the approach, the nerve is transected as far proximally as the incision allows, but care must be taken not to injure the common digital branches to the second or fourth web spaces. Several plantarly directed nerve branches run from the common digital nerve into the forefoot pad. They are found in the highest concentration in the distal aspect of the common digital nerve, just proximal to its bifurcation into the proper branches. If the nerve is not transected well proximal to the deep transverse intermetatarsal ligament, these plantarly directed nerve fibers can tether or prevent retraction of the common digital nerve, leading to its adherence near the metatarsal head and causing recurring symptoms. Careful localization of the most tender area, and presumably the neuroma stump, before surgery helps the surgeon to locate the nerve during the dissection. Mann recommended the dorsal approach for a recurrent neuroma and for the original excision.
The results of surgery for “recurrent” interdigital neuroma or resection of a neuroma stump are not as predictably satisfactory as the results of primary resection. Forty-three percent of patients in one study were dissatisfied with their clinical outcome or had major reservations about the efficacy of the procedure. Revision surgery for neuroma excision should be approached cautiously, and the patient should be counseled regarding the possibility of an unsatisfactory result after revision surgery for interdigital neuroma.
We currently do not use endoscopy for decompression (release of the transverse metatarsal ligament) or excision of an interdigital neuroma because of the lack of evidence regarding its efficacy and safety. However, Kubota et al. and Lui have reported encouraging case reports and techniques.
(Amis)
Make a dorsal longitudinal incision 3 to 4 cm proximal to the web and extend it distally, ending just proximal to the web space. It is important not to follow the extensor tendons because they take a more lateral direction. The incision, when properly made, is slightly oblique and medial in relation to the course of the extensor tendons.
Deepen the dissection and retract the dorsal sensory nerves to the side of least resistance.
Proximally identify the dorsal interosseous fascia. Follow the dorsal interosseous fascia and muscle distally directly to the bursa overlying the intermetatarsal ligament. Open the bursa and identify the intermetatarsal ligament.
Bluntly dissect proximally between the third and fourth metatarsals, retracting the corresponding dorsal interosseous muscle medially and opening the web space for better exposure proximally.
Place a small lamina spreader between the metatarsal necks and spread them apart ( Fig. 87.9A ).
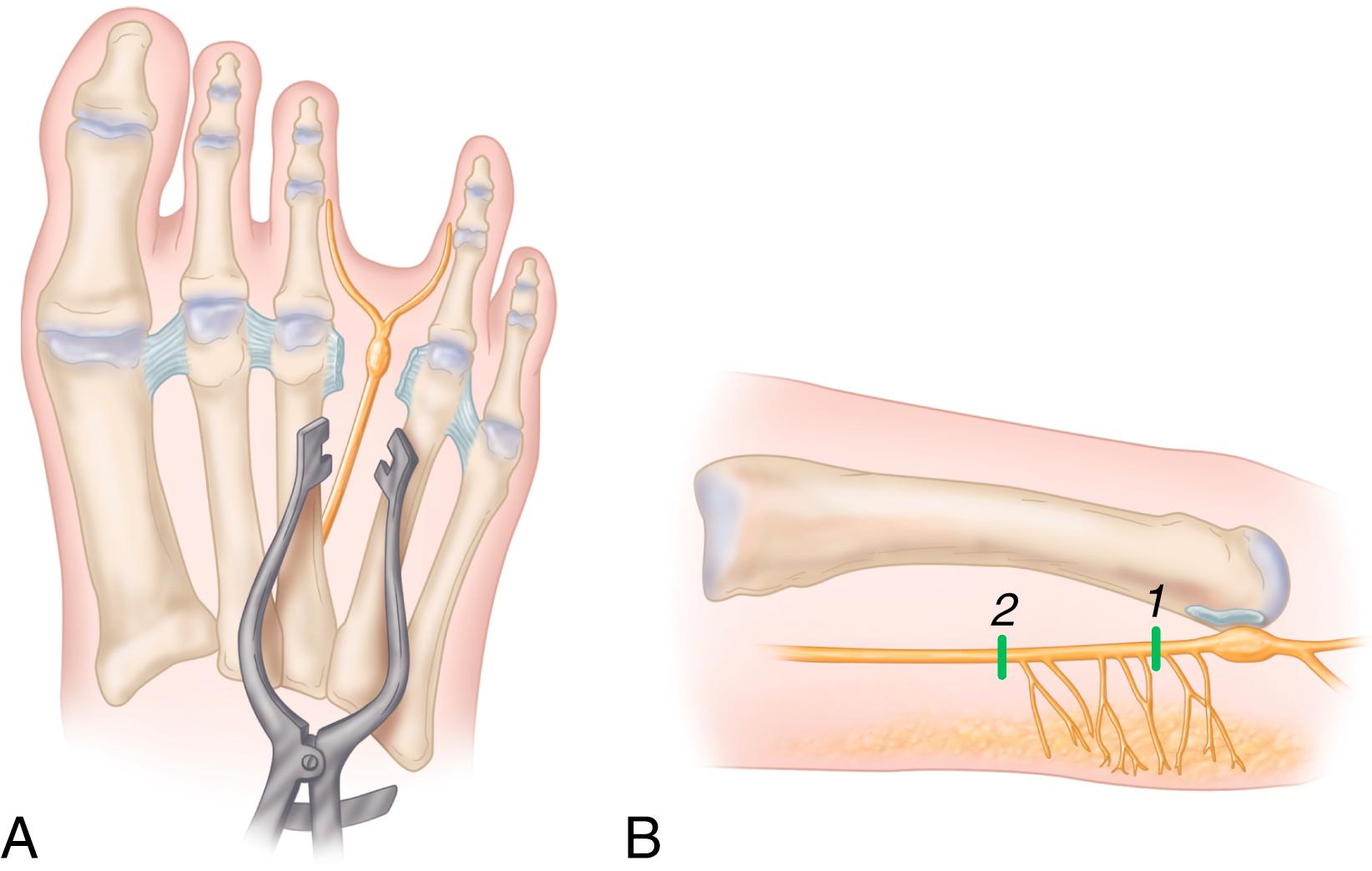
Retract the web space fat pad distally with the deep blade of a Senn retractor.
Sometimes a larger interdigital neuroma can be seen at this point ( Fig. 87.10 ). Do not press up beneath the foot to extrude the neuroma distal to the intermetatarsal ligament because this gives a false sense of exposure and probably is one reason why recurrence or incomplete resection occurs.
Dissect the intermetatarsal ligament at its distal portion and divide along the entire length longitudinally.
While protecting the underlying structures, release the intermetatarsal ligament using scissors or a No. 15 blade. Be careful not to damage the lumbrical tendon beneath.
When the distal portion of the intermetatarsal ligament is identified, free the neurovascular bundle, using scissors to spread under the intermetatarsal ligament from a distal to proximal direction.
Release the intermetatarsal ligament and remove it proximally with digital manipulation to identify any remaining intermetatarsal ligament that may not have been released. A complete release of the intermetatarsal ligament is essential to the success of this surgery.
Although the intermetatarsal nerve may not be enlarged to a great degree, the nerve should be dissected and resected as planned regardless of its size. Structures that could be mistaken for the nerve in the web space include the lumbrical tendon, which passes medially to the base of the fourth proximal phalanx, and the common digital artery, which usually crosses proximal medial to distal lateral lying dorsally over the nerve. Often the artery comes out from under the medial metatarsal neck. If identified, dissect the artery away from the nerve and preserve it.
