Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Skin disorders characterized by infiltrative lesions can be present at birth or develop during the first few months of life. Some represent frank neoplasms, both benign and malignant, whereas others are the result of metabolic errors. In most instances, diagnosis is facilitated by skin biopsy, in which certain cell types can be identified by special stains and immunologic markers. Others require special enzyme assays for definitive diagnosis.
Congenital leukemia is a rare hematologic disorder. Less than 1% of all childhood leukemia is diagnosed in newborns. The majority of neonates have acute myeloid leukemia (AML), while acute lymphoblastic leukemia (ALL) is less common. The most frequently occurring chromosomal abnormality in both congenital AML and ALL is a translocation involving the mixed lineage leukemia (MLL) gene. To differentiate congenital leukemia from the several infectious and proliferative disorders that can easily mimic this condition, the following diagnostic criteria are employed:
The presence of immature white cells in the blood
Infiltration of these cells into extrahematopoietic tissues
The absence of diseases that can cause leukemoid reactions (such as erythroblastosis fetalis and a variety of congenital infections)
The absence of chromosomal disorders that are associated with ‘unstable’ hematopoiesis (such as trisomy 21).
The cutaneous manifestations of congenital leukemia consist of petechiae, ecchymoses, and skin nodules. Leukemia cutis is seen in approximately 60% of patients with congenital leukemia. The firm nodules are usually 1–2.5 cm in diameter and blue to purple in color ( Fig. 28.1 ). They are often widely spread over the skin surface, although congenital leukemia may present as a single nodular cutaneous lesion. Diffuse calcinosis cutis in an infant with congenital AML has been reported. Congenital AML may be manifested by ‘blueberry muffin’ lesions, and Darier's sign has been observed. The clinical signs and symptoms include hepatosplenomegaly, pallor, lethargy, and respiratory distress. Lymphadenopathy occurs in some infants.
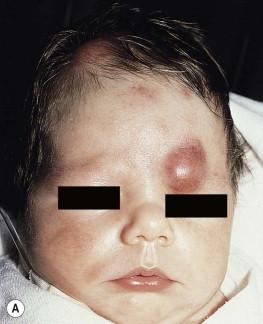
Biopsy of a cutaneous lesion reveals a dense pleomorphic mononuclear cell infiltrate in the dermis and subcutaneous fat. Atypical mitotic figures may be present. The diagnosis of congenital leukemia can usually be confirmed by a complete blood count, bone marrow aspirate, and radiographs of the skull and long bones. In every case, cytogenetic studies must be performed to exclude the possibility of transient myeloproliferative disorder, which is usually associated with trisomy 21. This disorder is also associated with a diffuse vesiculopustular eruption (see Chapter 10 ).
Congenital leukemia is often fatal, with overall survival ranging from 10–25%. Treatment consists of intensive multiagent chemotherapy. The benefit of hematopoietic stem cell transplantation remains uncertain. Spontaneous remission of congenital leukemia cutis, usually without involvement of blood or bone marrow, has been reported.
Leukemia occurring in infants under the age of 1 year bears many similarities to congenital and neonatal forms. Patients in this age group also have a high incidence of MLL rearrangements. Typical lesions include violaceous macules, nodules, and plaques. Patients with AML may present with larger purplish tumors, while those with ALL may develop numerous prurigo-like papules. The development of granulocytic sarcoma (formerly known as chloroma) in the periorbital skin of infants has also been reported.
Langerhans' cell histiocytosis (LCH), a rare proliferative disorder, may be present at birth or may develop during the first few months of life. The estimated incidence of neonatal LCH is 9/1 000 000 in infants <1 year of age, with disease presentation during the first month of life in about 6% of those affected. The spectrum of clinical presentations, and the clinical course, is extremely varied and ranges from the simple presence of one or several nodules, widespread crusted papules and vesicles, to severe, progressive multisystem disease.
