Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neuroendocrinology is the interactions between the nervous system and the endocrine system’s metabolic and hormonal homeostatic activities. One role the nervous system has is to connect the environment with the body. The most important environmental factors are temperature and light, which fluctuate in a predictable way each day. Humans have a circadian clock ( Chapter 374 ) to assure that physiology and behavior are attuned to the time of day. The nervous system senses temperature and light, and it uses them as clues to guide eating, activity, sleep, and other essential life functions. The microbiome ( Chapter 257 ) may also influence complex feeding behaviors via the neuroendocrine axis. The neurohypophysial neurons originate from the paraventricular and supraoptic nuclei and traverse the hypothalamic-pituitary stalk to the posterior pituitary, where nerve endings release vasopressin and oxytocin. Hypophysiotropic neurons are localized to specific hypothalamic nuclei, from which they project their axons to the median eminence, where they secrete hormones, which in turn stimulate or inhibit the release of peptides and bioamines into the proximal hypothalamic-pituitary vessels ( Fig. 204-1 ). The blood supply of the median eminence comes from the superior hypophysial artery and its richly arborized capillary beds that extend into the median eminence and then coalesce to form the portal veins that traverse the pituitary stalk and terminate in the pituitary gland. The neuroendocrine system relies on a series of feedback loops, which regulate pituitary hormones as well as the levels of target organ hormones. The target organ hormones regulate both the hypothalamus and the pituitary gland to complete the feedback loop. Perturbations in feedback loops can be altered by factors such as circadian rhythms (circadian periodicity), physiologic and psychological stress, nutritional status, and response to systemic illness.
