Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Traumatic brachial plexus injuries (BPIs) produce psychologically and functionally devastating handicaps afflicting, generally, a subset of young, healthy males in the prime of life. Seventy percent are caused by motor vehicle accidents, of which 70% are due to the use of two-wheelers. The disease burden is estimated to be about 2162 cases per annum in the United States; costs of treatment amount to about $34,733 per capita. It is prudent to mention at the outset that the management of traumatic BPI requires a complex multidisciplinary approach involving neurosurgeons, orthopedists, plastic/hand surgeons, neurologists, neuroradiologists, neurophysiologists, physiotherapists, and pain specialists.
Surgical repair for BPI involves either neurolysis (scar release) that may be external, internal, or both; nerve grafting using devascularized, vascularized, or pedicled nerve cables to bridge defects; or nerve transfers where healthy functioning donor nerve is coapted directly onto a damaged recipient nerve. Direct end-end repair can be performed for the occasional sharp injury to this region.
Specifically, nerve transfers involve reassigning an “expendable” or redundant working nerve, part of a nerve (fascicle), or a nerve branch (donor) to a more important, nonfunctioning nerve (recipient).
The use of and enthusiasm for nerve transfers have increased dramatically in the past 25 years, largely due to the creative contributions of innovators such as Drs. Christophe Oberlin and Susan Mackinnon. The introduction of a fascicular transfer by Oberlin et al. (1994) for upper trunk injuries catalyzed the transformation. This procedure introduced the concept of fascicular transfer by using functioning ulnar nerve fascicles supplying the flexor carpi ulnaris to be selectively transferred onto the nerve to biceps with excellent results.
The new era of nerve transfer has created a major controversy in brachial plexus surgery regarding the role of nerve grafts versus (distal) nerve transfers in postganglionic injuries. This issue remains largely unresolved, but the pendulum is now favoring nerve transfers. The advantages and disadvantages of performing nerve transfers over nerve grafts are listed in Tables 185.1 and 185.2 . As can be seen, the advantages of performing nerve transfers may outweigh those of brachial plexus nerve grafting. In fact, in the event of patients sustaining panplexal nerve injuries secondary to preganglionic injury with multiple root avulsions, nerve transfers may be the only viable form of repair.
| Nerve Transfer | Nerve Graft |
|---|---|
| A distal procedure is performed close to the motor point of muscles. This decreases the time to reinnervation. The direct repair is at a single suture line. This improves reinnervation as well. | Performed at the site of injury, typically proximally, and thus more anatomical. These are also more physiologic and make relearning when muscle power returns easier. |
| Surgical dissection occurs in uninvolved pristine tissue. | These repairs make available more donor motor axons as nerve stumps are typically largest proximally. |
| Directed (selective) neurotization makes targeting motor recipient axons easier as it is closer to the motor end plate. | As the entire cut surface is coapted with the injured stump, sensory reinnervation is also a possibility. |
| Is the only procedure possible after nerve root avulsion. | Grafts enable entire motor groups to recover as opposed to single targeted muscles. They also typically allow opportunities for sensory recovery. |
| Can be performed with minimal technology. |
| Nerve Transfer | Nerve Graft |
|---|---|
| Involves some loss of existing function, by definition. | Typically requires use of nerve grafts (e.g., non-degenerated sensory nerves like the sural). The harvest has cosmetic and functional sequelae. |
| Requires muscle re-education that can hamper autonomy because of co-contractions. | Has to be performed at the site of injury. Dissection is more difficult, especially in the presence of a concomitant vascular repair. |
| Number of available donor nerves is limited especially in patients with panplexal injuries. | Cannot be performed in patients with root avulsive injuries. |
| Number of available motor axons is necessarily limited. | Directed (motor donor to motor recipient) repair is seldom. All proximal components of the brachial plexus are mixed sensorimotor. |
| Longer grafts should be vascularized as free grafts may fibrose secondary to ischemic injury. | |
| Grafts require axonal sprouts to cross two suture lines. This further delays repair. If the distal suture line scars densely, the entire repair may fail here. | |
| Grafts ideally require the use of intraoperative sensory and motor-evoked potentials to determine the viability of the donor nerve stump (especially proximal cervical nerves), even if it appears structurally intact. These have several technical and observer-dependent confounding factors. |
“Irreparable” nerves that are avulsed from the spinal cord.