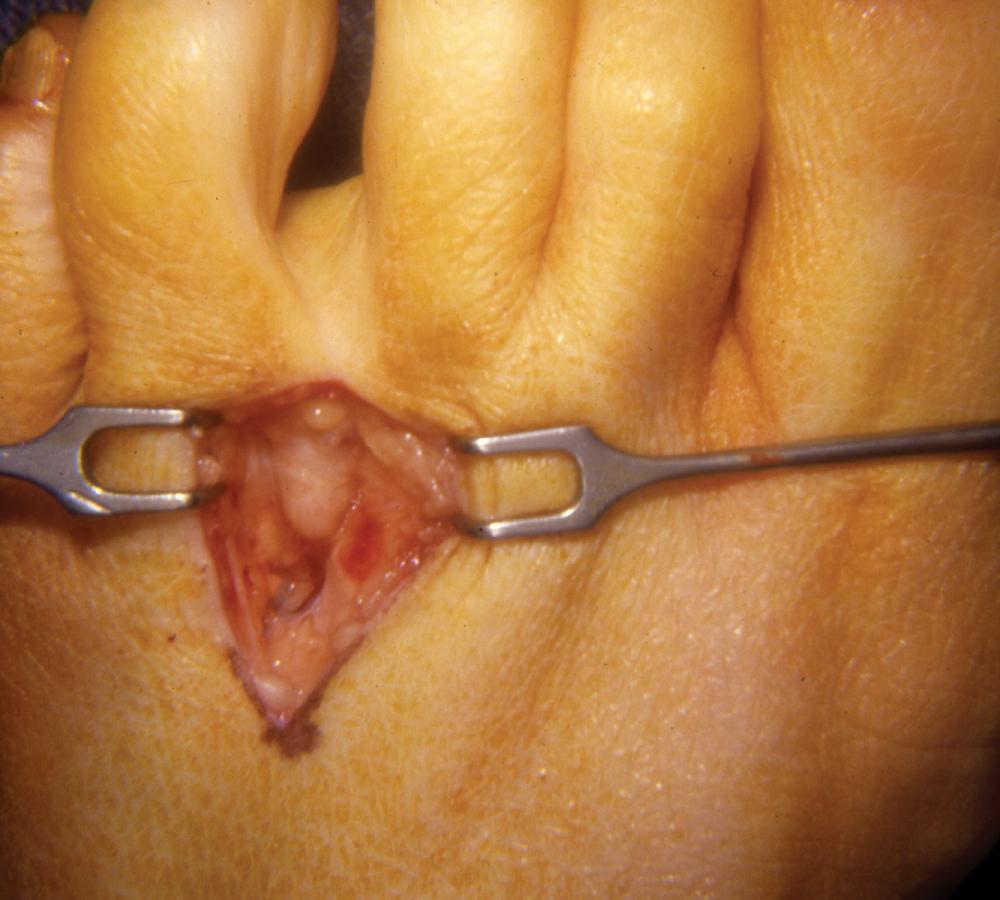
Dissect the nerve more distally, but not to the two proper digital branches ( Fig. 87.11 ).
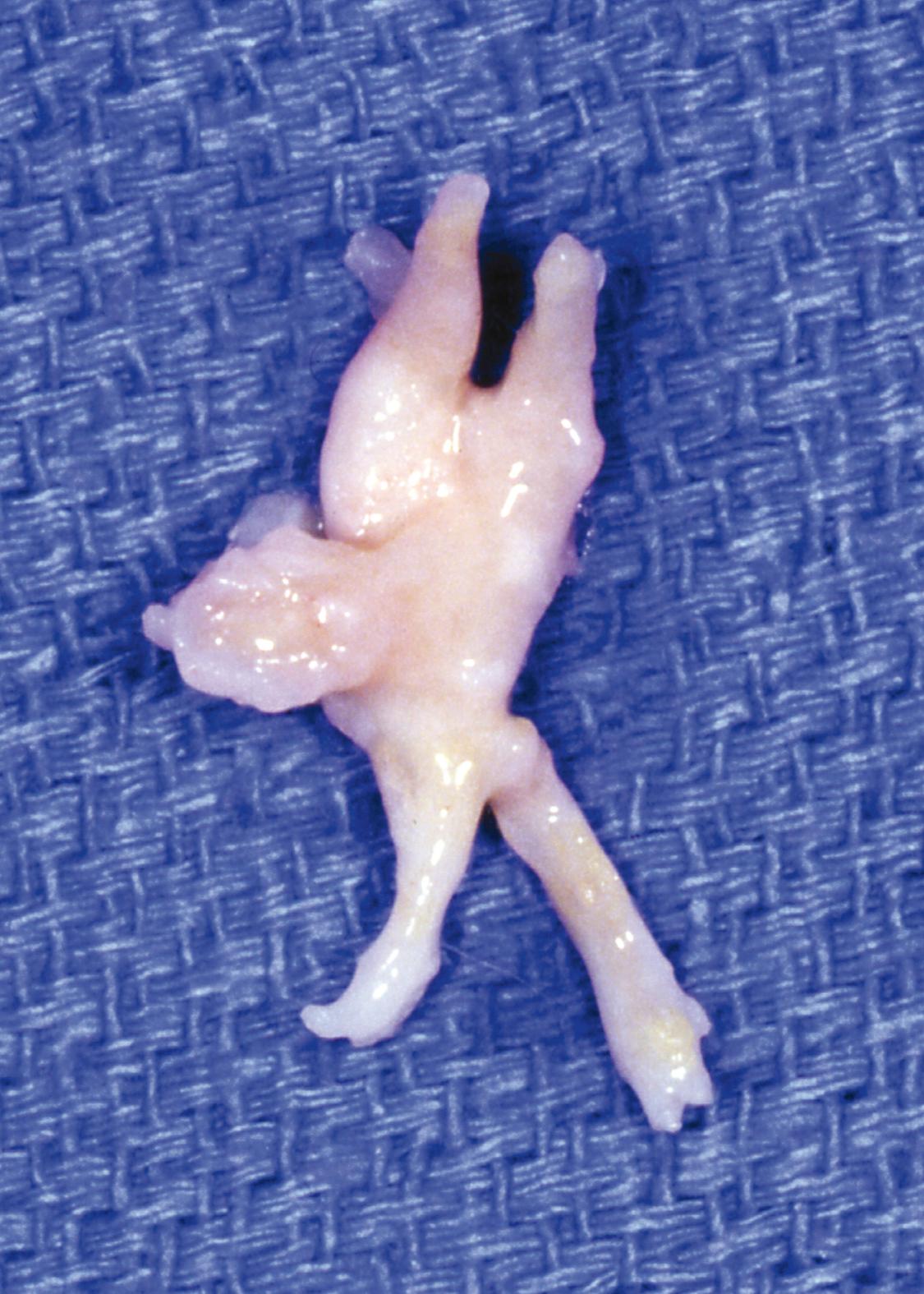
The neuroma usually is at or just distal to the intermetatarsal ligament. Extend the dissection to the distal aspect of the neuroma, dividing it at that point.