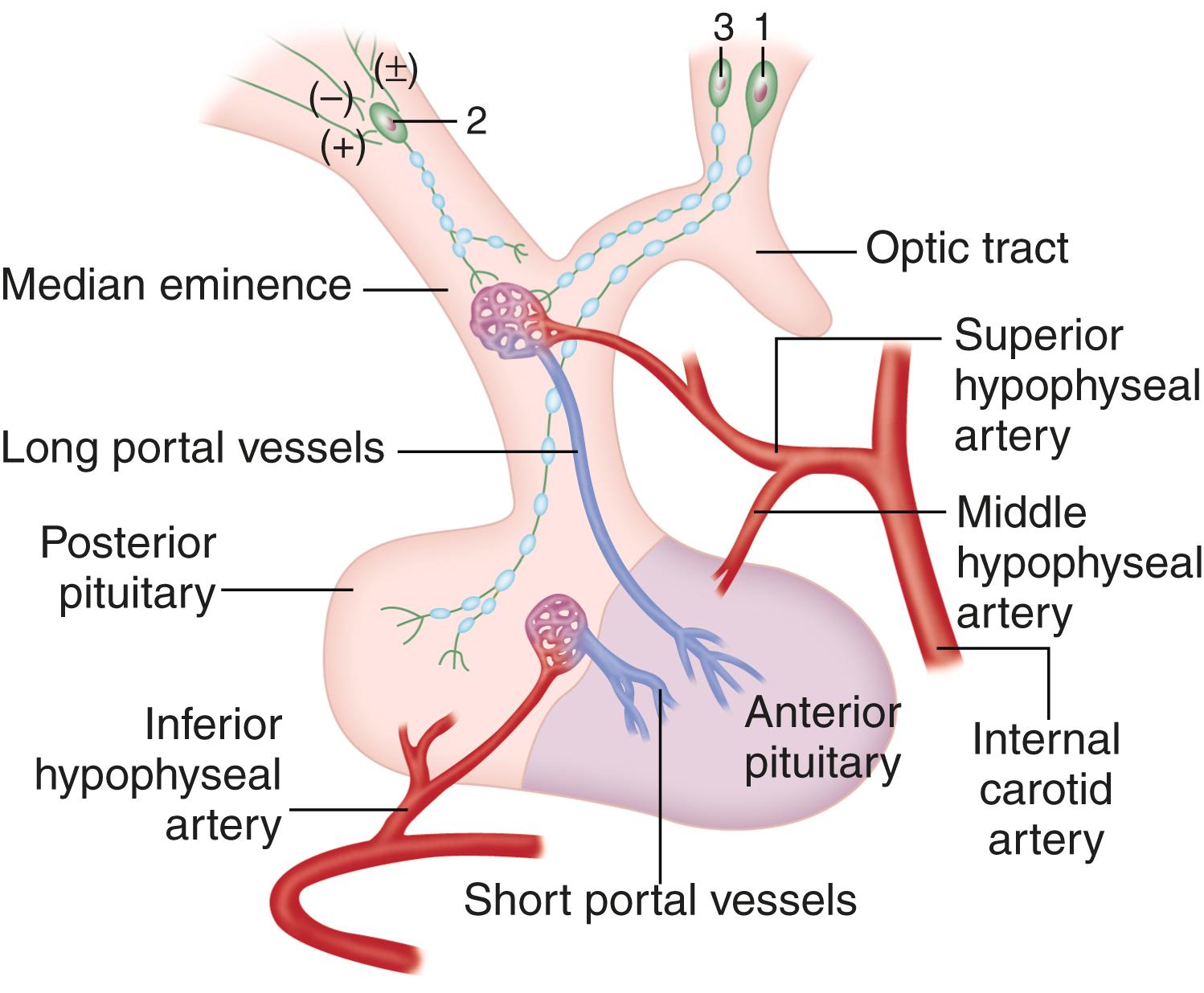
The hypophysiotropic hormones regulate the secretion of pituitary hormones. Some feedback loops are redundant, and some hypophysiotropic hormones exert effects on more than one pituitary hormone. Furthermore, the hypophysiotrophic hormones themselves are regulated by signals higher in the brain, such as the thalamus and the molecular clocks.
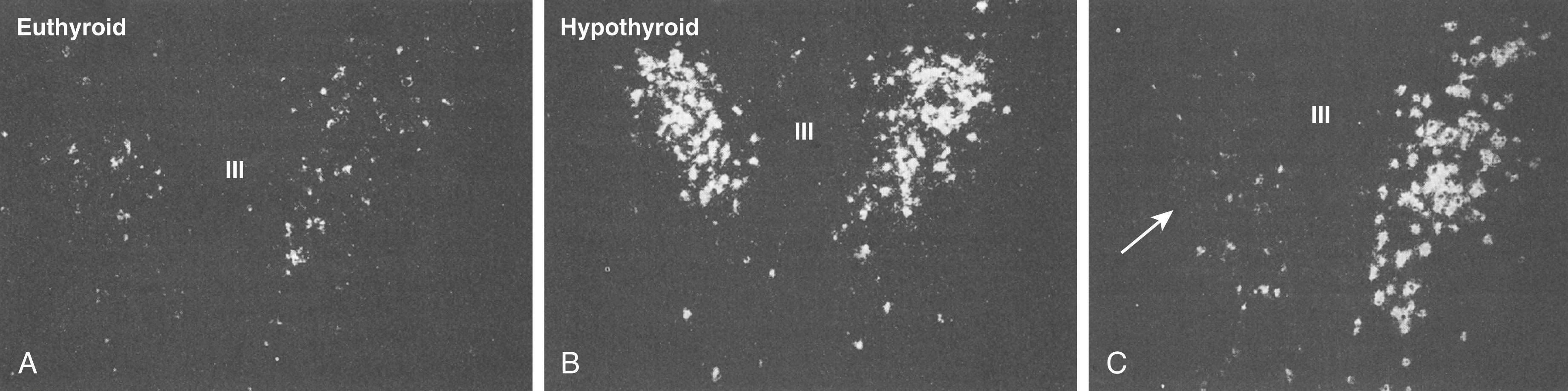
The primary neuroendocrine function of thyrotropin-releasing hormone (TRH) is to stimulate the synthesis and release of both thyroid-stimulating hormone (TSH) and prolactin. TRH is synthesized from the precursor prepro-TRH and then processed to biologically active TRH. Tanycytes that line the third ventricle contain an enzyme, pyroglutamyl peptidase II, that degrades TRH to regulate the amount of TRH that is transported to the pars distalis of the anterior pituitary gland. In hypothyroidism ( Chapter 207 ), the increased TRH synthesis and binding to the thyrocyte TRH receptors result in increased levels of TSH and prolactin. Correction of hypothyroidism with thyroid hormone replacement decreases the elevated levels of both TSH and prolactin. Conversely, in primary hyperthyroidism, TSH levels are markedly suppressed owing to a direct effect of thyroid hormone on mRNA expression of TRH in the paraventricular nucleus ( Fig. 204-2 ). TRH is the major regulator of the synthesis and secretion of TSH, but the role of TRH for regulating prolactin-releasing factor is less well understood.