Preganglionic injuries are not amenable to any other form of repair, as the motor axon has been physically disconnected from the neuronal cell body across the intervertebral foramen. Reconnection via axonal regeneration is not physically possible. In such patients, motor end plate degeneration starts at the time of injury and may be irreversible after 12 to 18 months. , To beat the biological clock, it is imperative to operate as soon as possible and transfer viable axons as close to the motor end point as possible. This is the procedure of choice in this form of injury.
More rapid or reliable recovery of motor function.
Most experts have now begun to recognize that nerve transfers allow a faster and perhaps more reliable way of regaining function, even in postganglionic injuries bucking the previous trend of using proximal nerve grafts ( Table 185.3 ).
To power free-functioning muscle transfers (FFMT).
| Recipient Nerve | Common Donor Nerves | Uncommon Donor Nerves |
|---|---|---|
| Suprascapular | Spinal accessory C5 nerve C6 nerve |
C4 nerve C7 nerve/middle trunk Phrenic nerve Contralateral C7 Dorsal scapular |
| Axillary | Triceps branch of radial Medial pectoral Motor intercostals |
Thoracodorsal Contralateral C7 |
| MCN, biceps, or brachialis branch | Ulnar nerve fascicle Median nerve fascicle Motor intercostal Medial pectoral |
Thoracodorsal Spinal accessory Phrenic Contralateral C7 Hemi-hypoglossal |
| Median | Sensory intercostals Contralateral C7 Ulnar nerve fascicle Brachialis branch of MCN |
Supinator/ECRB branch of radial |
| Radial | Motor intercostals Proximal branches of radial |
Contralateral C7 Dorsal scapular nerve Median nerve branches |
| Ulnar nerve | Anterior interosseous | Contralateral C7 |
FFMT is a reliable way to reconstruct the damaged upper extremity by moving a functioning muscle with its nerve and blood supply to another location where it can subserve a new function. This can occur after a successful nerve repair and anastomosis of the artery and vein have been accomplished. This microsurgical technique therefore does not have a time window like typical nerve reconstruction. Most commonly, FFMTs thereby have been performed for delayed cases to provide elbow flexion (such as in neglected injuries or those with poor or incomplete recovery). FFMTs in this setting can augment motor function. The native anatomy of the transferred muscle/tendon unit (gracilis, for example) has important features that allow FFMTs to achieve more distal function, ordinarily unachievable with standard techniques of nerve reconstruction (e.g., recovery of prehension of the hand in patients with flail limbs). The nerve supply is relatively close to the muscle and the tendon is long (and can be prolonged). This technique then can also be incorporated into an armamentarium in combination with other nerve techniques in the early setting.
The only absolute contraindications for a nerve transfer are the absence of a donor nerve and the presence of a fibrosed atrophic recipient nerve with no viable fascicles seen under magnification on sequential cut sections at the motor point. Relative contraindications would include significant time of denervation (roughly 12-18 months), the availability of a poor quality donor, as seen with direct electrical stimulation at surgery, the presence of a strongly stimulatable motor nerve recipient at surgery, or a short segment rupture/neuroma that can easily be repaired end-to-end without the use of a graft.
