Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
For heuristic purposes, we can distinguish the three mechanistically distinct sources of a pain state: (1) high intensity stimulation, (2) local tissue injury and products released secondary to injury and inflammation, and (3) injury to the peripheral nerve. These conditions may achieve further distinction by being characterized as acute or chronic, where chronic pain is defined as persistent or recurrent pain lasting longer than three months. Exposure of the organism to these conditions will typically lead to escape from the stimulus condition, emphasizing a hallmark of pain, which is that the stimulus condition is aversive and fraught with negative re-enforcing properties. This endows a painful state with two properties: (1) it supports avoidance behavior and (2) it can provide negative connotations to otherwise innocuous stimuli with which it is paired.
In the early 1900s, Sir Charles Sherrington designated high intensity stimuli that signaled a potential injury to the body as being nociceptive in character. The application of such stimuli to the body produces a syndrome that includes the withdrawal of the affected body part, signs of autonomic activation, and a complex set of behavioral responses that in intact animals include agitation and vocalization. In humans, an unconditioned high intensity stimulus evokes discrete sensations that have the assigned attribute of being painful (e.g. the sensation initiated by picking up a very hot cup of coffee). The reported sensation refers to the site of stimulation, and the magnitude of the report or response varies with the intensity of the stimulus. Termination of the stimulus before injury results in the cessation of sensation. Accordingly, nociception is a descriptor that refers to the physiologic response generated by high intensity, potentially tissue-injuring stimuli, whereas pain represents the interpretation of that event as a sensation with highly aversive properties. Such stimuli have powerful motivating effects that can support the generation of escape behavior when cued by innocuous stimuli that have previously been paired with a nociceptive stimulus. Thus a strong shock evokes a nociceptive state from which the animal will seek to escape. A light paired with that shock will become conditionally associated with the shock, such that in a short time, the light alone can initiate the same profile of nociception.
Suppose the stimulus is of sufficient magnitude to result in local injury (tissue disruption, plasma extravasation). In that case, a pain sensation will persist after removal of the stimulus, and the injury will be accompanied by increased sensitivity to subsequent stimuli applied to the injury site (primary hyperalgesia) and enhanced sensitivity to stimuli applied at sites adjacent to the injury site (e.g. secondary hyperalgesia or allodynia). Again, these states of enhanced sensation indicate that the non-tissue-injuring stimulus now acquires an aversive property (e.g. warm water on a sunburn). As will be considered below, the injury yields the release of chemical products that can, through eponymous receptors, directly increase activity in afferent systems that are typically activated by high intensity, nociceptive stimuli.
As an overview, the effects of a high intensity, tissue-injuring stimulus reflect an initial activation of the primary afferents that project to the dorsal horn of the spinal cord, from which transmitters are released that activate a complex dorsal horn circuitry. Under normal circumstances, these spinal neurons respond maximally to input arising from the root projecting to the spinal segments in which the cell lies. However, even though these afferents primarily activate these homosegmental neurons, they also send collaterals rostrally and caudally up to several spinal segments away, where they make synaptic contact with neurons in adjacent segments (heterosegmental). These distal neurons are less efficiently activated than the homosegmental cells (and may not be activated sufficiently to generate an action potential). However, these homosegmental and heterosegmental cells form the real or potential dimension of the dermatome of the spinal segment. As will be shown later, if the excitability of these higher-order spinal heterosegmental neurons is increased, cells that were not activated by a given input become depolarized, and the size of the dermatome of a given segment will be increased. These dorsal horn neurons then project by long tracts in the ventrolateral quadrant either (1) directly to diencephalic sites (e.g. thalamus, hypothalamus) or (2) indirectly through an intermediate synapse on neurons in the medulla, pons, or mesencephalon, which then project to a variety of diencephalic and limbic forebrain sites. The anatomic details of these pathways have been discussed previously (see Chapter 8).
In general, the processes leading to a pain state secondary to a high intensity peripheral stimulus reflect the frequency of traffic that appears in these spinofugal pathways. Activity in the spinofugal systems is primarily dependent on the intensity of the afferent stimulus, but as will be made evident, a variety of systems serve to increase the gain of the spinal input-output function and depress this gain. In the first case, we anticipated that there would be an increased pain sensation associated with any given stimulus, whereas in the second case, the pain sensation produced by a given stimulus would be diminished. This dynamic property of the input-output systems represents an important characteristic of systems that process nociceptive stimuli. In the following sections, we consider the transmitter pharmacology that defines these synaptic linkages.