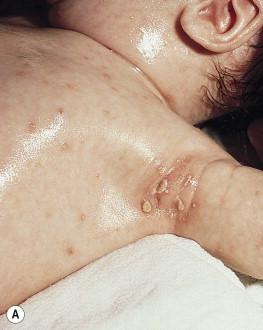
The most frequent cutaneous presentation – particularly in infants and newborns – consists of multiple widespread vesiculopustules with umbilication and a hemorrhagic crust. Lesions tend to favor the scalp, trunk, diaper area, and skin folds ( Fig. 28.2A ). They may begin as subtle brown or pink papules, and evolve into crusted or purpuric lesions ( Fig. 28.2B ). Papular lesions may coalesce into areas of superficial ulceration with oozing, especially in intertriginous areas. Characteristic lesions also include fissures behind the ears, and crusting and oozing of the external ear canals. The scalp is a frequent site of involvement, and the coalescence of crusted and scaling lesions may lead to partial alopecia. Presentation as a ‘blueberry muffin baby,’ with cutaneous hematopoiesis, has been reported. Nodules and petechiae, which may involve the palms and soles, are also observed. Oral lesions are relatively common, and can develop before skin lesions. They may appear as superficial ulcerations or erosions, but these are often associated with underlying alveolar bone disease. Other oral manifestations include gingival bleeding, facial swelling, and pain. Premature eruption and loss of teeth, and destruction of alveolar bone are characteristic. Involvement of the vulva and vagina may also occur. Nail involvement consists of subungual pustules, paronychia, onycholysis, and longitudinal grooving. Permanent nail dystrophy may result.
A multifocal congenital form of LCH, which has sometimes been called ‘Hashimoto–Pritzker disease’ is characterized by papulonodular and papulovesicular skin lesions. Cases presenting with a solitary nodule have also been reported. These solitary skin nodules are usually red-brown and often have a hemorrhagic quality, ulceration, or crusting. Most affected infants have lesions at the time of birth without evidence of other organ system involvement ( Figs 28.3 , 28.4 ). Skin lesions typically resolve spontaneously, without sequelae but localized areas of scar or atrophy can occur. However, skin relapse, bone disease, late-onset diabetes insipidus, and even progression to severe LCH has been reported in children who present with congenital skin lesions.
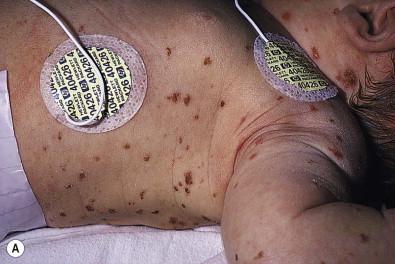
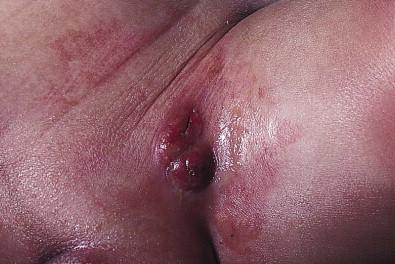
The majority of infants with all forms of congenital or neonatal Langerhans' cell histiocytosis will show evidence of multisystem disease. Bone lesions are the most frequent noncutaneous manifestation. Findings may include asymptomatic lytic lesions, deformation, fracture, or medullary compression. The skull is a common site, and disease in that location manifests on X-ray as punched-out lesions in the cranial vault. Mastoid involvement may lead to mastoid necrosis, and destruction of the ossicles may result in deafness. Intercranial disease may cause exophthalmos and diabetes insipidus. Lymph node involvement is seen in a significant percentage of patients, and tends to occur in the cervical chain. Pulmonary disease may be associated with cough and tachypnea. Other findings include bullae formation and diffuse interstitial fibrosis. Invasion of the liver may cause mild cholestasis, but eventually evolve to sclerosing cholangitis. Severe fibrosis of the liver results in ascites, jaundice, and liver failure. Involvement of the spleen may worsen the severity of thrombocytopenia. Gastrointestinal disease is reported to occur in a small percentage of patients. Characteristic findings are vomiting, diarrhea, protein-losing enteropathy, and failure to thrive secondary to malabsorption. The most common endocrine manifestation is diabetes insipidus. This occurs most often in children with extensive disease and involvement of the skull. Langerhans' cells may also infiltrate the thyroid and pancreas.