Gonadotropin-releasing hormone (GnRH) is a 10–amino acid peptide. The neurons that produce GnRH originate outside the central nervous system in the epithelium of the medial part of the olfactory placode. This location is relevant to Kallmann syndrome ( Chapter 214 ), in which GnRH deficiency is associated with congenital agenesis of the olfactory bulbs. One of its genetic forms is caused by a loss of anosmin, which is a protein that facilitates the embryologic migration of these GnRH-producing neurons.
The primary function of GnRH is to control the reproductive axis. GnRH, which is released in a pulsatile manner into the hypothalamohypophyseal portal circulation, subsequently reaches the anterior pituitary gland, where it stimulates the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH).
Once the GnRH neuron migrates to the hypothalamus, a GnRH pulse generator is activated. In early postnatal life, GnRH secretion increases, thereby leading to temporary activation of gonadal hormone production, referred to as “minipuberty.” During childhood, the gonads respond to exogenous LH and FSH stimulation but remain quiescent because the endogenous pulsatile GnRH release is suppressed until puberty.
GnRH results in the differential secretion of LH and FSH owing to variable sensitivity of the feedback loop for steroid and peptide hormones, as well as variable sensitivity to GnRH. The pulsatile secretion of GnRH directly increases its own receptor, whereas continuous administration of GnRH is associated with a decrease. In women, positive and negative regulation of steroid hormone feedback in the hypothalamic-pituitary-gonadal axis occurs at both the hypothalamic and pituitary levels. The hypothalamic effects depend on the amplitude and frequency of the pulsed release of GnRH, whereas the pituitary effects are modulated by the gonadotropin response to GnRH. In males, testosterone decreases pulsatile GnRH secretion, with a resulting decrease in the amplitude and frequency of the gonadotropin pulse as well as a decreased gonadotropin response to exogenous GnRH.
In addition to pulsatility and regulation of GnRH receptors, kisspeptin may indirectly stimulate LH and FSH release by stimulating GnRH neurons. Kisspeptin levels rise just before puberty. Mutations in the kisspeptin gene have been associated with hypothalamic hypogonadism and impaired pubertal development, thereby demonstrating its role in the reproductive axis.
The negative feedback effects of inhibin, a peptide produced by testicular Sertoli cells and ovarian granulosa cells, are predominantly on FSH at the pituitary level, where inhibin causes a decrease in the sensitivity of gonadotrophs to GnRH. A related ovarian protein, activin, stimulates the basal and GnRH-stimulated synthesis and release of FSH from the pituitary, but its primary effect is to promote the response of ovarian granulosa cells to FSH. Another gonadal peptide, follistatin, inhibits the GnRH-induced rise in FSH that follows oophorectomy, primarily by binding to activin. These ovarian peptides are also found in the pituitary; therefore they may have additional local effects on gonadotropin secretion.