Peripheral nerve injury may arise secondary to a variety of conditions, including physical injury (section, compression), changes in trophic functionality (ischemia, metabolic poisons), or immune interactions (as with autoantibodies). The functional phenotype of these changes is often an ongoing pain state, referring to the distribution of the injured nerve and an enhanced response to low-intensity mechanical or cold stimuli.
In the following sections, we provide an overview of the mechanisms underlying these pain states.
The systems underlying this acute psychophysical experience begin with the primary sensory neuron, the afferent fiber. The afferents possess several intrinsic attributes.
In the absence of a stimulus, most primary afferents show little or no ongoing activity.
Activity in these afferents is initiated by various mechanical, thermal, or chemical stimuli, which activate specific populations of primary afferents.
The nature of the sensory information encoded by a given primary afferent is dependent on the properties of the transduction channels or receptors that are expressed on the terminals of that sensory axon.
The frequency of firing in these afferents generated by a particular stimulus varies monotonically with the intensity of the stimulus (e.g. temperature for a thermal-sensitive afferent). Thus the specific population of primary afferents is activated, and the frequency of discharge encode the intensity of the stimulus.
Sensory afferents are morphologically categorized by diameter, state of myelination, conduction velocity, and stimulus properties that lead to their activation.
Aβ axons. These are large, myelinated, fast-conducting axons and exhibit specialized transducer elements (such as the Pacinian corpuscle). These afferents are typically activated by low-intensity mechanical stimuli.
Aδ axons. These are smaller, myelinated, fast-conducting afferents with subpopulations that preferentially respond to thermal or mechanical stimuli that may be preferentially activated by low-intensity or high intensity stimuli. Those responding only to high intensity thermal or mechanical stimuli are referred to as A∂ nociceptors. As larger myelinated axons, these A∂ often display specialized nerve endings.
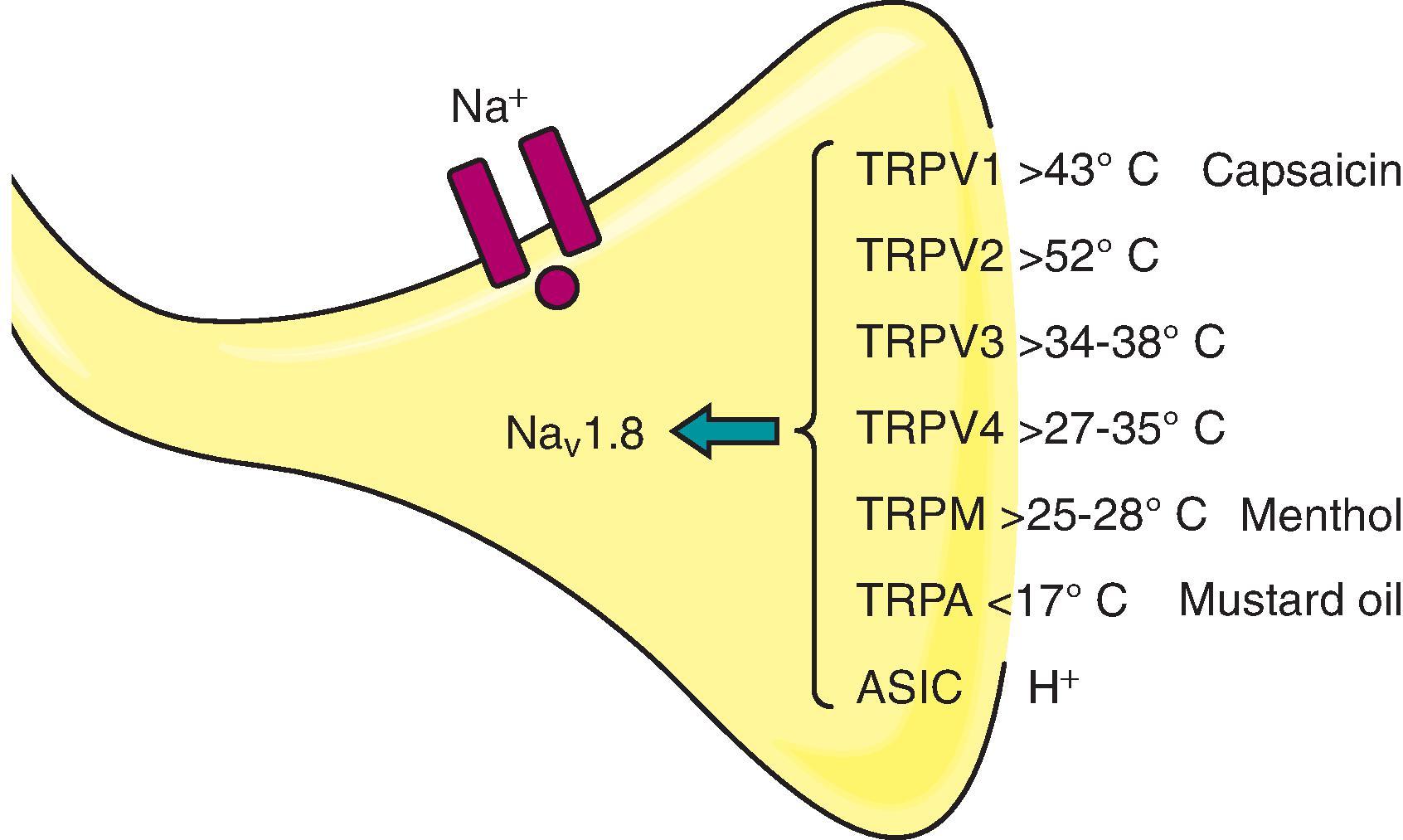
C axon. These are small, unmyelinated, very slowly conducting afferents that are activated by high intensity thermal, mechanical, or chemical stimuli. As a given C-fiber may be activated by high thermal, mechanical, and chemical stimuli, they are called polymodal nociceptors . In contrast to myelinated afferents, unmyelinated afferent axons typically show “free nerve endings.” That is, they display no evident morphologic specialization. However, these terminals express a myriad of specific