Dissect the nerve circumferentially to 3.0 to 3.5 cm proximal to the neuroma ( Fig. 87.9B ).
If the dissection is carried through to the adductor hallucis muscle, partially divide or retract it dorsally with a small right-angle (Ragnell) retractor.
Divide the plantar-directed branches of the intermetatarsal nerve to the forefoot pad so that the nerve is easily traced proximally. Ensure that the area of transection corresponds to the region of the non–weight-bearing part of the foot, 1 to 2 cm proximal to the weight-bearing pad of the forefoot, by placing a blunt instrument in the same location where the nerve will be transected and palpating it in the plantar aspect of the foot. Dissect more proximally if this non–weight-bearing area is not achieved.
Divide the nerve at its proximal resting place and remove the nerve that was circumferentially dissected and the neuroma.
Cauterize the neuroma stump and use a small hemostat to place the nerve stump well proximally and dorsally into the interosseous muscles, preventing it from reaching a weight-bearing area. Send the specimen to the pathology department for biopsy.
With the lamina spreader still in place, release the tourniquet to determine whether arteries or larger vessels have been transected during the procedure.
Remove the lamina spreader and place light compression on the forefoot for 5 to 10 minutes while the reactive hyperemia time passes. As an alternative, close the wound under tourniquet control and release the tourniquet after placement of sterile dressings.
Close the skin loosely using 4-0 nylon sutures in a vertical mattress suture fashion. Place a slightly bulky “sandwich” dressing over a petroleum jelly-impregnated gauze pad and wrap with an elastic bandage with only mild compression.
For the first few days, the patient rests with maximal elevation of the extremity but may bear weight as tolerated in a postoperative shoe as needed. After the first few days, the patient may increase walking as pain allows. Sutures are removed at 2 to 3 weeks, and plastic strips are placed across the wound for another week. A postoperative shoe usually is needed for 2 to 4 weeks, followed by a wide toe box, soft-vamp shoe for an additional 3 to 4 weeks.
Under tourniquet control with the patient supine and an assistant dorsiflexing the ankle to neutral position, begin a 3- to 4-cm plantar longitudinal incision just proximal to the web space and continue in a proximal direction ( Fig. 87.12A ). A small, self-retaining retractor is an excellent aid at this point because of fat overlying the plantar aponeurosis.
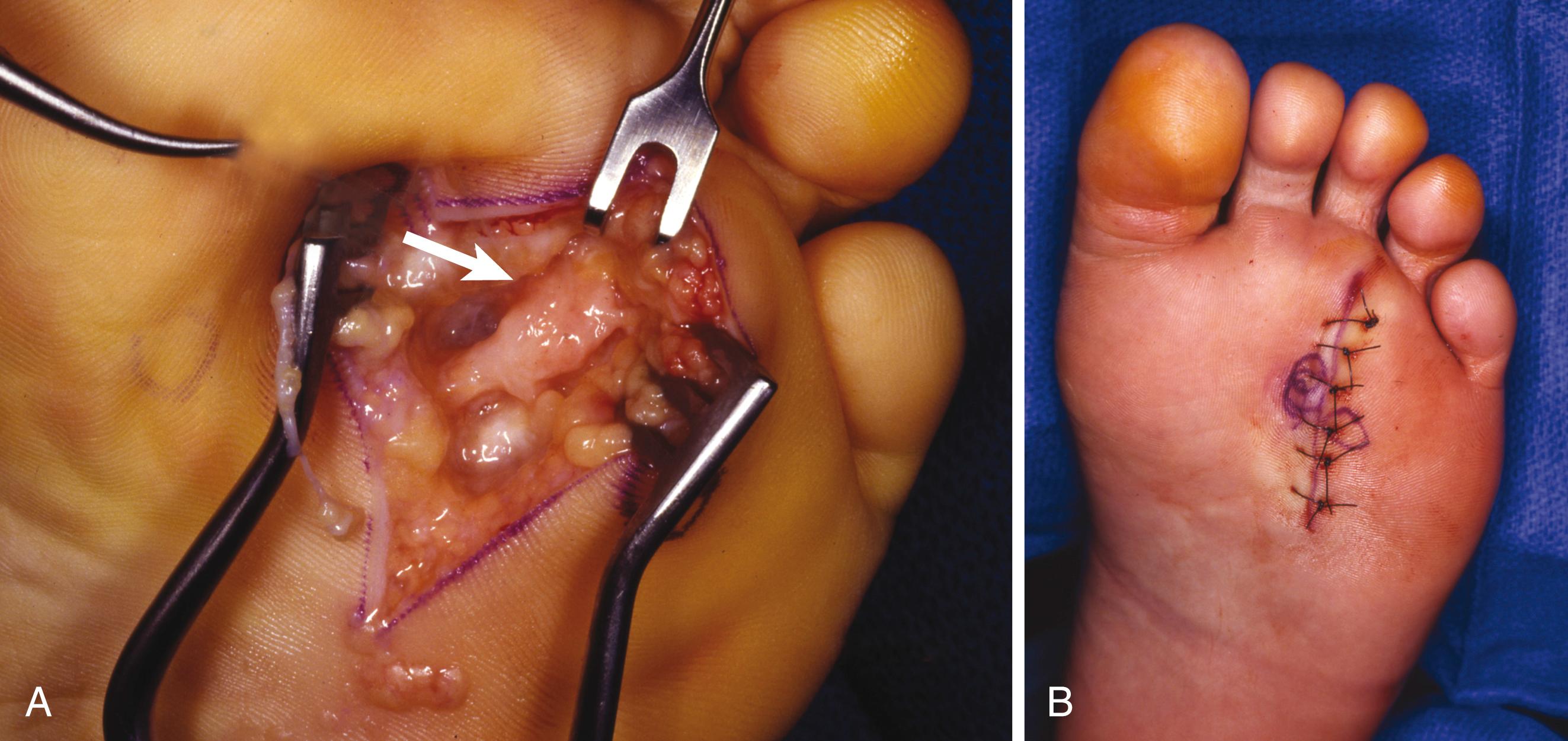
Make a longitudinal incision in line with the skin incision through the plantar aponeurosis.
Using blunt dissection in a longitudinal plane, identify the common digital nerve proximally; dissect it distally to the neuromatous enlargement just proximal to the emergence of the proper digital branches to the adjacent sides of the toes.
Excise the neuroma, being careful to resect the nerve 2 to 3 cm proximal to the deep transverse intermetatarsal ligament. Incising the deep transverse intermetatarsal ligament that lies dorsal to the neuroma is unnecessary.
Remove the tourniquet, achieve hemostasis, and close the skin with interrupted nonabsorbable sutures only ( Fig. 87.12B ).
A bulky compression dressing is applied to the forefoot, and the extremity is elevated for 24 hours before beginning ambulation in a wooden-soled shoe. We have not seen wound complications as a result of early weight bearing when a postoperative wooden-soled shoe has been used and the patient has been instructed to walk on the heel of the affected foot. If the patient cannot cooperate, however, crutch walking is encouraged until the sutures are removed 2 weeks later, at which time adhesive strips are applied to the wound for another week. The remaining management is the same as described for the dorsal incision.
