Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The diagnosis of a nerve entrapment lesion arising at the elbow can be relatively straightforward if the history, physical examination, electromyographic (EMG) and imaging studies, when indicated, all confirm the diagnosis and the localization of the lesion. However, when the history and physical examination do not correspond, or the electrophysiologic or imaging studies do not support a specific clinical diagnosis, then problems can arise. Therefore, one must apply the same systematic, thoughtful approach to the care of every patient. One can then put all of the data of the clinical puzzle together to offer appropriate treatment.
Sometimes, historical information can be misleading. For example, it is not uncommon for an elderly patient to say that his or her fingers are “stiff” when in reality the fingers are numb. Stiffness suggests an arthritic process, whereas numbness suggests involvement of the peripheral neurologic system in the pathologic process.
Other times, subjective complaints or physical findings may be difficult to interpret. Persistent pain or tenderness about the lateral aspect of the elbow that is resistant to all forms of conservative treatment, as well as operative treatment directed to the lateral epicondyle, may represent resistant lateral epicondylitis, entrapment of the posterior interosseous nerve alone, or both entities. In this instance EMG studies, more common than not, do not help establish the diagnosis of resistant “tennis elbow” due to posterior interosseous nerve compression, or localize the pathologic process. If both conditions are believed to be present on clinical grounds, then management of both lesions needs to be addressed simultaneously to relieve the patient's symptoms.
Other issues may confound the clinical picture and the treating physician:
Simultaneous peripheral nerve compressions may occur, whether from related or unrelated causes. For example, a diabetic patient may present with symptoms related to concurrent carpal tunnel syndrome and cubital tunnel syndrome. An active individual may present with well-defined symptoms of ulnar nerve compression at the elbow; electrical studies, however, may have normal results in the ulnar nerve but reveal changes of carpal tunnel syndrome (which may be either subclinical or less symptomatic to the patient). Posttraumatic thickening of the brachial fascia in the distal arm can produce a simultaneous median and lateral antebrachial nerve compression. When more than one nerve is suspected in the neural compression process, a more proximal lesion such as the brachial plexus must be ruled out as the site of the pathologic process.
A nerve can be compressed at more than one level; that is, a “double crush” lesion may exist. This most commonly occurs at the neck and the wrist but can also occur at other locations such as the thoracic outlet and the cubital tunnel.
Two separate neurologic processes may coexist. For example, a patient who is wheelchair-bound from a syrinx may develop hand atrophy, which represents new ulnar nerve compression rather than progression of the syrinx. Thus on occasion, it is necessary to direct one's conservative or surgical attention to two nerves, two sites on one nerve, or even two neurologic conditions to address the patient's presenting symptoms and new neurologic findings.
Some patients are prone or predisposed to sequential neural compression lesions. There are predisposing factors for multiple neural entrapment lesions. A group of substances, such as sorbitol, can cause an increase in intrafascicular pressure, which can predispose a patient with diabetes mellitus to a neural compression lesion. Hereditary neuropathies also occur; despite genetic advances, the mechanism of dysfunction is not fully understood. Congenital anomalies of the elbow, such as from a lacertus fibrosus variant or a ligament of Struthers, may result in nerve compression lesions. Developmental changes from hypertrophied muscles may lead to compressive neuropathies, such as a pronator syndrome in an athlete. Trauma may induce a nerve compression syndrome either acutely or chronically, either from bony or associated soft tissue changes.
Recurrent neural compression lesions also occur. A physician may successfully care for an individual's neural compression only for another nerve compression to arise a few months or years later, affecting the same peripheral nerve or another nerve. Usually, technical factors at surgery can prevent recurrent lesions. Free gliding of the nerve with elbow flexion and extension and forearm rotation helps prevent late postoperative symptoms. If a nerve is fixed by adhesions or scarring at a fracture site, it is not just a matter of entrapment. A traction neuritis can exist as well. As the joint moves, the nerve is tethered and can be stretched. If the ulnar nerve is transposed anteriorly (especially if it has not been transposed in a straight line), ulnar neuritis can develop at a later date. Similarly, if the medial epicondyle is resected and the nerve becomes adherent to the medial epicondylectomy site, resistant ulnar neuritis can develop after the primary surgery.
Certain principles apply to the surgical management of entrapment lesions. Wide exposure is often necessary to define the normal anatomy and the pathologic region. The nerve should be identified in a healthy region proximally and distally; only then should the nerve be traced to the pathologic region. Surgery should be guided by the use of internervous planes, and gentle handling of the nerve is critical. During the wide decompression, care must be given to the cutaneous nerves. Patients who have entrapment lesions are prone to develop symptomatic postoperative skin neuromata; their initial symptoms related to the nerve compression lesion may disappear postoperatively to be replaced by a different type of neuropathic pain. In particular, at the elbow level, the medial cutaneous nerve of the forearm is susceptible during ulnar nerve transposition and median nerve decompression, and the posterior cutaneous nerve of the forearm is at risk with posterior interosseous nerve neurolysis. In the majority of cases of nerve compression, external neurolysis is the usual operative intervention. Internal neurolysis, when indicated, should be limited to the neural segment and the internal region clinically involved. The perineurium should rarely, if ever, be violated. Nerves should be placed in healthy beds away from scar tissue. Intraoperative nerve action potentials may help in the management of more advanced lesions. Postoperative care should emphasize early mobilization. Early motion can improve neural gliding. The development of a stiff joint can undo an otherwise successful nerve decompression.
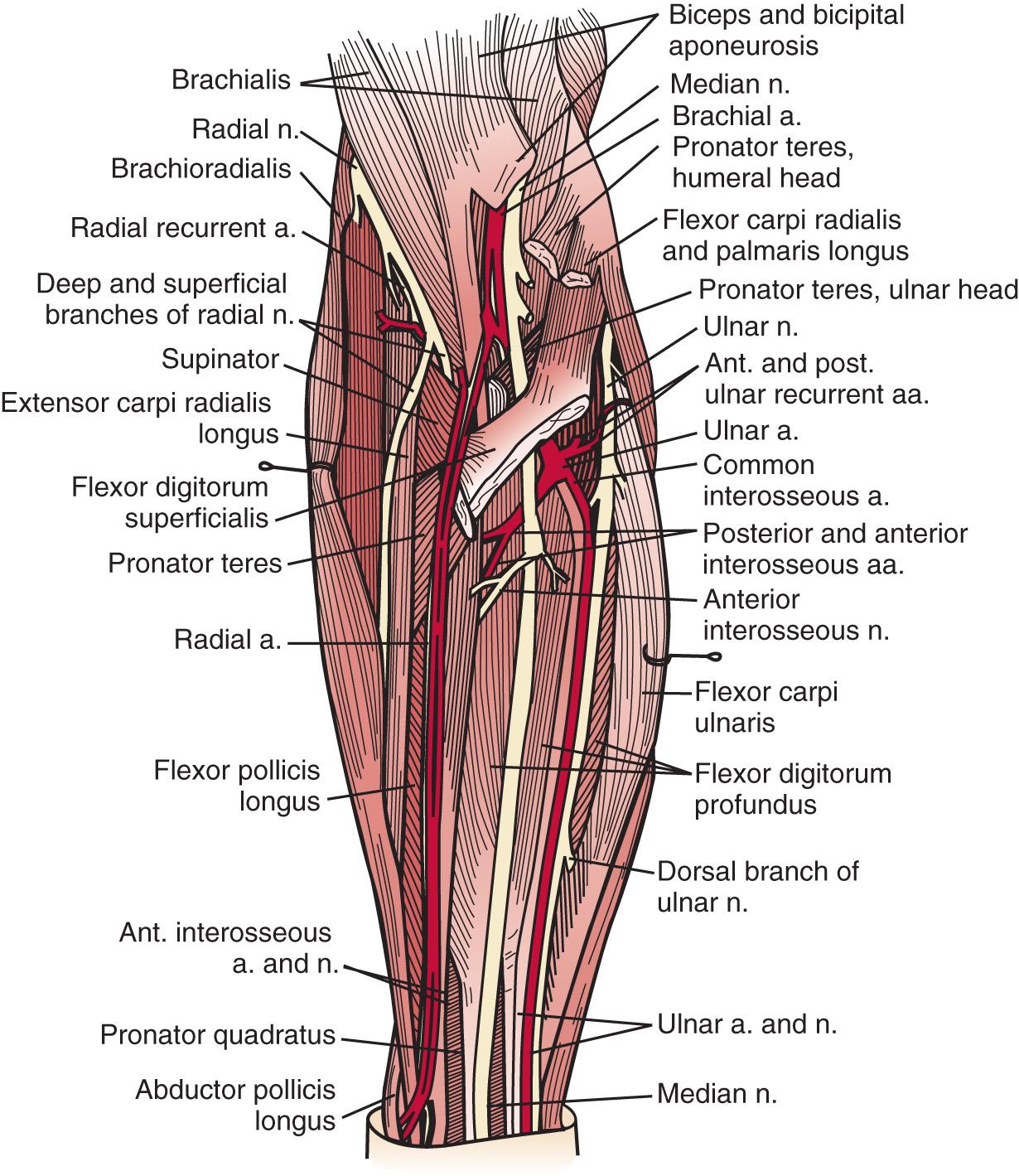
A detailed understanding of the complex normal anatomy of this region and the “common” variants is essential for proper diagnosis and treatment of these conditions ( Fig. 72.1 ). Careful history, serial examinations, and EMG studies, and at times, imaging modalities can usually localize the lesion or lesions. Early, accurate diagnosis and treatment are important for effective overall management of nerve compression lesions. Understanding the degree of nerve injury can help a physician predict recovery patterns and guide management.