Some basic principles must be respected while performing nerve transfers. These include the following:
Accurate documentation of preexisting muscle power, vascular injury, and joint contractures
Clear, coherent, and exhaustive discussion of options and priorities with the patient
Fallback planning and prior consent in case favored donors are poorly functioning
Elaboration of realistic goals prior to surgery, including risks related to possible donor morbidity (transient and permanent) and the relatively slow nature of recovery
Selection of the ideal donor nerve ( Table 185.4 )
| Pure | Motor or sensory. Example: Medial pectoral nerve (motor); sensory intercostal nerves. |
| Adjacent | Minimal donor dissection required to gain length. Example: Double fascicular transfer for elbow flexion. |
| Expendable | Unlikely to affect donor function. Example: Intercostal nerve transfer. |
| Uninjured | Donor fascicle muscle groups must have at least grade 4/5 MRC power. Example: Spinal accessory transfer is contraindicated when shoulder function is poor. |
| Remote | From zone of injury (dissection is easier). Example: Oberlin transfer in upper trunk injury (is performed in proximal arm). |
| Proximate | To motor point so reinnervation is faster and likely better. Example: Triceps to axillary nerve transfer. |
| Adequate | Diameter mismatch is prevented. Example: Three motor intercostals are used to neurotize the nerve to biceps. |
| Educatable | Same muscle compartment is ideal, as relearning and subsequent autonomy are better and faster as antagonistic co-contractions do not occur. |
Donor nerve dissected distal to recipient to gain length
Transection of recipient nerve as proximal as possible to determine adequacy before donor dissection
Selective neurotization based on knowledge of fascicular anatomy ( Table 185.5 )
| Nerve | Donor/Recipient | Fascicular Anatomy |
|---|---|---|
| CC7 | Donor | Posterior division has 2× motor axons. |
| Median (axilla) | Recipient | Lateral root is mainly sensory. |
| Median (arm) | Donor | FCR/FDS fascicle is medial. |
| Median (arm) | Recipient | Posterior fascicle (AIN). Anterior fascicle (PT, FCR). Middle fascicle (FDS, thenar, sensory). |
| Median (forearm) | Donor | Terminal AIN branch to PQ. |
| Nerve to biceps | Recipient | Lateral in musculocutaneous nerve. |
| Nerve to brachialis | Donor/recipient | Lateral to LABC. |
| Suprascapular | Recipient | Lateral within upper trunk. |
| Ulnar (arm) | Donor | FCU fascicle is posteromedial. |
Awareness of proximal and distal orientation of transected nerve segments
Tension-free direct repair under magnification
The common recipient nerves for transfers are the suprascapular, axillary, and musculocutaneous nerves. A list of donors is provided in Table 185.3 .
The brachial plexus is formed by the ventral rami of the fifth to eighth cervical and the first thoracic nerve roots. It is essential to remember that the nerves emerge between the scalenus anterior and medius (interscalene triangle) to form trunks in the supraclavicular fossa. The divisions of the brachial plexus are retroclavicular, while the cords are infraclavicular and named as such in relation to the axillary artery; terminal branches are located in the axilla.
The key muscles for the supraclavicular exploration of the brachial plexus are the omohyoid (deep to which lies the fat pad covering the plexus) and the scalenus anterior (deep to which lie the spinal nerves). The upper elements of the brachial plexus may be difficult to identify especially when there is extensive scarring. The phrenic nerve lies on the surface of the scalenus anterior. It may be identified visually as the only neural structure (other than the nerve to subclavius) that passes from lateral to medial in the root of the neck ( Fig. 185.1 ). If scarring is extensive, blind neural stimulation using 0.1 to 2 mA of current may provoke capnographic changes if not frank diaphragmatic contraction that may guide the surgeon to the approximate location of this key structure before sharp dissection is commenced. The phrenic nerve can then be traced back to the C5 nerve via its contribution to the former. From here, the C5 nerve can then be traced to the upper trunk. Proximal and distal dissection allows for the identification of C6 and the divisions of the upper trunk. The suprascapular nerve is the key target in this exploration and can be found superior, lateral, and posterior to the upper trunk. Its direction confirms its identity. It parallels the omohyoid and runs posteriorly obliquely to the scapular notch. In the event of a complete rupture of the C5 and C6 nerves, the distal components of the plexus can be found near, behind, or even below the clavicle.