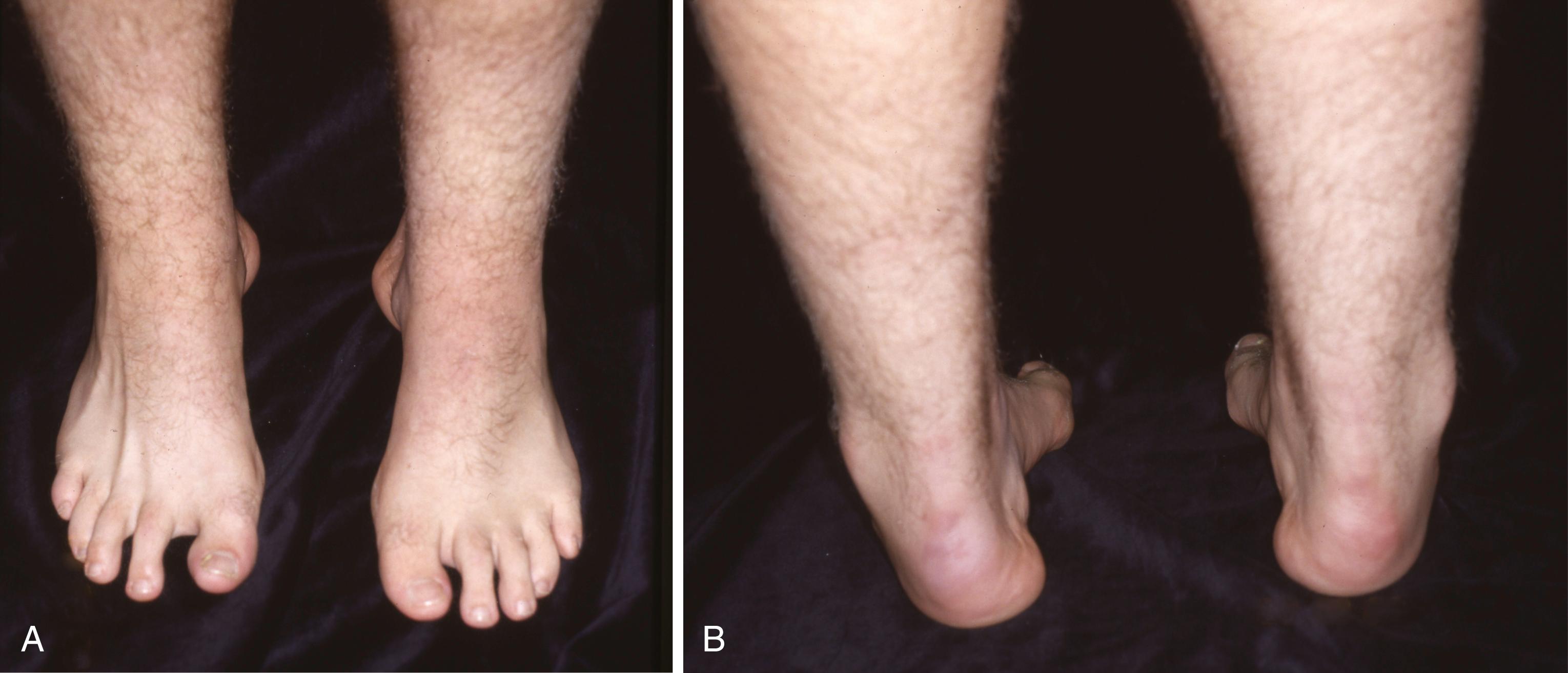
In its simplest form, a cavus foot is one with an abnormally high arch. This high arch usually accompanies a spectrum of deformities, including hyperextension of the toes at the metatarsophalangeal joints and hyperflexion at the interphalangeal joints, pronation and adduction of the forefoot (forefoot valgus), a “bony” dorsum of the midfoot with wrinkled skin folds on the medial plantar aspect, lengthened lateral border of the foot and shortened medial border, calluses beneath the metatarsal heads and lateral column, varied stiffness of the Chopart joint, fixed or flexible heel varus, and tightness of the Achilles tendon with or without an equinus contracture. Although pes cavus with multiple deformities is difficult to define, it is easily recognized ( Fig. 87.13 ); however, it is definitely not easy to treat operatively or nonoperatively.
A cavus foot may result from many different processes, all of which create muscle imbalances that result in deformity. The imbalance may occur from a progressive or static process, leading to variable severities and treatments. Underlying neuromuscular disease, such as Charcot-Marie-Tooth (CMT) or ataxia, leads to more progressive deformities. Other processes (postpoliomyelitis, cerebral palsy, club foot, etc.) are more static, but worsening contractures can still lead to varying cavus deformities. Spinal cord disorders (spinal cord tumors, spinal cord dysraphism, syrinx, etc.) lead to muscle imbalance causing cavus deformity. Even traumatic injuries, resulting in varus malunions or postcompartment syndrome imbalances, can lead to cavus deformity.
Charcot-Marie-Tooth disease is the most common neuromuscular disease causing pes cavus. This disease is actually a heterogeneous group of hereditary sensory and motor neuropathies (HSMN) caused by neural protein mutations affecting peripheral nerve conduction through axon or myelin irregularities. Our understanding and classification of this disease continues to advance. All types are inheritable, but nearly half of the cases result from a new mutation. Characterized by axon demyelination, CMT-1 is the most common type, occurring in over 50% of CMT patients. CMT-1 can be further subdivided into CMT-1A, CMT-1B, and CMT-1C. CMT-2 is the second most common type and may have near-normal nerve conduction velocities. There is striking variability of penetration and clinical expression of this autosomal-dominant disease, even in immediate family members and extended family. CMT-X is found in 10% to 20% and demonstrates an X-linked inheritance pattern. CMT-4 exhibits an autosomal recessive inheritance pattern and is very rare.