Nerve compression may be categorized as first-, second-, third-, or fourth-degree neural lesions. This method was first described by Sir Sydney Sunderland. The earlier classification of Sir Herbert Seddon (1943) uses the terms neurapraxia , axonotmesis , and neurotmesis and can be correlated with Sunderland's classification in the following manner. A first-degree lesion is a neurapractic lesion. A second-degree or mild third-degree lesion is an axonotmetic lesion. The neurotmetic lesion encompasses all of the fourth-degree lesions (the neuroma in continuity) and the advanced third-degree lesions. I prefer using Sunderland's classification when correlating clinical problems with the underlying nerve fiber pathologic condition present ( Table 72.1 ).
With neural compression lesions, it is rare to have a pure first-, second-, or third-degree lesion. Most often, these lesions are mixed. One of the degrees of injury usually predominates in a particular case. The lesion mix can be determined by serial physical examinations, preoperative and postoperative serial EMG studies, and knowledge of the duration of the partial or complete nerve compression lesions. A fourth-degree nerve compression lesion is found most often when motor and sensory complete paralysis of a particular nerve has existed for more than 18 months.
The factors that affect return of nerve function following entrapment lesions are (1) the nerve fiber pathology, (2) the duration of the lesion and whether it is complete or partial, (3) the status of the end organs (i.e., motor and sensory), and (4) the level of the lesion. When a nerve is entrapped, the peripheral fibers are the most vulnerable to the pathologic process. Similarly, the heavy myelinated fibers are more susceptible to compressive forces.
There appear to be several types of first-degree injury. These lesions are correlated best when both the nerve fiber pathologic processes and the clinical recovery following neurolysis are analyzed temporally. There are ionic and vascular lesions of nerve fibers that respond to release by prompt recovery within, at times, hours of surgery. There is a structural first-degree lesion, described by Gilliatt and colleagues and Ochoa, in which there is segmental injury to the nerve fiber consisting of segmental demyelination and remyelinization of just a few nodal segments of the fibers. In this instance, the entire recovery process takes 30 to 60 days. The clinical implications of this particular lesion are as follows: whether the lesion is high (proximal) or low (distal) in the nerve, it takes 30 to 60 days for neural function to be restored.
In contrast, in the second-degree lesion of nerve compression, there is degeneration from the point of injury distally. Regeneration of the nerve fibers occurs within the intact basement membrane. This usually progresses at the rate of 1 mm or more per day from the site of the lesion. A low second-degree lesion recovers much more rapidly than a high lesion with second-degree compression. A second-degree brachial plexus injury often takes at least 18 months before the intrinsic muscles in the hand recover, and they often do not fully recover. The more proximal extrinsic muscles of the forearm recover function at about the ninth to the 12th month following a second-degree entrapment lesion of the brachial plexus.