transducer channels that are sensitive to specific physical and chemical stimuli and increasingly depolarize the afferent terminal as the stimulus intensity increases. When activated by an appropriate stimulus, these channels in turn activate voltage-sensitive sodium channels that pass Na + and initiate action potentials. As indicated in Figure 9.1 , some of these channels that transduce a physical stimulus may also be activated by various chemicals. In this case, these chemicals produce a sensation that reflects the physical stimulus that is transduced by that channel (e.g. the transient receptor potential vanilloid-1 [TRPV1] receptor is activated by capsaicin, which produces a painful burning sensation, whereas menthol activates the TRPM8 cold receptor and initiates the sensation that would be generated by low temperature).
During development, the soma of the afferent neuron starts as a bipolar neuron. However, during development, the soma buds off from the axon, remaining attached by a long sinuous glomerulus. The primary afferent axons in the periphery typically display arborization in the target tissue. Importantly, action potentials traveling from the periphery continue to the spinal dorsal horn but also travel up the glomerulus to the dorsal root ganglion cell body. Thus the dorsal root ganglion (DRG) displays depolarization. As action potential can travel down the glomerulus, the DRG can be a source of action potential generation, as can occur when an avulsed disc fragment is compressed in the DRG in the vertebral foramen.
High intensity stimuli may result in local tissue injury. Such injury leads to cellular disruption, injury to local vascular integrity, and subsequent plasma extravasation, and migration of inflammatory cells such as macrophages and neutrophils. These events give rise to the release of various active factors from injured cells and the migration of inflammatory cells such as mast cells, macrophages, lymphocytes, and neutrophils ( Box 9.1 ). The contents of this local milieu of an injury, often referred to as the inflammatory soup, acts upon eponymous receptors that are present on the terminals of many unmyelinated axons. Activation of these receptors typically initiates two events: (1) an increase in intracellular calcium leading to depolarization of the terminal, leading to the discharge of the afferent, with the frequency of activation being dependent on factor concentration, and (2) activation of intra-terminal processes (phosphorylation of local membrane receptors and channels), which sensitizes the terminal such that the degree of depolarization for a given stimulus is enhanced. Such effects yield “spontaneous afferent activity,” and an enhanced response to a subsequent stimulus applied to the site of injury.