Pulsatile administration of exogenous GnRH is very successful for restoring normal sexual function and fertility in patients who have hypogonadotropic hypogonadism ( Chapter 216 ) secondary to GnRH deficiency. Long-acting GnRH agonists also are useful to downregulate GnRH receptors and gonadotropin secretion in a variety of conditions, including precocious puberty ( Chapter 214 ), prostate cancer ( Chapter 186 ), breast cancer ( Chapter 183 ), uterine fibroids ( Chapter 184 ), and endometriosis ( Chapter 218 ). Direct GnRH antagonists, which competitively bind to the GnRH receptor, are used for similar conditions.
Somatostatin (also known as somatotropin release–inhibiting factor) inhibits the secretion of growth hormone. The interactions between somatostatin and growth hormone–releasing hormone (GHRH) and their effects on the secretion of growth hormone are complex. Growth hormone secretory episodes are associated with increased secretion of GHRH, often accompanied by low somatostatin levels. By comparison, basal or trough levels of growth hormone are associated with lower levels of GHRH and more elevated levels of somatostatin. Somatostatin also inhibits both the basal and stimulated TSH secretion. However, growth hormone is about 10-fold more sensitive to inhibition by somatostatin than is TSH, thereby suggesting that the physiologic role of somatostatin in inhibiting TSH secretion is limited. Other tissues with somatostatin include the gut mucosa, D cells of the pancreatic islets, and the myenteric neural plexus. By its paracrine and endocrine actions, somatostatin suppresses the secretion of insulin, glucagon, gastrin, secretin, cholecystokinin, vasoactive intestinal polypeptide (VIP), and other gastrointestinal hormones, which in turn regulate functions such as gastric acid secretion, gastric emptying, gallbladder contraction, and splanchnic blood flow. Somatostatin analogs are used to treat acromegaly ( Chapter 205 ), carcinoid tumors ( Chapter 213 ), VIP-secreting tumors ( Chapter 213 ), TSH-secreting pituitary tumors ( Chapter 205 ), and islet cell tumors ( Chapter 211 ).
Corticotropin-releasing hormone (CRH) stimulates the release of equimolar amounts of adrenocorticotropic hormone (ACTH), β-endorphin, β-lipotropin, melanocyte-stimulating hormone (MSH), and other peptides that are generated from proopiomelanocortin. CRH and vasopressin have synergistic effects on the release of ACTH. For example, the release of ACTH in response to stress is mediated 75% via CRH and 25% by vasopressin. However, since the releases of CRH and vasopressin are not always coordinated, stress can selectively activate the vasopressin-containing subset of CRH neurons. In a feedback loop, cortisol decreases ACTH secretion at both the hypothalamic and the pituitary levels. ACTH and β-endorphin also feed back negatively to decrease the release of CRH by the hypothalamus. Central bioamines, opioids, and peptides also influence the secretion of CRH. Inflammatory monokines, including interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α, stimulate the synthesis and release of CRH and vasopressin from the hypothalamus. The resulting increase in cortisol then reduces the intensity of the inflammatory response and the related release of monokines, thereby completing the feedback loop. CRH receptors, which are widely distributed in the brain, are activated by the sympathetic nervous system and suppressed by the parasympathetic nervous system. CRH may also help regulate body weight, because overfeeding increases leptin levels, which stimulate CRH, which in turn inhibits appetite and increases energy expenditure.
Biosynthetic human CRH is helpful for differentiating Cushing disease from ectopic ACTH syndrome ( Chapter 208 ), with the finding that patients with Cushing disease respond with a greater than 35% increment, whereas those with ectopic ACTH secretion have a lesser response. If the results are equivocal, CRH testing during bilateral inferior petrosal sinus sampling for ACTH often provides additional discriminatory information.