Third-degree injury due to neural entrapment occurs most frequently when other mechanical factors affecting nerves, such as traction and friction, are superimposed on the compression neuropathologic process. In the third-degree lesion, there is increased fibrosis in and about the nerve fibers that causes further structural change and neural dysfunction. Nerves move with motion of the limb. If their mobility is restricted by adherence of the nerve about a joint, as, for example, at the site of a supracondylar fracture in which the ulnar nerve adheres to the posterior aspect of the distal humerus, movement of the joint without movement of the nerve can cause traction neuritis of the ulnar nerve, which, in turn, can produce a stovepipe appearance of the nerve. A markedly thickened ulnar nerve can be the source of chronic pain, even when it is intact and functioning.
The radial nerve and its major branches, the posterior interosseous nerve and the superficial radial nerve, are vulnerable to compression forces from the level of the lateral head of the triceps through the region of the elbow, proximal forearm, and even into the distal forearm. Depending on which branch of the nerve is involved at the elbow, either pure motor (posterior interosseous nerve) or sensory (superficial radial nerve) paralysis can occur; rarely, motor and sensory involvement can be due to a process in the proximal forearm affecting both branches rather than the radial nerve itself.
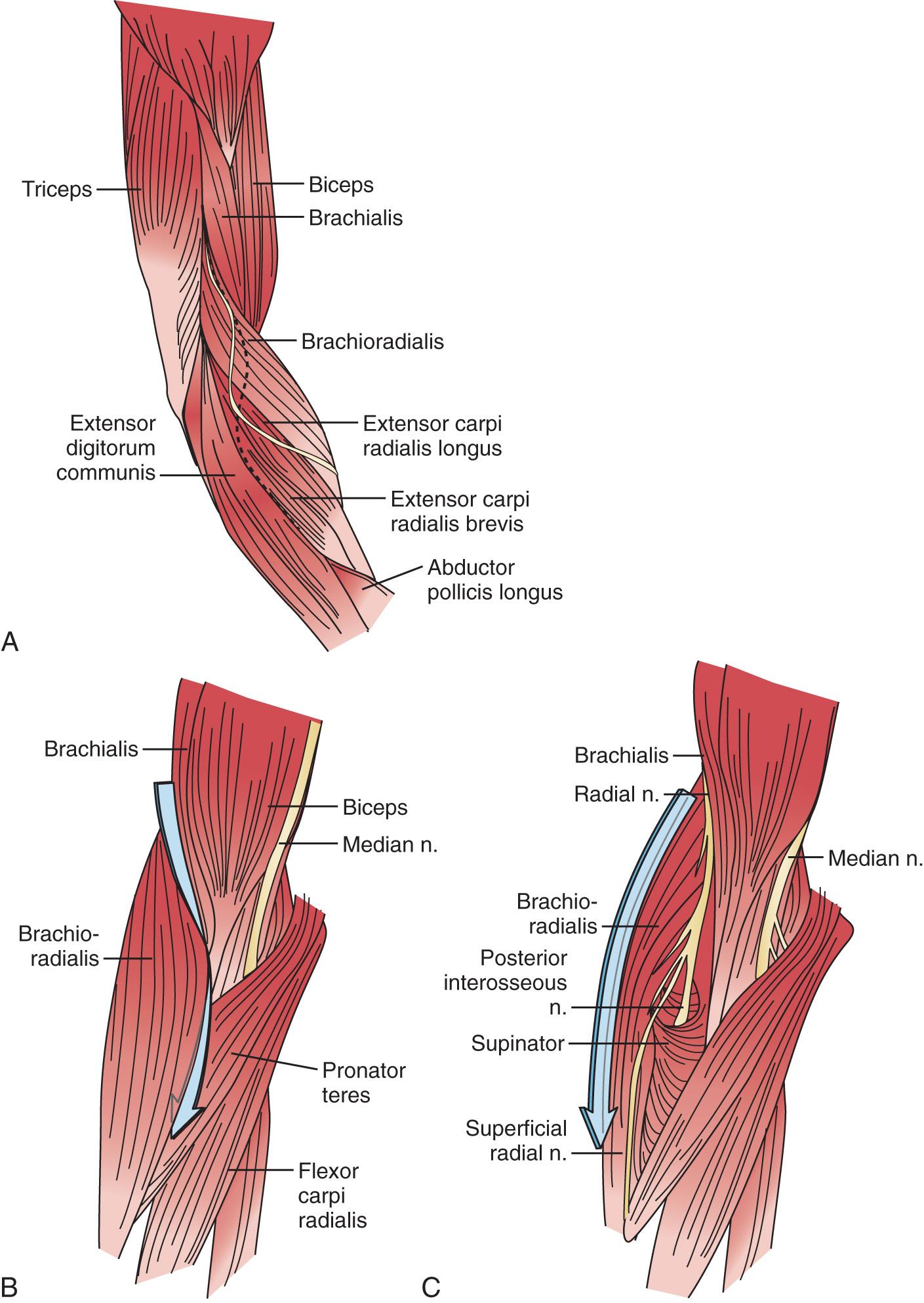
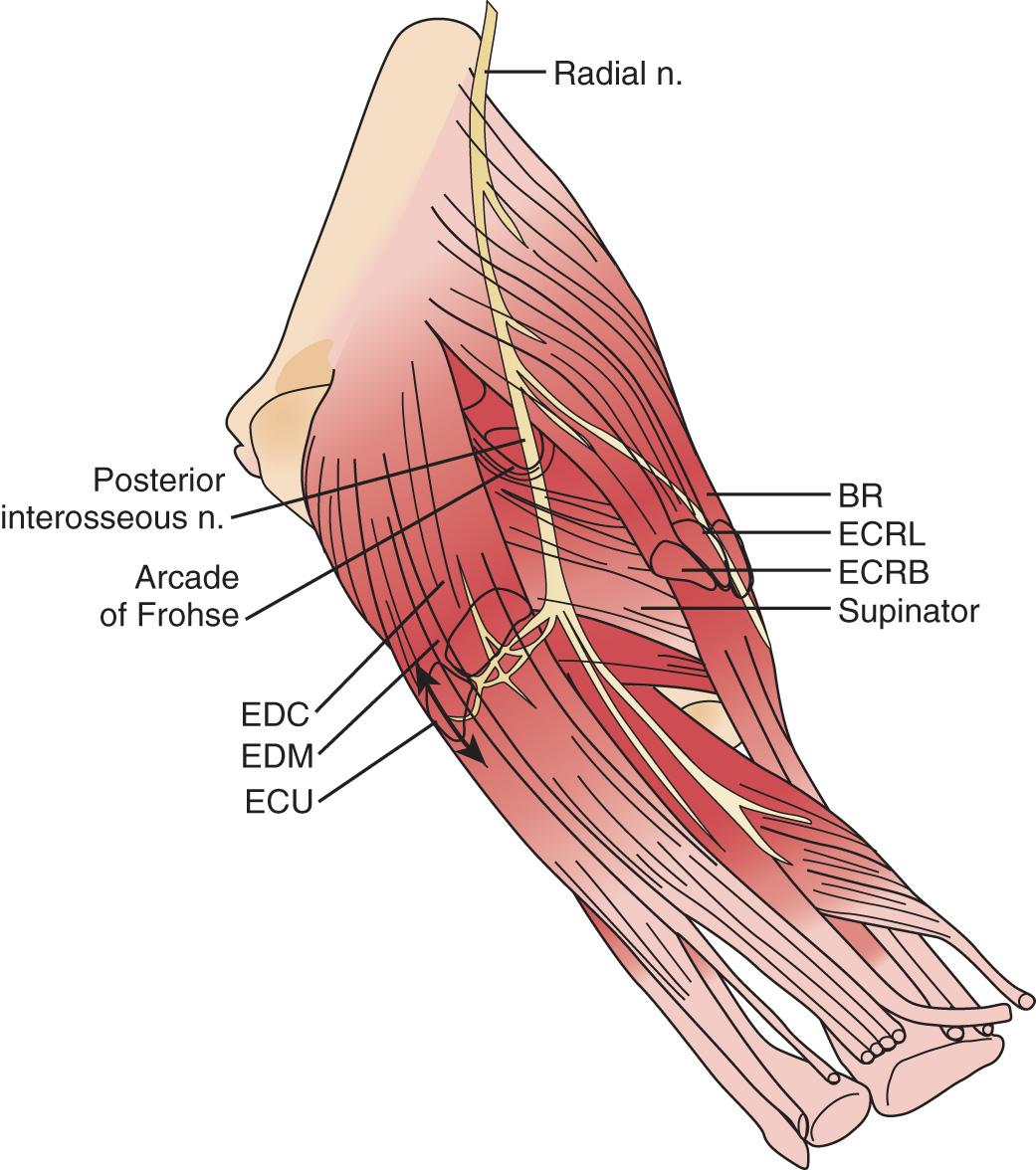
The radial nerve in the distal arm passes anteriorly, 10 cm proximal to the lateral epicondyle ( Fig. 72.2 ). At the level of the radiocapitellar joint, it divides into its major branches, the deep and the superficial radial nerves. In this passage, the radial nerve passes just deep to the fascia of the brachioradialis. Above the elbow, the radial nerve innervates the brachioradialis and the extensor carpi radialis longus. The motor branch to the extensor carpi radialis brevis arises from the superficial radial nerve in 58% of the population. It frequently arises as a separate terminal branch of the radial nerve with the posterior interosseous and superficial radial nerves.
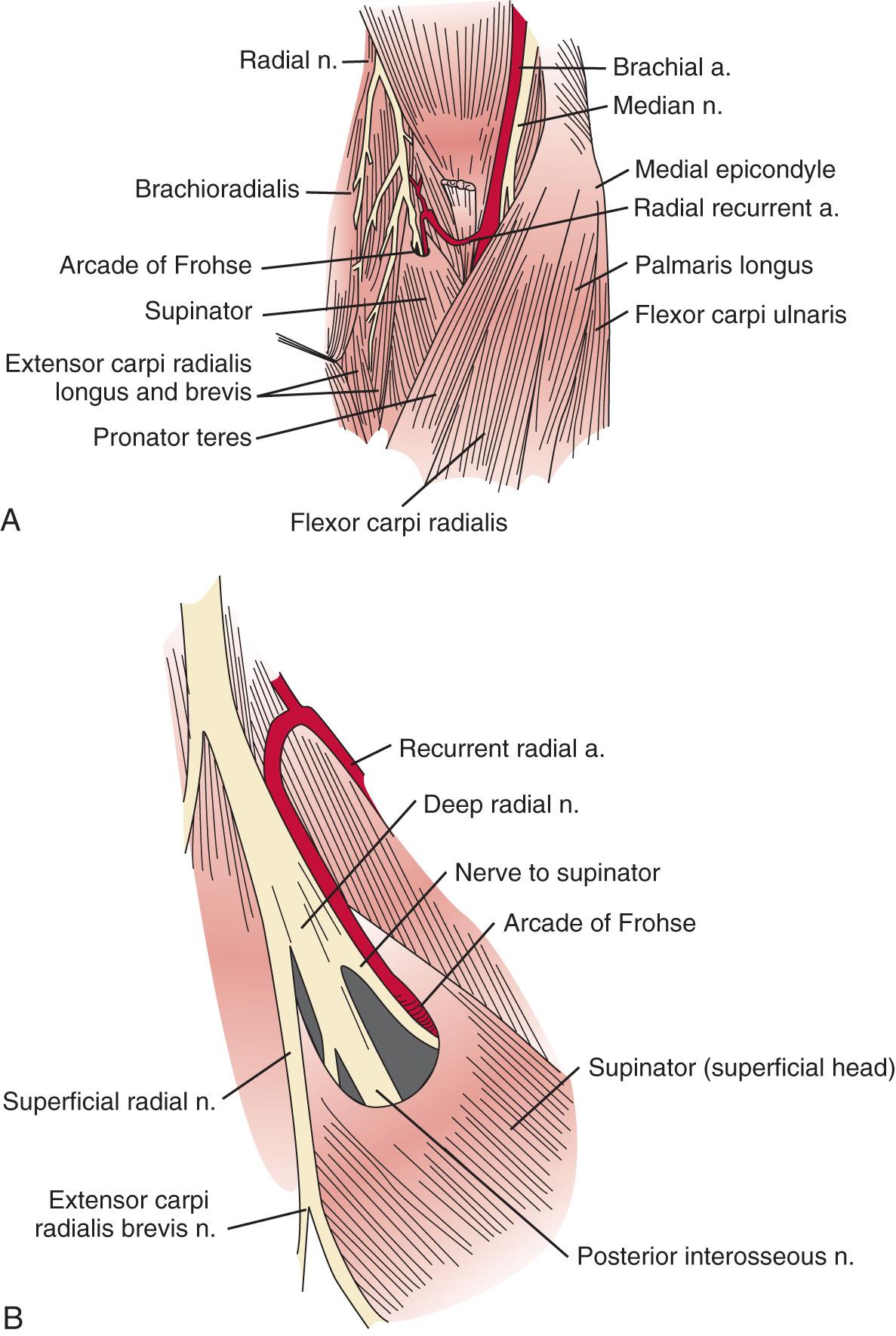
At the elbow, the deep branch passes between the two heads of the supinator muscle, where it becomes the posterior interosseous nerve. The proximal edge of the supinator forms an arch for the posterior interosseous nerve, the arcade of Frohse. The superficial radial nerve passes superficial to the supinator muscle. It is covered anteriorly by the brachioradialis. Recurrent vessels of the radial artery cross superficial and deep to these radial nerve branches. The posterior interosseous nerve courses in a dorsoradial direction in the proximal forearm. As it passes through the supinator, it innervates this muscle by multiple branches. Approximately 6 to 8 cm below the elbow joint, this nerve emerges from the supinator muscle, where it divides into its terminal motor branches to the extensor digitorum communis, extensor digiti minimi, extensor carpi ulnaris, abductor pollicis longus, extensor pollicis longus and brevis, and extensor indicis proprius.
In cases of a high bifurcation, the deep branch may be compressed at the lateral intermuscular septum, 4 cm proximal to the lateral epicondyle. There is focal tenderness and often wrist and finger extensor weakness. In these rare cases, the superficial radial nerve lies anterior to the lateral intermuscular septum.