The mediators listed below are released by injury from macrophages, mast cells, and blood vessels. These mediators evoke spontaneous activity in otherwise silent C-fibers and lower the threshold for activation in response to a ramped thermal stimulus (right: one vs.).
Amines. Histamine (mast cells, basophils, and platelets) and serotonin (mast cells and platelets) are released by various stimuli, including mechanical trauma, heat, radiation, and certain products of tissue damage.
Kinin. Bradykinin is synthesized by a cascade triggered by the activation of factor XII by agents such as kallikrein and trypsin and physical trauma. It acts through specific bradykinin receptors (B1/B2).
Lipidic acids. Tissue injury activates a variety of widely distributed phospholipases that are free of AA. AA is a substrate for a large family of enzymes, such as cyclooxygenase, to synthesize lipid mediators, including prostaglandin E 2 , prostacyclin, and thromboxane A 2 , all of which can facilitate the excitability of C-fibers through specific membrane receptors (EP-r, IPR, and Tx-R, respectively).
Cytokines. Cytokines such as tumor necrosis factor-α and interleukin such as IL-1β are released by inflammatory cells (macrophages) and sensitize C-fibers through eponymous binding sites.
Proteinases. Thrombin or trypsin is released from inflammatory cells and activates specific receptors (proteinase-activated receptors).
Neurotrophic factors. Nerve growth factor (NGF) activates primary afferent terminals by binding to TrkA tyrosine kinase. NGF is released from fibroblasts and mast cells via injury and inflammation.
[H] / [K] , Elevated H + (low pH), and high K + levels are found in the injured tissue. A variety of channels present on C-fibers (e.g. transient receptor potential vanilloid-1 [TRPV1]/acid-sensing ion channels [ASICs]) are activated by H + . Acid pH potentiates terminal activation by noxious heat and other chemical mediators.
Primary afferent peptides. CGRP and substance P are found in and released from the peripheral terminals of C-fibers. These peptides produce vasodilation, plasma extravasation, and degranulation of mast cells through their respective receptors. This leads to local reddening and swelling of the skin innervated by the stimulated sensory nerve.

The changes in the milieu of the peripheral terminal secondary to tissue damage and the accompanying extravasation of plasma occur because of increased permeability of the capillary wall (see Box 9.1 ). These events are responsible for the “triple response”: reddening at the site of the stimulus (reflecting local arterial dilation), local edema (increased capillary permeability), and a regional reduction in the magnitude of the stimulus required to elicit a pain response (i.e. hyperalgesia).
Following nerve injury arising from various insults, the organism will frequently display the development of a variety of highly aversive “spontaneous” sensations over time. This aversive component arises from aberrant ongoing afferent traffic. Sensory axons typically display little spontaneous activity in the absence of a stimulus. This is particularly true for small, high threshold afferents. However, after a chemical, immune, or mechanical injury to the nerve, afferent axons exhibit (1) an initial burst of afferent firing, (2) electrical silence for an interval of hours to days, and (3) the appearance over hours to days of “spontaneous” bursting activity in both myelinated and unmyelinated axons. This aberrant traffic may arise from several sources. This ongoing activity reflects the initial dying back of the injured axon (retrograde chromatolysis) and the initiation of sprouting. Collections of these sprouts form neuromas. Recording from the afferent axon indicates that the ongoing activity originates after an interval of days to weeks from the lesioned site (neuroma) and from the DRG of the injured nerve.
Several lines of evidence support the assertion that the ectopic activity in the afferent arising from the neuroma or the DRG of the injured axon is in part responsible for the observed pain behavior: (1) the onset of ectopic activity in the neuroma or DRG and the onset of pain behavior have parallel time courses, (2) pain behavior can be blocked by the application of tetrodotoxin (TTX) or local anesthetic to the neuroma or DRG, (3) dorsal rhizotomy transiently reverses the pain behavior, and (4) irritants applied to the DRG will initiate activity and yield pain behavior. Several changes can lead to prominent alterations in ongoing afferent activity.