Biopsy of a cutaneous lesion reveals a diffuse infiltration of histiocytes with abundant eosinophilic cytoplasm and eccentric, indented nuclei. In some cases, the nuclei may appear pleomorphic or atypical. Positive CD1a and/or CD207 (Langerin) staining of the lesional cells is needed for a definitive diagnosis. Electron microscopy is no longer required since it is now known that the expression of Langerin correlates with the ultrastructural presence of Birbeck granules.
Preliminary laboratory evaluation of the patient with biopsy-proven skin lesions must include a complete blood count (CBC), serum electrolytes, urine specific gravity, and assessment of liver function. Examination of the bone marrow may show the presence of increased histiocytes. Skeletal survey, chest radiographs, and magnetic resonance imaging (MRI) may be used to determine the extent of bone, pulmonary, liver, spleen, and CNS involvement.
The pustular and intertriginous lesions of LCH in neonates and infants may be confused with candidiasis, seborrheic dermatitis, psoriasis and scabies. Congenital LCH must also be differentiated from other neoplastic disorders, such as leukemia and lymphoma; from congenital infections, especially herpes simplex; and from those viral disorders associated with ‘blueberry muffin’ lesions.
The majority of children with infantile LCH develop multisystem disease. The prognosis seems to be better in congenital ‘self-healing’ LCH, but multisystem disease is still possible, and such neonates must be followed to assure that they do not develop extracutaneous disease later on. Survival of infantile LCH in the past few decades has improved from 57% to 74%. Single-system disease has a significantly higher rate of survival (94%), but patients with initial liver involvement have a much poorer prognosis, with a 25% 5-year survival. In children with LCH limited to the skin (<5% of cases), a ‘wait and see’ approach should be accompanied by careful monitoring for disease progression. Children in this category may benefit from therapy with mild to moderate-strength topical corticosteroids. Children with multisystem disease are treated with chemotherapy; standard treatment is based on steroids and vinblastine.
Mycosis fungoides is extremely rare in young children, but a number of cases have been well documented. The patients presented with widespread and persistent scaly plaques. Several cases were initially misdiagnosed as either atopic dermatitis or tinea corporis, leading to a significant delay in diagnosis.
This form of cutaneous T-cell lymphoma is characterized by large tumor cells that express CD30 antigen. Clinical presentations consists of papules, and plaques which may ulcerate. The rare occurrence of this disease during the first 2 years of life has been documented.
Lymphomatoid papulosis is a chronic, recurrent disorder characterized by the presence of reddish brown papules and nodules. Progression to malignant forms of lymphoma is rarely reported. In one series of pediatric patients, including children under the age of 2 years, a benign course, accompanied by a predominance of CD8+ lymphocytes was observed.
Primary cutaneous B-cell lymphoma is extremely rare in infancy. Primary cutaneous lymphoblastic lymphoma, presenting with firm violaceous nodules with overlying telangiectasia, has been reported to occur in some children under the age of 2 years. Subcutaneous panniculitis-like T-cell lymphoma, with purplish tender nodules in an acral distribution, has also been reported to occur in infancy.
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening condition characterized by hepatosplenomegaly, cytopenias and prolonged fever. Central nervous system (CNS) symptoms are frequent. Some children have been noted to have an evanescent macular and papular skin eruption, sometimes associated with episodes of fever ( Fig. 28.5 ). Genetic HLH occurs in familial forms (FHLH), and in association with a variety of immune deficiencies. Acquired and genetic forms may both be triggered by infections.
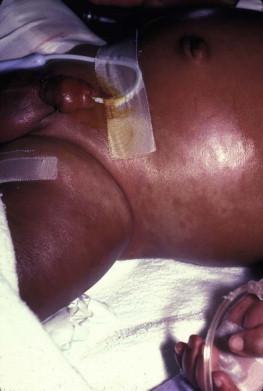
Diagnosis is made by the detection of a non-malignant mixed lymphohistiocytic proliferation in the reticuloendothelial system, with evidence of hemophagocytosis. Without treatment, FLH is usually rapidly fatal. The immediate goal of treatment is suppression of the increased inflammatory response by immunosuppressive/immunomodulatory agents and cytotoxic drugs. Genetic cases can only be cured with stem cell transplantation.