The deep branch can be compressed by a fibrous band or thickened proliferating rheumatoid synovium from the radiocapitellar joint, the recurrent radial artery, or the leading tendinous edge of the extensor carpi radialis brevis. Next, the posterior interosseous nerve can be compressed at the arcade of Frohse, a
a References .
the most common site of compression. It may also be compressed by fibrotic bands within the midportion of the supinator and its distal end. Other causes of compression include adhesions at the anterior aspect of the distal humerus, muscular anomalies, vascular aberrations, bursae, inflammatory thickening, and adherence of the extensor carpi radialis brevis tendinous origin to the proximal edge of the supinator on its radial side. Some have identified focal hourglass-type constriction(s) within the posterior interosseous nerve. Posterior interosseous nerve palsy may result from fractures or fracture–dislocations ( Fig. 72.3 ). Tardy posterior interosseous nerve palsy may also occur years after unreduced Monteggia fracture–dislocations or after radial osteomyelitis ( Fig. 72.4 ). Tumors may compress the nerve primarily, which can be secondarily compressed by a structure such as the arcade of Frohse.
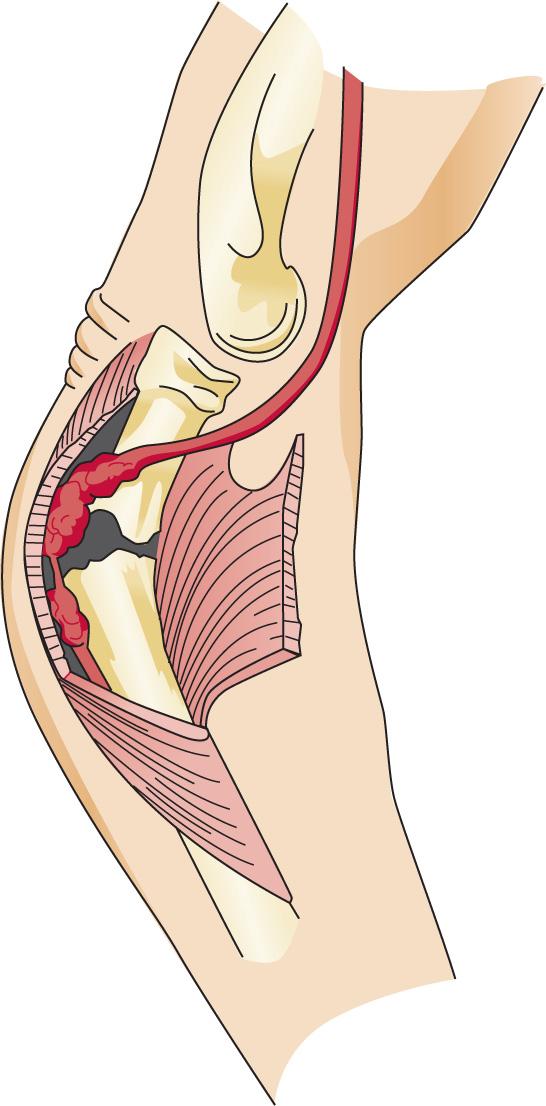
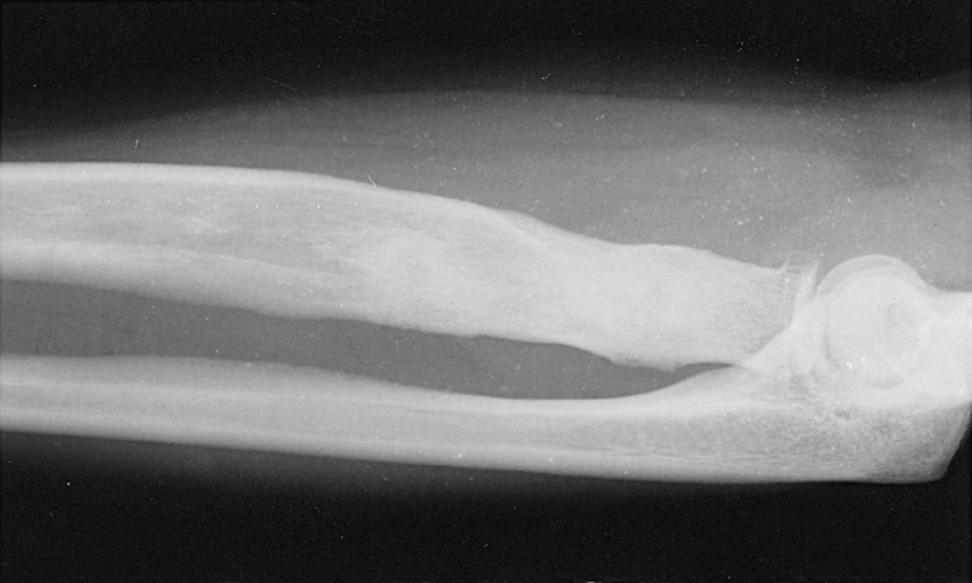
Classically, the clinical presentation of this nerve paralysis is thought to be typically motor because the posterior interosseous nerve basically carries motor fibers destined to innervate the extensor digitorum communis, extensor digiti minimi, extensor carpi ulnaris, abductor pollicis longus, extensor pollicis longus and brevis, and extensor indicis proprius. However, pain simulating lateral epicondylitis is also recognized as a common early presentation (see later discussion). Because of the segmental innervation of the supinator, the proximal or distal location of the compression of this nerve in the supinator can be determined by evaluating the electromyogram occasionally. Fibrillations in the supinator muscle suggest that the compression is proximal, at the arcade of Frohse. The pattern of involvement of this nerve varies depending on whether the entire nerve is compressed or whether there is a partial paralysis. When the entire posterior interosseous nerve is compressed, the fingers and thumb cannot extend at the metacarpophalangeal level, and the wrist deviates in a radial direction with wrist extension (because the branches to the extensor carpi radialis longus and brevis usually take off more proximally) ( Fig. 72.5 ). An untreated partial paralysis commonly evolves into a complete paralysis. Wrist drop signifies a lesion proximal to the posterior interosseous nerve branch ( Fig. 72.6 ).

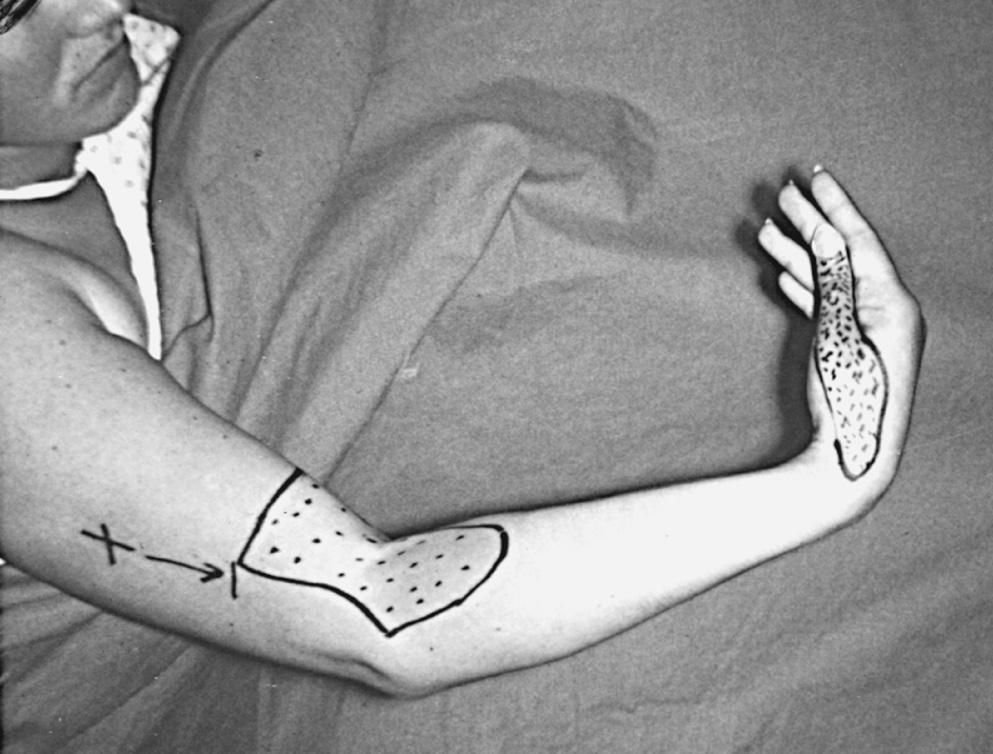
With partial paralysis, some of the digits, for example, the fourth and fifth fingers at the metacarpophalangeal joints, do not extend but the others do. This attitude looks like a “pseudoulnar” claw hand. In reality, there is no clawing but only a drop at the metacarpophalangeal joint and no true hyperextension of the metacarpophalangeal joints of the fourth and fifth fingers, as is present in a typical ulnar nerve palsy ( Fig. 72.7 ).