A variety of channels in the sensory afferents can modulate excitability. Two major classes are the sodium channel, which carries the primary current for axonal depolarization, and several potassium channels. The activation of these potassium channels can reduce axon excitability. Upregulation of sodium channels or downregulation of potassium channels would have a net effect of increasing axon excitability.
A large increase in the expression of sodium channels in the neuromas and DRGs occurs after nerve injury. Several sodium channel variants exist in primary afferent neurons, including subtypes designated as Na v 1.6, Na v 1.7, Na v 1.8, and Na v 1.9. Those designated as resistant to the sodium channel blocker TTX, Na v 1.8 and Na v 1.9, are found primarily in small DRG cells (C-fibers). These channels mediate slow activation and slow inactivation of sodium currents. The importance of some of these variants in nerve injury pain states is suggested by “knock-down” studies wherein reduction of, for example, Na v 1.8 has no effect on baseline pain thresholds but reverses nerve injury evoked pain states in animal models. In humans and animal models, systemic lidocaine at plasma concentrations that block ectopic activity can attenuate the hyperpathic state observed after nerve injury, thus confirming the importance of sodium channels in the post–nerve injury pain state. In humans, mutations in one sodium channel (Na v 1.7) can cause extremely painful conditions . Conversely, loss-of-function mutations lead to prominent insensitivity to pain generated by tissue-injuring stimuli. Conversely, other mutations can result in a “gain of function,” which Additionally correlates with syndromes such as erythromelalgia, characterized by severe episodic pain.
Following nerve injury, potassium currents (e.g. K2P, TWIK-related K + channels) have been shown to be reduced, thus suggesting downregulation of these channels. Potassium channel blockers increase ectopic firing after peripheral nerve injury.
Sprouted terminals of an injured axon display sensitivity to several humoral factors, including prostanoids, catecholamines, and chemokines or cytokines such as tumor necrosis factor (TNF). Several examples of how this sensitivity is related to the appearance of ongoing activities are noted.
After nerve injury, the release of various cytokines, including TNF, interleukins (IL), interferons (IFN), colony-stimulating factors, transforming growth factors, and chemo attractant cytokines, also called chemokines, have been noted in various local inflammatory cells. These cytokines directly activate the nerve and neuroma through eponymous receptors expressed in the membrane after nerve injury. For example, the mechanisms of TNF interactions are multiple and complicated. TNF decreases potassium conductance in neurons, whereas long-term effects may be initiated through activation of a variety of kinases (mitogen-activated protein kinases [MAPKs]). Behaviorally, the application of TNF to the nerve results in hyperalgesia, and systemic delivery of TNF-binding protein reduces free TNF and decreases pain behavior in animals with neuropathic pain.
Following nerve injury, post-ganglionic sympathetic efferents (cell bodies in the sympathetic ganglion) and sympathetic afferents (afferents that travel with the sympathetics but the cell body for which is located in the DRG) sprouts into the peripheral injury site and the DRG of the injured axons. In response to the release of nerve growth factors from local Schwann and inflammatory cells, these post-ganglionic terminals locally release catecholamines. Physiologic studies have shown that following nerve injury, stimulation of post-ganglionic axons will excite the injured axon and the DRG of the injured axon and that such activation is blocked by α-adrenergic antagonism. After nerve injury, upregulation of sympathetic fibers and α 1 -adrenergic receptors in the DRG and skin has been demonstrated. Accordingly, increased catecholamine concentrations in the vicinity of the DRG or injured neuroma can translate into enhanced activity.
Neuronal soma, post-ganglionic sympathetic axons, satellite glial cells, macrophages, and blood vessels in the DRG are densely packed into this small niche environment. In addition to neuronal somata (small, medium, and large fibers), macrophages and satellite glial cells have been shown to play a key role in pain processing. Macrophages produce a variety of chemokines and cytokines (e.g. IL-6) that can trigger neuronal sensitization. Satellite glial cells have been shown to enhance afferent signals by increasing interneuronal communication through gap junctions , and satellite glial cells are involved in IL-1B and prostanoid production.