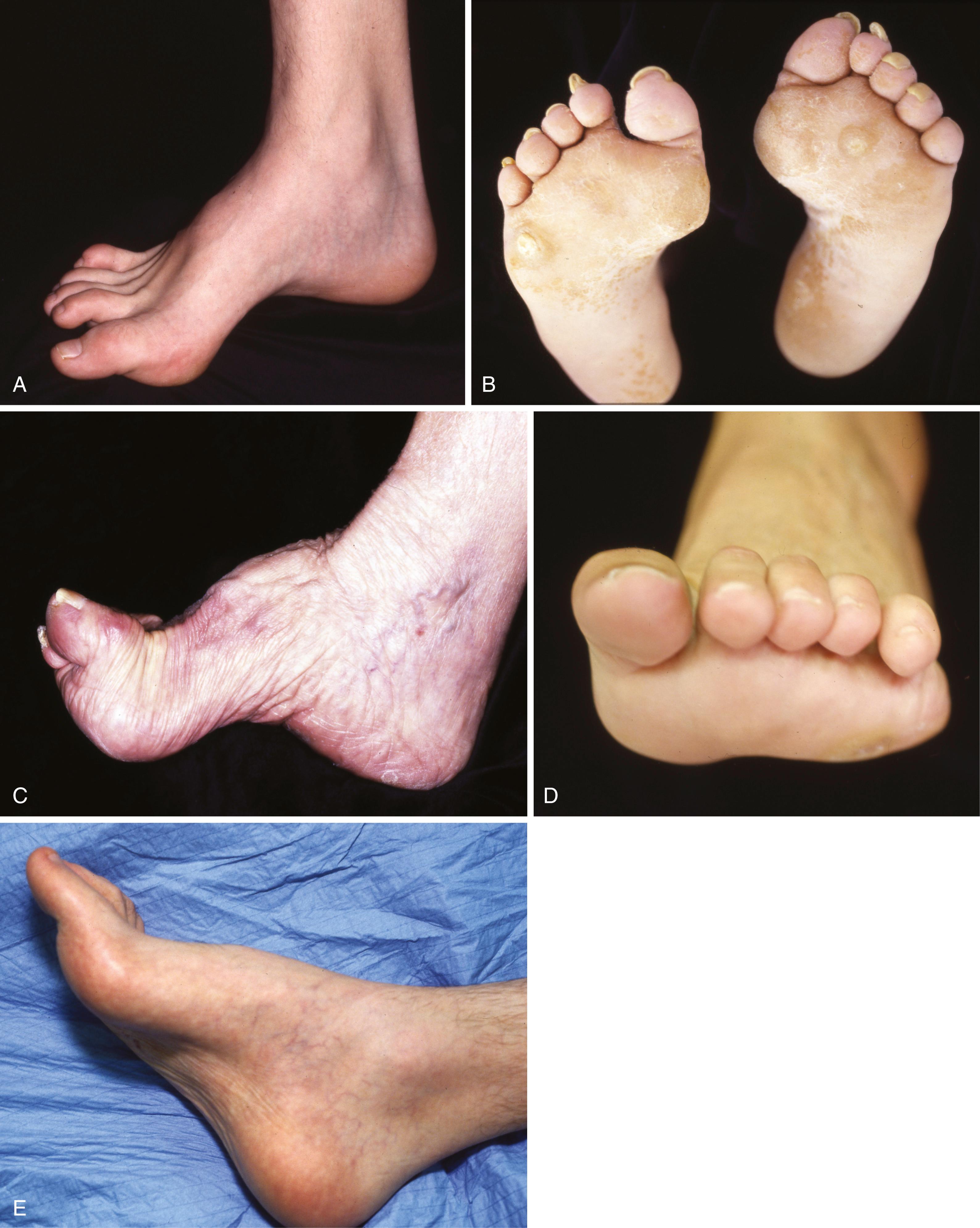
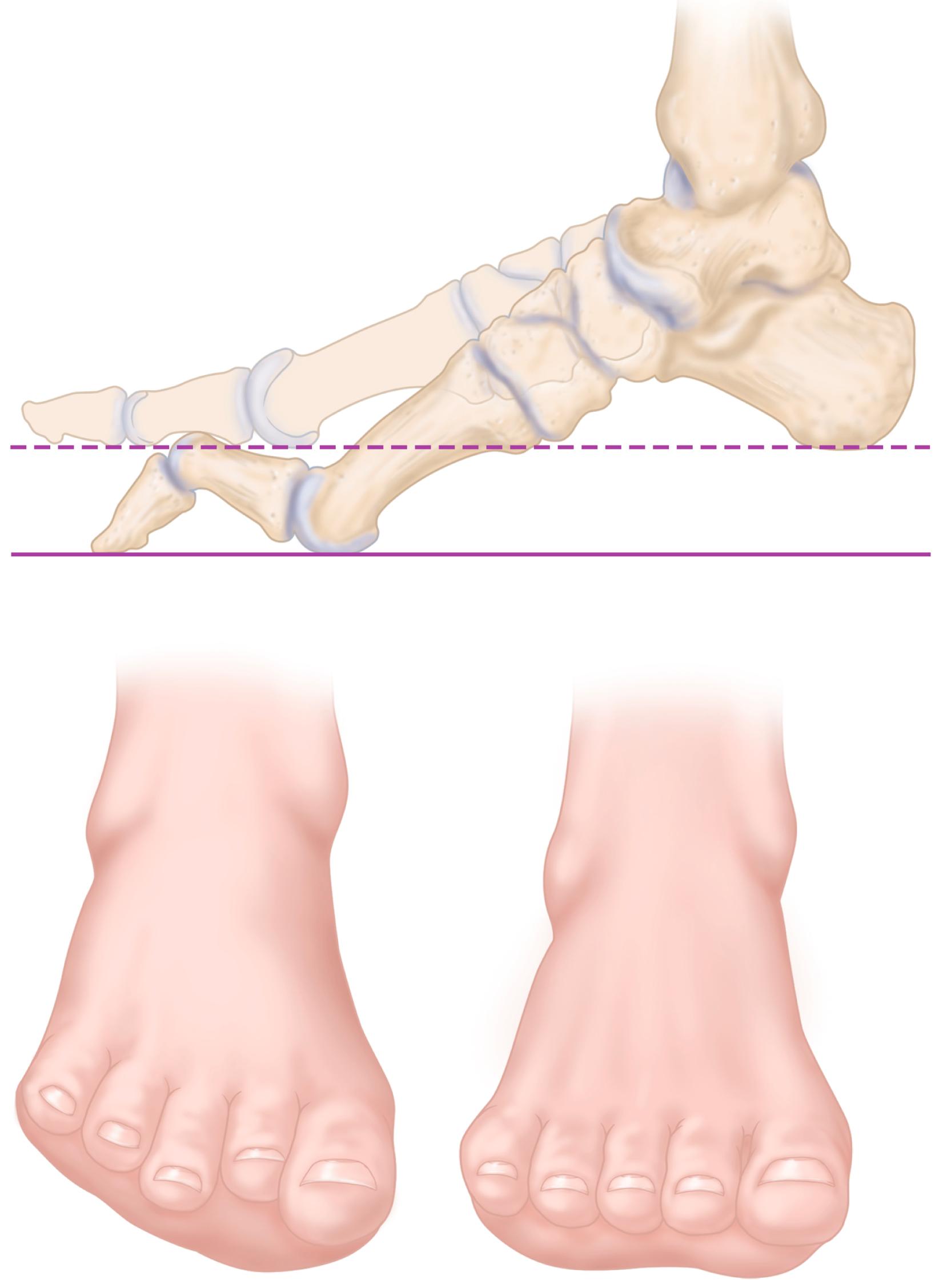
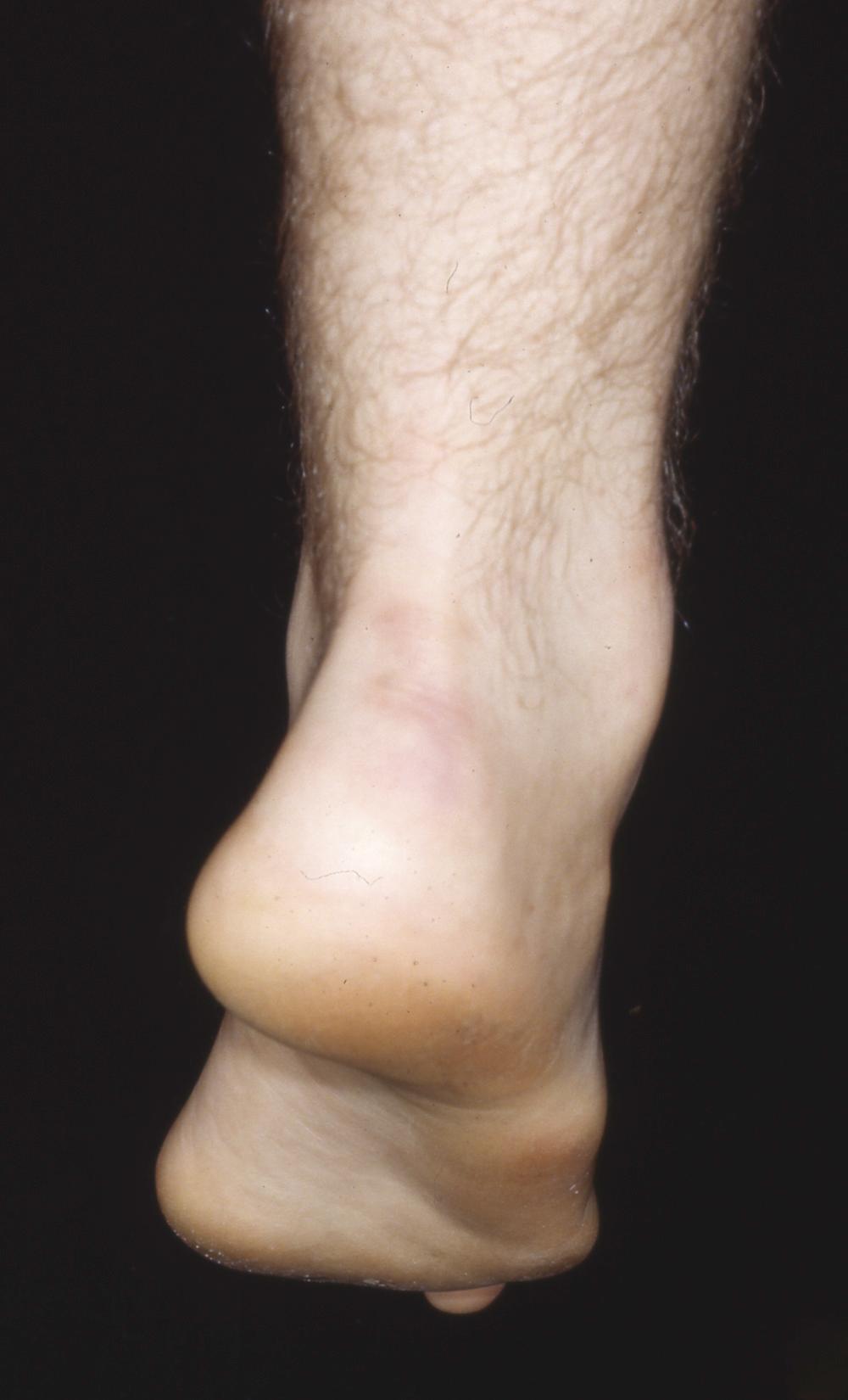
Regardless of the specific type, all types result in muscle imbalance affecting the lateral and anterior muscle compartments. The clinical expression varies from individual to individual, but the classic muscle imbalances involve weakness of the tibialis anterior while sparing the peroneus longus, resulting in a plantar flexed first metatarsal ( Fig. 87.14 ). To accommodate this forefoot pronation, the hindfoot assumes a varus posture ( Fig. 87.15 ). Additionally, peroneus brevis weakness countered by the near-normal tibialis posterior strength leads to worsening inversion of the midtarsal joints and hindfoot varus deformity. Developing contractures of the plantar fascia and the varus-oriented Achilles tendon further contribute to the deformity. Last, imbalance of the intrinsic-extrinsic muscles enhances the deformity. The intrinsic muscles in the plantar aspect of the foot generally flex the metatarsophalangeal joints and extend the interphalangeal joints. Any weakness of these muscles relative to their antagonists causes plantar flexion of the metatarsals, hyperextension of the metatarsophalangeal joints, and hyperflexion at the interphalangeal joints (toe clawing).
Patients with cavus deformity as a residual of poliomyelitis usually have different physical findings from patients with CMT disease. Fortunately, with the success of vaccination in the 1950s, postpoliomyelitis cavus is less commonly encountered. Depending on the level of injury and inconsistent recovery, poliomyelitis can cause differing patterns of muscle imbalance and subsequent deformity ( Fig. 87.16C ). Unlike CMT disease, a weak gastrocnemius-soleus muscle opposite a strong anterior tibial muscle may cause a calcaneal deformity of the hindfoot, with or without hindfoot varus or valgus, depending on which muscle groups have preserved strength. Because of intact sensation and the nonprogressive nature of the deformities, patients with postpoliomyelitis cavus feet typically have a better prognosis than patients with CMT disease.