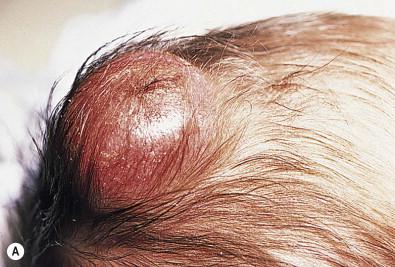
Congenital/infantile fibrosarcoma is a rare tumor that occurs most frequently on the extremities. Lesions are seen less commonly on the head, neck, and trunk, and may also occur in the retroperitoneum. The tumor may be present at birth, or may develop during early infancy.
Fibrosarcoma most often presents as a soft tissue mass, sometimes with rapid growth. The overlying skin may be tense, shiny, and erythematous, and ulceration may occur ( Fig. 28.6 ).
MRI is useful in defining the extent of the lesion, but the diagnosis is largely based on histology. Histologic examination reveals a highly cellular fibroblastic proliferation, with sizeable vascular clefts and occasional myxoid degeneration and hemorrhagic necrosis. Analysis of the biopsy sample for the recurrent translocation t(12;15)(p13;q25) is highly recommended.
Clinically, infantile fibrosarcomas are easily confused with either hemangiomas or vascular/lymphatic malformations. Transient consumptive coagulopathy mimicking Kasabach–Merritt phenomenon has been reported, causing confusion with other vascular tumors (see Chapters 21 and 22 ). The differential diagnosis also includes rhabdomyosarcoma and infantile myofibromatosis.
The risk of metastasis, especially in cutaneous lesions, is considerably lower in infants (approx. 8%) than in older patients. Treatment consists of wide local excision. Chemotherapy has been used in some patients to reduce the lesion mass preoperatively so as to avoid the need for mutilating surgery, and for lesions that are not resectable. Close follow-up to monitor for the presence of local recurrence and metastatic disease, is mandatory.
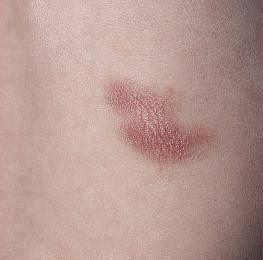
Dermatofibrosarcoma protuberans (DFSP) is a fibrohistiocytic tumor with low metastatic potential and a high incidence of local recurrence. Congenital DFSP is rare, but a number of cases have been reported. Another neoplastic disorder, giant cell fibroblastoma, can also present in early infancy ( Fig. 28.7 ). Some authors consider this to be a variant of DFSP; others consider it to be a separate entity, but believe that hybrids of giant cell fibroblastoma and DFSP may sometimes occur.
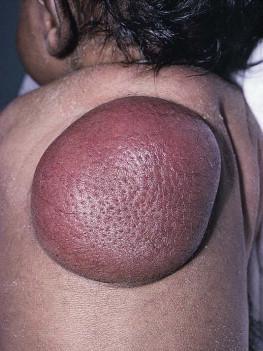
Characteristically, DFSP begins as an atrophic plaque surrounded by an area of bluish discoloration. Over time, as the cellular proliferation extends into the deep dermis and subcutaneous tissues, nodules develop on the plaque surface ( Fig. 28.8 ). Similar to the lesions seen in older patients, congenital DFSP occurs most commonly on the trunk and proximal extremities. Giant cell fibroblastoma more typically presents during infancy as a slow-growing soft-tissue mass.
Histologically, DFSP is characterized by the presence of spindle cells in a well-defined and uniform storiform pattern. Focal myxoid change and scarring may occur. Tumor cells express vimentin, but are negative for S100 protein, epithelial membrane antigen, and smooth muscle actin. Diagnosis can be facilitated by detection of the collagen type Iα1/platelet-derived growth factor β-chain fusion gene by means of reverse transcriptase–polymerase chain reaction or fluorescence in situ hybridization.
Clinically, the differential diagnosis of congenital DFSP includes fibrous hamartoma of infancy, infantile myofibromatosis, lipoblastoma (see Chapter 27 ), and vascular tumors and malformations. Neurofibroma and fibrous histiocytoma may mimic DFSP histologically.
Because of the high risk of recurrence, adequate surgical margins must be obtained. Mohs micrographic surgery is the preferred approach in older children. However, in young infants, this can be difficult to arrange logistically in a child requiring general anesthesia for the excision, so wide excision is generally performed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here