GHRH dose-dependently stimulates growth hormone secretion. Repetitive administration of GHRH can release enough growth hormone in children with GHRH deficiency to increase insulin-like growth factor (IGF)-I levels and accelerate growth. The negative feedback of both IGF-I and growth hormone on the secretion of growth hormone decreases GHRH and increases somatostatin. This feedback effect is clinically relevant, as evidenced by high circulating growth hormone levels in IGF-I–deficient states such as renal insufficiency ( Chapter 116 ) and cirrhosis ( Chapter 139 ). In children in whom mutations in the growth hormone receptor make them unresponsive to growth hormone (also known as Laron-type dwarfism), IGF-I levels are very low and levels of growth hormone are correspondingly elevated.
A separate growth hormone–stimulating system involves a distinct receptor, termed the growth hormone secretagogue receptor, which interacts with a 28–amino acid peptide called ghrelin, which initially was isolated from the stomach. Both growth hormone secretagogue receptor and ghrelin messenger RNA are present in the human hypothalamus and pituitary. The physiologic interaction of ghrelin with GHRH and somatostatin is complex. In addition to a direct stimulatory effect on the pituitary, ghrelin amplifies secretion of growth hormone by indirect hypothalamic actions. However, ghrelin appears to be more important for the regulation of appetite and food intake.
The inhibitory component of hypothalamic regulation of prolactin secretion predominates over the stimulatory component. Dopamine is the major factor that inhibits prolactin in most physiologic circumstances (e.g., lactation), such as when a rising prolactin level is accompanied by a simultaneous fall in dopamine, as well as a rise in prolactin-releasing factors such as VIP. When endogenous dopamine receptors are blocked by drugs, such as the antipsychotic agents, prolactin levels rise. Lesions that interrupt the basal hypothalamic neuronal pathways that carry dopamine to the median eminence or interrupt portal blood flow (e.g., craniopharyngiomas or other large mass lesions) decrease the amount of dopamine that reaches the pituitary and can cause hyperprolactinemia.
Hypothalamic peptides other than TRH also have prolactin-releasing factor activity. VIP stimulates the synthesis and release of prolactin in hypothalamic-pituitary portal blood. Within the VIP precursor, another similarly sized peptide (peptide histidine methionine) also has prolactin-releasing factor activity. Prolactin-releasing peptide, which is a hypothalamic factor with main effects on the brain, participates in the regulation of food intake and energy expenditure.
Endogenous opioid peptides, which have a common 5–amino acid sequence at their amino terminals (Tyr-Gly-Gly-Phe-Met [or Leu]), bind to endogenous opioid receptors. The opioid µ-receptor mediates most of the opioid endocrine effects and analgesia ( Chapter 26 ). Its primary peptide ligand is β-endorphin, which is derived from proopiomelanocortin. For the opioid δ-receptor, which mediates behavioral, analgesic, and endocrine effects, the principal peptide ligands are met- and leu-enkephalins that are derived from proenkephalin A. Naloxone is not as effective for blocking the δ-receptor as for blocking the µ-receptor. The κ-receptor, which mediates sedation and ataxia, binds principally to dynorphin and the neoendorphins. A fourth receptor, which is partly homologous with the δ-receptor, binds to nociception, an endogenous 17–amino acid peptide.
Proopiomelanocortin, which is a 31-kD precursor peptide, contains ACTH, β-lipotropin, and β-endorphin. Its major cleavage products in the anterior pituitary are ACTH and β-lipotropin, and much of the latter is then processed into β-endorphin. In the intermediate lobe of the pituitary, the major products are α-melanocyte stimulating hormone (α-MSH), corticotropin-like intermediate peptide, β-endorphin, and γ-lipotropin. In the brain, however, proopiomelanocortin is processed primarily to β-endorphin, γ-lipotropin, and ACTH, with most of the ACTH then further processed to corticotropin-like intermediate peptide and α-MSH. The pentapeptide enkephalins are derived from the 28-kD precursor proenkephalin A. Neuronal perikarya containing the enkephalins are widely distributed in the brain. Dynorphin is a 17–amino acid peptide that is derived from a 28-kD precursor called proenkephalin B or prodynorphin. This peptide, as well as shorter peptides called α-neoendorphin (with 10 amino acids) and β-neoendorphin (with 9 amino acids), react almost exclusively with the κ-receptor. Nociceptin, which is a 17–amino acid peptide that is derived from a κ precursor called pronociceptin, and its receptor, which also is present in the hypothalamus as well as in other areas of the brain, are sources of monoamine neurotransmitters. Nociceptin appears to have an antiopioid or antinociceptive effect. Opioid peptides that influence hormone secretion in the anterior pituitary are produced through modulation of hypothalamic bioamines and hypophysiotropic factors.