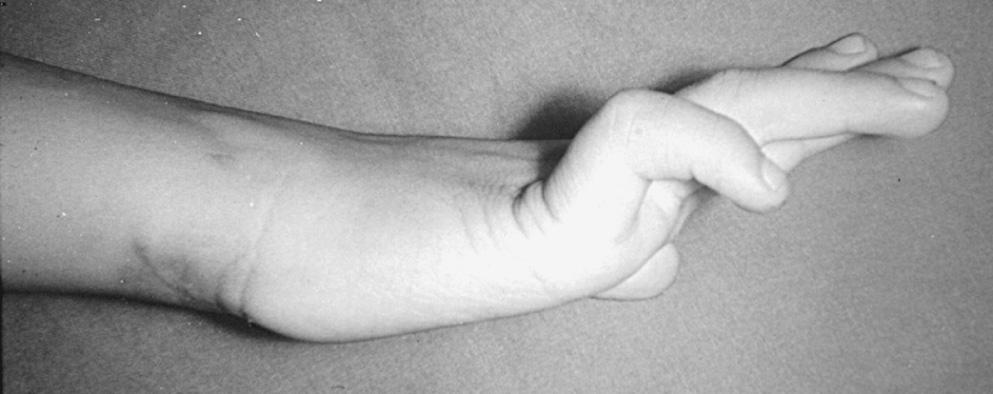
Understanding the branching pattern of the posterior interosseous nerve can assist in further localization of partial lesions. Different patterns of presentation have been described and localized. These include dropped finger (all) and thumb deformity; dropped long-ring, and little finger (and extensor carpi ulnaris) deformity; dropped thumb (abductor pollicis longus, extensor pollicis brevis and longus, and extensor indicis proprius) deformity; and dropped long and ring fingers only (“sign of the horns”). If surgery is entertained for incomplete lesions, the exit of the supinator may also need to be explored. In both partial and complete posterior interosseous nerve paralysis, sensation in the autonomous region on the dorsum of the first web space of the hand is uninvolved.
On occasion, isolated superficial radial nerve entrapment may occur in the elbow or proximal forearm region, but it is more commonly involved in the distal forearm or wrist. Isolated radial sensory paresthesias are usually secondary to irritations to the nerve in the region of the radial styloid. A compression neuropathy may occur in which this nerve penetrates the deep fascia in the midforearm between the brachioradialis and extensor carpi radialis longus. Focal tenderness usually identifies the involved site.
Because of the overlap of posterior interosseous nerve syndrome with many cases of Parsonage-Turner syndrome, EMG should examine other muscles (e.g., shoulder muscles) to identify a more diffuse neurologic process that would favor the diagnosis of an inflammatory disease. Imaging should be performed in cases of suspected nerve compression at unusual locations. Plain radiographs may be helpful in showing a fat stripe of a lipoma or a bony lesion in the vicinity of the radial neck (see Fig. 72.4 ). Ultrasound (US) or magnetic resonance imaging (MRI) may demonstrate an occult ganglion or elucidate a palpable mass by its imaging characteristics ( Fig. 72.8 ). US may reveal an enlarged nerve at the arcade of Frohse. MRI is helpful in demonstrating denervation atrophy and hyperintensity in the nerve, which may help confirm a diagnosis or localization of nerve compression, or both. Electrical studies typically demonstrate denervational changes in the muscles innervated by the posterior interosseous nerve. If there are no EMG abnormalities in the supinator, then one should have a suspicion that the compression lesion of the posterior interosseous nerve is at the distal end of this muscle rather than at its proximal end. The brachioradialis and the extensor carpi radialis longus and brevis should not reveal any abnormalities in the typical posterior interosseous nerve syndrome because these muscles are innervated by the radial nerve proximal to the arcade of Frohse.
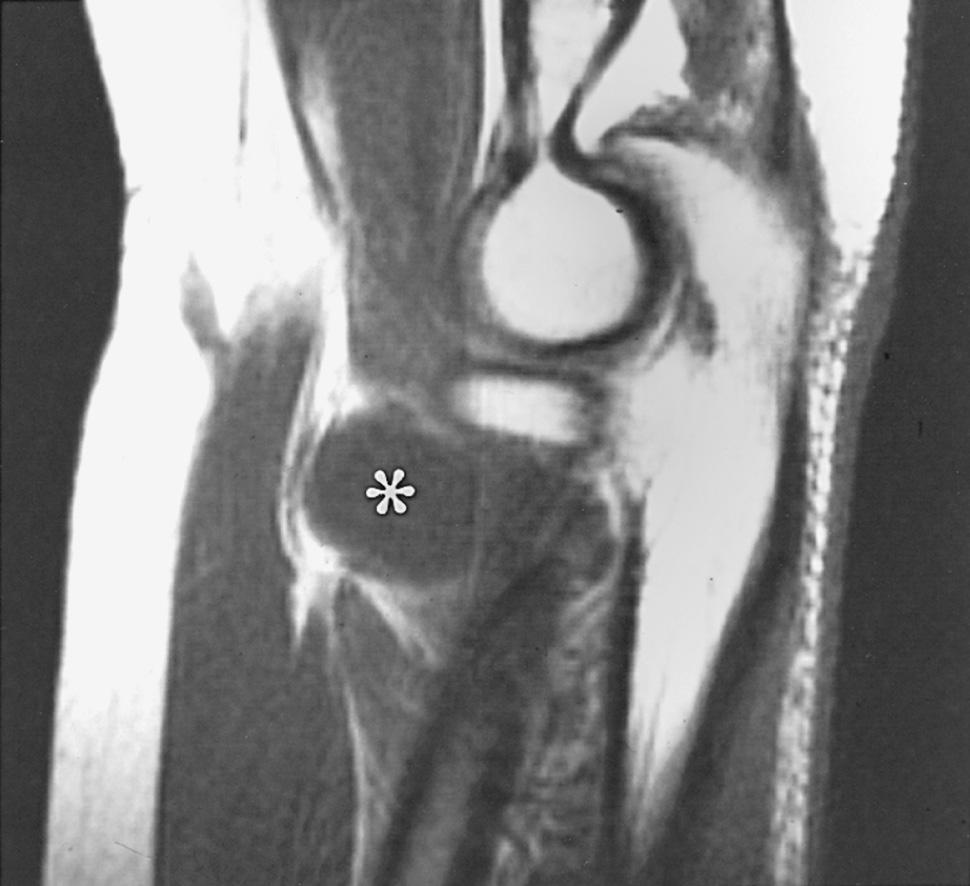
In the end, recovery following operative decompression in some patients with posterior interosseous nerve “entrapment” may well be due to a favorable natural history of Parsonage-Turner syndrome. I believe that Parsonage-Turner syndrome is underrecognized. For this reason, I recommend performing decompression after 6 months of observation in patients with spontaneous onset of symptoms in whom mass lesions are not discovered and there has not been any clinical recovery.
For the most part, resistant tennis elbow is caused by degeneration or fascial tears at the lateral epicondyle. On occasion, persistent complaints have been attributed to either compression of the posterior interosseous nerve or to a combination of nerve compression and persistent localized epicondylitis. Resistant pain localized to the proximal forearm should suggest that entrapment of the adjacent posterior interosseous nerve may be an unrecognized factor.
Physical findings frequently reveal tenderness, both over the lateral epicondyle and anteriorly over the course of the nerve as it passes through both heads of the supinator. On occasion, pain can be localized to the distal end of the supinator posteriorly at the junction of the middle and upper thirds of the proximal forearm. Pain may be increased with resisted active supination of the forearm or with resisted extension of the long finger with the elbow extended. There are no sensory abnormalities in the hand.
EMG studies in cases of resistant tennis elbow due to entrapment of the posterior interosseous nerve are often normal, even if the condition has been present for months and with definite clinical findings. Conduction delays are observed rarely. Stress testing as described by Werner has sometimes been helpful in confirming the diagnosis. Fibrillations in the muscles innervated by the posterior interosseous nerve are usually sparse, but if present, they are most likely in the extensor indicis proprius. If fibrillations are widespread in the more severe lesions, weakness of the finger extensors and extensor carpi ulnaris is also usually evident.
Patients suspected of having coexisting lateral epicondylitis and posterior interosseous nerve compression, who fail conservative treatment, should have both conditions treated simultaneously. Patients with persistent pain after surgery for lateral epicondylitis should be suspected of having posterior interosseous nerve compression.
When exposure of the entire posterior interosseous nerve is needed, the plane between the extensor carpi radialis brevis and the extensor digitorum communis ( Fig. 72.9 ) is developed. The incision begins 5 cm proximal to the lateral epicondyle and passes over the lateral epicondyle down to the region of the origin of the outcropping muscles (abductor pollicis longus, extensors pollicis longus and brevis). The aponeurotic plane between the extensor carpi radialis brevis and the extensor digitorum communis is developed from distal to proximal ( Fig. 72.10 ). Identification of the plane is facilitated by passive motion of the fingers while the wrist is held steady, and the plane can be developed by blunt dissection. The supinator muscle is seen in the depth of the wound as these muscles are liberated. To gain complete exposure to the proximal end of the supinator, the extensor carpi radialis brevis tendon can be detached from its origin at the lateral epicondyle. At times, the distal portion of the origin of the extensor carpi radialis longus is detached, if necessary, for complete exposure of the underlying arcade of Frohse. This is facilitated with elbow flexion. Adherence of the tendinous origin of this muscle to the lateral portion of the supinator muscle is frequently found and is freed to give exposure to the proximal end of the supinator. One can identify the posterior interosseous nerve by flexing the elbow and by palpating the nerve's course as it passes obliquely through the supinator in a dorsoradial direction. By gently spreading longitudinally through the fat on both sides of the nerve with a right-angled hemostat, the nerve can be isolated. Because there are recurrent vessels in the vicinity, dissection must be gentle. Any vessels crossing the nerve should be clamped and tied individually. When the posterior interosseous nerve is identified proximal to the arcade of Frohse, a vasoloop is passed around the nerve so that its identity and continuity are maintained. The arcade of Frohse may be found to be thickened. A hemostat is placed deep to the arcade but superficial to the nerve, and the arcade is incised, liberating the most proximal portion of the nerve. If further surgery is necessary, the entire posterior interosseous nerve can be traced and brought into direct view. Compression of the proximal and distal region has been described, as well as compression of the nerve in its midportion. Epineurotomy of the posterior interosseous nerve at the site of its compression on occasion may be deemed necessary. Microsurgical technique should be used when this is indicated.