Prostanoids are released by inflammatory cells secondary to tissue injury. Activation of prostanoid receptors leads to sensitization of the primary afferent nociceptors by phosphorylation of ion channels such as TRPA1 and TRPV1. Moreover, they can enhance the opening of TTX-insensitive sodium channels by acting through eponymous receptors on the afferent terminal. TTX-insensitive channels are typically found on small unmyelinated axons, which emphasizes the association of these observations with axons carrying information having “nociceptive” content.
Primary afferents enter the spinal dorsal horn. Large afferents (Aβ) terminate deep to the Rexed lamina III of the dorsal horn. Aδ afferent fibers terminate superficially and in deeper laminae, whereas C-fibers generally terminate superficially in laminae I and II (marginal layer and substantia gelatinosa).
It has classically been appreciated that primary afferent input results in a postsynaptic excitatory event, thus emphasizing that primary afferent transmitters are uniformly excitatory. In general, it appears that the principal primary afferent neurotransmitter evoking acute excitation is glutamate. It is present in the synaptic vesicles of most spinal afferent terminals, and its synthetic enzymes have been identified in virtually every primary afferent DRG cell body, regardless of the size or state of myelination. These acute effects are mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA)-type glutamate ionophores present on second-order neurons. This receptor produces a robust but short-lasting depolarization of the postsynaptic membrane by increasing sodium conductance ( Table 9.1 ).
 |
||
| Primary Afferent Transmitter | Receptor | Postsynaptic Action |
| Peptides | ||
| Substance P | Neurokinin 1 (NK1) | G Protein Coupled; Slow, Long-lasting Depolarization |
| CGRP | CGRP1 | |
| Prostaglandin | EP1-EP4 | |
| Purine | ||
| Adenosine triphosphate | P2X-r (P2X1-P2X7) | Ligand-gated ion channels that differ in ion selectivity and gating properties |
| P2Y1-P2Y14 | Metabotropic G protein coupled receptors | |
| Excitatory Amino Acid | ||
| Aspartate, glutamate | AMPA-r | Sodium ionophore; rapid short-lasting depolarization; gates sodium A subtype of the AMPA receptor can also gate calcium |
| NMDA-r | Calcium ionophore; slow onset, long-lasting; gates calcium | |
In addition to glutamate, populations of primary afferents may contain and release several neuropeptides, notably substance P (SP) and calcitonin gene-related peptide (CGRP), as well as certain growth factors such as brain-derived neurotrophic factor (BDNF). Given the complexity of coding, it is likely that a variety of transmitters processes nociceptive information. The transmitters in these small high threshold afferents display several general properties.
Consistent with their location in small afferent terminals, high levels of peptides are present in laminae I and II, and these levels are reduced by rhizotomy or ganglionectomy or by treatment with the small afferent neurotoxin capsaicin. The sensitivity of these afferents to capsaicin indicates that one important characteristic of many (but not all C-fibers) is the expression of the TRPV1 (capsaicin) receptor. A second population of C-fibers that do not express TRPV1 typically expresses a second marker (isolectin B4 [IB4]). These IB4-positive afferents characteristically project to the deeper layers of the dorsal horn and do not express neuropeptides.
Glutamate and many peptides are co-contained and co-released (e.g. glutamate, SP, and CGRP in the same C-fiber terminal).
Release is dependent on the opening of voltage-sensitive calcium channels, and the magnitude of release is proportional to the stimulus frequency.
Iontophoretic application of glutamate and the peptides found in primary afferents onto the dorsal horn will produce postsynaptic excitation. Amino acids produce a rapid, short-lasting depolarization, whereas peptides produce a delayed and long-lasting discharge (see Table 9.1 ).
As noted in the introduction, the intensity of the painful stimulus is encoded in the projection to higher centers by the frequency of the output function. Accordingly, factors that enhance the excitability of spinal afferent terminals (leading to increased release of the transmitter) or the excitability of the dorsal horn projection neuron will increase the apparent magnitude of a given stimulus. In contrast, factors that diminish the excitability of the primary afferent or the projection neuron will lead to a reduction in the apparent stimulus intensity. In the following section, we will consider substrates that enhance and diminish the response of the dorsal horn to a given stimulus.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here