The various opioid peptides and their receptors are linked to a number of bodily functions, including stress, mental illness, narcotic tolerance and dependence, pregnancy, eating, drinking, gastrointestinal function, learning, memory, reward, cardiovascular responses, respiration, thermoregulation, locomotor activity, seizures, brain electrical activity, and neuroimmune activity. Endogenous opioids inhibit gonadotropin secretion via their action on GnRH secretion. The exogenous administration of analogues of β-endorphin or enkephalin increase serum levels of growth hormone and prolactin, but blocking endogenous opioid pathways with naloxone does not alter basal or stimulated levels of growth hormone or prolactin. Opioids provide negative feedback on the secretion of ACTH and β-endorphin, and naloxone increases basal and stimulated levels of ACTH. Overall, the effects of the endogenous opioids on the normal physiologic regulation of the various pituitary hormones in humans is minimal. However, exogenous opioids in pharmacologic doses can impair the secretion of GnRH and gonadotropin, thereby causing hypogonadism (reduced libido, sexual function, and fertility) and adrenal insufficiency (by impairing the secretion of CRH and ACTH).
Pituitary hormones are secreted in a pulsatile fashion but in the context of underlying rhythms. A pituitary hormone’s pulse amplitude reflects the amount of releasing hormone as well as factors that alter the pituitary’s sensitivity to that releasing hormone. The pulse amplitude will be reduced by inhibitory factors (e.g., GHRH versus somatostatin), feedback from target organ hormones, nutritional factors, and prior stimulation that can deplete the releasable hormone pool. Pulse frequency is driven by the frequency at which the hypophysiotropic factor is released, which in turn is regulated by the hypothalamic pulse generator system.
The pituitary has an intrinsic rhythm of small amplitudes at a frequency of every 2 to 10 minutes. The pulsatile release of hypophysiotropic-releasing factors is superimposed on this intrinsic rhythm with or without the withdrawal of a corresponding inhibitory factor. Ultradian rhythms are less than 1 day, circadian rhythms have a periodicity of about 24 hours (synchronized by an environmental cue such as the light-dark cycle), and infradian rhythms have a periodicity of more than 24 hours. The suprachiasmatic nucleus functions as a circadian pacemaker and receives light-induced electrical impulses from the retina and then transmits these impulses to the pineal gland, where they are converted to hormonal signals. Infradian rhythms include the gravitational influence of the moon, which guides the menstrual cycle.