The detailed anatomy of the nerve supply to the extensor digitorum communis is important because this muscle obtains its innervation from branches of the terminal portion of the posterior interosseous nerve that run at right angles to the plane of the forearm in the distal portion of the proximal third of the forearm (see Fig. 72.10 ). The operating surgeon should not sweep the planes between the extensor digitorum communis and the supinator because these branches are vulnerable. Furthermore, strong retraction posteriorly of the extensor digitorum communis in this area could damage the nerve supply to this important muscle.
The tendinous origin of the extensor carpi radialis brevis tendon is not reattached. Increasingly, it has been recognized that the thick tendon of origin of this muscle in its distal prolongation passes directly over the arcade of Frohse. The combination of frictional and compressive irritation from this structure may be a major factor in the development of the radial tunnel syndrome. For this reason, a 3- to 4-cm portion of its most proximal tendinous origin may be excised. The tourniquet is released, and hemostasis is obtained. If the patient had lateral epicondylar pain and tenderness preoperatively, then at this time, the lateral epicondyle can be drilled or a small portion excised. The skin is closed, and the arm is immobilized in long arm plaster splints with the elbow at 90 degrees, the forearm in midposition, and the wrist in a functional position. The immobilization is continued for 2 weeks, and the limb is gradually mobilized.
If a limited approach to the proximal portion of the supinator is needed, I prefer an anterior exposure of the posterior interosseous nerve. The lesion localized to the arcade of Frohse is approached by developing the plane proximal to the elbow between the brachialis and brachioradialis (see Fig. 72.9 ). Distal to the elbow, the anatomic dissection is continued, and the plane between the brachioradialis and the pronator teres is developed. If the dissection is difficult because of scarring or muscle anomalies, the superficial radial nerve can be identified distally and traced proximally to the main radial nerve and then to the deep branch. Any obstructing collateral vessels are ligated. The proximal third of the posterior interosseous nerve can be best visualized with this exposure. If necessary, the rest of the nerve can be followed by a separate posterior approach.
A longitudinal transmuscular approach through the brachioradialis has been popularized by Lister and associates. It provides direct access to the nerve from the radiohumeral level to the midsupinator ( Fig. 72.11 ).
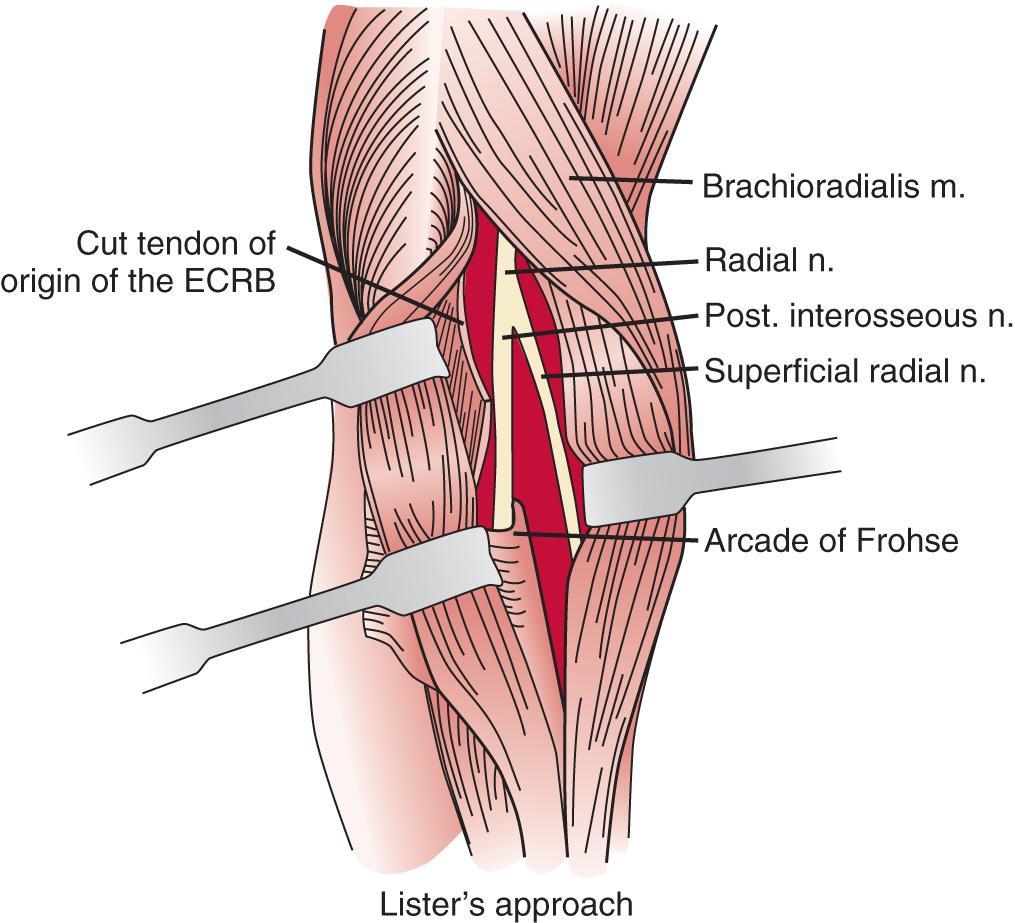
When there has been prior surgery in the region, an anterior and separate posterior incision may be necessary to expose the radial nerve and its branches safely.
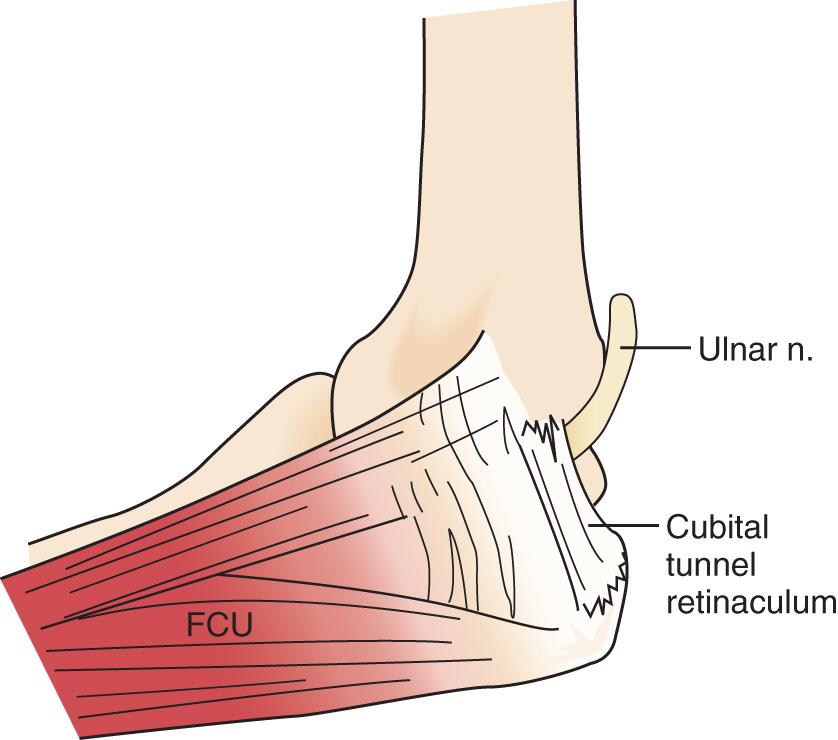
At the elbow, the ulnar nerve passes posterior to the medial epicondyle through the cubital tunnel. The cubital tunnel retinaculum ( Fig. 72.12 ) seems to be the predominant site of pathology for patients with primary symptoms. In the proximal arm, the nerve descends in the anterior compartment. In the majority of upper extremities, the ulnar nerve crosses from the anterior to the posterior compartment in the distal arm. The anatomy of the region (about 8 cm proximal to the medial epicondyle), corresponding to the so-called arcade of Struthers, is controversial. The ulnar nerve, similarly, passes from posterior to the medial epicondyle to the anterior compartment of the forearm a few centimeters distal to the medial epicondyle and the cubital tunnel.