Traumatic cavus deformity can be caused by deep posterior compartment syndrome or by malunion of midfoot and hindfoot fractures. The deformity may not appear for several months after the local ischemia and muscle fibrosis of the deep posterior compartment, or it may appear as a mild, barely perceptible abnormality that progresses relentlessly to a rigid cavovarus, claw toe deformity. Soft-tissue injuries from crush mechanisms or severe burns can lead to contractures and muscle imbalances that result in cavus deformity. Through altered bony anatomy and joint mechanics, hindfoot malunions (i.e., calcaneal and talar neck malunions) also can create posttraumatic cavus deformities.
In some patients with symptomatic cavus deformities, no definite cause is discovered ( Fig. 87.17 ). In patients with idiopathic deformities, the underlying pathologic mechanism of the cavus deformity is believed to be an imbalance of the extrinsic-intrinsic muscles. As previously discussed, the intrinsic minus foot demonstrates metatarsal plantar flexion and toe clawing in which the hindfoot assumes a varus posture to accommodate the rigid plantar flexed first ray (see Fig. 87.14 ). Furthermore, patients may display mild, subtle cavovarus as a normal variation within the bell curve for arch height and foot posture. Despite this “normal” variation, patient symptoms still require recognition and appropriate treatment.
Standard radiographic workup involves a standing series of the foot and ankle. Many angles and measurements have been described to help classify foot posture. These specific angles and values are likely most useful in research data collection rather than daily application, but the overall concept in recognizing these abnormal relationships is critical for understanding deformities and formulating treatment plans.