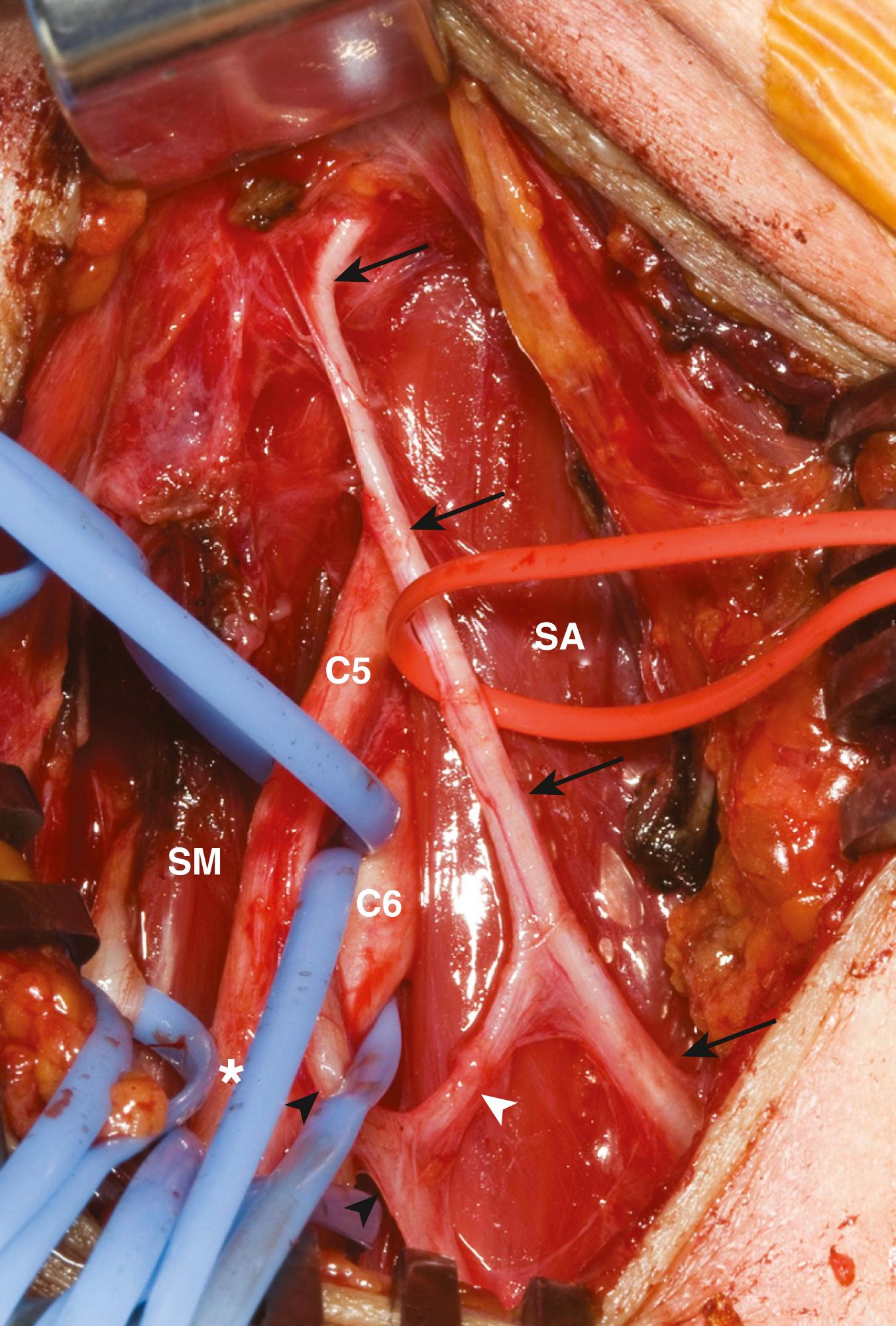
The spinal accessory nerve can be identified by exposing the lateral aspect of the supraclavicular incision. The nerve can be identified reliably a few centimeters above the clavicle on the anterior surface of the trapezius at its medial border. Other smaller, mostly sensory nerves frequently seen in this location represent neural elements of the cervical plexus. These may produce weak contractions of the trapezius. In contrast, direct stimulation of the spinal accessory nerve produces powerful trapezius contraction.
Care has to be taken, during dissection, not to injure the lymphatic duct on the right and the chyle carrying thoracic duct on the left side. These elements are at risk during medial dissection, such as with exposure of the lower trunk elements.
The key muscle in the infraclavicular exploration of the brachial plexus is the pectoralis minor. This may be approached either through the preferred corridor offered by the deltopectoral groove or a transpectoralis major (muscle splitting) exposure. The pectoralis minor is identified as the musculotendinous structure passing inferomedially from the coracoid process of the scapula to the third–fifth costochondral junctions. This anatomy also helps the surgeon define ribs and the underlying intercostal nerves with a number. Division of the pectoralis minor muscle reveals the cords, terminal branches, and axillary vessels within the axillary sheath. Fat encases these structures—especially the axillary and cephalic veins. Due care must be taken while retracting the pectoralis minor as the medial and lateral pectoral nerves arborize in the adjacent fat planes when penetrating this muscle.