In the arm, there usually are no branches of the ulnar nerve of significance. Occasionally there is a variant high take-off of a motor branch to the flexor carpi ulnaris in the distal arm. The dorsal cutaneous nerve of the forearm, the sensory branch to the dorsoulnar aspect of the hand, rarely has been observed to arise in the proximal rather than the distal forearm. At the elbow level, the first branch is usually an expendable articular branch that arises just distal to the medial epicondyle; next are usually varying branches of the flexor carpi ulnaris and the motor branch to the fourth and fifth flexor digitorum profundus muscles. Stimulation of these branches can help the physician in deciphering whether the branch is a motor branch to an end organ or an articular branch. In addition, fascicular mobilization of these branches can be performed safely for a distance up to 6 cm to facilitate ulnar nerve transposition.
Ulnar nerve compression lesions may be due to many factors. b
b References .
At the elbow level, spontaneous compression neuritis is well known as the cubital tunnel syndrome. It is second only to carpal tunnel syndrome in its frequency.
Ulnar nerve lesions may be due to compression, stretch, traction, friction, or a combination of these. Direct pressure on the posterior aspect of the elbow can compress the nerve and is seen in patients following coma, in surgical cases, or even in those who use wheelchairs. Flexion of the elbow may exacerbate symptoms because it causes tightening and narrowing of the cubital tunnel and traction-related deformation of the nerve. The tendinous origin of the flexor carpi ulnaris can compress this nerve between its ulnar and humeral heads with elbow flexion. Extrinsic pressure on the nerve may result from the anconeus epitrochlearis, a variant muscle crossing the ulnar nerve in the region of the medial epicondyle, or from adhesions. Tumors, such as ganglia, may also be a causative factor.
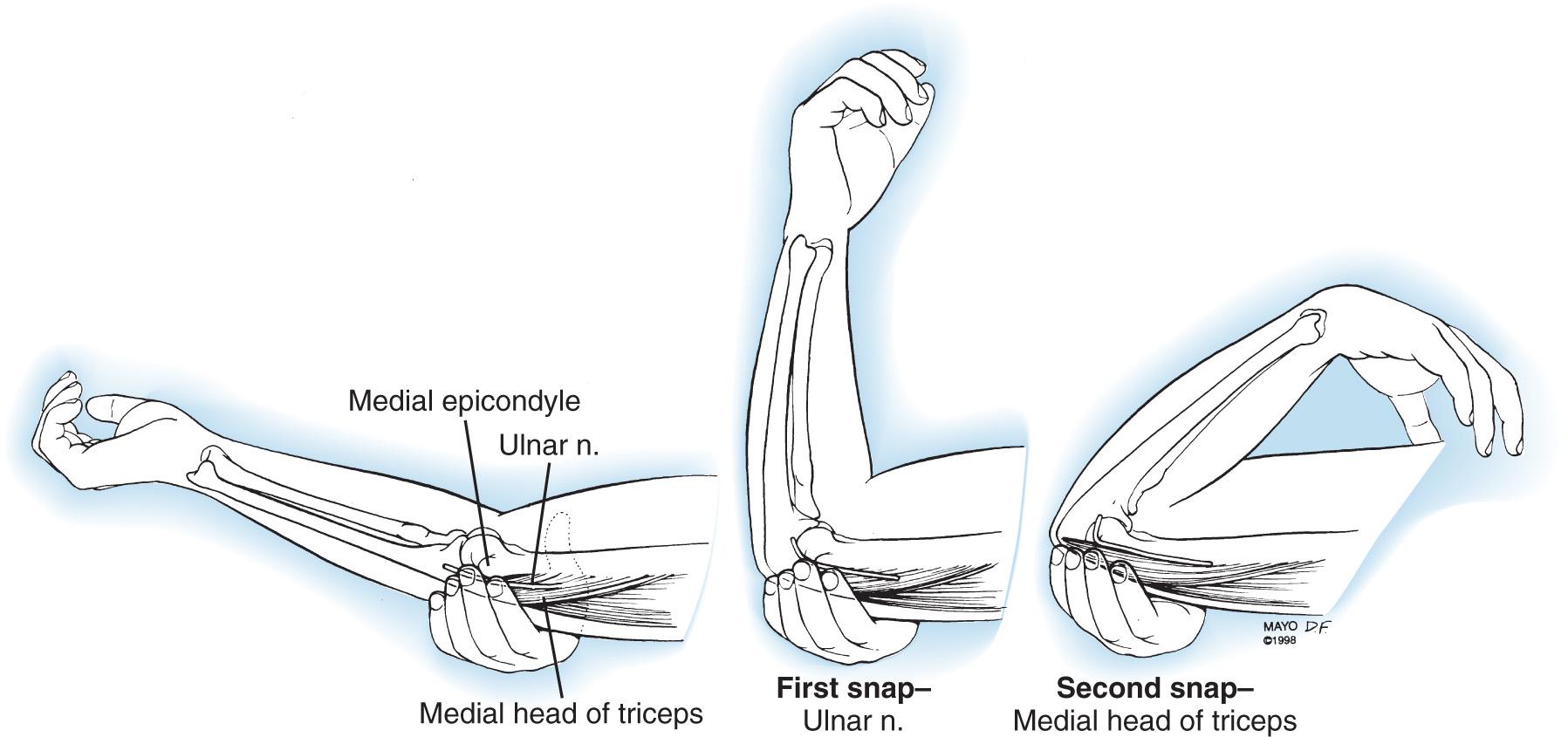
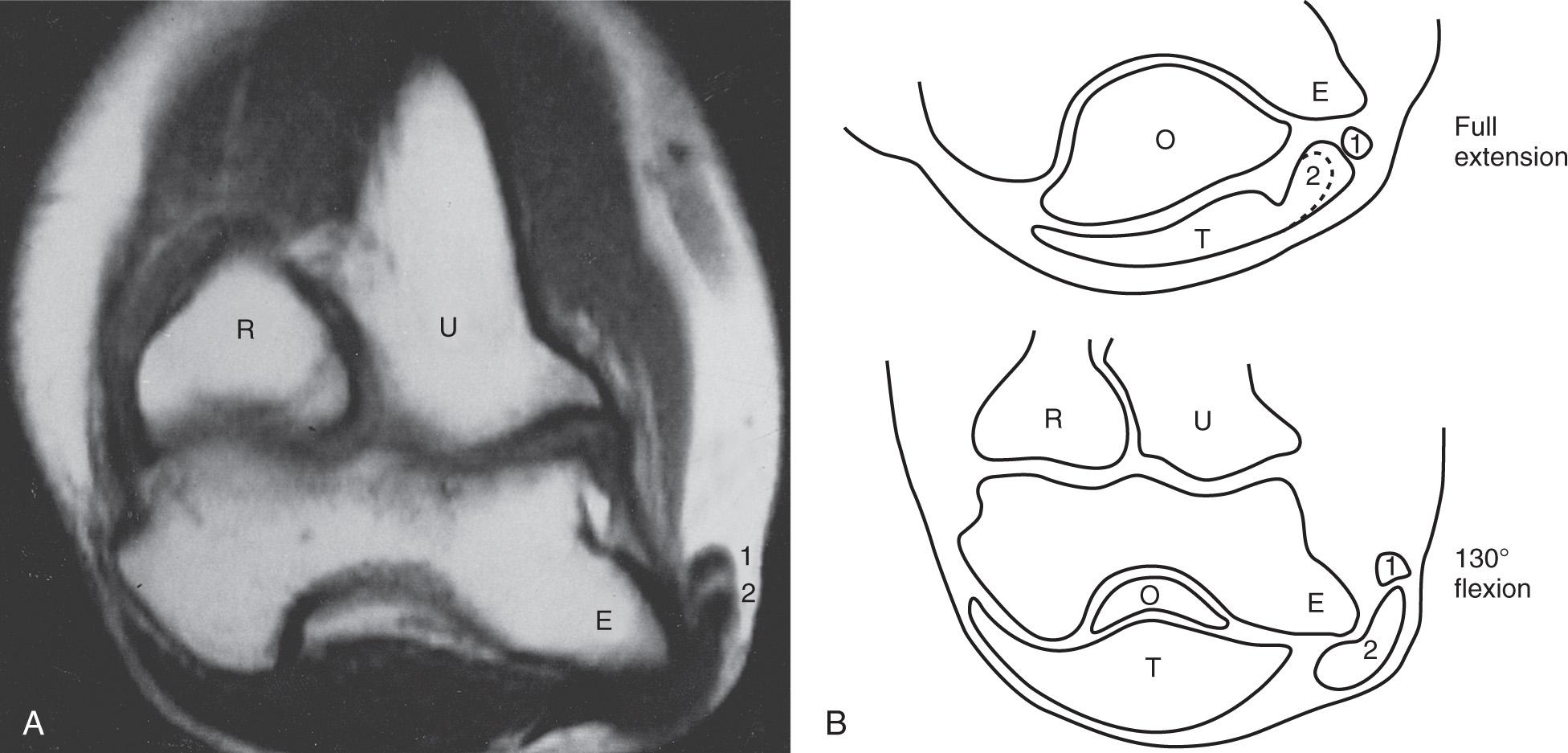
A hypermobile ulnar nerve can produce symptoms. This usually occurs during elbow flexion, as the nerve dislocates from the undersurface of the medial epicondyle to a position anterior to the epicondyle. Snapping of the medial triceps may be found in association with a dislocating ulnar nerve, and this can result in elbow pain, snapping, and ulnar nerve symptoms ( Figs. 72.13 and 72.14 ). Persistent pain after an otherwise successful ulnar nerve transposition may represent unrecognized snapping of the triceps.