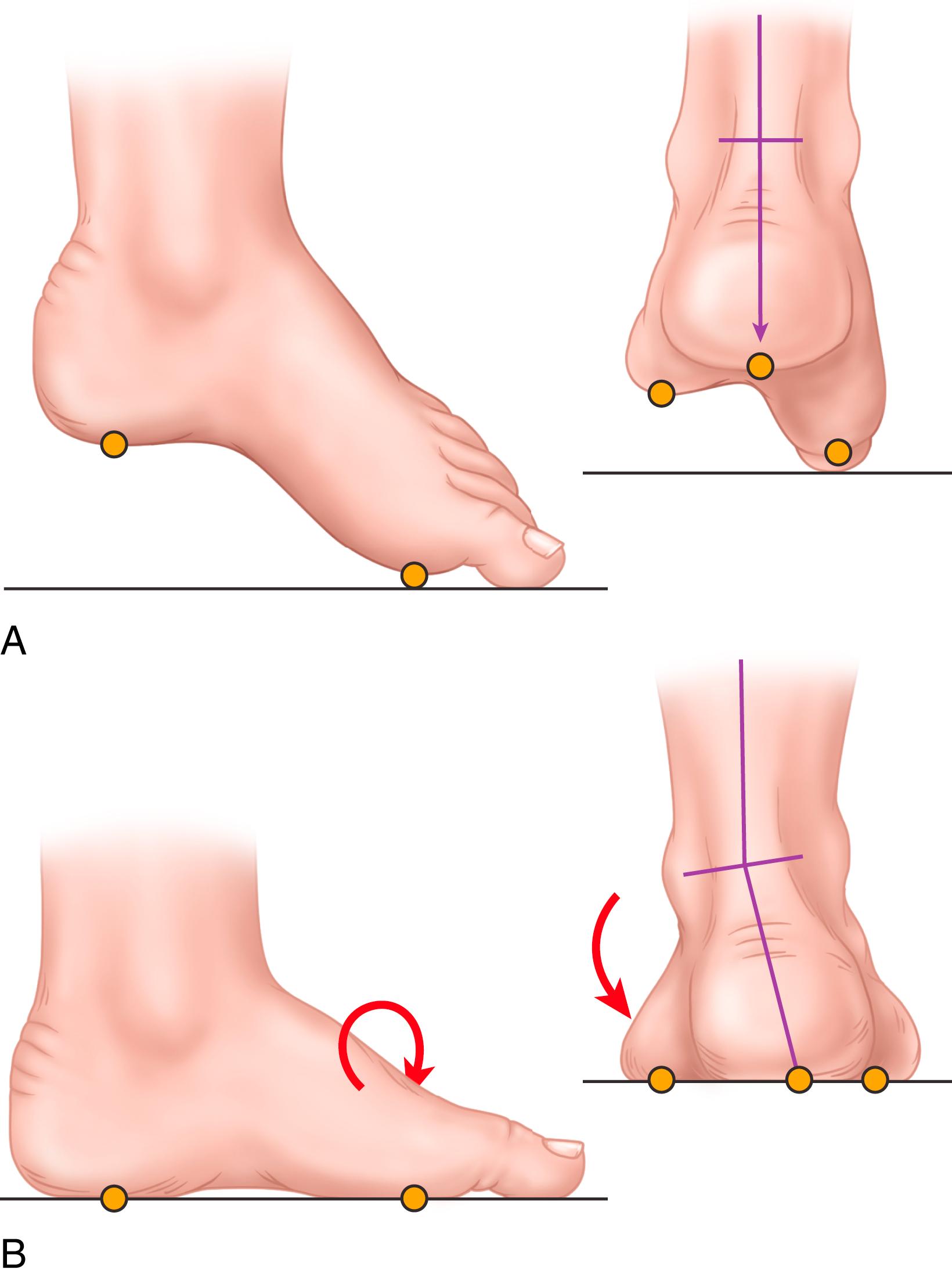
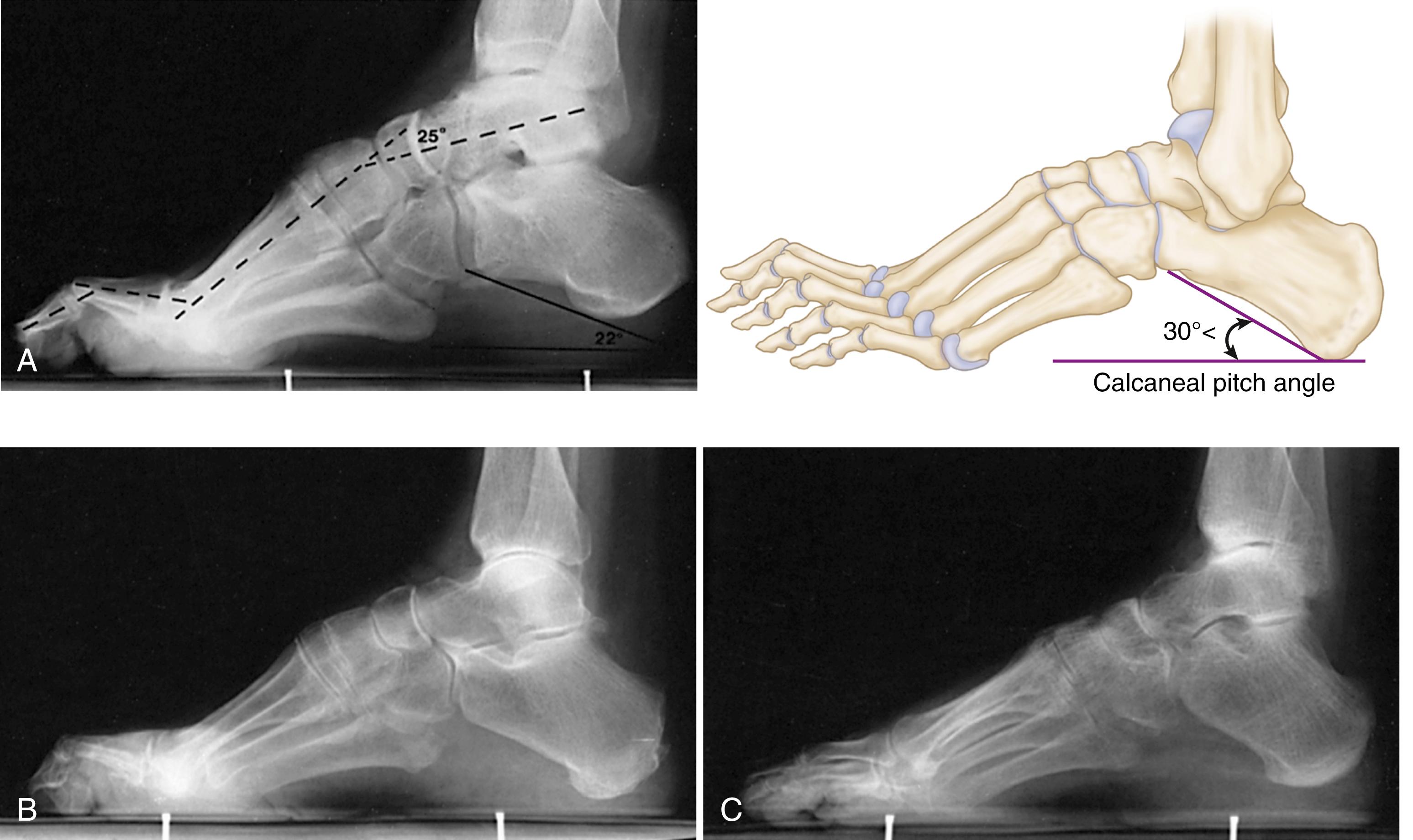
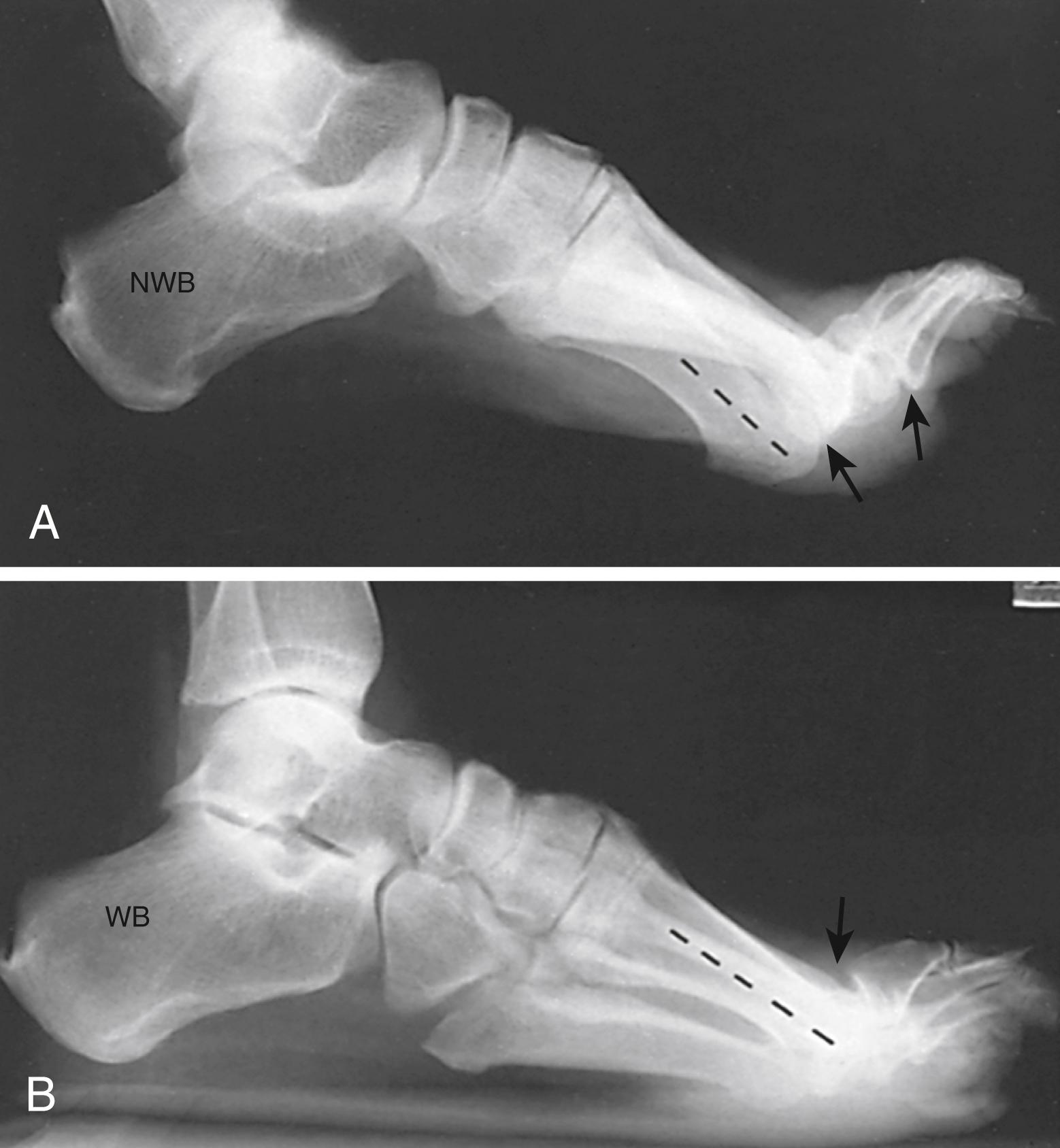
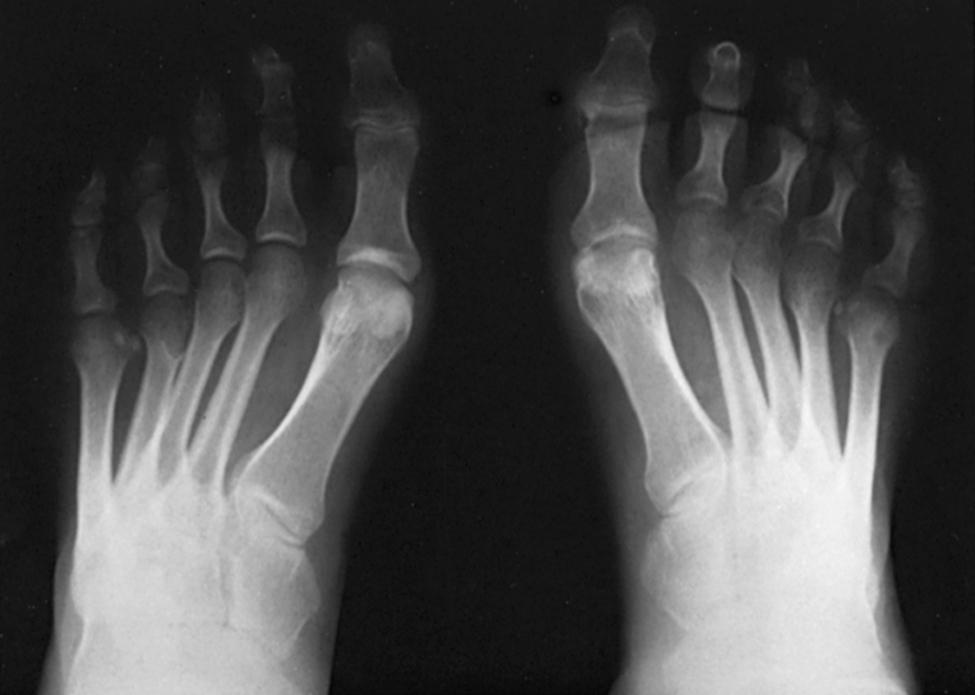
The standing lateral radiograph allows estimation of the contribution of the hindfoot (talus and calcaneus), midfoot (navicular and cuboid-cuneiform), and forefoot (Lisfranc) to the cavus deformity. The standing lateral view allows assessment of calcaneal pitch (see Fig. 87.16A ), talo–first metatarsal (Meary’s) angle, lateral talocalcaneal angle, and medial-cuneiform height. The extension deformity of the phalanges on the metatarsal heads during weight bearing helps determine the severity of the fixed forefoot deformity ( Figs. 87.18 and 87.19 ). The standing hindfoot alignment view displays the relationship of the calcaneus to the tibia, demonstrating varying degrees of hindfoot varus.
Similarly, the standing anteroposterior view allows estimation of the contribution from the hindfoot, midfoot, and forefoot. This view shows talar head overcoverage, metatarsus adductus, talocalcaneal angle, and the talo–first metatarsal angle. In flexible deformities, obtaining a standing anteroposterior foot radiograph with the hindfoot corrected (via Coleman block; see Fig. 87.22 ) helps corroborate any metatarsus adductus component suspected clinically ( Fig. 87.20 ). The talocalcaneal angle (Kite angle) is determined on this view. The closer the talocalcaneal angle approaches zero, the more parallel the talus is in relation to the calcaneus, indicating hindfoot varus.
Other radiographic findings that may be helpful include (1) degenerative changes in the tibiotalar, subtalar, or midtarsal joints; (2) rotation of the talus in the ankle mortise exhibited by a posterior fibula; and (3) dystrophic ossification in soft tissue suggesting tendon or ligament injury ( Fig. 87.21 ).
Although of questionable benefit for initial conservative treatment, CT scans are invaluable for surgical planning. The advancements in weight-bearing CT and three-dimensional reconstructions help surgeons conceptualize the deformity and localize the apex of the deformity for surgical correction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here