Circadian and infradian rhythms are modulated by the sleep-wake cycle. For example, the secretion of growth hormone, prolactin, ACTH, and pubertal LH are linked more to the sleep-wake cycle than to the dark-light cycle ( E-Fig. 204-1 ). Each of these hormones increases to its maximal level after the onset of sleep. The marked diurnal variation in ACTH and cortisol is often an indication of whether this system is functioning normally. Loss of this diurnal rhythm occurs when the regulation of CRH is abnormal, which may occur with depression, excessive alcohol intake, or autonomous secretion of ACTH in Cushing disease ( Chapter 208 ). In fact, a diagnostic test for Cushing syndrome is the presence of a disrupted diurnal cortisol rhythm. FLOAT NOT FOUND
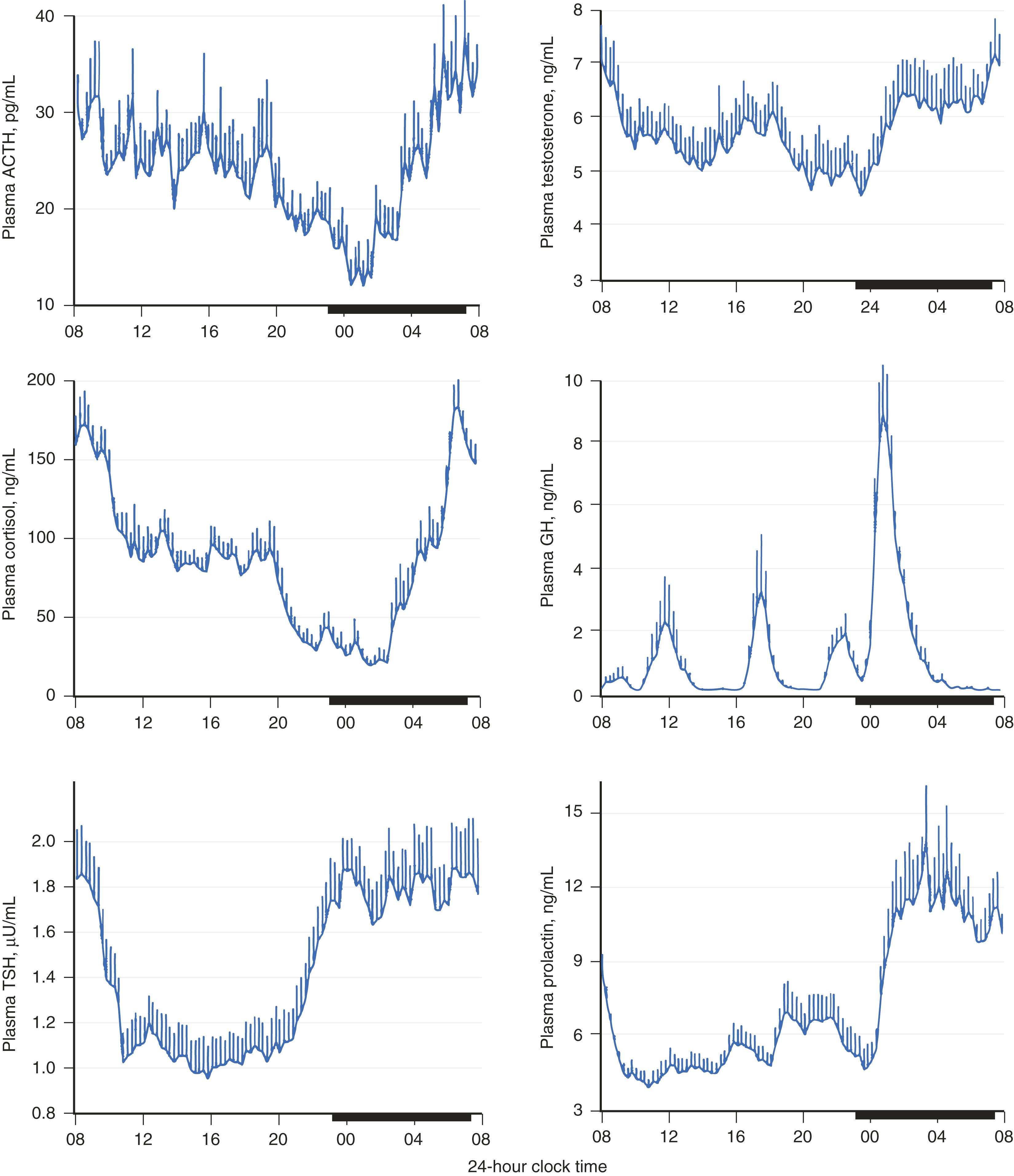
Hypothalamic-pituitary axes are blunted with aging. Furthermore, changes in the hormonal milieu associated with sleep deprivation and disruption of the axes result in significant metabolic sequelae, such as hypertension and impaired glucose tolerance.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here