Bony changes at the elbow, whether acute or chronic, can result in ulnar nerve symptoms. Fracture–dislocations, medial epicondylar fractures, arthritic changes from osteoarthritis or rheumatoid arthritis, callus, heterotopic bone, and spurs have been implicated. Both cubitus valgus and varus deformities may produce late ulnar nerve symptoms at the elbow.
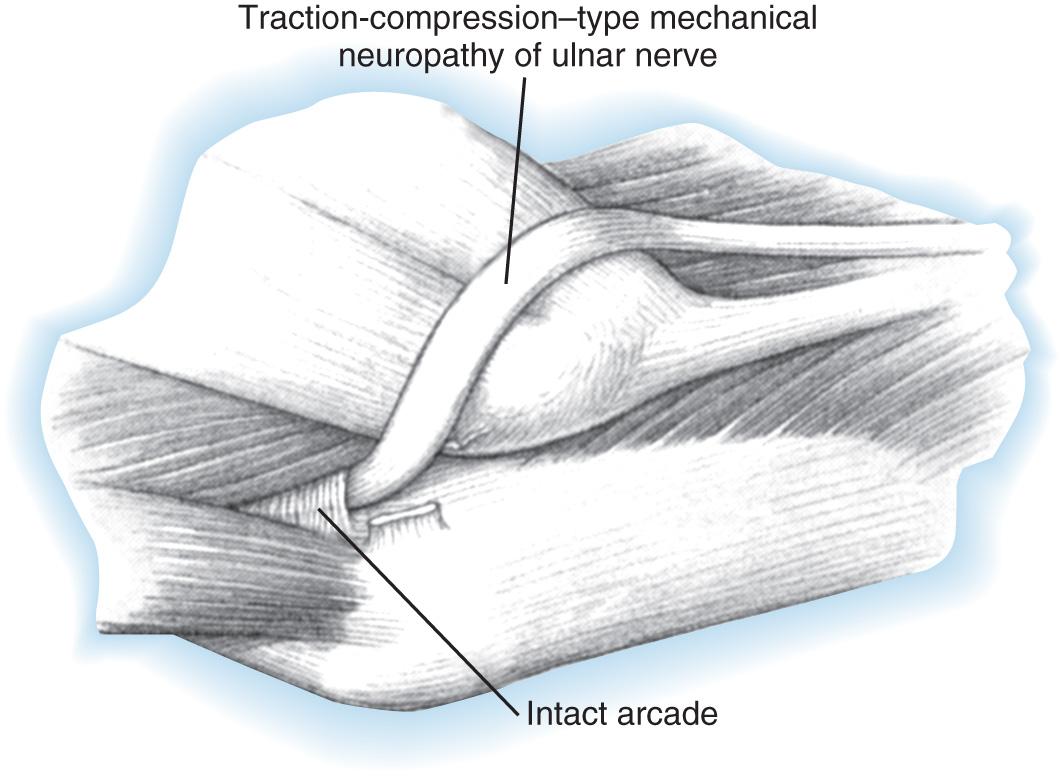
Iatrogenic causes of secondary ulnar nerve compression are numerous and related to technical factors. Compression may occur when the ulnar nerve is transposed anteriorly and is insufficiently mobilized, proximally or distally. After a previous transposition, secondary compression can be found proximally at the level of the so-called arcade of Struthers or distally where the ulnar nerve passes in the region of the common aponeurosis for the humeral head of the flexor carpi ulnaris and the origin of the flexor digitorum superficialis. If these aponeurotic areas are not released sufficiently both proximally and distally ( Fig. 72.15 ), then potential secondary sites of entrapment are created, which can produce symptoms. The medial intermuscular septum should be excised because it is also a common cause of secondary ulnar nerve entrapment. When the ulnar nerve is transposed, it should be transposed anteriorly without kinking. Tight slings used to maintain the nerve in an anterior position may result in secondary compression. Furthermore, traction neuritis can result when the nerve is transposed into a groove in the flexor-pronator group of muscles. When the nerve heals in the muscular groove, the longitudinal fibrotic aponeuroses of flexor muscles of the medial aspect of the elbow can produce secondary traction neuritis.
A patient with an ulnar nerve lesion at the elbow typically presents with a combination of elbow pain and sensory and motor complaints. The clinical presentation usually begins with intermittent paresthesias in the ring and little fingers that are aggravated by elbow flexion and frequently awaken the patient. Sensory loss in the ring and little fingers of the hand usually occurs later, but sensory loss in the dorsoulnar aspect of the hand is a classic localizing sign. Usually, there are no sensory abnormalities in the forearm. The sensory fibers and the intrinsic motor fibers lie more peripherally than the fibers of the flexor digitorum profundus or the flexor carpi ulnaris and may explain their vulnerability early on. Motor weakness may be progressive in both the extrinsics and the intrinsics; at times, significant motor findings can be present with minimal sensory symptoms. With paralysis of the flexor digitorum profundus to the ring and little fingers, there is usually minimal clawing or no clawing of the ring and little fingers. With partial lesions, clawing may be more pronounced if the flexor digitorum profundus muscles are intact and the intrinsic muscles are atrophic. However, if the metacarpophalangeal joints of the ring and little fingers cannot hyperextend because of innate tightness of the volar plates, then clawing will also not be observed.
A mechanical lesion of the ulnar nerve at the elbow may present with different clinical patterns in different patients because of the presence or absence of neural anomalies and the extent of involvement of the nerve. There are numerous variations in fibers carried within the ulnar nerve at the elbow level. In 15% of upper extremities, the median nerve will carry many of the intrinsic motor fibers to pass from the median nerve or the anterior interosseous nerve branch of the median nerve to the ulnar nerve in the midforearm.
The sensory pattern typical of an ulnar nerve lesion at the elbow with diminished or absent sensation on the dorsoulnar aspect of the hand may not be observed. This may occur when other sensory nerves take over the area usually supplied by the dorsal cutaneous branch of the forearm. One variant sensory pattern is observed when the superficial radial nerve not only innervates the dorsal radial aspect of the hand but also extends to supply the dorsoulnar aspect. Furthermore, sensation in all of the ring and middle fingers can be affected in some complete ulnar nerve lesions.
In the differential diagnosis, a nerve lesion that involves the cervical foramina, as in cervical arthritis, can present with ulnar nerve-type symptoms. Restriction and pain on movement of the neck, positive foraminal compression maneuvers, arthritic changes seen radiographically, and cervical paravertebral muscle electrical abnormalities are usually noted. Short segment stimulation may be effective in isolating the level of the compression to the ulnar nerve at the elbow. MRI may show focal pathology, such as foraminal stenosis or a disk herniation with spinal nerve compression.
Another frequent site for exclusion is the thoracic outlet. The medial components of the plexus (lower trunk, or C8 and T1) are most frequently involved. Radiation of paresthesias along the inner aspect of the arm with symptoms extending to the ring and little fingers is a common neural presentation. Clinical signs characteristic of thoracic outlet syndrome, including a positive percussion sign or a positive Adson or Wright test, hyperabduction maneuver, or the presence of an arterial bruit with shoulder abduction or extension, may help localize the pathologic process to the thoracic outlet, but one should also be aware of the presence of false-positive provocative maneuvers on physical examination in the normal population. EMG studies in patients with neurogenic (or true) thoracic outlet syndrome have abnormalities beyond the ulnar nerve territory, whereas those with thoracic outlet symptoms (or disputed thoracic outlet syndrome) have normal EMG studies. The absence of ulnar F-wave abnormalities and cervical paravertebral fibrillations and the presence of an ulnar nerve conduction delay distally across the elbow can be suggestive of findings of a lesion at the elbow. High-resolution MRI or US of the brachial plexus can be helpful in detecting subtleties (hyperintensity or enlarged lower trunk elements). On occasion, double crush lesions can occur, and patients who have persistent symptoms after elbow surgery may have a more proximal lesion.
Entrapment in the hand is much less common than entrapment at the elbow; entrapment in the forearm is even rarer. Depending on the level of nerve involvement, varying clinical signs and symptoms become manifest. In a full-blown lesion in the Guyon canal, there is usually more significant (“paradoxical”) clawing of these digits because the flexor digitorum profundus is functioning (see Fig. 72.7 ). The sensation on the dorsoulnar aspect of the hand is intact, whereas the palmar aspect of the hand may have some hypesthesia. Lesions of the ulnar nerve in the proximal forearm have findings similar to those at the elbow, whereas in the middle and distal forearm, symptoms depend on the relationship of the lesion to the motor branch of the flexor digitorum profundus and the dorsal cutaneous branch of the forearm. A lesion distal to the take-off of the motor branch of the flexor digitorum profundus is usually seen in patients with clawing. A lesion proximal to the dorsal cutaneous branch presents with numbness in the dorsoulnar aspect of the hand. EMG and high-resolution imaging (MRI or US) would also be useful to establish entrapment of the ulnar nerve at rarer sites.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here