The key neural structure to be identified here is the median nerve, which is characterized by a Y-shape at its takeoff from both the lateral and medial cords. The lateral cord is the first neural structure seen typically during the infraclavicular exposure. The lateral cord can then be followed distally into the musculocutaneous nerve and to the median nerve via its lateral root. The median nerve helps navigation. The medial root of the median nerve can be traced proximally into the medial cord, and the medial cord can be traced to the ulnar nerve in the arm, which lies in the biceps–triceps groove. The proximal median nerve is at first lateral to the brachial artery and crosses it ventrally in the mid-arm to gain its medial relation. At this point, the ulnar nerve, which lies medial to the brachial artery in the upper arm, begins descending posteriorly to eventually penetrate the medial intermuscular septum and pass behind the medial epicondyle.
It is important to realize that the musculocutaneous nerve provides its first branch to the coracobrachialis muscle and then penetrates this muscle. This must not be mistaken for the nerve to the biceps, which is given from its lateral aspect in the mid-arm (typically 12 cm distal to the acromion). The nerve to the brachialis is given off further distally (approximately 17 cm distal to the acromion) and typically lies lateral to the terminal branch—the lateral antebrachial cutaneous nerve. The former is a pure motor branch, while the latter is a sensory nerve. The authors verify the nature of both these terminal divisions by tracing the brachialis branch until it arborizes on the muscle surface. A variation that has to be considered is the low takeoff of the musculocutaneous nerve from the median nerve. This is important to consider, especially when the median nerve is being considered as a donor for a simultaneous nerve transfer.
The suprascapular and axillary nerves can be approached either anteriorly or posteriorly. The suprascapular nerve is typically identified in the supraclavicular dissection. Occasionally it can be identified and dissected further distally in the infraclavicular region. It lies lateral to the cords/divisions. It runs near the coracoid process as it heads toward the suprascapular notch.
A separate posterior incision can be done along the lateral aspect of the scapular spine. The trapezius can be elevated from the scapular spine or its fibers split in line with their course. The atrophic supraspinatus can be mobilized inferiorly. The shiny transverse scapular ligament can be seen and divided. The suprascapular nerve is deep to the ligament. This posterior approach can be used if a second site of injury is suspected or if a distal nerve transfer is performed (distal spinal accessory nerve to the distal suprascapular nerve).
The infraclavicular exposure allows anterior exposure of the axillary nerve. It can be located superior and lateral to the posterior cord at the level of the humeral head. For identifying the posterior cord, the axillary artery has to be mobilized, generally ventrally and laterally off the axillary vein. This is a delicate procedure, and the access is made more difficult by the operating depth or by previous vascular injury and/or repair. The conjoined tendon can be taken down from the coracoid to allow more lateral and distal exposure—that is, more removed from the typical vascular injury. The conjoined tendon would need to be reapproximated at the end of the case.
A posterior approach allows easy dissection of the axillary nerve in the quadrangular space formed laterally by the long head of the triceps, medially by the proximal humerus, superiorly by the teres minor, and inferiorly by the teres major. The axillary nerve here follows the posterior circumflex humeral artery and splits into the anterior and posterior divisions. The anterior division is generally targeted, as it supplies the anterior and middle deltoid. The posterior division innervates the less important posterior deltoid and also gives rise to the superior lateral cutaneous nerve of the arm, which need not be reinnervated. The importance of the superior lateral cutaneous nerve of the arm lies in that it can be used, when traced retrogradely from the skin incision, for navigating to the posterior division and thereon to the anterior division of the axillary nerve. Sometimes, a combined anterior and posterior approach may be required to satisfactorily visualize the axillary nerve in its entirety or to find a healthy recipient nerve (the pull-through procedure). The radial nerve exits the axilla inferior (more inferiorly) to the axillary nerve. It courses through the triangular interval formed by the teres major superiorly, the long head of the triceps inferomedially, and the shaft of the humerus laterally.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here